Abstract
Few studies have investigated the link between putative biomarkers of attention-deficit/hyperactivity disorder (ADHD) symptomatology and genetic risk for ADHD. To address this, we investigate the degree to which ADHD symptomatology is associated with white matter microstructure and cerebral cortical thickness in a large population-based sample of adolescents. Critically, we then test the extent to which multimodal correlates of ADHD symptomatology are related to ADHD polygenic risk score (PRS). Neuroimaging, genetic, and behavioral data were obtained from the IMAGEN study. A dimensional ADHD composite score was derived from multi-informant ratings of ADHD symptomatology. Using tract-based spatial statistics, whole brain voxel-wise regressions between fractional anisotropy (FA) and ADHD composite score were calculated. Local cortical thickness was regressed on ADHD composite score. ADHD PRS was based on a very recent genome-wide association study, and calculated using PRSice. ADHD composite score was negatively associated with FA in several white matter pathways, including bilateral superior and inferior longitudinal fasciculi (p < 0.05, corrected). ADHD composite score was negatively associated with orbitofrontal cortical thickness (p < 0.05, corrected). The ADHD composite score was correlated with ADHD PRS (p < 0.001). FA correlates of ADHD symptomatology were significantly associated with ADHD PRS, whereas cortical thickness correlates of ADHD symptomatology were unrelated to ADHD PRS. Variation in hyperactive/inattentive symptomatology was associated with white matter microstructure, which, in turn, was related to ADHD PRS. Results suggest that genetic risk for ADHD symptomatology may be tied to biological processes affecting white matter microstructure.
Subject terms: Neuroscience, Diseases of the nervous system, Developmental disorders
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most prevalent neuropsychiatric conditions among children and adolescents, and symptoms include a developmentally inappropriate pattern of inattention and/or hyperactivity/impulsivity [1, 2]. The overall prevalence of ADHD is approximately 7.2% in children, and 2.5% in adults [3]. Importantly, heritability estimates for ADHD range from 0.70 to 0.80, with little evidence of shared environmental factors influencing ADHD symptomatology [4]. Despite such findings, there has been little research on the degree to which putative brain-based biomarkers of ADHD symptomatology are associated with genetic risk for ADHD. The advent of large population-based neuroimaging studies affords the opportunity to delineate relations between polygenic risk, brain structure, and ADHD psychopathology. Such efforts are greatly needed in order to further elucidate the neurodevelopmental underpinnings of ADHD symptomatology.
More accurate phenotypic characterizations of ADHD symptomatology are essential in linking genetic risk, brain structure, and behavior. Among clinicians and researchers, ADHD has been traditionally conceptualized as a categorical, or dichotomous, construct. Such views are consistent with the prevailing diagnostic taxonomy, the Diagnostic and Statistical Manual of Mental Disorders [1, 2]. In the past decade, however, empirically based assessment of psychopathology has revealed aspects of dimensionality with regard to many psychiatric disorders, including ADHD [5]. Intriguingly, evidence for dimensionality of ADHD has also been observed at a genetic level. For example, it has been previously reported that polygenic risk for ADHD predicts dimensional measures of hyperactivity and inattention in population-based samples of children [6]. Such findings indicate genetic overlap between clinical and normative levels of ADHD symptomatology, and provide additional support for a dimensional conceptualization of ADHD [6, 7].
Paralleling genetic findings, a number of neuroimaging studies indicate largely overlapping neuroanatomical correlates with regard to clinical and subclinical levels of ADHD symptomatology [8–12]. Ducharme et al. [8] revealed that subclinical attention problems in typically developing youths were associated with a decreased rate of cerebral cortical thinning in frontal regions—largely overlapping with cortical areas in which delayed cortical thickness maturation has been observed in ADHD participants [8, 9, 13, 14]. Similarly, Shaw et al. [9] reported that clinical and subclinical symptoms of hyperactivity and impulsivity were associated with delayed cortical thickness maturation, predominantly in prefrontal cortical regions. Shaw et al. [9] found that youths with higher levels of hyperactivity/impulsivity had a slower rate of cortical thinning—and youths with ADHD had the slowest rate of cortical thinning. Taken together, there is compelling evidence that both normative and clinical levels of ADHD symptomatology share overlapping neural and genetic substrates—and that these associations may be obfuscated by strict categorical characterization of psychopathology.
