Abstract
The differentiation of areas of the mammalian neocortex has been hypothesized to be controlled by intrinsic genetic programs and extrinsic influences such as those mediated by thalamocortical afferents (TCAs). To address the interplay between these intrinsic and extrinsic mechanisms in the process of arealization, we have analyzed the requirement of TCAs in establishing or maintaining graded or areal patterns of gene expression in the developing mouse neocortex. We describe the differential expression of Lhx2,SCIP, and Emx1, representatives of three different classes of transcription factors, and the type II classical cadherins Cad6, Cad8, andCad11, which are expressed in graded or areal patterns, as well as layer-specific patterns, in the cortical plate. The differential expression of Lhx2, SCIP,Emx1, and Cad8 in the cortical plate is not evident until after TCAs reach the cortex, whereasCad6 and Cad11 show subtle graded patterns of expression before the arrival of TCAs, which later become stronger. We find that these genes exhibit normal-appearing graded or areal expression patterns in Mash-1 mutant mice that fail to develop a TCA projection. These findings show that TCAs are not required for the establishment or maintenance of the graded and areal expression patterns of these genes and strongly suggest that their regulation is intrinsic to the developing neocortex.
Keywords: cerebral cortex, area specification, transcription factors, cadherins, Lhx2, Emx1, SCIP
The neocortex, a major region of the cerebral cortex, is divided into functionally specialized areas characterized by a unique architecture and distinct sets of input and output projections. Areas gradually differentiate within the cortical plate (CP), which initially does not exhibit the anatomical features that later distinguish different areas. The mechanisms that control neocortical arealization have been a debated issue, focusing on the roles of intrinsic mechanisms, such as differential gene regulation autonomous to the developing neocortex, versus extrinsic mechanisms, such as the influence of thalamocortical afferents (TCAs), the principal input to the neocortex (Rakic, 1988; O'Leary, 1989). A role of TCAs in controlling specific features associated with arealization, including differential gene expression ranging from graded to area specific, and anatomical properties that characterize neocortical areas would be suggested if these features become apparent in the CP after TCAs reach the neocortex. A role of genetic regulation would be suggested if genes are differentially expressed in graded or areal patterns before TCAs arrive and before other area-specific properties appear.
The developing neocortex exhibits considerable plasticity in the differentiation of area-specific properties, and TCAs seem to be a key regulator of this plasticity. For example, studies of the rodent primary somatosensory area have demonstrated a critical role of TCAs in the differentiation of barrels, a functional grouping unique to this area (for review, see Woolsey, 1990; Schlaggar and O'Leary, 1993). Transplant experiments have shown that pieces of embryonic neocortex grafted heterotopically to a different neocortical area can acquire the area-specific architecture and connections characteristic of the new area (O'Leary and Stanfield, 1989; Schlaggar and O'Leary, 1991). In addition, reductions in TCAs arising from the lateral geniculate nucleus have been correlated with a corresponding reduction in the extent of the neocortex that differentiates the architecture characteristic of primary visual cortex, the target area of that thalamic nucleus (Dehay et al., 1989, 1991; Rakic et al., 1991). The influence of TCAs on areal plasticity, and by inference on normal arealization, could be caused in part by its control of differential gene expression in the developing CP.
Recent reports have described the differential expression of several EphA receptor tyrosine kinases and their ephrin-A ligands in the CP before the arrival of TCAs. In embryonic monkeys, the receptorsEphA3, A4, A6, and A7 are expressed in graded or areal patterns before TCAs reach the cortex (Donoghue and Rakic, 1999). On the other hand, the receptorEphA5 and the ligands ephrin-A2, A3,and A5, which are graded or areal at later stages, are either not expressed when TCAs arrive or their expression is uniform. In rodents, EphA5 and ephrin-A5 also exhibit substantial differences in their expression patterns before and after TCAs arrive in the cortex (Zhang et al., 1997; Mackarehtschian et al., 1999).
Although both intrinsic and extrinsic mechanisms contribute to the process of arealization (O'Leary et al., 1994; Chenn et al., 1997;Levitt et al., 1997), little is known about how these mechanisms cooperate to establish area-specific properties. The purpose of this study was to assess the potential interplay between these mechanisms by examining the role of TCAs in influencing differential gene expression in the CP. We focused on genes that are differentially expressed tangentially across the developing CP and encode either nuclear proteins that regulate gene expression or cell-surface proteins that mediate cell–cell interactions. We wished to identify genes whose expression becomes graded or areal in the CP either before or after TCAs reach the cortex. We then assessed the role of TCAs in establishing and maintaining differential gene expression patterns by examining them in mice deficient for the basic helix–loop–helix transcription factor gene Mash-1 (Guillemot et al., 1993), which fail to develop a TCA projection (Tuttle et al., 1999).
MATERIALS AND METHODS
Animals. Embryos and postnatal pups obtained from timed pregnant ICR mice (Harlan Sprague Dawley, Indianapolis, IN) were used for all analyses except those examining the role of TCAs, which used Mash-1−/− mice (Guillemot et al., 1993) and their wild-type littermates outcrossed into the CD1 strain. The day of insemination is designated embryonic day 0.5 (E0.5). The day of birth is designated postnatal day 0 (P0). Maintenance and genotyping of the Mash-1 mutant embryos were done as described previously (Guillemot et al., 1993; Tuttle et al., 1999). Analysis of expression patterns was done blinded to genotype (although the null mutant brains can be distinguished by their smaller olfactory bulbs). Three to four brains per genotype were analyzed byin situ hybridization.
