Abstract
Psychosis is strongly related to increased striatal dopamine. However, the neural consequences of increased striatal dopamine in psychosis risk are still not fully understood. Consistent with an increase in striatal dopamine, in previous research, psychosis risk has been associated with neural EEG evidence of a greater response to unexpected reward than unexpected punishment feedback on a reversal-learning task. However, previous research has not directly examined whether psychosis risk is associated with altered striatal activation when receiving unexpected feedback on this task. There were two groups of participants: an antipsychotic medication-naive psychosis risk group (n = 21) who had both (a) extreme levels of self-reported psychotic-like beliefs and experiences and (b) interview-rated current-attenuated psychotic symptoms; and a comparison group (n = 20) who had average levels of self-reported psychotic-like beliefs and experiences. Participants completed a reversal-leaning task during fMRI scanning. As expected, in both ROI and whole-brain analyses, the psychosis risk group exhibited greater striatal activation (for whole-brain analyses, the peak was located in the right caudate) to unexpected reward than unexpected punishment feedback relative to the comparison group. These results indicate that psychosis risk is associated with a relatively increased neural sensitivity to unexpected reward than unexpected punishment outcomes and appears consistent with increased striatal dopamine. The results may help us better understand and detect striatal dysfunction in psychosis risk.
Subject terms: Reward, Risk factors
Introduction
The most frequently replicated pathophysiological correlate of psychotic symptoms (e.g., delusions and hallucinations, with schizophrenia-spectrum disorders being the most common psychotic disorder) is increased striatal dopamine [1, 2], with current evidence most consistent with increased presynaptic striatal dopamine. Psychotic disorders are usually preceded by a significant elevation of subthreshold psychotic symptoms (i.e., attenuated psychotic symptoms, APS [3]), during which individuals are considered at risk for psychosis. Using PET, psychosis risk is also associated with increased dopamine [4, 5]. However, PET imaging is probably not feasible for clinical use. Further, the neural consequences of increased striatal dopamine in psychosis risk remain unclear. Understanding these neural consequences could help better understand the nature of psychosis risk and better detect and prevent psychotic disorders. A long line of evidence from studies involving human and nonhuman subjects suggests that striatal dopamine is involved in feedback-related learning, with dopamine differentially released for unexpected reward versus punishment feedback [6–9]. The current study used fMRI to examine striatal activation in psychosis risk when receiving unexpected reward versus unexpected punishment feedback.
With regard to the neurobiology of the striatum, 90–95% of striatal neurons are GABAergic medium spiny neurons (MSNs) [10–12]. In the striatum, these MSNs project to other basal ganglia regions via two main pathways: the Go (i.e., direct) pathway and the No Go (indirect) pathway [8, 13, 14]. The Go pathway involves primarily dopamine D1 receptors, and is activated by phasic bursts of dopamine, especially after better-than-expected reward feedback (i.e., positive prediction error) [11, 12, 15]. The No Go pathway involves primarily inhibitory dopamine D2 receptors, and is activated by phasic dips of dopamine (i.e., decreased activation of the inhibitory D2 receptor), especially after worse-than-expected punishment feedback (i.e., negative prediction error) [15]. Hence, higher striatal dopamine levels lead to increased activation in the Go pathway through D1 receptors, whereas higher dopamine leads to decreased activation (i.e., increased inhibition) of the indirect pathway through D2 receptors [6, 14].
Therefore, if psychosis risk is associated with increased striatal dopamine, then psychosis risk may be associated with increased striatal activation after unexpected reward than after unexpected punishment feedback (i.e., Go > No Go pathway activation). Consistent with this, we previously used EEG to examine neural responses to unexpected reward versus unexpected punishment feedback [16]. We used a reversal-learning task (RLT) developed by Cools et al. [17] that involved periodic unexpected reward and unexpected punishment feedback. In particular, we examined the feedback-related negativity (FRN), and the subsequent P3a, components critical to feedback learning and reward processing [18], and found that psychosis risk was associated with an increase of both the FRN and P3a to unexpected reward versus unexpected punishment feedback. However, our previous EEG study could not examine neural responses directly in the striatum. Conversely, there may be an inverted-U effect, whereby with increased dopamine, there is initially greater Go pathway than No Go pathway activation; however, with even further increased dopamine, there may be greater “noise” (i.e., imbuing too many signals with significance), thereby impairing reinforcement learning and appropriate behavioral responses (and perhaps leading to impaired striatal activation) [15, 19, 20]. Consistent with this, previous research has found reduced ventral striatum activation during reward anticipation associated with psychosis, including evidence for a specific association with negative symptoms of psychosis [21, 22]. In contrast, other research indicates that risk for positive symptoms of psychosis may be associated with heightened reward sensitivity [23], consistent with increased striatal dopamine. The current study is the first to examine whether attenuated psychotic symptoms in an antipsychotic-naive population are associated with neural evidence of increased sensitivity to unexpected reward, which would be expected with increased striatal dopamine.
Methods and materials
Participants
Research procedures were approved by the University of Missouri’s Institutional Review Board, and all participants provided informed consent. Participants were undergraduate students recruited as in previous research (see Supplement for details) [24]. Data from these participants performing another task (one that could not specifically distinguish between response to positive/reward versus negative/punishment feedback) have been previously reported [25].