Whereas most structural studies of ADHD have focused on gray matter structure, relatively few have investigated white matter correlates of ADHD symptomatology. Even fewer studies have examined associations between white matter microstructure and dimensional measures of psychopathology in population-based samples [15]. With regard to white matter microstructure, prior research indicates that the metric of fractional anisotropy (FA) reflects several properties of cerebral white matter, including packing density of axons, axonal diameter, and degree of myelination [16, 17]. Given that myelination is a protracted developmental process that continues into adulthood, and that genetic investigations of ADHD have implicated genes involved in axonal development, diffusion imaging may help to elucidate relations between genetic risk and adolescent ADHD symptomatology [18, 19]. It is also possible that altered FA may reflect other aspects of white matter microarchitecture, independent of myelination, among individuals with ADHD symptomatology. Nonetheless, a growing body of literature indicates altered white matter microstructure among ADHD patients relative to typically developing controls. In particular, recent diffusion imaging studies of adolescent and adult participants with ADHD, utilizing relatively large samples (i.e., >200), have reported aberrant white matter microstructure in the corpus callosum (CC), superior longitudinal fasciculus (SLF), and the internal capsule [20, 21]. To the best of our knowledge, no prior studies have examined putative associations between white matter microstructure and dimensional assessments of ADHD symptomatology using a population-based sample of youths.
In the present study, we first test the extent to which ADHD polygenic risk score (PRS) is associated with dimensional assessments of ADHD symptomatology in a population-based sample of youths. Next, we investigate the degree to which ADHD symptomatology is associated with white matter microstructure and cerebral cortical thickness in the same population-based sample of adolescents. Importantly, we then test the extent to which white matter and cerebral cortical correlates of ADHD symptomatology are associated with ADHD PRS.
Methods and materials
Sample
Neuroimaging and behavioral data were obtained from the IMAGEN study conducted across eight European sites in France, the United Kingdom, Ireland, and Germany, which includes 2223 adolescents recruited from schools at age 14 years (age range = 12.9–15.7 years). Recruitment into the IMAGEN study targeted adolescents for whom all four grandparents were the same nationality as the participant (i.e., participants were required to have four Western European grandparents); as such, the sample is racially and ethnically homogenous. Local ethics research committees approved the study at each site. Written consent was obtained from the parent or guardian as well as verbal assent from the adolescent. A detailed description of recruitment and assessment procedures has been published elsewhere [22]. Only participants possessing multi-informant psychopathology data, quality controlled neuroimaging data, and complete demographic data were included in the present study.
Psychopathology assessment
The Development and Well-Being Assessment (DAWBA) [23] is a computer-based package of questionnaires, interviews, and rating techniques used to assess adolescent psychopathology. In the present study, ADHD symptom scores were derived from the parent version of the DAWBA—youths did not complete the DAWBA ADHD module. In addition to total symptom score, the parent version of the DAWBA yielded separate scores for both Hyperactivity/Impulsivity and Inattention.
Self-report and parent report versions of the Strengths and Difficulties Questionnaire (SDQ) were also used to assess symptoms of hyperactivity and inattention [24]. In addition to the Hyperactive/Inattentive scale, the Emotional scale on the youth SDQ was utilized to assess mood and anxiety symptomatology. The SDQ is a reliable and valid measure of youth emotional and behavior symptoms, on which scores are predictive of increased probability of clinician-rated psychiatric disorders and retest stability over 4–6 months [25].
A dimensional, multi-informant ADHD composite was derived by standardizing DAWBA ADHD total symptom score, as well as parent and youth SDQ Hyperactive/Inattentive scores, and summing the standardized z-scores.
ADHD PRS
ADHD PRS in the present study was based on a very recent ADHD genome-wide association (GWAS) [26]. Importantly, this GWAS by Demontis et al. [26] represents the first report of genome-wide significant risk loci for ADHD, with 12 independent loci surpassing genome-wide significance. Of note, genotype data from IMAGEN were not used in this study.
In order to calculate the ADHD PRS score, summary statistics were downloaded from the Psychiatric Genomics Consortium (http://www.med.unc.edu/pgc/results-and-downloads; 18,381 cases and 27,969 controls, all European descent). Calculating a PRS score requires the use of a significance threshold for inclusion of single-nucleotide polymorphisms (SNPs) in that score. In cases where a researcher is only interested in the strength of the relation between the score and the phenotype, the threshold for an SNP to be included in the PRS score can be selected as that which leads to the highest variance explained between the phenotype and the score [27, 28]. However, in the present investigation, we were interested in associations of the PRS scores with both the ADHD behavioral phenotype, and neuroimaging measures. In this case, varying the threshold for inclusion in the score can lead to issues of circularity in analyzing the neuroimaging data, as the PRS score would then already be optimized to associate with the ADHD composite score used here. For this reason, we pre-selected the use of a nominal p = 0.05 threshold for inclusion of SNPs in the calculation of the PRS score, which avoids this overfitting/circularity problem. All genetic data processing and analyses were performed using the R package PRSice [28] and PLINK version 1.90.
ADHD PRS was available for 1597 of the 1753 participants (91.1%) included in cortical surface-based analyses, and 1341 of the 1471 participants (91.2%) included in diffusion imaging analyses.
MRI acquisition
Magnetic resonance imaging scanning was conducted at the eight IMAGEN assessment sites using 3 T whole-body MRI systems [22]. See Supplementary Materials for further details.