In situ hybridization. In situhybridization and counterstaining on 20 μm cryostat sections were done according to the methods of Tuttle et al. (1999). The following digoxigenin-labeled RNA probes were used: Lhx2 (897–1482 of mouse Lhx2; GenBank accession number AF124734; a gift from S. Bertuzzi) (Xu et al., 1993); SCIP (rat full-length clone; a gift from G. Lemke) (Monuki et al., 1989); Emx1 (mouse full-length clone) (R. H. Dyck, J. Richards, J. J. A. Contos, C. Akazawa, J. Chun, D. D. M. O'Leary, unpublished observations); Cad6 (mouse full-length clone; a gift from S. Mah and C. Kintner) (Inoue et al., 1997); Cad8[241–1481 of mouse Cad8; GenBank accession number X95600; obtained by reverse transcription (RT)–PCR] (Korematsu and Redies, 1997); and Cad11 (1278–2121 of mouseCad11; GenBank accession number D31963; obtained by RT-PCR) (Kimura et al., 1995). Hybridization using the rat SCIPprobe identified the same cell populations in rat and mouse tissues. Whole-mount in situ hybridization was based on the method ofWilkinson (1993), with the following modifications: brain were pretreated with 10 μg/ml proteinase K for 30 min, and the prehybridization, hybridization, and posthybridization washes were done at 70°C. In addition, we used 3 μg/ml digoxygenin-labeled probes for hybridization. Although the whole-mount analysis is useful to survey the global gene expression patterns, we found that expression in deeper layers of the cortical wall is often not detected, presumably because of the poor penetration of riboprobes; for example, layer 5 expression of Cad8 along the whole rostrocaudal axis of the neocortex at E18.5 is not detected with our whole-mount protocols.
RESULTS
For this analysis, we performed in situ hybridization using digoxigenin-labeled riboprobes on E10.5–P2 mouse cerebral cortex. TCAs pass from the internal capsule into the neocortex at E14.5; by E15.5 they have spread across much of the neocortex and have begun to extend branches toward the CP (Bicknese et al., 1994). We use the term “areal” to describe restricted tangential patterns of gene expression and do not intend to imply that these patterns directly relate to specific areas.
Graded expression of Lhx2, SCIP, andEmx1 in developing neocortex
Lhx2 encodes an LIM-homeodomain transcription factor postulated to control cortical neuron differentiation (Xu et al., 1993). Lhx2 has been shown recently to be expressed in mouse neocortex in both proliferating and nonproliferating cells (Retaux et al., 1999) and to be involved in the proliferation of cortical neuroepithelial cells (Porter et al., 1997). However, the differential expression of Lhx2 along the tangential extent of the neocortex has not been described.
Lhx2 is expressed in the dorsal telencephalic wall as early as E10.5, but its expression is not graded (data not shown). In contrast, at E12.5, Lhx2 expression is graded in high-medial-to-low-lateral (Fig.1A) and high-caudal-to-low-rostral (data not shown) patterns. Expression in the preplate (PP) does not decline rostrally as much as that in the ventricular zone (VZ), resulting in much higher expression in the PP than in the VZ at rostral levels (data not shown).
Fig. 1.

Graded expression of regulatory genes in the E10.5–E15.5 mouse neocortex. Coronal (A–C, E–K) and sagittal (D) sections of mouse forebrain show the expression of Lhx2(A–D), SCIP(E–G), and Emx1(H–K). A, Lhx2shows a graded pattern in the dorsal telencephalon at E12.5 with higher expression medially than laterally (arrowheads).B, At E14.5, Lhx2 expression is high in the VZ and SVZ and graded with a high-medial-to-low-lateral pattern but is very low in the CP with no obviously graded patterns along the tangential axes. C, D, At E15.5,Lhx2 expression exhibits a dramatic increase in the upper CP of the caudolateral neocortex (arrowheads), strongly graded in high-lateral-to-low-medial (B) and high-caudal-to-low-rostral (C) patterns.E, At E12.5, SCIP expression is detected in the PP of the rostral cortex (arrowheads). SCIP is expressed in a more ventral region, which appears to be the lateral ganglionic eminence (E, arrows). F, G, At E14.5, SCIP expression is limited to the IZ and is graded in a high-lateral-to-low-medial pattern (F;arrowheads) that is still present at E15.5 (G). At E15.5, it is also detected in the upper CP (G; arrowheads), but the caudolateral part of the neocortex, where Lhx2 is highly expressed, shows much weaker expression (G; arrow).H, Emx1 is in a slightly graded pattern as early as E10.5, with higher expression more medially than laterally (arrowheads). I, The same graded expression is detected at E12.5 (arrowheads).J, At E14.5, Emx1 is expressed in the VZ/SVZ and IZ of the neocortex in a slightly graded, high-medial-to-low-lateral pattern, whereas the level of expression in the CP is very low. K, At E15.5, Emx1 is most highly expressed in the upper CP of the caudolateral neocortex (arrowheads). B and J are from adjacent sections, and F is caudal to them.C and K are from adjacent sections, andG is rostral to them. A is slightly caudal to I. In this and all subsequent figures, dorsal is to the top and midline is to the rightin coronal sections, and dorsal is to the top and caudal is to the right in sagittal sections, except forH, which is a coronal section showing both sides of the telencephalon. Scale bar, 100 μm.
At E14.5, we find that Lhx2 expression is high in the VZ and subventricular zone (SVZ) but very low in the CP (Fig.1B). Within the VZ/SVZ, Lhx2expression is graded in a high-medial-to-low-lateral, as well as a high-caudal-to-low-rostral (data not shown), pattern, whereas graded expression is not detected in the CP (Fig. 1B). At E15.5, Lhx2 expression is increased substantially in the upper CP of the caudolateral neocortex and is strongly graded in high-lateral-to-low-medial and high-caudal-to-low-rostral patterns (Fig. 1C,D). The graded expressions in the VZ/SVZ at E15.5 are the same as those at E14.5. Interestingly, the strongly gradedLhx2 expression in the CP is in a countergradient along the mediolateral axis compared with its expression in the VZ and SVZ (Fig.1B,C). At both E18.5 (see Fig.4A–C) and P2 (see Fig. 3A),Lhx2 continues to be differentially expressed, with higher expression laterally, in the putative auditory cortex, than medially, in the putative visual cortex. The transition in expression level along this axis shows a relatively abrupt decline. Even more medially, expression is again slightly higher than that in the putative visual area (see Fig. 3A).
Fig. 4.