The current study involved antipsychotic medication-naive people with increased risk for psychosis (i.e., psychosis risk group; n = 20 with useable imaging data; n = 21 with behavioral data) and a comparison group not at increased risk for psychosis (n = 20). Our previous EEG study comparing psychosis risk and a non-risk comparison group on the same RLT found a large neural effect size difference between groups, d = 1.14 [16]. In the current study, given our sample size, our power to detect that same effect size was 0.94. Further, the current study had power of 0.80 to detect a between-group difference effect size = 0.91.
The psychosis risk group (n = 21; Table 1) both (a) scored 1.96 (note, Z = 1.96 is ~97.5% percentile of a normal distribution) sex-normed standard deviations above the mean on the Perceptual Aberration (PerAb) [26] or Magical Ideation (MagicId) [27] Wisconsin Positive Schizotypy Scales that assess self-reported psychotic-like beliefs and experiences (see Supplement for details), or a combined three sex-normed standard deviations above the mean on the PerAb and MagicId scales; and (b) had interview-rated current APS [i.e., a rating of at least moderate on the Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms (SIPS/SOPS), see Supplement for details] [28], with all participants exhibiting past week APS. Previous research has found that people with both elevated self-reported psychotic-like beliefs and experiences and interview-rated APS have a 14% rate of psychotic disorders at 10-year follow-up [29], with an estimated lifetime risk of psychotic disorders >20% [30], a lifetime risk of psychotic disorder as high as relatives with a first-degree family history of psychosis [31]. Hence, the psychosis risk group in the current study had current APS, was antipsychotic medication-naive and had a level of psychotic disorder risk comparable with a first-degree relative sample. One psychosis risk group participant was excluded from brain imaging analyses due to excessive artifacts (i.e., dental braces), leaving 20 psychosis risk group participants for brain imaging analyses (this subject is included in behavioral data analyses).
Table 1.
Sample characteristics of participants
| Psychosis risk group (n = 21) | Comparison group (n = 20) | Test statistic (χ2a or tb) | p-value | |
|---|---|---|---|---|
| Ethnicityc | 7.48a | 0.06 | ||
| Caucasian (%) | 66.7% | 90.0% | ||
| African American (%) | 28.6% | – | ||
| Asian American (%) | 4.8% | 5.0% | ||
| Biracial (%) | – | 5.0% | ||
| Age | 18.29 (0.56) | 18.35 (0.59) | −0.36b | 0.72 |
| Year of education | 13.14 (0.36) | 13.10 (0.31) | 0.18a | 0.68 |
| Sex (% female) | 71.4% | 70.0% | 0.01a | 0.92 |
| Handedness (% left-handed)d | 9.5% | 5% | 0.36a | 0.55 |
| Psychotropic medication use (%)e | 23.8% | 15.0% | 0.51a | 0.48 |
| Stimulants | 9.5% | 5.0% | 0.31a | 0.58 |
| Antidepressants | 19.0% | 10.0% | 0.67a | 0.41 |
| Anxiolytics | 14.3% | 5.0% | 1.00a | 0.32 |
| Wisconsin Schizotypy Scales | ||||
| Magical ideation | 20.05 (3.41) | 7.80 (1.58) | 14.87b | <0.001 |
| Perceptual aberration | 16.90 (6.53) | 4.15 (1.35) | 8.75b | <0.001 |
| SIPS/SOPS Scalesf (% APS) | ||||
| Unusual thought content | 71.43% | 0.00% | 22.53a | <0.001 |
| Suspiciousness | 38.10% | 0.00% | 9.47a | <0.005 |
| Grandiosity | 9.52% | 0.00% | 2.002a | 0.16 |
| Perceptual aberrations | 66.67% | 0.00% | 18.13a | <0.001 |
| Disorganized communication | 33.33% | 0.00% | 8.04a | <0.01 |
SIPS/SOPS Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms
aχ2 tests were used to compare ordinal/binary variables across groups
bMeans (SDs). Independent-samples t tests were used to compare means for the psychosis risk and comparison groups
cPerformance in the psychosis risk group did not significantly differ by ethnicity [i.e., there was neither a main effect of ethnicity for trial type × reaction time analyses, p = 0.32, nor for accuracy, p = 0.57; for striatal ROI analyses, p = 0.49]
dResults when excluding left-handed individuals were extremely similar for all analyses (see Supplement)
eResults when excluding medicated individuals were extremely similar for all analyses (see Supplement)
fSymptoms are rated on a 0–6 scale, ranging from “absent” to “severe and psychotic”; reported percentage having a rating of 3–5, signifying the presence of attenuated psychotic symptoms (APS)
Participants in the comparison group (n = 20; Table 1) scored between −0.6 and 0.6 sex-normed standard deviations around the mean on both the PerAb and MagicId scales. Hence, the comparison group’s mean scores were close to population averages and were not an extreme low-scoring group. In addition, comparison participants had to be rated as not having APS on the SIPS/SOPS.
Reversal-learning task (RLT)
Participants completed a task previously found to activate the striatum, a RLT [16, 17, 32]. In general, on RLTs, participants first learn that one stimulus is rewarded and that a different second stimulus is punished. Subsequently, associations with reward and punishment are switched (i.e., an unexpected feedback trial) and participants have to learn that the first stimulus is now punished and that the second stimulus is now rewarded, with this pattern repeating through a number of reversals. There are two trial types that are most important for this task: (1) unexpected reward trials, an unexpected feedback trial where a stimulus that was previously associated with punishment is now associated with reward; and (2) unexpected punishment trials, an unexpected feedback trial where a stimulus previously associated with reward is now associated with punishment.