Diffusion MRI
Diffusion data preprocessing was implemented with the FMRIB Diffusion Toolbox in FSL (http://www.fmrib.ox.ac.uk/fsl). Preprocessing of the data included the following steps: (1) affine registration to the first b = 0 image in order to correct for head motion and eddy current distortion; (2) brain extraction using the FSL brain extraction tool [29]; and (3) voxel-wise diffusion tensor fitting in order to create FA images. Voxel-wise statistical analysis of subjects’ FA data was performed using TBSS (Tract-Based Spatial Statistics), which is part of the FSL software package [30, 31]. All participants’ FA data were aligned into a common space using a tensor-based registration with the Diffusion Tensor Imaging Toolkit [32]. Next, the mean FA image was created and thinned to create a mean FA skeleton, which represents the centers of all tracts common to the group. This skeleton was then thresholded at FA > 0.2 in order to keep only main white matter tracts. Each adolescent’s aligned FA data were then projected onto the skeleton and the resulting data fed into voxel-wise cross-individual statistics. Images corrupted by head movement or with defective spatial normalization were excluded from subsequent analysis. See Supplementary Materials for further details.
Cerebral cortical morphometry
High-resolution anatomical MRIs were acquired with a three-dimensional T1-weighted magnetization prepared gradient echo sequence (MPRAGE) based on the ADNI protocol (http://adni.loni.usc.edu/methods/documents/mri-protocols/). Quality-controlled native MR images were processed through the CIVET pipeline (version 2.1.0) using the CBRAIN platform [33]. See Supplementary Materials for further information.
Statistical analysis
Cortical thickness analysis was implemented using SurfStat, a toolbox created for MATLAB 7 (The MathWorks Inc., Natick, MA, USA) by Dr. Keith Worsley (http://www.math.mcgill.ca/keith/surfstat/). Age, total brain volume, sex, site, socioeconomic status (SES), and pubertal development were controlled for in a vertex-wise surface-based analysis. To account for multiple comparisons, random field theory (RFT) correction was applied to the entire cortical surface [34]. In order to identify significant clusters, an initial height threshold of p ≤ 0.001 was implemented at the vertex level, and a corrected family-wise error (p ≤ 0.05) was subsequently applied. In addition, vertex-level RFT thresholding was implemented using the vertex-wise RFT critical t-value, which was calculated from the expected Euler characteristic and number of resolution elements, or resels [34].
Voxel-wise linear regression of ADHD composite score on FA maps was tested in the framework of the general linear model using a randomization-based method (5000 permutations) with age, total brain volume, sex, site, SES, and pubertal stage entered as confounding covariates. Statistical thresholds were set at p < 0.05 family-wise error corrected and threshold-free cluster enhancement corrected (TFCE) [35].
Finally, multiple linear regression was conducted in order to test the degree to which cerebral cortical and white matter correlates of ADHD psychopathology were related to ADHD PRS. Mean cortical thickness was calculated for the area of cortex that was significantly associated with ADHD symptomatology (p < 0.05, RFT cluster corrected). Similarly, mean FA was calculated for the portion of the white matter skeleton that was significantly associated with ADHD symptomatology (p < 0.05, TFCE corrected). The effects of sex, site, age, total brain volume, pubertal stage, and SES were controlled for in these regression analyses.
It should be noted that, in the above analyses, IQ was not included as a covariate. This decision was based on the following: (1) that overall cognitive ability is significantly lower among individuals with ADHD relative to controls, and (2) evidence of IQ and ADHD being underpinned by overlapping genetic factors [26, 36–38]. As written by others, when a covariate is intrinsic to the condition being studied (e.g., IQ and ADHD), it is problematic to statistically “adjust” for differences in the covariate—potentially leading to spurious results [39, 40].
Results
Demographic measures
Demographic information for participants is summarized in Table 1.
Table 1.
Summary statistics for demographic and psychometric variables
| Diffusion (n = 1471) | Cortical surface based (n = 1753) | |
|---|---|---|
| Age (in years), mean (SD) | 14.42 (0.42) | 14.43 (0.41) |
| Sex (%) |
766 females (52.1%) 705 males (47.9%) |
905 females (51.6%) 848 males (48.4%) |
| SES, mean (SD) | 18.00 (3.87) | 17.87 (3.91) |
| Pubertal development score, mean (SD) | 2.91 (0.55) | 2.91 (0.56) |
| H/I score on youth SDQ, mean (SD) | 3.90 (2.17) | 3.98 (2.16) |
| H/I score on parent SDQ, mean (SD) | 2.89 (2.30) | 2.94 (2.27) |
| DAWBA ADHD score, mean (SD) | 3.93 (5.84) | 3.94 (5.80) |
SES socioeconomic status, H/I Hyperactive/Inattentive scale, SDQ Strengths and Difficulties Questionnaire, DAWBA Development and Well-Being Assessment, ADHD attention-deficit/hyperactivity disorder
ADHD PRS and ADHD symptomatology
Correlation analysis revealed that ADHD composite score was significantly associated with ADHD PRS in participants with available cortical thickness data (r = 0.125, p < 0.001; N = 1597), as well as participants with available diffusion data (r = 0.137, p < 0.001; N = 1341). These associations remained significant when controlling for the effects of age, sex, site, total brain volume, SES, and pubertal stage.