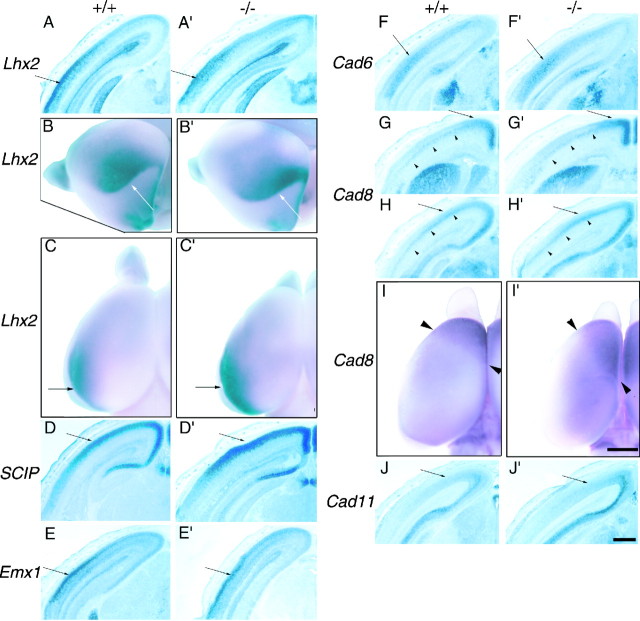
Graded and/or areal distributions of regulatory genes and cadherins are not altered in the E18.5 Mash-1mutant neocortex. Coronal sections (A, D–H, J, A′, D′–H′, J′) and whole mounts (B, C, I, B′, C′, I′) of wild-type (A–J) and Mash-1 null (A′–J′) mutant forebrains show the comparison ofLhx2 (A–C, A′–C′), SCIP(D, D′), Emx1 (E, E′),Cad6 (F, F′), Cad8(G–I, G′–I′), and Cad11 (J, J′) expression between the two genotypes. TheMash-1 mutant does not show any significant differences from wild type in graded, areal, or laminar cortical expression patterns of the genes examined. The whole-mount panelsshow the left hemispheres; B and B′ are the caudolateral view with the olfactory bulbs on theleft, and C, I, C′, and I′are dorsal views with the midline to the right.A–C, A′–C′, Lhx2 is highly expressed in the caudolateral neocortex in both genotypes (arrows).D–F, J, D′–F′, J′, Tangentially graded patterns ofSCIP, Emx1, Cad6, andCad11 are similar between the wild type and the mutant (arrows show areas with higher expression). G, H, G′, H′, Cad8 expression in layer 5 is graded in a high-medial-to-low-lateral pattern in both genotypes (arrowheads), and expression in the upper layers of the frontal (G, G′; arrows) and occipital (H, H′; arrows) cortex is also unchanged in the mutant. I, I′, The whole-mount pictures show theCad8 expression in the frontal cortex, with thearrowheads showing the caudal boundary of the expression domains. In the Mash-1 mutant, the medial portion of this expression domain extends further caudally than in the wild type. Scale bars: sections, 500 μm; whole mounts, 1 mm.
Fig. 3.
Differential expression of regulatory genes and cadherins in the P2 mouse neocortex. A–L, Coronal sections of mouse forebrain show graded expression and areal patterns (A–G) and layer specificity (H–L) of the expression ofLhx2 (A, H), SCIP(B, I), Emx1 (C, J), Cad6 (D, K),Cad8 (E, F, L; E is rostral to F), and Cad11(G). H′–L′, These panels show 4,6-diamidino-2-phenylindole counterstaining ofH–L, respectively. The boxes inA–D and F show the approximate locations of panels of a higher magnification, H–L. The graded patterns of gene expression that are observed at E15.5 are maintained at P2. A, H, Lhx2 is highly expressed in layers 2/3, 5, and 6 of the caudolateral neocortex, corresponding to the putative auditory area (A; box,H), whereas its expression level is much lower in the more medial, putative visual area (A;singlearrow); expression increases again more medially (A; doublearrows). B, I, SCIPexpression is mainly detected in layers 2/3 and layer 5 (I) and is lowest in the auditory area (B; singlearrow) and much higher in the visual area (B; box)—a graded expression pattern opposite to that of Lhx2. The area indicated by doublearrows (B), where Lhx2 expression is slightly higher than that in the putative visual area, exhibits a lower expression level ofSCIP compared with that in the visual area. C, J, Emx1 is expressed in a tangential pattern similar to that of Lhx2 (C;box for the auditory area with higher expression,singlearrow for the visual area with lower expression, and doublearrows for the area with slightly higher expression) and is higher in layers 2/3, 4, and 6 than in layer 5 (J). D, K, Cad6 expression is graded in a high-lateral-to-low-medial pattern (D) with the highest level in layer 5 and the lower aspect in layers 2/3 (K); the putative auditory area shows a high level of expression (D; box), whereas the visual area shows much lower expression (D;doublearrows) with a different layer specificity (mainly in layer 4). Expression between these areas is at lower levels in all layers (D; singlearrow). E, F, L, Cad8 is more highly expressed medially than laterally in layer 5 (E, F; arrowheads) and, in addition, is expressed in the upper layers of the putative motor (E;arrow) and visual (F; box,L) areas. G, Cad11 is expressed higher in the putative visual (doublearrows) than in the auditory (singlearrow) area, without much layer preference. A–D, F, and G are from adjacent sections. Scale bars:A–G, 1 mm; H–L, H′–L′, 100 μm. iz, Intermediate zone; mz,marginal zone; sp, subplate.
The graded differential expression of Lhx2 occurs in a layer-specific manner. At P2, Lhx2 is most highly expressed in layers 2/3, 5, and 6 (see Fig. 3H,H′). Expression by the deep layer neurons is evident as early as E15.5, at which time the CP has strong expression and is mainly populated by future layer 5 and 6 neurons (Caviness, 1982; Frantz et al., 1994). The upper layer expression is already present at E18.5 (see Fig. 4A), which approximately matches the time when the future layer 2/3 neurons start to reach the CP (Caviness, 1982; Frantz et al., 1994).
