Abstract
Long-term potentiation (LTP) of unitary EPSPs, generated by pairs of monosynaptically connected CA3 and CA1 pyramidal cells, was compared with LTP of extracellularly evoked, multi-unitary EPSPs in rat hippocampal slice cultures. LTP was induced by repeated, synchronous pairing of low-frequency presynaptic and postsynaptic activity. Three differences were observed. First, LTP of multi-unitary EPSPs displayed two phases: transient (<5 min) and sustained. Potentiation of unitary EPSPs displayed both phases in 42% of experiments; the remainder showed sustained potentiation only. Unitary EPSPs displaying transient-sustained and only sustained potentiation could be recorded from single postsynaptic cells, indicating that excitatory synapses on a given cell are heterogeneous with respect to short-term plasticity. Second, whereas LTP of multi-unitary EPSPs never resulted in greater than twofold increases in amplitude (mean potentiation of 175% of control), maximal LTP of unitary EPSPs was as great as 13-fold (mean potentiation of 250%). Third, LTP could not be induced in 24% of unitary EPSPs. We provide here the first evidence for the coexistence of potentiatable and nonpotentiatable synapses on individual postsynaptic neurons. Thirty-seven percent of connections not displaying LTP exhibited long-term depression (LTD), suggesting that the connections were already maximally potentiated. In the remaining 63% of these pairs, neither LTP nor LTD could be induced, despite the existence of a pharmacologically identified, NMDA receptor-mediated EPSP component. In conclusion, there is considerable heterogeneity in the amplitude and time course of LTP expression at different synaptic connections. A substantial proportion of apparently nonplastic synapses probably accounts for the weaker potentiation displayed by compound EPSPs.
Keywords: excitatory synapse, hippocampus, long-term potentiation, long-term depression, synaptic plasticity
Associational learning is thought to result from use-dependent increases in the strength of the synaptic connections between cells encoding different aspects of the relevant stimuli, as proposed by Hebb (1949). Long-term potentiation (LTP) has provided an important experimental model of the cellular changes underlying associative increases in synaptic strength. LTP of synapses between hippocampal pyramidal cells is induced when a depolarization of a postsynaptic cell is repeatedly coincident with activity in a presynaptic cell. The simultaneous depolarization of the postsynaptic cell and activation of synaptic NMDA receptors allows Ca2+ to enter the postsynaptic cell and activate the biochemical pathways ultimately responsible for increased synaptic efficacy (Bliss and Collingridge, 1993; Sanes and Lichtman, 1999).
LTP has been studied extensively in acutely prepared ex vivohippocampal slices, primarily using extracellular stimulation to activate large numbers of synapses. These studies have revealed that induction of LTP results in an average potentiation of 150–200% of the control EPSP amplitude (Sastry et al., 1986; Gustafsson et al., 1987; Bliss and Collingridge, 1993). More recently, it has become possible to study the plasticity of single or very few synapsesa by using so-called minimal stimulation techniques (Isaac et al., 1996; Dobrunz and Stevens, 1997) or by recording from pairs of monosynaptically coupled cells (Debanne et al., 1996a, 1998). These studies have raised the prospect that not all synapses or connections display LTP (Petersen et al., 1998) and that the magnitude of LTP of unitary EPSPs can be remarkably large (Malinow, 1991).
In the present study, we have taken advantage of the ease with which pairs of monosynaptically coupled cell pairs may be recorded in organotypic hippocampal slice cultures, and our previous characterization of the induction of synaptic plasticity at these connections (Debanne et al., 1995, 1996a, 1998), to ask the following questions. How does LTP of unitary EPSPs compare with LTP of more conventionally studied extracellularly evoked EPSPs? Are the time course and magnitude of LTP the same for all synapses? Are all synapses capable of expressing LTP? Is there evidence for coexistence of potentiatable and nonpotentiatable synapses on individual pyramidal cells? Our results indicate that there is a considerable heterogeneity in the plasticity displayed at the connections formed by single presynaptic and postsynaptic hippocampal pyramidal cells.
MATERIALS AND METHODS
Slice culture preparation. Hippocampal slice cultures were prepared and maintained as described previously (Gähwiler, 1981). In brief, hippocampi were dissected from 5- to 7-d-old rat pups. Slices were cut with a tissue chopper at 400 μm and attached to glass coverslips in a chicken plasma clot. The coverslips and slices were placed in individual sealed test tubes containing semisynthetic medium and maintained on a roller drum in an incubator for 2–4 weeks.
Electrophysiology. For electrophysiological recordings, cultures were transferred to a recording chamber mounted on an inverted microscope and continuously superfused with a warmed (32°C) saline containing (in mm): Na+ 149, Cl−149, K+ 2.7, Ca2+ 2.8, Mg2+ 2.0, HCO3− 11.6, H2PO4−0.4, glucose 5.6, and Phenol red 10 mg/l, at pH 7.4. Presynaptic and postsynaptic cells were impaled in stratum pyramidale using sharp microelectrodes filled with 1 m potassium methylsulphate (40–70 MΩ), and their membrane potential was amplified 100× (Axoclamp-2A; Axon Instruments, Foster City, CA). EPSPs were recorded from CA3 or CA1 pyramidal cells in current-clamp mode. Pyramidal cells were identified electrophysiologically, as described previously (Debanne et al., 1995). The criteria for establishing that EPSPs between cell pairs were monosynaptic included relatively short and invariant onset latencies and the ability to follow brief high-frequency stimulus trains, as described in detail previously (Debanne et al., 1995).