Diffusion imaging
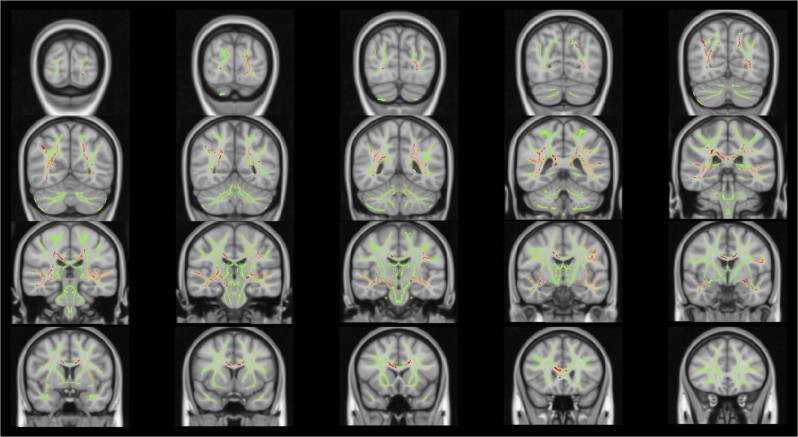
ADHD composite score was negatively associated with FA in numerous brain regions at p < 0.05, TFCE corrected, including portions of the CC, bilateral uncinate, inferior longitudinal, and superior longitudinal fasciculi (Fig. 1). The most statistically significant associations between FA and ADHD composite score (p < 0.01, TFCE corrected) were found in portions of the left inferior fronto-occipital, inferior longitudinal, and uncinate fasciculi (Supplementary Fig. 1). A similar pattern of results emerged when controlling for IQ (Supplementary Figs. 2 and 3), and self-reported anxious/depressed symptoms (using the Youth SDQ Emotion score). The association between ADHD composite score and FA was not significantly moderated by sex.
Fig. 1.
White matter regions in which fractional anisotropy is negatively associated with attention-deficit/hyperactivity disorder (ADHD) composite score (p < 0.05, threshold-free cluster enhancement corrected), controlling for age, sex, site, socioeconomic status (SES), pubertal stage, and total brain volume. Shown in radiological perspective
Exploratory post hoc analysis revealed that the above findings were largely unchanged by removing participants with ADHD composite scores >2 standard deviations above the mean (N = 71; 4.8% of total sample). Further, when examining mean FA of white matter regions that were significantly associated with ADHD symptomatology (p < 0.05, TFCE corrected), we found that the extreme symptom group (>2 SDs above mean ADHD composite score) exhibited significantly reduced FA relative to the remainder of the sample (p = 1.76 × 10−4). Thus, when compared to the remainder of the sample, the extreme symptom group evidenced reduced FA in white matter regions that were dimensionally related to the ADHD composite score in the total sample.
Cortical thickness
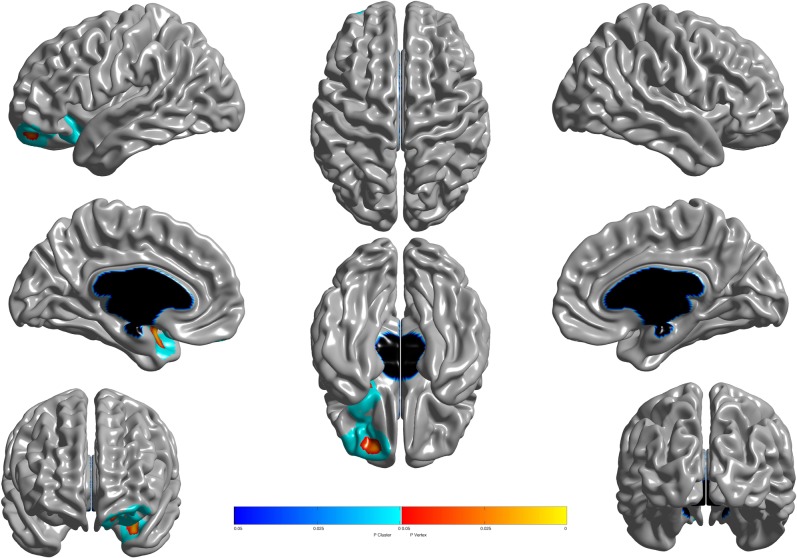
ADHD composite score was negatively associated with cortical thickness in left orbital and ventromedial prefrontal cortices, extending into left anterior insula and left anterior temporal cortices (Fig. 2). The association between ADHD composite score and cortical thickness was not significantly moderated by sex. No other associations survived correction for multiple comparisons.