SCIP, which encodes a POU domain-containing transcription factor (He et al., 1989; Monuki et al., 1989; Suzuki et al., 1990), is expressed in a layer-specific manner in the developing rat neocortex (Frantz et al., 1994), but differential tangential expression has not been reported. At E10.5, SCIP is not expressed in the dorsal telencephalic wall. At E12.5, it is expressed in the PP of the rostral part of the dorsal telencephalon (Fig.1E), as described previously (Frantz et al., 1994). At E14.5, we find that SCIP expression is mostly limited to the IZ and is graded in a high-lateral-to-low-medial pattern (Fig.1F). At E15.5, SCIP expression is also detected in the upper CP throughout most of the neocortex (Fig. 1G), but in a graded pattern opposite to that observed at E14.5 in the IZ, with much lower levels in the caudolateral part of the CP (Fig.1G). Thus, SCIP expression in the CP is complementary to that of Lhx2. At P2, the graded pattern ofSCIP expression is similar to that at E15.5 (see Fig.3B) and in addition has a clear layer specificity (see Fig.3I,I′; high in layer 2/3 and layer 5) as reported previously (Frantz et al., 1994). In addition, a medial part of the caudal neocortex, where Lhx2 expression is at a slightly higher level than in the putative visual area, exhibits lower SCIPexpression, reinforcing the conclusion that graded patterns ofSCIP and Lhx2 expression in the CP are complimentary (see Fig. 3A,B).
Emx1 is a homeodomain transcription factor expressed in embryonic and postnatal mouse neocortex, in both proliferating cells and postmitotic neurons (Simeone et al., 1992; Gulisano et al., 1996). A graded distribution of Emx1 transcripts has not been described, but immunostaining has revealed a graded pattern with higher expression caudolaterally and lower expression rostromedially in postnatal mouse neocortex (Briata et al., 1996). We find thatEmx1 expression in the neocortex, both embryonic and postnatal, is graded. At both E10.5 and E12.5, Emx1expression in the dorsal telencephalic wall is slightly higher medially than laterally (Fig. 1H,I). A high-caudal-to-low-rostral-graded expression pattern is also found at E12.5 (data not shown). At E14.5, Emx1 is expressed highly in the VZ/SVZ and IZ and is still slightly graded in a high-medial-to-low-lateral pattern, whereas the expression in the CP is very low, and graded expression is not detected (Fig.1J). At E15.5, however, Emx1 is expressed most highly in caudolateral neocortex in a pattern similar to that inLhx2 (Fig. 1K); this pattern is still present at P2 (see Fig. 3C). Emx1 expression is high in the putative auditory area, declines medial to it in the putative visual area, but appears to increase further medially (see Fig. 3C). The layer specificity of Emx1 is not as evident as that of Lhx2 or SCIP, but its expression appears to be higher in layers 2/3, 4, and 6 than in layer 5 (see Fig. 3J,J′), which is consistent with the results ofGulisano et al. (1996).
In summary, Lhx2, SCIP, and Emx1 are expressed in graded patterns in the developing neocortex, including the CP, with different degrees of layer specificity. Because the expression of these genes in the CP becomes differential only after TCAs arrive in the cortex (Bicknese et al., 1994), TCAs could play a role in controlling the establishment and maintenance of these differential expression patterns. In addition, the finding that Lhx2 andSCIP expression in the CP is in countergradients to their expression in the VZ/SVZ and IZ, respectively, makes them particularly promising candidates to be potentially regulated by TCAs.
Temporal differences in the onset of areal expression of cadherins
Cad6, Cad8, and Cad11 have been reported to be expressed in area-specific manners in P2 mouse cortex by whole-mountin situ hybridization (Suzuki et al., 1997), andCad6 expression has been reported to be graded in the CP at E14.5 (Inoue et al., 1998). We have examined the expression ofCad6, Cad8, and Cad11 at embryonic and postnatal ages, using both cryosections and whole-mount brains, to determine when they take on their graded and areal patterns of expression.
In agreement with Inoue et al. (1998), we find that Cad6expression is graded in a high-lateral-to-low-medial pattern in the CP as early as E14.5 (Fig.2A). However, in addition, we find that Cad6 is also expressed in the VZ and SVZ at E14.5 in a similarly graded manner to that in the CP (Fig.2A). The expression in the VZ is detected at E12.5, but no expression is evident at E10.5 (data not shown). Cad6expression in the CP appears more clearly graded at E15.5, because of an apparent increase in Cad6 expression in the lateral neocortex, whereas expression in the VZ/SVZ appears to be substantially diminished (Fig. 2B,C). The graded expression in the CP is still present at P2. Caudally, the putative auditory area expresses the highest level of the Cad6 transcript, with the highest expression in layers 2/3 and 5 (Fig.3D,K,K′). The expression level in these layers declines medially (Fig. 3D); although even more medially in the putative visual area, a detectable level ofCad6 is found, but in contrast to that in the auditory area, expression is mainly in layer 4.
Fig. 2.
Graded expression of cadherin genes in the E14.5 and E15.5 mouse neocortex. Coronal sections of mouse forebrain show the expression of Cad6 (A–C) andCad8 (D–F), and sagittal sections show that of Cad11 (G, H).A, At E14.5, expression of Cad6 is already graded in a high-lateral-to-low-medial pattern both in the CP (arrowheads) and in the VZ/SVZ (arrows).B, C, At E15.5, this graded expression becomes more pronounced in the CP (B, C; arrowheads), but the expression declines in the VZ/SVZ, especially at rostral levels (B). The medial part of the neocortex expresses a very low level of Cad6 (B, C;arrows). D, Cad8expression is detected only in the intermediate zone at E14.5 and is not graded (arrowheads). E, F, But at E15.5, it is graded in a high-medial-to-low-lateral pattern in the upper CP (arrowheads for high-medial expression). Rostrocaudal differences in expression are not clear for eitherCad6 or Cad8 (data not shown).G, Cad11 expression in the CP is slightly graded in a high-caudal-to-low-rostral pattern at E14.5 (arrowheads). H, At E15.5, this graded expression becomes more evident (arrowheads).A and D as well as C andF are from adjacent sections. E is slightly caudal to B. Scale bar, 100 μm.
