Abstract
Mesenchymal Stem/stromal Cells (MSCs) may be able to improve ischemic conditions as they can actively seek out areas of low oxygen and secrete pro-angiogenic factors. In more severe trauma and chronic cases, however, cells alone may not be enough. Therefore, we have combined the stem cell and angiogenic factor approaches to make a more potent therapy. We developed an engineered stem cell therapy product designed to treat critical limb ischemia (CLI), that could also be used in trauma-induced scarring and fibrosis where additional collateral bloodflow is needed following damage to and blockage of the primary vessels. We used MSCs from normal human donor bone marrow, and engineered them to produce high levels of the angiogenic factor Vascular Endothelial Growth Factor (VEGF). The MSC/VEGF product has been successfully developed and characterized using Good Manufacturing Practice (GMP) - compliant methods, and we have completed experiments showing that MSC/VEGF significantly increased blood flow in the ischemic limb of immune deficient mice, as compared to the saline controls in each study. We also performed safety studies, demonstrating that the injected product does not cause harm and that the cells remain around the injection site for over a month after hypoxic pre-conditioning. An on-demand formulation system for delivery of the product to clinical sites that lack cell processing facilities is in development.
Keywords: Mesenchymal Stem Cells, Critical Limb Ischemia, Vascular Endothelial Growth Factor, Gene Therapy, Cell Therapy, Angiogenesis, Collateral Vessels
Clinical Trials of Gene and Cell therapy for CLI
Prior clinical trials have investigated injection of Vascular Endothelial Growth Factor gene therapy agents to promote angiogenesis and restore blood flow in chronic conditions such as CLI. These therapeutic agents seemed promising in early stage-clinical trials, but were not significantly better than controls in Phase III trials. Other clinical trials using the patient’s own stem cells from the bone marrow and injected into the ischemic leg had shown some benefit, although the final assessments are not yet completed, and patients fall into two categories, “Responders” and “Non-responders”, making it difficult to meet overall endpoints.
One approach to treatment of CLI is inducing formation of collateral blood vessels that bypass the primary blockage and restore tissue perfusion to initiate healing. Proteins in the vascular endothelial growth factor (VEGF) family have long been considered prime candidates for promoting such therapeutic angiogenesis, and several clinical trials have been carried out to test these molecules as CLI therapeutics. Proteins, however, have a short half-life and are usually extremely expensive. Gene therapy for therapeutic angiogenesis presents an alternative to recombinant protein administration. Clinical studies that delivered pro-angiogenic cytokines as genes began later than many of the studies involving recombinant protein formulations of these cytokines, in part due to issues regarding the appropriate vectors and promoter sequences to be employed, as well as additional safety concerns surrounding the field of human gene therapy.
FDA approved phase I and phase II clinical trials involving intramuscular (IM) injection of naked DNA or viral vectors have tested the delivery of angiogenic factors, including VEGF, indicated a potential benefit of VEGF administration for CLI1-3. Patients with advanced myocardial ischemia have been treated with a VEGF-A165 plasmid delivered through the endocardium via a percutaneous approach for refractory coronary artery disease (CAD) and PAD 4-7. Another open label study, treated 30 patients with “no option” advanced CAD and refractory angina, with a VEGF-A165 coding plasmid, found a significant improvement in symptoms, and a modest improvement in exercise treadmill time 8. The RAVE study 9 was a large (107 patient), randomized Phase II, double blind, placebo-controlled clinical study using an adenovirus coding for VEGF-A121 in patients with unilateral advanced intermittent claudication. Unfortunately, no differences in outcome could be identified between Ad/VEGF-A121 and placebo treated patients. However, no major safety issues associated with Ad/VEGF-A121 were identified.
Although Phase I and II trials of VEGF delivered via plasmid and as purified protein injections showed potential clinical benefit in treatment of CLI, Phase III studies in this indication did not achieve significant efficacy. There was, however, benefit observed for administration of the pCMV-vegf165 plasmid in intramuscular gene transfer for treatment of moderate to severe claudication due to chronic lower limb ischemia.10, 11 The failure of these agents to significantly affect therapeutic angiogenesis in the most severe forms of CLI (Rutherford Category IV/V) has been attributed to the mechanisms of VEGF delivery, because expression from plasmids is suboptimal and transient and the VEGF protein has a short half-life. We hope to circumvent this issue by delivering VEGF from MSCs, which could work in synergy with the other paracrine angiogenic factors naturally produced by MSCs.