Fig. 2.
Brain areas where local cortical thickness is negatively associated with the dimensional multi-informant ADHD composite score (n = 1753). Random field theory (RFT) was used to correct for multiple comparisons over the entire cortical mantle. Figure is shown at p ≤ 0.05, RFT corrected. Blue areas are significant at the cluster level and red color corresponds to areas significant at the vertex level. Controlled for age, total brain volume, sex, site, socioeconomic status (SES), and pubertal development
ADHD PRS and brain structure
ADHD PRS predicted mean FA of white matter regions in which FA was significantly associated with dimensional ADHD symptom score, b = −0.044, p = 0.045. Importantly, this association remained significant when the threshold for inclusion of SNPs in the calculation of the PRS score was changed to p < 0.01 and <0.10. Similarly, the association remained significant when controlling for IQ. Sex did not significantly moderate the association between PRS score and mean FA. In a follow-up voxel-wise analysis, the relationship between ADHD PRS and FA was examined within regions of the white matter skeleton in which FA was significantly associated with ADHD symptomatology (Supplementary Fig. 4). Peak associations (p < 0.001, uncorrected) were revealed in portions of the left inferior fronto-occipital, inferior longitudinal, and superior longitudinal fasciculi. ADHD PRS was not associated with cortical thickness in the areas of cortex that were significantly associated with ADHD symptomatology.
Discussion
To our knowledge, this is the first study to reveal dimensional relations between ADHD symptomatology, white matter microstructure, and ADHD polygenic risk in a population-based sample of adolescents. Specifically, variation in hyperactive/inattentive symptomatology was associated with white matter microstructure, which in turn was related to genetic risk for ADHD. Cerebral cortical thickness correlates of ADHD symptomatology were not related to ADHD PRS. Taken together, these findings suggest that ADHD polygenic risk is tied, in part, to biological processes affecting white matter microstructure during adolescence.
The PRS used in the present study was derived from the first report of genome-wide significant risk loci for ADHD [26]. Importantly, the present study serves to replicate this prior report, and, further, suggests that risk loci identified in Demontis et al. [26] influence clinical and subclinical levels of ADHD symptomatology. Several of the genome-wide significant loci that inform ADHD PRS have been implicated in neurodevelopmental processes, including FOXP2, DUSP6, and SEMA6D. FOXP2, in particular, is active during stages of embryonic development and is known to play a vital role in neurogenesis and formation of synapses [41]. FOXP2 has also been implicated in the development of language and cognition—a particularly intriguing finding given the co-occurrence of ADHD and learning disorders [42–44]. What is more, in the present study, PRS was negatively associated with FA in portions of the extreme capsule of the left hemisphere—a fiber pathway that is related to cortical regions in the left inferior frontal gyrus that subserve language in humans. Future research will be needed to elucidate the neurobiological processes serving to mediate the association between ADHD PRS and reduced FA.
It is noteworthy that white matter microstructure was related to ADHD symptomatology in widespread regions. Specifically, a dimensional ADHD composite score was negatively associated with FA in areas, including the CC, bilateral uncinate, inferior longitudinal, inferior fronto-occipital, and superior longitudinal fasciculi. The most statistically significant associations (p < 0.01, TFCE- corrected) were revealed in portions of the left fronto-occipital, inferior longitudinal, and uncinate fasciculi. Surface-based cortical analysis revealed significant negative associations between cortical thickness and ADHD composite score in left orbital and ventromedial prefrontal cortices, extending into left anterior insula and left anterior temporal cortices. Reduced FA within the inferior fronto-occipital fasciculus represents a consistent finding among diffusion imaging studies of adult ADHD [45–47]. In a recent study, Leng et al. [48] employed structural equation modeling in order to assess associations between FA within the inferior fronto-occipital fasciculus and attention. Executive control of attention was particularly tied to FA in the frontal component of the left inferior fronto-occipital tract, whereas alerting was associated with FA in the insular and parietal components of the left inferior fronto-occipital tract. Results of the present study are strikingly similar to a report by Shaw et al. [47] in which dimensional measures of inattention were negatively associated with FA in the left inferior fronto-occipital and uncinate fasciculi among young adults. Further, Shaw et al. [47] reported that young adults with persistent ADHD symptoms possessed lower FA relative to young adults with remitted ADHD symptoms in the bilateral inferior fronto-occipital fasciculus, right SLF, and left uncinate fasciculus. Findings from the present study further implicate the inferior fronto-occipital fasciculus in the pathophysiology of ADHD symptoms.