In contrast to the graded expression of Cad6,Cad8 is more uniformly expressed at E12.5 and E14.5 along both the mediolateral (Fig. 2D for E14.5) and rostrocaudal (data not shown) axes, and its expression is most pronounced in the intermediate zone at E14.5 (Fig.2D) and in the PP at E12.5 (data not shown). However, at E15.5 Cad8 expression exhibits a graded pattern with a higher level medially than laterally in the CP (Fig.2E,F). At P2, Cad8 expression has a graded pattern similar to that at E15.5 (Fig. 3E,F) and is strong in layer 5 (Fig. 3L,L′). Interestingly, the laminar pattern of Cad8 expression also shows areal differences. In most of the neocortex, Cad8 expression is primarily limited to layer 5. However, in addition to the layer 5 expression, the putative motor (Fig. 3E) and visual (Fig.3F) areas have higher levels of Cad8expression in layers 2/3 and 4 (Fig. 3L,L′). This upper layer expression is observed as early as E18.5 (Fig.4G,H), which approximately coincides with the arrival of upper layer neurons at the CP (Caviness, 1982; Frantz et al., 1994).
Cad11 expression is detected in the CP, but not in the VZ/SVZ, at all ages examined (E14.5, E15.5, E18.5, and P2). Expression is not detected at E10.5 or E12.5 (data not shown). Cad11exhibits a graded expression in a high-caudal-to-low-rostral pattern in the CP (Fig. 2G,H) as well as a high-medial-to-low-lateral one (Fig. 3G), which are subtle at E14.5 but pronounced by E15.5.
In conclusion, our findings reveal temporal differences in the onset of the graded expression of Cad6, Cad8, andCad11. Because the areal pattern of expression ofCad6 observed postnatally is reflected by its graded expression as early as E14.5 in the CP, as well as in the proliferative layers that give rise to it, the initial establishment of this patterned expression appears to be independent of TCAs. The later onset of the graded expressions of Cad8 and Cad11suggests that TCAs could control the establishment of their expression patterns or be involved in refining and maintaining the patterned expression of all three cadherins.
Graded or areal patterns of gene expression in the neocortex ofMash-1 mutant mice
We next examined whether TCAs are required to establish and/or maintain the graded or areal gene expression patterns described above. For this, we analyzed gene expression in mice deficient forMash-1 (Guillemot et al., 1993), which fail to develop a TCA projection (Tuttle et al., 1999). Because Mash-1 itself is not expressed at detectable levels in the cortex (Torii et al., 1999;Tuttle et al., 1999), differences in the expression patterns between wild-type and mutant littermates would strongly imply a role of TCAs in regulating these patterns. We performed these comparisons of gene expression at E18.5 (which coincides with the day of birth), becauseMash-1 mutant mice die soon after birth (Guillemot et al., 1993).
As shown in Figure 4, Mash-1 mutant mice do not exhibit any significant differences from wild-type mice in the graded, areal, or laminar cortical expression patterns of Lhx2 (Fig.4A–C,A′–C′), SCIP (Fig.4D,D′), Emx1 (Fig.4E,E′), Cad6 (Fig.4F,F′), or Cad11 (Fig.4J,J′). In addition, Cad8 expression in wild-type and mutant mice shows a similarly graded pattern in layer 5 with a higher level medially than laterally (Fig.4G,H,G′,H′) and exhibits area-specific laminar differences in expression, characterized by increased expression in the upper layers of frontal (Fig. 4G,G′) and occipital (Fig.4H,H′) cortex. We did note, however, that the shape and extent of the Cad8 expression domain in rostral cortex differ between wild-type (Fig. 4I) and mutant (Fig.4I′) brains. Specifically, we find that the medial portion of this Cad8 expression domain extends further caudally than normal. This change is also seen in sections and appears to be mainly caused by an abnormal caudal extension of the upper layerCad8 expression typical of frontal cortex (data not shown). These results show that the establishment and maintenance of the graded or areal gene expression patterns described here, with the possible exception of some features of Cad8 expression, do not require TCAs.
DISCUSSION
The primary goal of our study was to determine the requirement of TCAs for establishing and maintaining graded or areal patterns of gene expression in the CP of the developing neocortex. As a prerequisite, we have described several novel patterns of gene expression that may be relevant to neocortical arealization. Among these, we have shown that the regulatory genes Lhx2, SCIP, andEmx1 are expressed in graded patterns in the developing neocortex, including the CP, have defined the onset of the areal expression of Cad8 and Cad11, and have described some unique features of Cad6 expression.
The differential gene expression patterns that we describe become evident at different ages. Graded expression of Lhx2,SCIP, Emx1, and Cad8 in the CP is not detected until E15.5, by which time TCAs have already entered the cortex (Bicknese et al., 1994). Therefore, the time of emergence of these patterns is consistent with the hypothesis that they require TCAs for their establishment. Cad6 and Cad11 show slightly graded patterns of expression in the CP at E14.5, which are more robust at E15.5. The earlier onset of these patterns suggests that they do not require TCAs to be established but may nonetheless require TCAs to be refined and maintained. However, we find that each of these genes exhibits normal-appearing graded or areal expression patterns inMash-1 mutant mice that fail to develop a TCA projection (Tuttle et al., 1999). These findings indicate that TCAs are not required for the establishment or maintenance of the graded and areal expression patterns of the genes analyzed here. The possible exception is the relatively minor change in the rostral expression domain ofCad8, which shows a caudalward extension along its medial edge.