Single action potentials were elicited in the presynaptic cell with short depolarizing current pulses (10–20 msec duration, 0.2–0.7 nA), delivered every 3.3 sec. The membrane resistance of the postsynaptic cell was monitored throughout experiments with short hyperpolarizing current pulses (40 msec duration, −0.1 nA).
All drugs were prepared freshly from frozen stock solutions and applied by bath perfusion. 6-Cyano-7-nitroquinoxaline-2,3,-dione (CNQX) andd-2-amino-5-phosphono-valerate (AP-5) were purchased from Tocris Cookson (Bristol, UK).
Induction of LTP and long-term depression. LTP of unitary and multi-unitary EPSPs was induced by repeatedly pairing (30–100 times) presynaptic stimulation with a postsynaptic burst of 5–12 action potentials induced by a 240 msec depolarizing current pulse (Debanne et al., 1994, 1996a). Long-term depression (LTD) of unitary EPSPs was induced by tetanization of the presynaptic neuron at 3 Hz for 3 min (Debanne et al., 1996a, 1998).
Data analysis. The analog signals from the two electrodes were digitized at 18 kHz and recorded on video tape. Off-line acquisition of 200–500 msec sequences was performed on a personal computer at a digitization rate of 8–10 kHz (Acquis1; Bio-Logic, Claix, France). Postsynaptic responses were averaged after aligning the presynaptic action potential using automatic peak detection. EPSP amplitudes were measured in all cases at fixed latency from the peak of the presynaptic action potential. Values of potentiation and depression reported in this study are based on averages (50–80 traces) of EPSPs recorded 10 min after the end of the pairing procedure or the 3 Hz tetanus.
Results are expressed as mean ± SEM. The Mann–WhitneyU test, Snedecor F test, and Student's pairedt test were used to test statistical significance, as stated in the text.
RESULTS
Heterogeneity of short-term plasticity
We first compared the time course of potentiation of multi-unitary EPSPs evoked with extracellular stimulation with that of unitary EPSPs elicited in pairs of individual neurons. In both cases, LTP was induced by a synchronous pairing of the synaptic input activated continuously at 0.3 Hz, with a burst of 5–12 postsynaptic action potentials elicited with a depolarizing current pulse. Two phases in the time course of potentiation induced with synchronous pairing of extracellularly evoked EPSPs in CA1 pyramidal cells (n= 36) (Fig. 1A,open circles) could be distinguished: a transient phase, which lasted for 2–4 min, and a sustained plateau level (dotted line), which lasted for as long as the recordings could be stably maintained (up to 140 min).
Fig. 1.
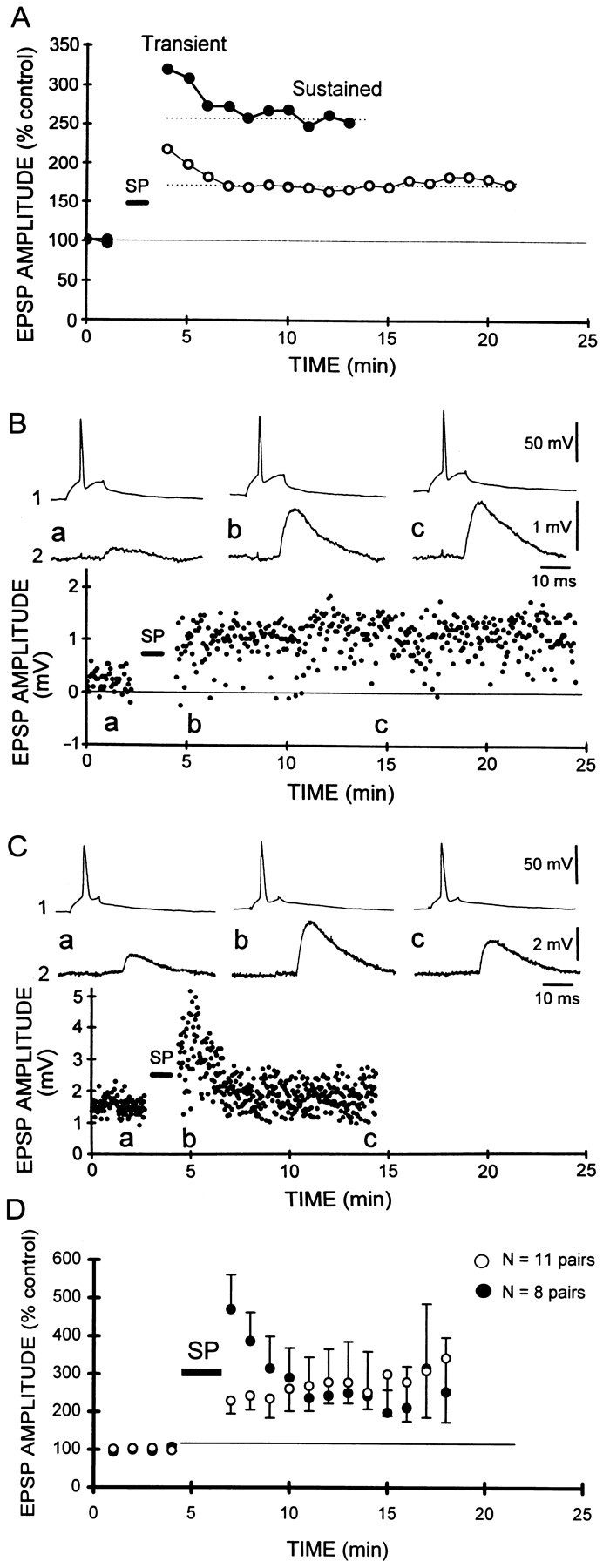
Transient and sustained potentiation of unitary CA3–CA1 EPSPs. A, Two components were apparent in the time course of potentiation induced by synchronous pairing (see Results for details), of extracellularly evoked, multi-unitary EPSPs (thin line): a transient phase lasting 2–4 min, and a sustained component remaining >20 min after the pairing. A similar time course was apparent when all unitary CA3–CA1 EPSPs were averaged (thick line). B, Sustained potentiation of an individual CA3–CA1 EPSP. In 58% of experiments, potentiation displayed no transient phase and was sustained at a relatively constant level through out the recording period (19 min after pairing in this experiment). Responses above were taken at the indicated times in this and subsequent figures. Seventy-five synchronous pairings.C, Transient and sustained potentiation of another CA3–CA1 unitary EPSP. Transient potentiation lasting only 3 min was observed, followed by a sustained phase without any apparent decrement. Sixty synchronous pairings. D, Pooled data from cell pairs displaying only the sustained phase of potentiation (○;n = 11) and cell pairs exhibiting both transient and sustained phases (●; n = 8). Note that the level of sustained potentiation was similar for both classes.