Numerous clinical trials have demonstrated the biosafety of systemic infusion of allogeneic Mesenchymal Stem/Stromal Cells (MSCs) into patients with various diseases 12, 13. These cells are ideal for this application due to their ease of isolation and expansion, low immunogenicity in allogeneic settings 14, and ability to secrete paracrine factors that stimulate regeneration 12. Furthermore, MSCs show tropism toward sites of hypoxia 15 and are stimulated to express angiogenic factors in hypoxic environments 16, which make them particularly advantageous for application in ischemic disease.
Clinical studies have provided extensive safety data for non-matched allogeneic bone marrow-derived MSCs administration in patients through FDA-approved clinical trials. Liew and O'Brien reviewed the progress of using MSCs to treat CLI 17. Early phase trials for heart and limb revascularization have shown safety and early indication of efficacy (REVASCOR-Mesoblast, RESTORE-CLI-Aastrom, Multistem-Athersys, and others). The lack of infusion-related serious adverse events in these trials demonstrates the safety of MSCs and other expanded adherent marrow-derived cell infusion, when performed carefully and according to specific clinical regimens. Cell therapy for CLI was recently reviewed, with an excellent integration of the preclinical and clinical studies. 18
Potency assays for MSCs in angiogenesis
In contrast to features such as their osteogenic potential or their ability to suppress the immune system, the pro-angiogenic activity of MSCs remains difficult to predict prior to experimental use. The common and simple methods to isolate and expand MSCs from the bone marrow lead to a rather heterogeneous population19, 20. Nonetheless, many characteristics of the cells are consistent and only minimally vary between donors or culture conditions. For example, their surface phenotype is homogenous regarding the absence of hematopoietic markers (CD45, CD34, CD14) and presence of mesenchymal markers CD73, CD90 and CD105.
Bone marrow-derived MSCs may be better at promoting blood flow restoration than MSCs derived from adipose tissue 21. Studies have demonstrated that the therapeutic potency of MSCs can vary greatly depending on the donor 12. Marrow-derived MSCs have a long safety history and have shown efficacy in some clinical trials, while other late-stage trials have failed. Expansion and conditioning procedures are important for optimizing their survival in vivo after delivery, as are identifying the best cell batches prior to clinical use. It is thus imperative to define surrogate potency assays, such as rate of expansion, viability and identity, sterility, cytokine release, in vitro vascularization tube-forming assay, and in vitro induction of target cell mobility, as assessed by videomicroscopy.22
Development of the MSC/VEGF Product
The rationale for delivering VEGF from MSC in our novel product is to extend the duration of local expression of VEGF and thereby promote angiogenesis. Through interaction with the FDA and pioneering scientists in the field of gene therapy, we developed a protocol to generate the MSC/VEGF product to be as safe and efficient as possible, using lentiviral vector technology. The keys to our approach include the use of a third generation lentiviral vector 23, no other transgenes to minimize immunogenicity, and a limit of 1-2 proviral insertions per cell, to minimize the risk of insertional mutagenesis. A strong promoter (MNDU3; previously used in human gene therapy 24) and an enhancer element acting in cis (woodchuck hepatitis post-transcriptional regulatory element; WPRE 25) maximize transgene expression, while minimizing viral load. The MSC/VEGF was produced in a GMP-compliant manner in the laboratory using the same standard operating procedures, reagents and formulations to be used in future GMP batches. Cells were then cryopreserved as several “tox batches” for future in vivo testing.
The preclinical studies published by our group 26, 27 demonstrate that MSC/VEGF may be useful in promoting angiogenesis, because the cells show tropism toward hypoxic sites and deliver high levels of VEGF from the introduced transgene at the sites of ischemia, promoting reperfusion of the ischemic tissue. MSC/VEGF are more effective than unmodified MSC (without the VEGF transgene) in restoring blood flow in animal models 26, 27. MSC/VEGF cells do not persist indefinitely at the site of ischemia. MSC/VEGF can initiate angiogenesis that should be carried on by endogenous mechanisms once tissue perfusion is restored.
We published proof of concept data showing that engineering MSCs over-expressing VEGF-A165 (hereafter VEGF) show a much greater potential to restore blood flow in a murine model of hind limb ischemia (HLI), as compared to control MSCs 26. We then manufactured and tested the efficacy of clinically-compliant MSC/VEGF, made under current good manufacturing practice (cGMP) conditions 27. We are currently testing MSC/VEGF in a large animal model (rabbits), to further determine the optimal implementation of the cell/gene therapy.