The graded patterns of gene expression that we observe in the CP are layer specific and become apparent soon after the appropriate set of neurons reaches the CP. The graded patterns observed in the CP are unlikely to be maturation dependent, because they are maintained over a long time period extending from the peak of cortical neurogenesis until after neurogenesis has ceased and most, if not all, neurons have reached the CP. In addition, the graded expression of Lhx2and Emx1 would be difficult to explain on the basis solely of gradients of maturation because along the medial–lateral axis the oldest neurons express the highest levels, whereas along the rostral–caudal axis the youngest neurons express the highest levels. For the genes analyzed here, with the exception of Cad6, the graded expression observed in the CP is not seen in the VZ/SVZ or as the postmitotic neurons migrate through the IZ to the CP. Thus, these expression patterns exhibited by CP neurons are not simply a maintenance of the relative expression levels found in their progenitor cells in the VZ/SVZ. This difference between the CP and VZ/SVZ in expression patterns is particularly interesting for Lhx2 andEmx1, because their graded pattern of expression in the CP is the opposite of those in the VZ/SVZ. Likewise, the graded expression of SCIP in the CP is the opposite of that in the IZ. Thus, the regulation of these genes in the CP differs from that in the VZ/SVZ and IZ.
Our findings also suggest that potentially different regulatory mechanisms control the expression of the cadherin genes that we have analyzed. For example, Cad6 is in similarly graded patterns in the VZ and the CP, and therefore its expression pattern is expected to be determined early. In contrast, Cad8 expression appears to be mediolaterally graded only in the CP at E15.5. Later, at E18.5, this graded CP expression is found in layer 5 throughout the neocortex, and layered onto it is Cad8 expression in the upper layers, but only in restricted domains in rostral and occipital cortex. This finding suggests that the graded and areal expression ofCad8 is regulated by mechanisms that are linked to those regulating the layer specificity of cortical cells.
As we were preparing to submit this paper, a study by Miyashita-Lin et al. (1999) was published that addresses a similar issue, although they used a different mutant mouse (deficient for the homeodomain transcription factor gene Gbx2) and examined a different set of genes (Id-2, a helix–loop–helix transcription factor;Tbr-1, a T-box transcription factor; RZR-β, an orphan nuclear receptor; EphA7; and Cad6). As in the Mash-1 mutants (Tuttle et al., 1999), TCAs also fail to project to the neocortex in the Gbx2 mutants (Miyashita-Lin et al., 1999). They found that the graded or differential gene expression patterns, as well as the layer-specific patterns, observed at P0 appear normal in the Gbx2 mutants. The study by Miyahsita-Lin et al. (1999) and our study complement one another well, because the two groups analyzed different genes in different mutants but obtained the similar finding that TCAs are not required to establish or maintain the differential gene expression patterns normally observed in the developing neocortex. When we consider the diverse and large set of genes analyzed in the two studies together, they reinforce one another and strongly support the conclusion that much of differential gene expression in the embryonic neocortex is established by mechanisms intrinsic to the telencephalon.
However, despite the findings presented in our study and that ofMiyashita-Lin et al. (1999), it may be premature to conclude that all differential gene expression in the developing neocortex is TCA independent. Both studies are limited by the fact that theMash-1 and Gbx2 mutants die on the day of birth (E18.5/P0); therefore it was not possible to assess effects that TCAs may have on gene expression at later stages of development. This is a fairly significant caveat because most aspects of area-specific architecture and connectivity emerge postnatally. In addition, a “rerouting” of TCAs to inappropriate target areas of the neocortex, rather than removing TCAs, may provide a more revealing test of their potential influences on cortical gene expression. A late influence of TCAs has been described for maintaining the area-specific distribution of the α1 subunit of the GABAA receptor observed in the somatosensory area of P7 rats, which only begins to emerge a day or two before birth (Paysan et al., 1997). Even the relatively late removal of TCAs by ablation of the dorsal thalamus at P0 results in the loss of expression of this receptor subunit in the somatosensory area (Paysan et al., 1997). This influence of TCAs appears to be activity independent (Penschuck et al., 1999). A particularly intriguing example is the expression of the H-2Z1 transgene that is primarily restricted to layer 4 of the granular parts of somatosensory cortex (Cohen-Tannoudji et al., 1994). Although the identity of an endogenous gene regulated in this manner is not known, findings obtained from cortical slice cultures and heterotopic cortical transplantation suggest that the area-specific expression of the transgene is specified early in embryonic cortical development, even though the transgene itself is not expressed until P2 (Cohen-Tannoudji et al., 1994; Gitton et al., 1999a). Curiously, although cortical slices removed from embryonic mice before TCA ingrowth and cultured for a long term will later express the transgene, transgene expressionin vivo is dramatically attenuated in mice with a neonatal thalamic ablation. Thus, although the early area-specific determination of the transgene expression is independent of extrinsic influences including TCAs, in vivo expression of the transgene does seem to require TCAs (Gitton et al., 1999b).
Similarly, in vivo and in vitro studies on the regional expression of the limbic system-associated protein (LAMP; which is preferential for limbic cortex) and latexin (which is found in the infragranular layers of lateral cortex, including the neocortex and the archicortex) have provided evidence that the regional specification of the cerebral cortex, as measured by the commitment to differential gene expression, occurs early during corticogenesis, probably within the ventricular zone (Barbe and Levitt, 1991; Arimatsu et al., 1992;Ferri and Levitt, 1993), although it is not known whether the in vivo expression of LAMP and latexin requires TCAs. Nevertheless, it is not clear whether it is valid to extrapolate the mechanisms controlling regionalization of the cerebral cortex to the process of arealization of the neocortex.
The graded and areal expression of the genes analyzed here is likely controlled by a combinatorial action of regulatory genes that are differentially expressed at earlier stages in the dorsal telencephalic neuroepithelium, which gives rise to the CP. Candidates include the homeodomain gene Emx2 and the paired domain genePax6 that are expressed at the onset of cortical neurogenesis in countergradients along the rostrolateral-to-caudomedial extent of the dorsal telencephalic neuroepithelium (Walther and Gruss, 1991; Gulisano et al., 1996; Dyck et al., 1997; Mallamaci et al., 1998). These genes have been proposed to be involved in regulating the expression of axon guidance molecules that control the area-specific targeting of TCAs (O'Leary et al., 1994), as well as imparting areal identities to cortical neurons reflected by their gene expression profiles and the axonal connections that they subsequently form (Chenn et al., 1997). In turn, the differential expression of these early regulatory genes is likely controlled by patterning centers localized to the telencephalon (for review, see Rubenstein and Beachy, 1998). A better understanding of the control of differential gene expression patterns intrinsic to the neocortex, as well as other features related to neocortical arealization, will require defining the action and downstream targets of early-expressed regulatory genes such asEmx2 and Pax6 and the even earlier patterning mechanisms that establish their differential expression across the dorsal telencephalic neuroepithelium.