When all of the data from unitary connections between CA3 and CA1 cells were averaged (n = 24) (Fig. 1A,filled circles), the time course appeared identical to that seen for multi-unitary EPSPs. When the unitary EPSPs were considered individually, however, they could be divided into two distinct classes. In 11 of 19 experiments (58%) exhibiting a significant long-lasting potentiation (>110% of the control amplitude 10 min after pairing), no transient phase of potentiation was apparent (Fig.1B). In the remaining experiments (8 of 19, 42%), the time course of potentiation showed a transient phase, followed by sustained potentiation (Fig. 1C). Comparison of the potentiation for both classes (Fig. 1D) revealed that the average amount of sustained potentiation was comparable (249 ± 55 and 285 ± 42% of the control for the sustained and transient categories; Mann–Whitney U test;p > 0.1). Furthermore, there was no significant difference in either the mean EPSP amplitude (1.41 ± 0.42 and 0.94 ± 0.4 mV; Mann–Whitney U test; p> 0.1) or time-to-peak (5.55 ± 0.64 and 5.13 ± 1.25 msec; Mann–Whitney U test; p > 0.1) for the sustained and transient-sustained classes. There was no significant correlation between the number of pairings between presynaptic and postsynaptic depolarizations (range of 30–100) and the presence or absence of a transient phase (linear regression, r2 = 0.1; data not shown). When multiple presynaptic cells were recorded sequentially while maintaining a recording from a single common postsynaptic cell (see below), examples of both classes of synapses, those with and without transient potentiation, were observed at different synapses formed on a single postsynaptic cell (see Fig. 3C). The mechanisms underlying this difference are therefore likely to be synapse- rather than cell-specific.
Fig. 3.
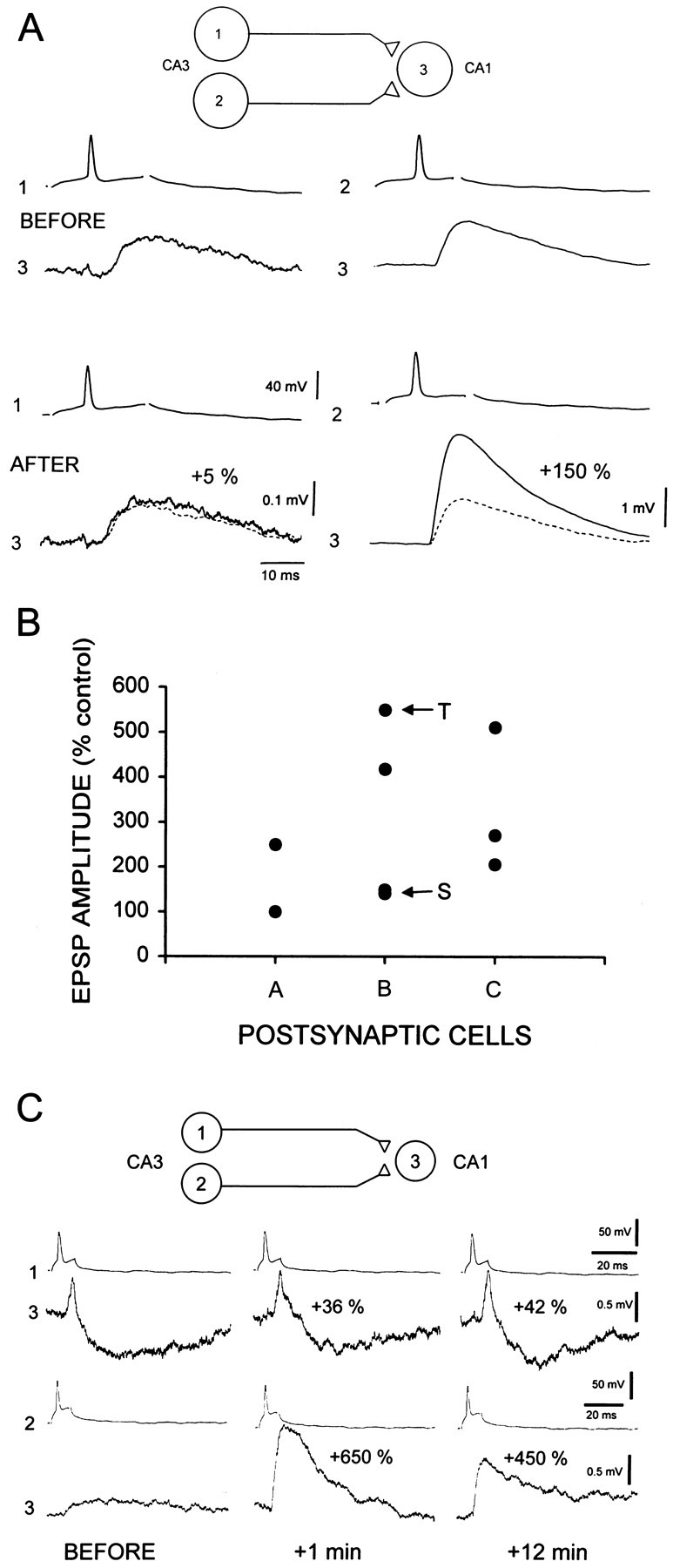
Potentiatable and nonpotentiatable EPSPs produced by different presynaptic cells with a common postsynaptic cell.A, Sixty synchronous pairings failed to induce potentiation of the connection formed by neurons 1 and 3 (105% of control), whereas the same pairing procedure applied later to a synapse formed by another neuron (2) with the same postsynaptic cell (3) resulted in robust potentiation (250%). B, Graph displaying the amount of potentiation induced by synchronous pairing with different presynaptic cells and a given postsynaptic cell (n= 3). Note the variability of potentiation among different presynaptic partners (vertical differences). Unitary connections displaying sustained (S) and transient-sustained potentiation (T) were found on the postsynaptic