MSC/VEGF On - Demand Product Formulation
Gene-modified allogeneic mesenchymal stem cells (MSCs) are highly relevant products for clinical cell and gene therapies. To transport these cellular products for clinical administration, cryopreservation, formulation and transportation methods must be developed. There is evidence that MSCs need a refractory phase after cryopreservation, prior to injection, to allow best in vivo survival and function.28, 29 It is possible that some MSC trials may not be meeting endpoints because the cells are cleared too rapidly due to effects from cryopreservation.28-30
If, rather than the “thaw and infuse” method, culture-recovered and hypoxic conditioned cells could be used, the therapy could have higher likelihood to be successful. We have shown retention of MSCs prepared in this manner at higher levels than unconditioned cells.31 Ten percent of the human MSCs are retained at the one month time point using our methods, after IM injection, as compared to only 1% without the preconditioning.31 Another key post-thaw conditioning protocol could be lowering the sugars in the preconditioning media, since MSCs are slow to switch to glycolysis in times of glucose deprivation, such as in ischemic tissues.32
The final certified lot of MSC/VEGF will be banked in liquid nitrogen in the GMP facility. When the patient’s clinic date is identified, the GMP facility will receive notice that the cells should be thawed for recovery and formulation 48 hours prior to injection. Final release criteria are done and the FDA does not require a 14 day sterility test to be repeated if culture is under four days. In the last steps of manufacturing of MSC/VEGF, the cell product is thawed on demand, cultured, exposed to hypoxic conditions, and then formulated to be delivered and released for administration without refreezing (Figure 1). We have demonstrated that such procedures increase the survival and retention of MSC in vivo 33 Consequently, it is anticipated that MSC/VEGF will be more active in promoting therapeutic angiogenesis in CLI than unmodified MSCs or VEGF (gene or protein).
Figure 1. On demand formulation strategy.
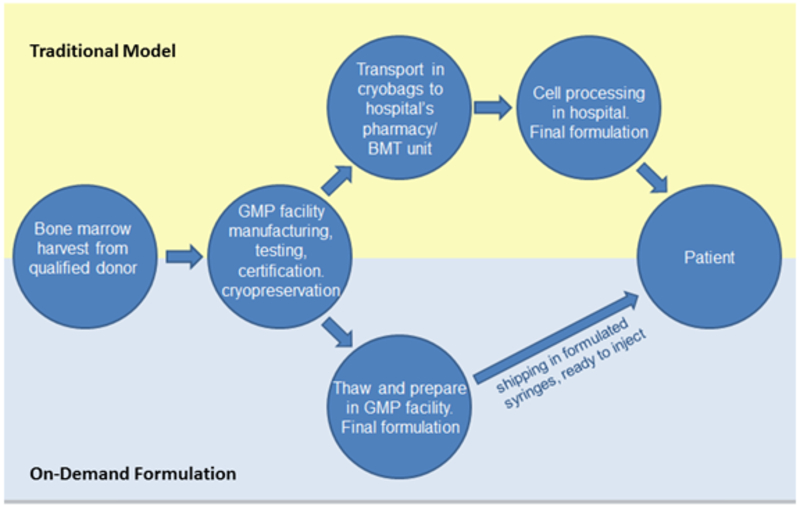
In the traditional model, cells are manufactured in a GMP facility and shipped to the clinical site as cryopreserved units. At the clinical site, the units are checked in through the pharmacy and transferred in the cryoshipper to the Hospital Cell Processing Laboratory. The cells are held until the patient is in clinic, then thawed, washed, checked for viability, and formulated into the batch of syringes needed for intramuscular injection in the patient’s limb, following a grid. There is much room for inconsistency and variability in cell handling, due to human factors and site-specific conditions.
In the proposed model, all formulation is done in a consistent manner in the GMP facility, closely following established Standard Operating Procedures. Orders are placed, vials of cryopreserved VEGF/MSCs to fulfill the order are thawed, preconditioned, and formulated into batches of 28 syringes in the GMP facility. Syringes are shipped overnight to the clinic for receipt, transfer to the vascular clinic, and injection into the patient.
Pre-formulated product will be received at the administering center in 28 1 cc syringes, proper chain of custody documentation will be in place, and our stability studies indicate that the product is stable for 24 hours during shipping and administration.34 The product will be administered as 28 intramuscular injections to the index limb isolated to the injection grid within the lower and upper anterior, medial and posterior aspect of the index leg.