Present findings appear to support the notion that both clinical and subclinical ADHD symptomatology are associated with white matter microstructure in largely overlapping areas. In particular, the association between ADHD symptomatology and white matter FA was not meaningfully altered when participants with extreme ADHD composite scores (>2 SDs above mean; 4.8% of the total sample) were excluded. Further, this extreme group (>2 SDs above mean) exhibited significantly reduced FA relative to the remainder of the sample in white matter regions that were dimensionally related to the ADHD composite score in the total sample. These findings extend prior research on gray matter correlates of ADHD symptomatology in non-clinical samples [8, 9], and lend further credence to dimensional conceptualizations of ADHD psychopathology. Present results also dovetail with reports of ADHD polygenic risk predicting dimensional measures of clinical and subclinical attention problems in population-based samples of children [6].
It has been argued that cerebral cortical volume should not be used in genetically informative studies, or as an endophenotype for a given disorder or psychopathology [49]. Given the genetic focus of the present study, we chose to reexamine relations between cortical gray matter and ADHD symptomatology with more recently developed surface-based methods that assess thickness as a distinct property of the cortex [49]. That being said, the surface-based cortical morphometry findings of the present study largely recapitulate previous voxel-based morphometry findings on this cohort of adolescents [11].
Interestingly, follow-up analyses revealed that cortical thickness correlates of ADHD symptomatology were not significantly associated with FA correlates of ADHD symptomatology—suggesting, perhaps, that these two biomarkers possess differing neurodevelopmental origins. Our results suggest that the ADHD PRS, derived from Demontis et al. [26], is more closely tied to white matter integrity during adolescence as opposed to cerebral cortical morphology. Although speculative, it is possible that the cortical correlates revealed in the present study represent compensatory changes in response to altered white matter development, or separate genetic influences. It is also possible that cortical correlates of ADHD PRS may be present earlier in the course of development, prior to age-related cortical thickness changes that accompany the transition from early childhood to adolescence. Population-based longitudinal studies, beginning at younger ages, are desperately needed to address such questions.
Although the present study possesses a number of significant strengths, several limitations need to be addressed. As is true for most diffusion imaging studies, we can only speculate as to the neurobiological underpinnings of our FA findings. Future studies will likely benefit from characterizing white matter microstructure using a number of diffusion metrics—not simply FA. Further, the observed effect size for the association between PRS and FA was relatively small. It is likely that the white matter microstructure findings of the present study represent one facet by which ADHD PRS influences brain structure.
In conclusion, this is the first study to reveal dimensional relations between ADHD polygenic risk, white matter microstructure, and ADHD symptomatology in a population-based sample of adolescents. Our findings suggest that ADHD polygenic risk is associated with clinical and subclinical ADHD symptomatology and, further, that genetic risk for ADHD symptoms may be tied to processes affecting the structural integrity of cerebral white matter pathways.
Funding and disclosure
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT-2007-037286), the Horizon 2020 funded ERC Advanced Grant “‘STRATIFY’” (Brain network-based stratification of reinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological, and Subjective Factors in Drug Use Pathways) (PR-ST-0416-10004), BRIDGET (JPND: BRain Imaging, cognition Dementia and next-generation GEnomics) (MR/N027558/1), the FP7 projects IMAGEMEND (602450; IMAging GEnetics for MENtal Disorders) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grant “‘c-VEDA”’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the Swedish Research Council FORMAS, the Medical Research Council, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL 01EE1406A, 01EE1406B), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-2, SFB 940/2), the Medical Research Foundation and Medical research council (grant MR/R00465X/1). Further support was provided by grants from: ANR (project AF12-NEUR0008-01-WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), U.S.A. (Axon, Testosterone, and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centers of Excellence. In addition, Drs. Garavan and Potter are supported P20GM103644 (PI: Stephen T. Higgins), Agency: NIGMS Vermont Center on Behavior and Health. Dr. Banaschewski has served as an advisor or consultant to Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire; the present work is unrelated to these relationships. The other authors report no biomedical financial interests or no competing interests.
Supplementary information
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0383-y).