cell B (see C). C, Sustained and transient potentiation on the same postsynaptic cell. The connection between neurons 1 and 3 exhibited a level of potentiation immediately after pairing (+1 min, +36%) that did not change significantly 12 min after the pairing (+42%). Note the disynaptic IPSP in this unitary response (Debanne et al., 1995). In contrast, the connection between neuron 2 and the same postsynaptic neuron (3) exhibited a large increase in amplitude after the pairing (+650%) that declined to a lower, stable level (+ 450%) 12 min after the end of the pairing.
We conclude that the population of synapses on CA1 pyramidal neurons is not homogeneous with respect to their ability to express short-term potentiation.
Heterogeneity in the magnitude of LTP
We next compared the magnitude of the steady-state level of potentiation between multi-unitary and unitary EPSPs. The steady-state level of potentiation was measured in all experiments 5–10 min after pairing, i.e., after full decay of the transient phase (Fig. 1). The variability of potentiation was significantly greater for unitary EPSPs than for multi-unitary EPSPs (variance F test;p < 0.0001). Potentiation of multi-unitary EPSPs never exceeded 250% of the control amplitude in CA1 or CA3 cells (Fig.2A, top). In contrast, potentiation of unitary EPSPs to as much as 1300% of the control amplitude was observed at CA3–CA1 or CA3–CA3 connections (Fig. 2A, bottom). Unitary EPSPs exhibiting potentiation of >250% of the control amplitude were observed in 24% of experiments (11 of 45).
Fig. 2.
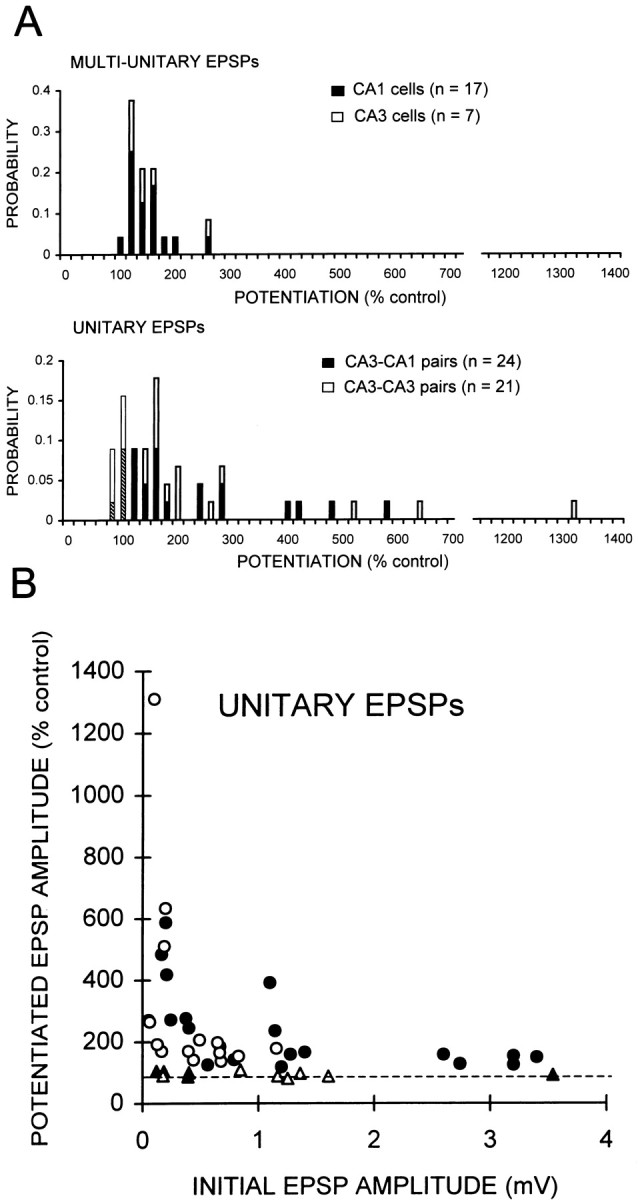
Heterogeneous plasticity of unitary EPSPs.A, Histograms of the level of potentiation of multi-unitary (top) and unitary EPSPs (bottom), measured in all experiments 10 min after synchronous pairing. Filled bars, Schaffer collateral–CA1 pyramidal cell connections; open bars, recurrent excitatory connections between CA3 cells. Potentiation of multi-unitary EPSPs did not exceed 250% of the control amplitude, whereas potentiation of unitary EPSPs was as much as 13-fold. One third of unitary EPSPs displayed potentiation of >250%. Unitary synapses that did not express potentiation to >110% of the control amplitude comprise the first two bars of the bottom histogram. B, Amount of potentiation plotted as a function of initial CA3–CA1 (●) and CA3–CA3 (○) unitary EPSP amplitude. Potentiation of small unitary EPSPs was more variable and could be greater than for large EPSPs. Unitary connections that did not display potentiation >110% are indicated with open and filled triangles for CA3–CA3 and CA3–CA1 connections, respectively.