During the task, on each trial, two horizontally adjacent stimuli were presented simultaneously, one face and one nature scene (i.e., depicting a mountain range), one of which was highlighted with a yellow border. The participant predicted whether the highlighted stimulus would lead to a reward or a punishment, indicating their prediction by pressing one of two buttons on a response box (see Supplement for timing information). The participant’s response was followed by a feedback message. Reward consisted of a green smiley face and the words ‘You Win!’ and ‘+100’. Punishment consisted of a red sad face and the words ‘You Lose’ and ‘−100’. Through trial and error, participants learned which image was associated with reward or with punishment (note that participants were not actually rewarded or punished, beyond receipt of this visual feedback). Reward and punishment associations would change after the participant got a random number (between 5 and 9) of consecutive trials correct (i.e., unexpected reward and unexpected punishment trials; [17]). Participants completed a total of 448 RLT trials.
Imaging data analysis
Imaging took place on a 3T Siemens Trio scanner with an eight-channel head coil. T1-weighted structural images were acquired for every participant using a high-resolution T1-weighted sagittal scan (MPRAGE sequence, 176 sagittal slices, 1-mm slice thickness, TR = 1920 ms, TE = 2.93, and flip angle = 9). Functional images were acquired for every participant using a T2*-weighted gradient-echo planar pulse sequence (TR = 2500, TE = 25, flip angle = 70, FOV = 256 × 256 mm, and in-plane resolution 3.75 × 3.75 × 4.00 mm; see Supplement for additional details). Imaging data were analyzed using a mixed-effect single-subject general linear model using FSL (http://www.fmrib.ox.ac.uk/fsl) [33]. Motion correction was performed using Motion Correction in FMRIB’s Linear Image Registration Tool [34].
The analyses focused on striatum-related activation associated with feedback from joint Unexpected Reward and Unexpected Punishment, Unexpected Reward, Unexpected Punishment, Unexpected Reward versus Unexpected Punishment, and Unexpected Punishment versus Unexpected Reward [fMRI analyses only included incorrect unexpected trials; see Supplement for all non-striatum whole-brain analyses for each of these trial types; see Supplement for Expected Reward and Expected Punishment analyses (i.e., trials that did not involve a change in reward or punishment stimulus mappings, where, as expected, the groups did not differ in striatal activation)]. In whole-brain analyses, cluster thresholding was used in which contiguous clusters were first identified with a threshold of Z = 3.10 and then used Gaussian random field theory to estimate each cluster’s estimated significance level, with significant clusters having a p < 0.01 FWE-corrected for multiple comparisons [34, 35]. The Yeo atlas [36] was used to identify the networks associated with peak MNI coordinates for significant whole-brain activation.
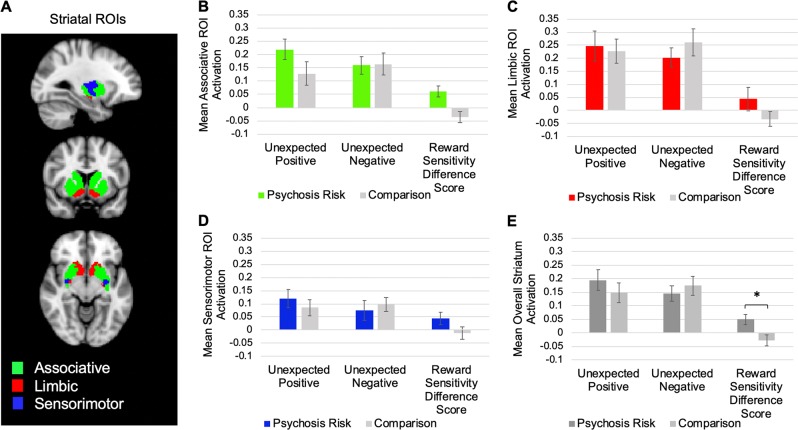
In addition to whole-brain analyses, striatal region of interest (ROI) analyses were conducted. We first examined whether as expected, the striatum was activated both for Unexpected Reward and for Unexpected Punishment trials. We then computed Reward Sensitivity Difference Scores (Unexpected Reward feedback activation minus Unexpected Punishment feedback activation) [16], with higher Reward Sensitivity Difference Scores indicating greater activation for Unexpected Reward than for Unexpected Punishment. The whole striatum was defined as in previous psychosis risk striatum research [25] as the sum of the activation in associative, limbic, and sensorimotor striatal ROIs [37] (Fig. 1a). These ROIs were created based on replicated large sample-resting-state analyses [37] (our associative striatum analyses aggregated the striatal subregions associated with the frontoparietal network (FPN), default mode (DMN), and ventral attention networks; collectively, these subregions have been previously labeled as the associative striatum [5]).
Fig. 1.
a The three striatum regions of interest (ROIs). b Mean associative striatum activation for Unexpected Reward trials, Unexpected Punishment trials, and Reward Sensitivity Difference Scores (Unexpected Reward minus Unexpected Punishment trials) for the psychosis risk and comparison groups. c Mean limbic striatum activation for Unexpected Reward trials, Unexpected Punishment trials, and Reward Sensitivity Difference Scores for the psychosis risk and comparison groups. d Mean sensorimotor striatum activation for Unexpected Reward trials, Unexpected Punishment trials, and Reward Sensitivity Difference Scores for the psychosis risk and comparison groups. e Mean overall striatum activation for Unexpected Reward trials, Unexpected Punishment trials, and Reward Sensitivity Difference Scores for the psychosis risk and comparison groups. For all charts, error bars reflect standard errors. *p < 0.05
For behavioral analyses, for unexpected trials, we examined accuracy and reaction time (RT) on the trial immediately following unexpected feedback (see Supplement for overall RT, overall accuracy, and expected trial analyses). We computed Reward Sensitivity Difference Scores (for accuracy: post unexpected reward trial minus post unexpected punishment trial; for RT: post unexpected punishment trial minus post-unexpected reward trial), with higher Reward Sensitivity Difference Scores indicating more accurate and faster learning after unexpected reward than after unexpected punishment [16]. We focused on comparing the groups on a composite Reversal Learning Score, which was the standardized sum of difference scores for RT and accuracy (see Supplement for correlations between psychosis risk symptoms [i.e., Wisconsin Positive Schizotypy Scales, SIPS subscales] and both striatal activation and behavioral performance).