Footnotes
This work was supported by National Institutes of Health Grants NS31558 (D.D.M.O) and NS32817 (J.E.J). Y.N. was supported by the Human Frontier Science Program and the Uehara Memorial Foundation. We thank T. Savage for genotyping Mash-1 mutant embryos, S. Bertuzzi, G. Lemke, S. Mah, and C. Kintner for providing cDNAs, K. Bishop, A. Butler, N. Dwyer, and L. Krubitzer for helpful comments on this manuscript, and K. Yee for help in the blind analysis ofMash-1 mutant embryos.
Correspondence should be addressed to Dr. Dennis D. M. O'Leary, Molecular Neurobiology Laboratory, The Salk Institute, 10010 North Torrey Pines Road, La Jolla, CA 92037. E-mail:dennis_oleary@qm.salk.edu.
REFERENCES
- 1.Arimatsu Y, Miyamoto M, Nihonmatsu I, Hirata K, Uratani Y, Hatanaka Y, Takiguchi-Hayashi K. Early regional specification for a molecular neuronal phenotype in the rat neocortex. Proc Natl Acad Sci USA. 1992;89:8879–8883. doi: 10.1073/pnas.89.19.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbe MF, Levitt P. The early commitment of fetal neurons to the limbic cortex. J Neurosci. 1991;11:519–533. doi: 10.1523/JNEUROSCI.11-02-00519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bicknese AR, Sheppard AM, O'Leary DDM, Pearlman AL. Thalamocortical axons extend along a chondroitin sulfate proteoglycan-enriched pathway coincident with the neocortical subplate and distinct from the efferent path. J Neurosci. 1994;14:3500–3510. doi: 10.1523/JNEUROSCI.14-06-03500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briata P, Di Blas E, Gulisano M, Mallamaci A, Iannone R, Boncinelli E, Corte G. EMX1 homeoprotein is expressed in cell nuclei of the developing cerebral cortex and in the axons of the olfactory sensory neurons. Mech Dev. 1996;57:169–180. doi: 10.1016/0925-4773(96)00544-8. [DOI] [PubMed] [Google Scholar]
- 5.Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- 6.Chenn A, Braisted JE, McConnell SK, O'Leary DDM. Development of the cerebral cortex: mechanisms controlling cell fate, laminar and area patterning, and axonal connectivity. In: Cowan L, Zipursky L, Jessell T, editors. Molecular and cellular approaches to neural development. Oxford UP; Oxford: 1997. pp. 440–473. [Google Scholar]
- 7.Cohen-Tannoudji M, Babinet C, Wassef M. Early determination of a mouse somatosensory cortex marker. Nature. 1994;368:460–463. doi: 10.1038/368460a0. [DOI] [PubMed] [Google Scholar]
- 8.Dehay C, Horsburgh G, Berland M, Killackey H, Kennedy H. Maturation and connectivity of the visual cortex in monkey is altered by prenatal removal of retinal input. Nature. 1989;337:265–267. doi: 10.1038/337265a0. [DOI] [PubMed] [Google Scholar]
- 9.Dehay C, Horsburgh G, Berland M, Killackey H, Kennedy H. The effects of bilateral enucleation in the primate fetus on the parcellation of visual cortex. Brain Res Dev Brain Res. 1991;62:137–141. doi: 10.1016/0165-3806(91)90199-s. [DOI] [PubMed] [Google Scholar]
- 10.Donoghue MJ, Rakic P. Molecular evidence for the early specification of presumptive functional domains in the embryonic primate cerebral cortex. J Neurosci. 1999;19:5967–5979. doi: 10.1523/JNEUROSCI.19-14-05967.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyck RH, Richards LJ, Akazawa C, Contos JJA, Chun J, O'Leary DDM. Graded expression of Emx-1 and Emx-2 in developing rat cortex. Soc Neurosci Abstr. 1997;23:872. [Google Scholar]
- 12.Ferri RT, Levitt P. Cerebral cortical progenitors are fated to produce region-specific neuronal populations. Cereb Cortex. 1993;3:187–198. doi: 10.1093/cercor/3.3.187. [DOI] [PubMed] [Google Scholar]
- 13.Frantz GD, Bohner AP, Akers RM, McConnell SK. Regulation of the POU domain gene SCIP during cerebral cortical development. J Neurosci. 1994;14:472–485. doi: 10.1523/JNEUROSCI.14-02-00472.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gitton Y, Cohen-Tannoudji M, Wassef M. Specification of somatosensory area identity in cortical explants. J Neurosci. 1999a;19:4889–4898. doi: 10.1523/JNEUROSCI.19-12-04889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gitton Y, Cohen-Tannoudji M, Wassef M. Role of thalamic axons in the expression of H 2Z1, a mouse somatosensory cortex specific marker. Cereb Cortex. 1999b;9:611–616. doi: 10.1093/cercor/9.6.611. [DOI] [PubMed] [Google Scholar]
- 16.Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- 17.Gulisano M, Broccoli V, Pardini C, Boncinelli E. Emx1 and Emx2 show different patterns of expression during proliferation and differentiation of the developing cerebral cortex in the mouse. Eur J Neurosci. 1996;8:1037–1050. doi: 10.1111/j.1460-9568.1996.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 18.He X, Treacy MN, Simmons DM, Ingraham HA, Swanson LW, Rosenfeld MG. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989;340:35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- 19.Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev Biol. 1997;183:183–194. doi: 10.1006/dbio.1996.8501. [DOI] [PubMed] [Google Scholar]