The magnitude of potentiation was correlated strongly with the amplitude of the EPSP before potentiation (Fig. 2B). A significant difference in the amplitude of potentiation was observed between initially small and large unitary EPSPs. Small unitary EPSPs (0.1–0.5 mV) displayed greater potentiation on average and more variability than large unitary EPSPs (2.5–3.5 mV; varianceF test; p < 0.0001).
Failure to induce LTP of some unitary EPSPs
Potentiation of unitary EPSPs to >110% of the control amplitude could not be induced in 11 of 45 cell pairs (5 of 24 unitary CA3–CA1 EPSPs and 6 of 21 unitary CA3–CA3 EPSPs). In these experiments, the unitary EPSP amplitude averaged 95 ± 3% of the control amplitude 10 min after pairing (n = 11). There was no correlation between the control EPSP amplitude and the failure to display potentiation (Fig. 2B). Some of these synapses did display the transient form of potentiation (see Fig. 5A). We next considered several potential explanations for the failure of these EPSPs to display potentiation.
Fig. 5.

Nonpotentiatable and nonplastic connections.A, Synchronous pairing (SP; repeated 30 times) produced only a transient (∼5 min) potentiation (a+b) of the unitary CA3–CA3 EPSP. Subsequent tetanization of the presynaptic cell (1) at 3 Hz for 3 min, however, induced depression to 61% of the control EPSP amplitude (b+c) in the postsynaptic cell (2). B, Change in unitary EPSP amplitude after synchronous pairing (SP) and 3 Hz tetani in eight experiments in which the unitary EPSP was <110% of the control amplitude after synchronous pairing. Three unitary EPSPs were depressed significantly after the 3 Hz tetanus (saturated synapses), whereas five EPSPs were not depressed (nonplastic synapses).
First, failure to induce LTP could reflect damage or disturbance to the postsynaptic cell and its intracellular biochemistry. Second, the postsynaptic cell may be lacking in the necessary biochemical intermediaries underlying LTP induction and/or expression. These hypotheses were tested by recording sequentially from two cells forming synapses with a common postsynaptic cell. Connections at which potentiation could and could not be induced could be recorded from a single postsynaptic neuron. In the example illustrated in Figure3A, no significant potentiation (105% of the control amplitude) was induced at the connection formed between neurons 1 and 3. When the electrode was removed from cell 1 and cell 2 was impaled, however, robust potentiation (250% of the control amplitude) of the unitary EPSP was induced with the same stimulation protocol. Substantial variations in the amount of potentiation of unitary EPSPs in single postsynaptic cells was observed in three experiments in which an identical number of pairings was used (Fig. 3B).
Although identical LTP induction protocols were used for both synapses in these experiments, the possibility that some cellular properties may have changed in the ∼20 min between the two induction attempts cannot be excluded. We therefore took advantage of the fact that unitary EPSPs between pairs of cells in hippocampal slice cultures can sometimes be recorded, which appear to result from distinct groups of synapses (Fig.4). Such unitary EPSPs were observed in only 12% of the CA3–CA1 and 24% of the CA3–CA3 connections (from 41 and 59 pairs, respectively). The evidence that both components of such EPSPs were monosynaptic is, first, that their latencies were fixed within 1 msec (see histograms in Fig. 4). Second, both components were elicited consistently during high frequencies of presynaptic stimulation (data not shown). Third, monophasic events with the same two fixed latencies could be elicited in isolation when the release probability was decreased by increasing extracellular magnesium to 5.0 mm (n = 3) or by applying low concentrations of adenosine (1–2 μm) (n = 2). We believe that such unitary EPSPs were produced when two collaterals of the presynaptic cell formed distinct groups of synapses with the postsynaptic cell. The difference in the latencies of the two components results presumably from unequal lengths of the two axon collaterals and/or from unequal conduction velocities, as might result if there were differences in their diameter or degree of myelination. The range of latencies for CA3–CA3 and CA3–CA1 monosynaptic connections (7 and 11 msec, respectively) (Debanne et al., 1995) was compatible with the latencies of the double monosynaptic EPSPs observed.
Fig. 4.

Differential potentiation expressed by different EPSP components in single cell pairs. Differing magnitudes of potentiation at different components of a unitary EPSP in a CA3–CA1 cell pair. Two monosynaptic EPSP components (a andb) were elicited in neuron 2 in response to single action potentials in neuron 1 (before). Both EPSP components had relatively invariant latencies (within 1 msec, inset). Sixty-five synchronous pairings resulted in a potentiation of EPSPb to 220% of the control amplitude but not of EPSPa (100% of control). The graph displays the amount of potentiation induced by synchronous pairing at different subsets of synapses in single cell pairs. Note the variability of potentiation among different unitary EPSP components (vertical differences).
An example of an experiment in which LTP induction was attempted on a double monosynaptic EPSP is provided in Figure 4. After repeated (70 times) pairing of suprathreshold 240 msec depolarizing pulses with single presynaptic action potentials, potentiation of the long-latency EPSP (Fig. 4, b) was observed to 220% of the control amplitude but not of the short-latency EPSP (a; 100% of the control amplitude). Such variability in the amount of potentiation was also observed with double monosynaptic EPSPs in two other pairs of cells, as illustrated in Figure 4C. Because both EPSP components were paired simultaneously with the same postsynaptic depolarization, these experiments provide additional evidence that not all synapses on a single postsynaptic cell are equally capable of demonstrating LTP.