Results
Imaging analyses
Whole-brain fMRI analyses
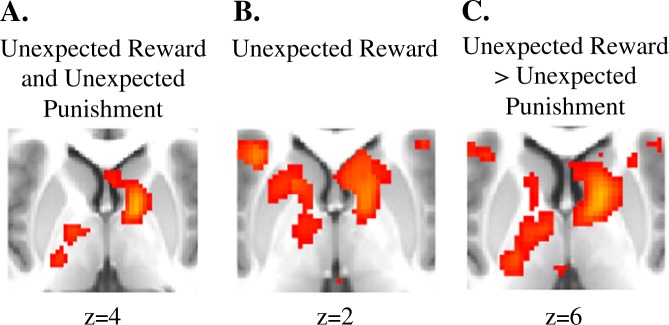
First, we examined striatal/basal ganglia regions (see Supplement for non-striatal/basal ganglia regions) that were significantly jointly activated for both Unexpected Reward and Unexpected Punishment (Unexpected Reward and Unexpected Punishment). As can be seen in Table 2 and Fig. 2a, the psychosis risk group showed significantly greater activation in the striatum/basal ganglia than the comparison participants, with a group difference peak in the right pallidum. For the control group, no striatal regions survived thresholding in whole-brain analyses. Next, we examined striatal regions significantly active on Unexpected Reward trials. As can be seen in Table 2 and Fig. 2b, the psychosis risk group showed significantly greater activation in the bilateral caudate and the bilateral globus pallidus (extending into the nucleus accumbens and putamen) striatal in regions functionally connected to the DMN, FPN, and Limbic networks. For Unexpected Punishment trials, as can be seen in Table 2, while overall the putamen was significantly active, no striatal regions survived thresholding in whole-brain analyses for either the control group or the psychosis risk group.
Table 2.
Whole-brain analyses for basal ganglia/striatal regions significantly active on the reversal-learning task and group differences
| Region | Volume of voxels (mm3) | Peak activity MNI coordinates | Maximum Z statistic | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Overall Unexpected Reward and Unexpected Punishment | |||||
| Right putamen | 629 | 18 | 14 | −6 | 14.6 |
| Regions Significantly More Active for the Comparison Group than Psychosis Risk for Unexpected Reward and Unexpected Punishment | |||||
| – | |||||
| Regions Significantly More Active for Psychosis Risk than the Comparison Group for Unexpected Reward and Unexpected Punishment | |||||
| Right pallidum | 278 | 14 | 2 | 4 | 7.13 |
| Overall Unexpected Reward | |||||
| Right caudate | 16,106 | 14 | 12 | 4 | 17.52 |
| Regions Significantly More Active for the Comparison Group than Psychosis Risk for Unexpected Reward | |||||
| – | |||||
| Regions Significantly More Active for Psychosis Risk than the Comparison Group for Unexpected Reward | |||||
| Right pallidum | 345 | 14 | 8 | 2 | 7.9 |
| Left caudate | 66 | −12 | 12 | 2 | 6.2 |
| Overall Unexpected Punishment | |||||
| Left putamen | 233 | −22 | 10 | 0 | 9.87 |
| Regions Significantly More Active for the Comparison Group than Psychosis Risk for Unexpected Punishment | |||||
| – | |||||
| Regions Significantly More Active for Psychosis Risk than the Comparison Group for Unexpected Punishment | |||||
| – | |||||
| Overall Unexpected Reward > Unexpected Punishment | |||||
| Right caudate | 1893 | 14 | 10 | 8 | 4.43 |
| Right globus pallidus | 2015 | 14 | 4 | 4 | 6.36 |
| Regions Significantly More Active for Psychosis Risk than the Comparison Group for Unexpected Reward > Unexpected Punishment | |||||
| Right globus pallidus | 398 | 14 | 4 | 4 | 6.36 |
| Right caudate | 12 | 10 | 6 | 4.78 | |
| Regions Significantly More Active for the Comparison Group than Psychosis Risk for Unexpected Reward > Unexpected Punishment | |||||
| – | |||||
| Overall Unexpected Punishment > Unexpected Reward | |||||
| – | |||||
| Regions Significantly More Active for the Comparison Group than Psychosis Risk for Unexpected Punishment > Unexpected Reward | |||||
| – | |||||
| Regions Significantly More Active for Psychosis Risk than the Comparison Group for Unexpected Punishment > Unexpected Reward | |||||
| – | |||||
Fig. 2.
a Regions significantly more active for the psychosis risk group than the comparison group for Unexpected Reward and Unexpected Punishment. b Regions significantly more active for the psychosis risk group than the comparison group for Unexpected Reward. c Regions significantly more active for the psychosis risk group than the comparison group for Unexpected Reward > Unexpected Punishment
Next, we examined regions that were both (a) significantly active on Unexpected Reward trials and (b) significantly more active on Unexpected Reward than Unexpected Punishment trials (Unexpected Reward > Unexpected Punishment). As can be seen in Table 2 and Fig. 2c and consistent with the ROI analyses, the psychosis risk group showed significantly greater activation in the bilateral caudate and the bilateral globus pallidus. In the caudate, the peak was in the right associative striatum, more specifically in a subregion previously found to be most functionally connected to the cortical FPN [37] (for regions activated outside of the striatum, see the Supplement). Lastly, we examined regions that were both (a) significantly active on Unexpected Punishment trials and (b) significantly more active on Unexpected Punishment than Unexpected Reward trials (Unexpected Punishment > Unexpected Reward). For both the comparison group and psychosis risk groups, no striatal regions survived thresholding in whole-brain analyses.