References
- 1.APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Arlington, VA Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 3.Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135:e994–1001. doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- 4.Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Prim. 2015;1:15020. doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- 5.Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. Int J Methods Psychiatr Res. 2007;16(Suppl 1):S16–23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groen-Blokhuis MM, Middeldorp CM, Kan KJ, Abdellaoui A, van Beijsterveldt CE, Ehli EA, et al. Attention-deficit/hyperactivity disorder polygenic risk scores predict attention problems in a population-based sample of children. J Am Acad Child Adolesc Psychiatry. 2014;53:1123–9. doi: 10.1016/j.jaac.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Morris S, Cuthbert B. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialog Clin Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducharme S, Hudziak JJ, Botteron KN, Albaugh MD, Nguyen TV, Karama S, et al. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J Am Acad Child Adolesc Psychiatry. 2012;51:18–27. doi: 10.1016/j.jaac.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, et al. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 2011;168:143–51. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albaugh Matthew D, Ivanova Masha, Chaarani Bader, Orr Catherine, Allgaier Nicholas, Althoff Robert R, D’ Alberto Nicholas, Hudson Kelsey, Mackey Scott, Spechler Philip A, Banaschewski Tobias, Brühl Rüdiger, Bokde Arun L W, Bromberg Uli, Büchel Christian, Cattrell Anna, Conrod Patricia J, Desrivières Sylvane, Flor Herta, Frouin Vincent, Gallinat Jürgen, Goodman Robert, Gowland Penny, Grimmer Yvonne, Heinz Andreas, Kappel Viola, Martinot Jean-Luc, Martinot Marie-Laure Paillère, Nees Frauke, Papadopoulos Orfanos Dimitri, Penttilä Jani, Poustka Luise, Paus Tomáš, Smolka Michael N, Struve Maren, Walter Henrik, Whelan Robert, Schumann Gunter, Garavan Hugh, Potter Alexandra S. Ventromedial Prefrontal Volume in Adolescence Predicts Hyperactive/Inattentive Symptoms in Adulthood. Cerebral Cortex. 2018;29(5):1866–1874. doi: 10.1093/cercor/bhy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albaugh Matthew D., Orr Catherine, Chaarani Bader, Althoff Robert R., Allgaier Nicholas, D’Alberto Nicholas, Hudson Kelsey, Mackey Scott, Spechler Philip A., Banaschewski Tobias, Brühl Rüdiger, Bokde Arun L.W., Bromberg Uli, Büchel Christian, Cattrell Anna, Conrod Patricia J., Desrivières Sylvane, Flor Herta, Frouin Vincent, Gallinat Jürgen, Goodman Robert, Gowland Penny, Grimmer Yvonne, Heinz Andreas, Kappel Viola, Martinot Jean-Luc, Paillère Martinot Marie-Laure, Nees Frauke, Orfanos Dimitri Papadopoulos, Penttila¨ Jani, Poustka Luise, Paus Tomáš, Smolka Michael N., Struve Maren, Walter Henrik, Whelan Robert, Schumann Gunter, Garavan Hugh, Potter Alexandra S. Inattention and Reaction Time Variability Are Linked to Ventromedial Prefrontal Volume in Adolescents. Biological Psychiatry. 2017;82(9):660–668. doi: 10.1016/j.biopsych.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albaugh Matthew D., Potter Alexandra S. The ventromedial prefrontal cortex: a putative locus for trait inattention. Neuropsychopharmacology. 2018;44(1):226–227. doi: 10.1038/s41386-018-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–54. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–9. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 15.Albaugh MD, Ducharme S, Karama S, Watts R, Lewis JD, Orr C, et al. Anxious/depressed symptoms are related to microstructural maturation of white matter in typically developing youths. Dev Psychopathol. 2017;29:751–58. doi: 10.1017/S0954579416000444. [DOI] [PubMed] [Google Scholar]
- 16.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 17.Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45:191–5. doi: 10.1002/1522-2594(200102)45:2<191::AID-MRM1025>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Poelmans G, Pauls DL, Buitelaar JK, Franke B. Integrated genome-wide association study findings: identification of a neurodevelopmental network for attention deficit hyperactivity disorder. Am J psychiatry. 2011;168:365–77. doi: 10.1176/appi.ajp.2010.10070948. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Neale BM, Liu L, Lee SH, Wray NR, Ji N, et al. Polygenic transmission and complex neuro developmental network for attention deficit hyperactivity disorder: genome-wide association study of both common and rare variants. Am J Med Genet B. 2013;162B:419–30. doi: 10.1002/ajmg.b.32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Ewijk H, Heslenfeld DJ, Zwiers MP, Faraone SV, Luman M, Hartman CA, et al. Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2014;53:790–9. doi: 10.1016/j.jaac.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Onnink AM, Zwiers MP, Hoogman M, Mostert JC, Dammers J, Kan CC, et al. Deviant white matter structure in adults with attention-deficit/hyperactivity disorder points to aberrant myelination and affects neuropsychological performance. Prog Neuropsychopharmacol Biol Psychiatry. 2015;63:14–22. doi: 10.1016/j.pnpbp.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–39. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 23.Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–55. doi: 10.1111/j.1469-7610.2000.tb02345.x. [DOI] [PubMed] [Google Scholar]