Depression, but not potentiation, of some unitary EPSPs
Two explanations may be advanced for the failure of a synapse to demonstrate LTP. First, the LTP expression mechanism(s) at that synapse could have been saturated previously at the maximally potentiated level (Petersen et al., 1998). If true, then it should be possible to “depotentiate” the synapse using standard LTD induction protocols (Barrionuevo et al., 1980). Alternatively, the synapse may be incapable of demonstrating either LTP or LTD.
Of the 11 unitary EPSPs that did not exhibit potentiation to >110% of the control amplitude, eight were tested for depression using a 3 Hz tetanus for 3 min. Three unitary EPSPs could be significantly depressed to 53 ± 4% of the control amplitude. We conclude that LTP had been saturated previously at the synapses underlying these EPSPs (saturated synapses).
The time course of the depression of a saturated unitary EPSP is illustrated in Figure 5. Synchronous pairing produced only a transient potentiation (Fig. 5A,a and b), whereas tetanization of the presynaptic cell at 3 Hz for 3 min induced a long-lasting decrease in the amplitude of the unitary EPSP (Fig. 5A, b andc).
The remaining five unitary EPSPs did not show depression to <90% of the control amplitude. The EPSP amplitude after the 3 Hz tetanus averaged 95 ± 1% of the control amplitude. We conclude that these EPSPs arise out of nonplastic synapses (Figs.5B, 6A).
Fig. 6.
Nonplastic synapses express NMDA receptors.A, Synchronous pairing (SP; repeated 50 times) did not induce potentiation of this unitary CA3–CA3 EPSP (a+b). Likewise, 3 Hz tetanization of the presynaptic cell (1) failed to depress the EPSP in cell 2 (b+c). B, In the same pair, bath application of the non-NMDA receptor antagonist CNQX (20 μm) blocked the fast EPSP component and unmasked a slow EPSP when the postsynaptic cell was depolarized to −55 mV. The NMDA receptor antagonist AP-5 (25 μm) abolished the slow EPSP, indicating that functional NMDA receptors were present at this nonplastic synapse.
Synaptic activation of NMDA receptors at nonplastic connections
Induction of LTP and LTD at CA3–CA1 and CA3–CA3 synapses with our stimulation protocols requires the activation of postsynaptic NMDA receptors (Dudek and Bear, 1992; Debanne et al., 1994, 1998). One readily testable explanation for an absence of synaptic plasticity would be a lack of postsynaptic NMDA receptor activation at nonplastic synapses. We therefore investigated whether an NMDA receptor-mediated EPSP component could be pharmacologically isolated in nonplastic unitary connections. An example of a unitary EPSP that was unaffected by either the LTP or LTD induction protocols is provided in Figure 6. Application of the non-NMDA receptor antagonist CNQX (20 μm) and depolarization of the postsynaptic cell to −55 mV revealed a slow depolarizing potential. Subsequent addition of the NMDA receptor antagonist AP-5 (25 μm) fully blocked this slow potential. Similar results were obtained in the two other experiments in which these manipulations could be performed. We conclude that lack of plasticity does not result solely from an absence of an NMDA receptor-mediated component of the unitary EPSP.
DISCUSSION
Transient potentiation occurs at only some connections
We have studied the plasticity of unitary EPSPs between pairs of cells in hippocampal slice cultures. We have distinguished two classes of connections on the basis of whether or not they display a transient phase of potentiation. Some unitary EPSPs (42%) expressed a transient potentiation lasting 2–4 min, which then decayed to a sustained level. Other unitary EPSPs became potentiated to a level that remained essentially constant for as long as the recordings could be maintained.
The mechanisms underlying short-term potentiation have not yet been fully established, rendering it difficult to know what cellular properties are different at these two classes of connections. An NMDA receptor-dependent form of short-term potentiation has been described that can be activated with pairing protocols similar to the ones used here (Malenka, 1991; Colino et al., 1992). It was suggested that this form of potentiation may represent a transient, incomplete activation of the same biochemical machinery responsible for lasting potentiation. The occurrence of stable LTP in the absence of transient potentiation would appear to rule this possibility out, however, particularly because the magnitude of the stable LTP was not different for the two classes of connections.
Alternatively, postsynaptic depolarizing pulses, such as those used to induce LTP in the present experiments, induce a nonassociative transient potentiation of synaptic transmission that requires an L-type Ca2+ channel-mediated, but NMDA receptor-independent, influx of Ca2+(Kullmann et al., 1992). We have not tested the effects of L-type Ca2+ channel antagonists on transient potentiation in our system. If this mechanism underlies the transient potentiation reported here, however, then there may be heterogeneity in the postsynaptic expression of L-type Ca2+channels (Hell et al., 1993). Because no significant difference was seen in the amplitude and time-to-peak of EPSPs expressing or lacking transient potentiation, these differences may result from a nonuniform distribution of dendritic Ca2+ channels, as shown previously in hippocampal cell cultures (Segal, 1995).
Not all connections are equally plastic
Connections between different presynaptic cells and a single postsynaptic neuron, or even between different subsets of synapses formed by the same presynaptic neuron with a given postsynaptic cell, were found to display varying amounts of potentiation, ranging from 13-fold increases in amplitude to no potentiation. Similar observations of nonpotentiatable synapses (Petersen et al., 1998) and very large potentiation (Malinow, 1991) have been made in acutely preparedex vivo hippocampal slices. These phenomena are thus not unique to cultured hippocampal tissue.