Striatal ROIs
Next, we examined whether participants activated the striatum on Unexpected Reward and Unexpected Punishment feedback trials. Overall, participants did exhibit significant striatal activity on both Unexpected Reward, t(39) = 6.50, p < 0.01, d = 1.03, 95% CI [0.64, 1.41], and Unexpected Punishment trials, t(39) = 7.14, p < 0.01, d = 1.13, 95% CI [0.73, 1.52]. Hence, as expected, participants did strongly activate the striatum when they received unexpected feedback on the RLT. However, the groups did not significantly differ on either overall unexpected reward (overall: t(38) = 0.91, p = 0.37, d = 0.29; associative: t(38) = 1.55, p = 0.13, d = 0.49; limbic: t(38) = 0.25, p = 0.80, d = 0.08; sensorimotor: t(38) = 0.74, p = 0.47, d = 0.23; see Table 3 and Fig. 1) or unexpected punishment (overall: t(38) = −0.63, p = 0.53, d = 0.10; associative: t(38) = −0.09, p = 0.93, d = 0.03; limbic: t(38) = −0.91, p = 0.37, d = 0.29; sensorimotor: t(38) = −0.47, p = 0.64, d = 0.15), although if anything, numerically, the psychosis risk showed greater striatal activation for unexpected rewards (especially for the associative striatum) and reduced activation for unexpected punishments.
Table 3.
Striatal region of interest (ROI) means (and standard deviations) for each group for the reversal-learning task (RLT)
| Group | ||||
|---|---|---|---|---|
| Striatal region | Psychosis risk | Comparison | t Statistic | p-value |
| Unexpected Reward | ||||
| Associative | 0.219 (0.174) | 0.128 (0.197) | 1.55 | 0.13 |
| Limbic | 0.246 (0.256) | 0.228 (0.209) | 0.25 | 0.80 |
| Sensorimotor | 0.119 (0.153) | 0.085 (0.138) | 0.74 | 0.47 |
| Overall striatum | 0.195 (0.173) | 0.147 (0.160) | 0.91 | 0.37 |
| Unexpected Punishment | ||||
| Associative | 0.159 (0.149) | 0.164 (0.183) | −0.09 | 0.93 |
| Limbic | 0.203 (0.166) | 0.261 (0.229) | −0.91 | 0.37 |
| Sensorimotor | 0.075 (0.173) | 0.097 (0.123) | −0.47 | 0.64 |
| Overall striatum | 0.146 (0.129) | 0.174 (0.155) | −0.63 | 0.53 |
| Reward Sensitivity Difference Score | 0.049 (0.089)* | −0.027 (0.090) | 2.69 | 0.01 |
Next, we examined whether the psychosis risk group exhibited altered neural activation, such that they tended to exhibit relatively increased activation for Unexpected Reward than for Unexpected Punishment trials (Unexpected Reward > Unexpected Punishment). As expected, using a 2 (trial type: Unexpected Reward, Unexpected Punishment) × 2 (group: psychosis risk, comparison) ANOVA, there was a significant trial type by group interaction of a large effect size, F(1,38) = 7.22, p = 0.011, d = 0.85, 95% CI [0.19, 1.48]. As can be seen in Table 3 and Fig. 1, the difference in overall striatal activation between Unexpected Reward and Unexpected Punishment trials (i.e., the Reward Sensitivity Difference Score) was significantly larger in the psychosis risk group than in the comparison group, such that the psychosis risk group demonstrated greater Unexpected Reward > Unexpected Punishment striatal activation relative to the comparison group [note there was not a significant group × striatal region (i.e., associative, limbic, and sensorimotor) interaction, p = 0.23; see Supplement]. Hence, the psychosis risk group showed greater neural sensitivity to unexpected reward versus unexpected punishment feedback than the comparison group.
Behavioral results
For the Reward Learning Score (i.e., standardized sum of Reward Sensitivity Difference Scores for RT and accuracy), there was not a significant difference between the groups, t(39) = 1.34, p = 0.19, d = 0.41, 95% CI [−0.20, 1.04], although if anything, the psychosis risk group showed nonsignificantly greater sensitivity to Unexpected Reward (i.e., better learning for Unexpected Reward > Unexpected Punishment; see Supplemental Table 1) than the comparison group (and note that the effect size in the current study, d = 0.41, is similar to the effect size in our previous study, d = 0.44) [16]; further, note that if we meta-analyze results for the two studies [38], there is a trend for a significant group difference in the Reward Learning Score, d = 0.43 [0.02, 0.87].