- 20.Inoue T, Tanaka T, Suzuki SC, Takeichi M. Cadherin-6 in the developing mouse brain: expression along restricted connection systems and synaptic localization suggest a potential role in neuronal circuitry. Dev Dyn. 1998;211:338–351. doi: 10.1002/(SICI)1097-0177(199804)211:4<338::AID-AJA5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol. 1995;169:347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- 22.Korematsu K, Redies C. Restricted expression of cadherin-8 in segmental and functional subdivisions of the embryonic mouse brain. Dev Dyn. 1997;208:178–189. doi: 10.1002/(SICI)1097-0177(199702)208:2<178::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.Levitt P, Barbe MF, Eagleson KL. Patterning and specification of the cerebral cortex. Annu Rev Neurosci. 1997;20:1–24. doi: 10.1146/annurev.neuro.20.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Mackarehtschian K, Lau CK, Caras I, McConnell SK. Regional differences in the developing cerebral cortex revealed by Ephrin-A5 expression. Cereb Cortex. 1999;9:601–610. doi: 10.1093/cercor/9.6.601. [DOI] [PubMed] [Google Scholar]
- 25.Mallamaci A, Iannone R, Briata P, Pintonello L, Mercurio S, Boncinelli E, Corte G. EMX2 protein in the developing mouse brain and olfactory area. Mech Dev. 1998;77:165–172. doi: 10.1016/s0925-4773(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 26.Miyashita-Lin EM, Hevner R, Wassarman KM, Martinez S, Rubenstein JL. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- 27.Monuki ES, Weinmaster G, Kuhn R, Lemke G. SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron. 1989;3:783–793. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- 28.O'Leary DDM. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989;12:400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- 29.O'Leary DDM, Stanfield BB. Selective elimination of axons extended by developing cortical neurons is dependent on regional locale: experiments utilizing fetal cortical transplants. J Neurosci. 1989;9:2230–2246. doi: 10.1523/JNEUROSCI.09-07-02230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Leary DDM, Schlaggar BL, Tuttle R. Specification of neocortical areas and thalamocortical connections. Annu Rev Neurosci. 1994;17:419–439. doi: 10.1146/annurev.ne.17.030194.002223. [DOI] [PubMed] [Google Scholar]
- 31.Paysan J, Kossel A, Bolz J, Fritschy JM. Area-specific regulation of gamma-aminobutyric acid type A receptor subtypes by thalamic afferents in developing rat neocortex. Proc Natl Acad Sci USA. 1997;94:6995–7000. doi: 10.1073/pnas.94.13.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penschuck S, Giorgetta O, Fritschy JM. Neuronal activity influences the growth of barrels in developing rat primary somatosensory cortex without affecting the expression pattern of four major GABAA receptor alpha subunits. Brain Res Dev Brain Res. 1999;112:117–127. doi: 10.1016/s0165-3806(98)00171-0. [DOI] [PubMed] [Google Scholar]
- 33.Porter FD, Drago J, Xu Y, Cheema SS, Wassif C, Huang SP, Lee E, Grinberg A, Massalas JS, Bodine D, Alt F, Westphal H. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development. 1997;124:2935–2944. doi: 10.1242/dev.124.15.2935. [DOI] [PubMed] [Google Scholar]
- 34.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 35.Rakic P, Suner I, Williams RW. A novel cytoarchitectonic area induced experimentally within the primate visual cortex. Proc Natl Acad Sci USA. 1991;88:2083–2087. doi: 10.1073/pnas.88.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Retaux S, Rogard M, Bach I, Failli V, Besson MJ. Lhx9: a novel LIM-homeodomain gene expressed in the developing forebrain. J Neurosci. 1999;19:783–793. doi: 10.1523/JNEUROSCI.19-02-00783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubenstein JL, Beachy PA. Patterning of the embryonic forebrain. Curr Opin Neurobiol. 1998;8:18–26. doi: 10.1016/s0959-4388(98)80004-4. [DOI] [PubMed] [Google Scholar]
- 38.Schlaggar BL, O'Leary DDM. Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science. 1991;252:1556–1560. doi: 10.1126/science.2047863. [DOI] [PubMed] [Google Scholar]
- 39.Schlaggar BL, O'Leary DDM. Patterning of the barrel field in somatosensory cortex with implications for the specification of neocortical areas. Perspect Dev Neurobiol. 1993;1:81–91. [PubMed] [Google Scholar]
- 40.Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki N, Rohdewohld H, Neuman T, Gruss P, Scholer HR. Oct-6: a POU transcription factor expressed in embryonal stem cells and in the developing brain. EMBO J. 1990;9:3723–3732. doi: 10.1002/j.1460-2075.1990.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 43.Torii M, Matsuzaki F, Osumi N, Kaibuchi K, Nakamura S, Casarosa S, Guillemot F, Nakafuku M. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development. 1999;126:443–456. doi: 10.1242/dev.126.3.443. [DOI] [PubMed] [Google Scholar]
- 44.Tuttle R, Nakagawa Y, Johnson JE, O'Leary DDM. Defects in thalamocortical axon pathfinding correlate with altered cell domains in Mash-1-deficient mice. Development. 1999;126:1903–1916. doi: 10.1242/dev.126.9.1903. [DOI] [PubMed] [Google Scholar]
- 45.Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson DG. In situ hybridization. In: Stern CD, Holland PWH, editors. Essential developmental biology. Oxford UP; Oxford: 1993. pp. 257–274. [Google Scholar]
- 47.Woolsey TA. Peripheral alteration and somatosensory development. In: Coleman J, editor. Development of sensory systems in mammals. Wiley; New York: 1990. [Google Scholar]
- 48.Xu Y, Baldassare M, Fisher P, Rathbun G, Oltz EM, Yancopoulos GD, Jessell TM, Alt FW. LH-2: a LIM/homeodomain gene expressed in developing lymphocytes and neural cells. Proc Natl Acad Sci USA. 1993;90:227–231. doi: 10.1073/pnas.90.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J-H, Pimenta AF, Levitt P, Zhou R. Dynamic expression suggests multiple roles of the eph family receptor brain-specific kinase (Bsk) during mouse neurogenesis. Mol Brain Res. 1997;47:202–214. doi: 10.1016/s0169-328x(97)00051-x. [DOI] [PubMed] [Google Scholar]