Our data allow us to suggest an explanation for this variability. We have shown previously that unitary EPSPs result from the release of transmitter at multiple synaptic contacts comprising a given connection (Debanne et al., 1996b). We assume that the size of the unitary EPSP is, at least in part, a function of the number of synapses activated by the presynaptic cell. Smaller unitary EPSPs displayed the greatest increases in amplitude, expressed as percent change from the control amplitude, and also the greatest variability in the amount of potentiation (Fig. 2). One possible explanation for the smaller potentiation of large unitary EPSPs is that they are larger because they have already been potentiated. If so, then the amount of depression of large EPSPs should be greater than that obtained for small EPSPs. The amount of depression of naive unitary CA3–CA1 EPSPs was found previously to be independent of EPSP amplitude (Debanne et al., 1996a, their Fig. 3A), however, rendering this hypothesis unlikely.
Alternatively, the variability may arise because some individual synapses in a given connection have a high intrinsic plasticity and capability for large increases in synaptic strength after induction of LTP, whereas other synapses are either capable of only modest amounts of potentiation or are not potentiatable. Evidence of this variable range in the magnitude of potentiation is apparent in the data obtained with small unitary EPSPs (Fig. 2), because these are likely to result from activation of the smallest number of synapses. In contrast, large unitary EPSPs, likely to result from activation of a larger number of synapses, displayed a comparatively smaller average potentiation. Indeed, the average potentiation of unitary CA3–CA1 EPSPs <1 mV was 241%, whereas the average potentiation of unitary CA3–CA1 EPSPs >2 mV was 134%. Extracellularly evoked responses will be comprised of unitary EPSPs of different amplitudes and would thus be expected to display an average potentiation between the two values mentioned before, which is indeed the case. So-called silent synapses in which the induction of LTP results in the appearance of a detectable non-NMDA receptor-mediated EPSP at connections in which none was previously detectable (Isaac et al., 1995; Liao et al., 1995; Durand et al., 1996), represent an essentially infinite increase in synaptic strength. Although it appears that the level of potentiation is all-or-none at a given synapse (Petersen et al., 1998), differences in the relative number or efficacy of non-NMDA receptors activated before and after LTP induction at different synapses may account for the observed range in the level of potentiation. In addition, connections are likely to be composed of varying proportions of plastic and nonplastic synapses.
Nonpotentiatable and nonplastic connections
Twenty-four percent of unitary EPSPs studied failed to display potentiation after 50–100 synchronous pairings of presynaptic and postsynaptic depolarization. An identical proportion of nonpotentiatable synapses has been reported in area CA1 of ex vivo hippocampal slices with the use of minimal stimulation techniques (Petersen et al., 1998). In this study, however, it was not clear whether the lack of plasticity resulted from the condition of the postsynaptic cell, because only one synapse per cell was tested. We provide here the first evidence for the coexistence of potentiatable and nonpotentiatable connections on individual postsynaptic neurons. Moreover, potentiatable and nonpotentiatable EPSP components were identified in single cell pairs, demonstrating that this variability does not result from some global property of the presynaptic cell.
Of these nonpotentiatable synapses, approximately half became decreased in amplitude after low-frequency tetanization of the presynaptic cell. We can therefore conclude that these connections had been potentiated previously to the saturating level, so that no further potentiation was induced by the LTP induction protocol. Previous potentiation may have occurred during cell impalement or as a result of spontaneous activity. It should be noted that such depotentiation should have allowed induction of LTP at previously nonpotentiatable synapses. Unfortunately, we were unable to maintain any of our recordings from these synapses long enough to test this hypothesis.
The remaining unitary EPSPs (15% of all connections studied) could be neither potentiated by pairing nor depressed by 3 Hz tetanization. What could account for the lack of plasticity at these synapses? Because induction of LTP and depotentiation both require an NMDA receptor-mediated elevation of intracellular Ca2+ in the postsynaptic cell, the simplest explanation is to suggest that a failure in some common process results in an inability to induce either form of plasticity. Several possibilities may be raised. First, some unitary EPSPs might not have appeared plastic because they were generated at electrotonically remote sites, so that action potentials may have failed to backpropagate in some dendrites but not in others (Spruston et al., 1995), and the postsynaptic depolarization failed to sufficiently depolarize the synapses for relief of the Mg2+ block of NMDA receptor-gated ion channels. In none of the nonplastic synapses we examined, however, did synaptically released glutamate fail to activate postsynaptic NMDA receptors (Fig. 6). Alternatively, the Ca2+ influx may have been adequate for synaptic plasticity, but the biochemical pathways responsible for changes in synaptic strength may not have been activated because of rapid Ca2+ buffering or lack of some critical protein. Finally, the diversity of plasticity at synapses on a single neuron might arise from diversity in the distribution of postsynaptic glutamate receptors (Rubio and Wenthold, 1997; Tóth and McBain, 1998).
It should also be noted that synapses that are not plastic at one particular time may become plastic later as a result of different physiological and developmental conditions (Abraham and Bear, 1996). Various patterns of activity, for example, have been shown to regulate the subsequent induction of synaptic plasticity. Weak tetanization of Schaffer collateral synapses can prevent subsequent induction of LTP by high-frequency stimulation (Huang et al., 1992), and a brief priming stimulation can facilitate LTD induction in the dentate gyrus (Wang et al., 1998). Interestingly, depotentiation by theta pulse stimulation in area CA1 can be induced only within the first 20 min after induction of LTP (Stäubli and Chen, 1996). It is thus possible that previous spontaneous activity in the slice culture may have led to the induction of stable, irreversible potentiation of these unitary EPSPs before our attempts to induce potentiation.