Discussion
Increased striatal dopamine in psychosis risk is expected to result in differential neural activation for unexpected reward compared with unexpected punishment feedback [6, 39]. For the first time, the current study used fMRI to examine striatal activation in an antipsychotic-naive psychosis risk group and found that psychosis risk was associated with relatively greater striatal/basal ganglia activation for unexpected reward than for unexpected punishment feedback relative to a non-risk comparison group in both ROI and whole-brain analyses (hence ROI analyses replicated patterns of striatal activation seen in whole-brain analyses). In whole-brain analyses, the psychosis risk group showed greater unexpected reward than unexpected punishment striatal activation in both the associative striatum portion of the right caudate and globus pallidus. There was also evidence that greater unexpected reward than unexpected punishment striatal activation was specifically associated with psychosis risk symptoms (see Supplement). The increased striatal activation to unexpected rewards is consistent with increased striatal dopamine [15] and greater Go than No Go pathway activation [6]. These findings contribute to the wealth of research, indicating the importance of the striatum in psychosis and indicate that altered striatal functioning can be detected in a group experiencing attenuated psychotic symptoms.
The current results for psychosis risk striatal activation for unexpected reward compared with unexpected punishment feedback are generally consistent with previous research. For example, our results are consistent with the previous EEG study which found that psychosis risk was associated with relatively greater sensitivity to unexpected reward than negative feedback on the RLT [16]. The current results are also consistent with imaging research examining psychosis risk. In particular, one previous study found that increased psychosis polygenetic risk scores are associated with increased striatal activation during reward anticipation [40]. Similarly, another study found that positive symptoms of psychosis (i.e., delusions and hallucinations) were positively correlated with striatal activation for reward anticipation in a psychosis risk group [41]. Recent psychosis risk research also indicates that increased striatal-to-midbrain connectivity positively correlates with severity of delusional ideation [42]. Thus, the current study’s findings are generally consistent with a previous research finding that psychosis risk is associated with increased reward sensitivity [23].
However, the current results finding increased neural activation for unexpected reward than unexpected punishment feedback in psychosis risk appear generally different from what has been reported in psychotic disorders. In psychotic disorders, there is evidence of a blunted responses to reward feedback [22, 43] and blunted striatal activation to prediction errors [44, 45], including in unmedicated samples [46, 47]. However, the results for prediction errors in ultra-high-risk groups are mixed, with some evidence of increased striatal prediction error processing [42], although other research finds blunted striatal prediction error signals [48] or no significant evidence of striatal impairment [19], indicating that perhaps the degree of striatum-related reinforcement learning impairments depends on the degree of current dopaminergic dysfunction [19]. Furthermore, these previous studies did not specifically examine striatal activation for reward versus punishment on a RLT, as we did in the current study.
We have previously reported that psychosis risk is associated with decreased striatal activation on a different feedback-driven learning task, the Probabilistic Category Learning Task (PCLT) [25]. The current study reported that psychosis risk was associated with differential striatal activation for unexpected reward versus unexpected punishment feedback, whereas for the PCLT, psychosis risk is associated with decreased striatal activation. Together, these results appear very consistent with previous research on Parkinson’s disease [49]. Among other neural changes [50], Parkinson’s disease involves degeneration in the ventral substantia nigra, resulting in decreased dorsal striatum dopamine. In an unmedicated, low-dopamine state, people with Parkinson’s disease exhibit better learning from negative than positive outcomes [8]. Nevertheless, despite exhibiting sensitivity to negative outcomes, when people with Parkinson’s disease perform the PCLT, there is evidence of decreased striatal activation [51]. Therefore, the current psychosis risk RLT results (i.e., differential striatal activation for unexpected reward compared with unexpected punishment feedback) when combined with prior psychosis risk PCLT results (i.e., decreased striatal activation for feedback) appear generally consistent with Parkinson’s disease research.
One strength of the current study is that the results cannot be attributed to antipsychotic medication effects, as all participants were antipsychotic-naive. The results of previous psychotic disorder studies may have potentially been conflated with medication use [45] (although as previously mentioned, some research indicates that unmedicated psychosis risk may be associated with attenuated striatum activation when learning from rewards [47]), as dopamine antagonist antipsychotic medication permanently alters striatal functioning. For example, medication usage is associated with both a significantly increased number of striatal dopamine receptors [52] and exerting a strong influence on caudate volume and therefore caudate neural activation [53, 54]. Thus, antipsychotic medication may potentially preclude finding evidence of a relationship between current psychosis and striatal functioning [55]. Another possibility is that while medication explains much of these differences, as previously mentioned, perhaps, these results can be partially explained by an inverted U of dopaminergic functioning, whereby further elevations in dopamine (as seen in full-blown psychotic disorders) might result in impaired reinforcement learning [15]. In addition, another difference between the current study and previous psychotic disorder studies is that previous psychotic disorder studies tend to use chronic samples and find that blunted reward responding is associated with negative symptomology (whereas the current study focused on recruiting a psychosis risk group elevated in current positive symptomology) [15].
Furthermore, finding different results in unmedicated versus medicated samples seems consistent with previous research on Parkinson’s disease. As previously mentioned, people with Parkinson’s disease when unmedicated (i.e., low dopamine state) exhibit increased sensitivity to negative versus positive feedback. However, when medicated (i.e., high dopamine state), this is reversed and now they exhibit increased sensitivity to positive versus negative feedback [8]. Similar to the results in Parkinson’s disease, the results in psychosis risk and in people with psychotic disorders appear to vary depending on medication status. Unmedicated psychosis risk (high dopamine state) individuals generally exhibit increased neural activity for positive relative to negative feedback, but medicated people with psychotic disorders (with dopamine D2 receptor blockade) generally exhibit blunted neural activity for rewards.