- 24.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 25.Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40:1337–45. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Demontis Ditte, Walters Raymond K., Martin Joanna, Mattheisen Manuel, Als Thomas D., Agerbo Esben, Baldursson Gísli, Belliveau Rich, Bybjerg-Grauholm Jonas, Bækvad-Hansen Marie, Cerrato Felecia, Chambert Kimberly, Churchhouse Claire, Dumont Ashley, Eriksson Nicholas, Gandal Michael, Goldstein Jacqueline I., Grasby Katrina L., Grove Jakob, Gudmundsson Olafur O., Hansen Christine S., Hauberg Mads Engel, Hollegaard Mads V., Howrigan Daniel P., Huang Hailiang, Maller Julian B., Martin Alicia R., Martin Nicholas G., Moran Jennifer, Pallesen Jonatan, Palmer Duncan S., Pedersen Carsten Bøcker, Pedersen Marianne Giørtz, Poterba Timothy, Poulsen Jesper Buchhave, Ripke Stephan, Robinson Elise B., Satterstrom F. Kyle, Stefansson Hreinn, Stevens Christine, Turley Patrick, Walters G. Bragi, Won Hyejung, Wright Margaret J., Andreassen Ole A., Asherson Philip, Burton Christie L., Boomsma Dorret I., Cormand Bru, Dalsgaard Søren, Franke Barbara, Gelernter Joel, Geschwind Daniel, Hakonarson Hakon, Haavik Jan, Kranzler Henry R., Kuntsi Jonna, Langley Kate, Lesch Klaus-Peter, Middeldorp Christel, Reif Andreas, Rohde Luis Augusto, Roussos Panos, Schachar Russell, Sklar Pamela, Sonuga-Barke Edmund J. S., Sullivan Patrick F., Thapar Anita, Tung Joyce Y., Waldman Irwin D., Medland Sarah E., Stefansson Kari, Nordentoft Merete, Hougaard David M., Werge Thomas, Mors Ole, Mortensen Preben Bo, Daly Mark J., Faraone Stephen V., Børglum Anders D., Neale Benjamin M. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics. 2018;51(1):63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Euesden J, Lewis CM, O’Reilly PF. PRSice: Polygenic Risk Score software. Bioinformatics. 2015;31:1466–8. doi: 10.1093/bioinformatics/btu848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Avants BB, Yushkevich PA, Woo JH, Wang S, McCluskey LF, et al. High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: an example study using amyotrophic lateral sclerosis. IEEE Trans Med Imaging. 2007;26:1585–97. doi: 10.1109/TMI.2007.906784. [DOI] [PubMed] [Google Scholar]
- 33.Sherif T, Rioux P, Rousseau ME, Kassis N, Beck N, Adalat R, et al. CBRAIN: a web-based, distributed computing platform for collaborative neuroimaging research. Front Neuroinform. 2014;8:54. doi: 10.3389/fninf.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. Neuroimage. 2004;23(Suppl 1):S189–95. doi: 10.1016/j.neuroimage.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 36.Faraone Stephen V., Larsson Henrik. Genetics of attention deficit hyperactivity disorder. Molecular Psychiatry. 2018;24(4):562–575. doi: 10.1038/s41380-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:543–55. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 38.Kuntsi J, Eley TC, Taylor A, Hughes C, Asherson P, Caspi A, et al. Co-occurrence of ADHD and low IQ has genetic origins. Am J Med Genet B. 2004;124B:41–7. doi: 10.1002/ajmg.b.20076. [DOI] [PubMed] [Google Scholar]
- 39.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15:331–43. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubia K, Halari R, Mohammad AM, Taylor E, Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;70:255–62. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsui D, Vessey JP, Tomita H, Kaplan DR, Miller FD. FoxP2 regulates neurogenesis during embryonic cortical development. J Neurosci. 2013;33:244–58. doi: 10.1523/JNEUROSCI.1665-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen CM, Steinhausen HC. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. Atten Defic Hyperact Disord. 2015;7:27–38. doi: 10.1007/s12402-014-0142-1. [DOI] [PubMed] [Google Scholar]
- 43.Schreiweis C, Bornschein U, Burguiere E, Kerimoglu C, Schreiter S, Dannemann M, et al. Humanized Foxp2 accelerates learning by enhancing transitions from declarative to procedural performance. Proc Natl Acad Sci USA. 2014;111:14253–8. doi: 10.1073/pnas.1414542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sia GM, Clem RL, Huganir RL. The human language-associated gene SRPX2 regulates synapse formation and vocalization in mice. Science. 2013;342:987–91. doi: 10.1126/science.1245079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortese S, Imperati D, Zhou J, Proal E, Klein RG, Mannuzza S, et al. White matter alterations at 33-year follow-up in adults with childhood attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74:591–8. doi: 10.1016/j.biopsych.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, et al. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur J Neurosci. 2010;31:912–9. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- 47.Shaw P, Sudre G, Wharton A, Weingart D, Sharp W, Sarlls J. White matter microstructure and the variable adult outcome of childhood attention deficit hyperactivity disorder. Neuropsychopharmacology. 2015;40:746–54. doi: 10.1038/npp.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leng Y, Shi Y, Yu Q, Van Horn JD, Tang H, Li J, et al. Phenotypic and genetic correlations between the lobar segments of the inferior fronto-occipital fasciculus and attention. Sci Rep. 2016;6:33015. doi: 10.1038/srep33015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.