In summary, our experiments have revealed an unexpectedly large degree of heterogeneity in the plasticity of the connections formed between individual pyramidal cells. The existence of nonplastic synapses may be important for maintaining reliable transmission of information in the brain, providing an essential element of stability and limiting the range within which synaptic gain may vary.
Footnotes
This work was supported by the Roche and Dr. Eric Slack-Gyr Foundations, and Swiss National Science Foundation Grant 31–42174.94. We thank L. Heeb, E. Hochreutener, R. Kägi, H. Kasper, L. Rietschin, and R. Schöb for excellent technical assistance.
Correspondence should be addressed to Dominique Debanne, Unité de Neurocybernétique Cellulaire, Centre National de la Recherche Scientifique, Unité Propre de Recherche 9041, 280 Boulevard Sainte-Marguerite, 13009 Marseille, France. E-mail:debanne@marseille.inserm.fr.
aWe shall refer to the EPSP elicited in paired recordings as a “unitary” response and those elicited by stimulation of large numbers of presynaptic axons with extracellular stimulation as “multi-unitary” responses. Similarly, a synapse is defined as a contact between a presynaptic release site and a postsynaptic cell. Minimal stimulation is reputed to result in activation of a single synapse (for detailed criteria, seeExperimental Procedures in Dobrunz and Stevens, 1997) (but see Larkman et al., 1997). Unitary responses in hippocampal slice cultures typically reflect activation of several synapses (Debanne et al., 1996b).
REFERENCES
- 1.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 2.Barrionuevo G, Schottler F, Lynch G. The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- 3.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 4.Colino A, Huang YY, Malenka RC. Characterization of the integration time for the stabilization of long-term potentiation in area CA1 of the hippocampus. J Neurosci. 1992;12:180–187. doi: 10.1523/JNEUROSCI.12-01-00180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debanne D, Gähwiler BH, Thompson SM. Asynchronous pre- and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. Proc Natl Acad Sci USA. 1994;91:1148–1152. doi: 10.1073/pnas.91.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Physiology and pharmacology of synaptic connections between cell pairs in areas CA3 and CA1 of the rat hippocampal slice cultures. J Neurophysiol. 1995;73:1282–1294. doi: 10.1152/jn.1995.73.3.1282. [DOI] [PubMed] [Google Scholar]
- 7.Debanne D, Gähwiler BH, Thompson SM. Cooperative interactions in the induction of long-term potentiation and depression of synaptic excitation between hippocampal CA3-CA1 cell-pairs in vitro. Proc Natl Acad Sci USA. 1996a;93:11225–11230. doi: 10.1073/pnas.93.20.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol (Lond) 1996b;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debanne D, Gähwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol (Lond) 1998;507:237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;16:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 11.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapses induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 13.Gähwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson B, Wigström H, Abraham WC, Huang Y-Y. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley of synaptic potentials. J Neurosci. 1987;7:774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebb DO. The Organization of behavior. Wiley; New York: 1949. [Google Scholar]
- 16.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y-Y, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- 18.Isaac JTR, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Isaac JTR, Hjelmstad GO, Nicoll RA, Malenka RC. Long-term potentiation at single fiber inputs to hippocampal CA1 pyramidal cells. Proc Natl Acad Sci USA. 1996;93:8710–8715. doi: 10.1073/pnas.93.16.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kullmann DM, Perkel DJ, Manabe T, Nicoll RA. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. Neuron. 1992;9:1175–1193. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- 21.Larkman AU, Jack JJB, Stratford KJ. Assessment of the reliability of amplitude histograms from excitatory synapses in rat hippocampal CA1 in vitro. J Physiol (Lond) 1997;505:443–456. doi: 10.1111/j.1469-7793.1997.443bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 23.Malenka RC. Postsynaptic factors control the duration of synaptic enhancement in area CA1 of the hippocampus. Neuron. 1991;6:53–60. doi: 10.1016/0896-6273(91)90121-f. [DOI] [PubMed] [Google Scholar]
- 24.Malinow R. Transmission between pairs of hippocampal slice neurons: quantal levels, oscillations, and LTP. Science. 1991;252:722–724. doi: 10.1126/science.1850871. [DOI] [PubMed] [Google Scholar]
- 25.Petersen CCH, Malenka RC, Nicoll RA, Hopfield JJ. All-or-none potentiation at CA3-CA1 synapses. Proc Natl Acad Sci USA. 1998;95:4732–4737. doi: 10.1073/pnas.95.8.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- 27.Sanes JR, Lichtman JW. Can molecules explain long-term potentiation? Nat Neurosci. 1999;2:597–604. doi: 10.1038/10154. [DOI] [PubMed] [Google Scholar]
- 28.Sastry BR, Goh JW, Auyeung A. Associative induction of posttetanic and long-term potentiation in CA1 neurons of rat hippocampus. Science. 1986;232:988–990. doi: 10.1126/science.3010459. [DOI] [PubMed] [Google Scholar]
- 29.Segal M. Imaging of calcium variations in living dendritic spines of cultured rat hippocampal neurons. J Physiol (Lond) 1995;486:283–295. doi: 10.1113/jphysiol.1995.sp020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- 31.Stäubli U, Chen D. Factors regulating the reversibility of long-term potentiation. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tóth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Wu J, Rowan MJ, Anwyl R. Role of protein kinase C in the induction of homosynaptic long-term depression by brief low frequency stimulation in the dentate gyrus of the rat hippocampus in vitro. J Physiol (Lond) 1998;513:467–475. doi: 10.1111/j.1469-7793.1998.467bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