Several limitations of the current study should be noted. First, a limitation of the study is the relatively small sample size (n = 20 per group), although, as previously mentioned, the imaging analyses examining striatal activation were well powered. Regardless, these results should be considered preliminary and replication is required [56]. Second, the current study did not assess several variables previously linked to striatal functioning, including psychiatric diagnoses and recreational drug use, including cannabis use [57]. Future research should examine how other dimensions of psychopathology (e.g., substance abuse and current functioning) relate to striatal functioning in psychosis risk. Third, although the current study examined striatal activation associated with psychosis risk, the results cannot directly speak to whether these activation differences are the result of increased striatal dopamine. Fourth, we failed to find significant behavioral evidence for greater sensitivity to reward than punishments. This lack of significant behavioral findings may indicate that in this sample, the increased striatal dopamine was sufficient to detect via fMRI but insufficient to result in significant behavioral effects. It is also possible that activation in regions associated with FPN, especially during unexpected punishment feedback, may have “overridden” any effects that striatal dopamine had on performance. These speculative hypotheses should be further tested in future work. In addition, to further characterize Go pathway activation, future research should examine cortico-striatal functional connectivity to examine whether patterns of activation are consistent with activation of the Go pathway and inhibition of the No Go pathway in psychosis risk. Lastly, the current study used a complex RLT to examine responses to unexpected reward and punishments. However, this is arguably not ideal because reversal learning involves complex processes that go beyond mere responses to unexpected rewards and punishments. Other tasks (including simpler tasks examining rewards and punishments using actual monetary rewards) should examine whether psychosis risk is associated with increased striatal activation to unexpected rewards than punishments.
Our study demonstrates that psychosis risk is associated with relatively increased sensitivity to unexpected reward than to unexpected punishment feedback, as indicated by greater striatal activation. This increased sensitivity predates the emergence of a psychotic disorder, and therefore cannot be attributed to chronic illness or medication effects. These results are also consistent with increased striatal dopamine in psychosis risk. Overall, the current study suggests that altered striatal activation to unexpected reward compared with unexpected punishment outcomes may be a potential biomarker of psychosis risk.
Supplementary information
Funding and Disclosure
The authors declare that there are no conflicts of interest in relation to the subject of this study. This research was supported by the National Institute of Mental Health (T32 MH014677 to NRK and MH100359 to JGK) and by the University of Missouri research funds (to JGK).
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at (10.1038/s41386-019-0455-z).
References
- 1.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III-the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesby JP, Eyles DW, McGrath JJ, Scott JG. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl Psychiatry. 2018;8:30. doi: 10.1038/s41398-017-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGlashan TH. Commentary: progress, issues, and implications of prodromal research: an inside view. Schizophr Bull. 2003;29:851–8. doi: 10.1093/oxfordjournals.schbul.a007051. [DOI] [PubMed] [Google Scholar]
- 4.Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 5.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 6.Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cognit Neurosci. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- 7.Klanker M, Sandberg T, Joosten R, Willuhn I, Feenstra M, Denys D. Phasic dopamine release induced by positive feedback predicts individual differences in reversal learning. Neurobiol Learn Mem. 2015;125:135–45. doi: 10.1016/j.nlm.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–3. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 9.Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 2012;15:1281–9. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerfen Charles R. The Rat Nervous System. 2004. Basal Ganglia; pp. 455–508. [Google Scholar]
- 11.Nelson AB, Kreitzer AC. Reassessing models of basal ganglia function and dysfunction. Annu Rev Neurosci. 2014;37:117–35. doi: 10.1146/annurev-neuro-071013-013916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–66. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcott PF, Gong S, Donthamsetti P, Grinnell SG, Nelson MN, Newman AH, et al. Regional heterogeneity of D2-receptor signaling in the dorsal striatum and nucleus accumbens. Neuron. 2018;98:575–87.e4. doi: 10.1016/j.neuron.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP, Kreitzer AC. Striatal cholinergic interneurons drive GABA release from dopamine terminals. Neuron. 2014;82:63–70. doi: 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maia TV, Frank MJ. An integrative perspective on the role of dopamine in schizophrenia. Biol Psychiatry. 2017;81:52–66. doi: 10.1016/j.biopsych.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karcher NR, Bartholow BD, Martin EA, Kerns JG. Associations between electrophysiological evidence of reward and punishment-based learning and psychotic experiences and social anhedonia in at-risk groups. Neuropsychopharmacology. 2017;42:925–32. doi: 10.1038/npp.2016.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–43. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.San Martin R. Event-related potential studies of outcome processing and feedback-guided learning. Front Hum Neurosci. 2012;6:304. doi: 10.3389/fnhum.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ermakova OA, Knolle F, Justicia A, Bullmore ET, Jones PB, Robbins TW, et al. Abnormal reward prediction-error signalling in antipsychotic naive individuals with first-episode psychosis or clinical risk for psychosis. Neuropsychopharmacology. 2018;43:1619–99. doi: 10.1038/s41386-018-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt A, Antoniades M, Allen P, Egerton A, Chaddock CA, Borgwardt S, et al. Longitudinal alterations in motivational salience processing in ultra-high-risk subjects for psychosis. Psychol Med. 2017;47:243–54. doi: 10.1017/S0033291716002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72:1243–51. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- 22.Strauss GP, Cohen AS. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull. 2017;43:712–9. doi: 10.1093/schbul/sbx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ermel JA, Moran EK, Culbreth AJ, Barch DM. Psychotic like experiences as part of a continuum of psychosis: associations with effort-based decision-making and reward responsivity. Schizophr Res. 2018;206:307–312. doi: 10.1016/j.schres.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Karcher NR, Martin EA, Kerns JG. Examining associations between psychosis risk, social anhedonia, and performance of striatum-related behavioral tasks. J Abnorm Psychol. 2015;124:507–18. doi: 10.1037/abn0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karcher NR, Hua JPY, Kerns JG. Probabilistic category learning and striatal functional activation in psychosis risk. Schizophr Bull. 2018;45:396–404. doi: 10.1093/schbul/sby033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. J Abnorm Psychol. 1978;87:399–407. doi: 10.1037/0021-843X.87.4.399. [DOI] [PubMed] [Google Scholar]
- 27.Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. J Consult Clin Psychol. 1983;51:215–25. doi: 10.1037/0022-006X.51.2.215. [DOI] [PubMed] [Google Scholar]
- 28.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–15. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 29.Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. J Abnorm Psychol. 1994;103:171–83. doi: 10.1037/0021-843X.103.2.171. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen CB, Mors O, Bertelsen A, Waltoft BL, Agerbo E, McGrath JJ, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry. 2014;71:573–81. doi: 10.1001/jamapsychiatry.2014.16. [DOI] [PubMed] [Google Scholar]
- 31.Faridi K, Pawliuk N, King S, Joober R, Malla AK. Prevalence of psychotic and non-psychotic disorders in relatives of patients with a first episode psychosis. Schizophr Res. 2009;114:57–63. doi: 10.1016/j.schres.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Karcher NR, Cicero DC, Kerns JG. An experimental examination of the aberrant salience hypothesis using a salience manipulation and a behavioral magical thinking task. J Exp Psychopathol. 2015;6.jep.041814.
- 33.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 34.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- 35.Woo C-W, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–9. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–63. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. http://www.jstatsoft.org/v36/i03/.
- 39.Cox SM, Frank MJ, Larcher K, Fellows LK, Clark CA, Leyton M, et al. Striatal D1 and D2 signaling differentially predict learning from positive and negative outcomes. Neuroimage. 2015;109:95–101. doi: 10.1016/j.neuroimage.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 40.Lancaster TM, Linden DE, Tansey KE, Banaschewski T, Bokde AL, Bromberg U, et al. Polygenic risk of psychosis and ventral striatal activation during reward processing in healthy adolescents. JAMA Psychiatry. 2016;73:852–61. doi: 10.1001/jamapsychiatry.2016.1135. [DOI] [PubMed] [Google Scholar]
- 41.Wotruba D, Heekeren K, Michels L, Buechler R, Simon JJ, Theodoridou A, et al. Symptom dimensions are associated with reward processing in unmedicated persons at risk for psychosis. Front Behav Neurosci. 2014;8:382. doi: 10.3389/fnbeh.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winton-Brown T, Schmidt A, Roiser JP, Howes OD, Egerton A, Fusar-Poli P, et al. Altered activation and connectivity in a hippocampal-basal ganglia-midbrain circuit during salience processing in subjects at ultra high risk for psychosis. Transl Psychiatry. 2017;7:e1245. doi: 10.1038/tp.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor N, Hollis JP, Corcoran S, Gross R, Cuthbert B, Swails LW, et al. Impaired reward responsiveness in schizophrenia. Schizophr Res. 2018;199:46–52. doi: 10.1016/j.schres.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 44.Morris RW, Vercammen A, Lenroot R, Moore L, Langton JM, Short B, et al. Disambiguating ventral striatum fMRI-related BOLD signal during reward prediction in schizophrenia. Mol Psychiatry. 2012;17:235. doi: 10.1038/mp.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13:239. doi: 10.1038/sj.mp.4002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlagenhauf F, Huys QJ, Deserno L, Rapp MA, Beck A, Heinze HJ, et al. Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. Neuroimage. 2014;89:171–80. doi: 10.1016/j.neuroimage.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reinen JM, Van Snellenberg JX, Horga G, Abi-Dargham A, Daw ND, Shohamy D. Motivational context modulates prediction error response in schizophrenia. Schizophr Bull. 2016;42:1467–75. doi: 10.1093/schbul/sbw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millman ZB, Gallagher K, Demro C, Schiffman J, Reeves GM, Gold JM, et al. Evidence of reward system dysfunction in youth at clinical high-risk for psychosis from two event-related fMRI paradigms. Schizophr Res. (pii: S0920-9964(19)30111-2 2019. [E-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 49.Vo A, Seergobin KN, Morrow SA, MacDonald PA. Levodopa impairs probabilistic reversal learning in healthy young adults. Psychopharmacology. 2016;233:2753–63. doi: 10.1007/s00213-016-4322-x. [DOI] [PubMed] [Google Scholar]
- 50.Robbins TW, Cools R. Cognitive deficits in Parkinson’s disease: a cognitive neuroscience perspective. Mov Disord. 2014;29:597–607. doi: 10.1002/mds.25853. [DOI] [PubMed] [Google Scholar]
- 51.Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson’s disease. Behav Neurosci. 2004;118:438–42. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- 52.Samaha AN, Seeman P, Stewart J, Rajabi H, Kapur S. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27:2979–86. doi: 10.1523/JNEUROSCI.5416-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Zoltick B, et al. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry. 2008;63:475–83. doi: 10.1016/j.biopsych.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 54.van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, et al. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–66. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- 55.Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–10. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- 56.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 57.Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, et al. Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry. 2016;73:838–44. doi: 10.1001/jamapsychiatry.2016.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.