By using the on - demand product thaw, condition and formulation method, in theory the syringes can be sent out to centers that do not have a bone marrow transplant unit, which traditionally processes and formulates cells for cell therapy applications, or a formal cell processing facility. The clinician at the receiving end would have the syringes ready to administer upon receipt through the investigational pharmacy. As we look to the future of cell therapies, the formulation and packaging, and the area of the hospital or pharmacy where cell processing for administration is done at each clinical site will be increasingly important parameters for successful and consistent usage of the cellular product.
Acknowledgements
This project was funded by Disease Team Grant DR2A-05423 for Critical Limb Ischemia from the California Institute for Regenerative Medicine (CIRM), CIRM Early Translational Grant TR2-01787 (JN) and NIH Transformative Grant 1R01GM099688 (JN). Our team is supported by a Donation from the Dickensons and PZ is the 2016 Wing Fat Fellow and 2017 Howard and Abby Milstein Foundation awardee. NM and HD are scholars of the Bridges to Stem Cell Research Program (#TB1-01190 and #TB1-01184) from CIRM to promote undergraduate training in stem cell biology and regenerative medicine.
Footnotes
The work presented in this manuscript was performed at the Institute for Regenerative Cures, University of California Davis: 2921 Stockton Blvd. Sacramento, CA. 95817. United States.
The authors declare no conflicts of interest.
References
- 1.Birk DM, Barbato J, Mureebe L, Chaer RA. Current insights on the biology and clinical aspects of VEGF regulation. Vase Endovascular Surg 2008; 42(6): 517–530. e-pub ahead of print 2008/09/19; doi: 1538574408322755 [pii] 10.1177/1538574408322755 [DOI] [PubMed] [Google Scholar]
- 2.Mughal NA, Russell DA, Ponnambalam S, Homer-Vanniasinkam S. Gene therapy in the treatment of peripheral arterial disease. Br J Surg 2012; 99(1): 6–15. doi: 10.1002/bjs.7743 [DOI] [PubMed] [Google Scholar]
- 3.Shimamura M, Nakagami H, Koriyama H, Morishita R. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. BioMed research International 2013; 2013: 186215. doi: 10.1155/2013/186215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vale PR, Losordo DW, Milliken CE, Maysky M, Esakof DD, Symes JF et al. Left ventricular electromechanical mapping to assess efficacy of phVEGF(165) gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation 2000; 102(9): 965–974. [DOI] [PubMed] [Google Scholar]
- 5.Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N et al. Phase ½ placebocontrolled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation 2002; 105(17): 2012–2018. [DOI] [PubMed] [Google Scholar]
- 6.Freedman SB, Vale P, Kalka C, Kearney M, Pieczek A, Symes J et al. Plasma vascular endothelial growth factor (VEGF) levels after intramuscular and intramyocardial gene transfer of VEGF-1 plasmid DNA. Human gene therapy 2002; 13(13): 1595–1603. doi: 10.1089/10430340260201680 [DOI] [PubMed] [Google Scholar]
- 7.Shyu KG, Chang H, Isner JM. Synergistic effect of angiopoietin-1 and vascular endothelial growth factor on neoangiogenesis in hypercholesterolemic rabbit model with acute hindlimb ischemia. LlfeSci 2003; 73(5): 563–579. e-pub ahead of print 2003/05/29; doi: S0024320503003187 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Fortuin FD, Vale P, Losordo DW, Symes J, DeLaria GA, Tyner JJ et al. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am J Cardiol 2003; 92(4): 436–439. e-pub ahead of print 2003/08/14; doi: S0002914903006611 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Rajagopalan S, Mohler ER 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 2003;108(16):1933–1938. e-pub ahead of print 2003/09/25; doi: 10.1161/01.CIR.0000093398.16124.29 01.CIR.0000093398.16124.29 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Deev RV, Bozo IY, Mzhavanadze ND, Voronov DA, Gavrilenko AV, Chervyakov YV et al. pCMV-vegfl65 Intramuscular Gene Transfer is an Effective Method of Treatment for Patients With Chronic Lower Limb Ischemia. J Cardiovasc Pharmacol Ther 2015; 20(5): 473–482. e-pub ahead of print 2015/03/15; doi: 10.1177/1074248415574336 [DOI] [PubMed] [Google Scholar]
- 11.Deev R, Plaksa I, Bozo I, Isaev A. Results of an International Postmarketing Surveillance Study of pl-VEGF165 Safety and Efficacy in 210 Patients with Peripheral Arterial Disease. Am J Cardiovasc Drugs 2017; 17(3): 235–242. e-pub ahead of print 2017/01/05; doi: 10.1007/s40256-016-0210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 2014; 14(2): 141–145. doi: 10.1016/j.stem.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 13.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015; 17(1): 11–22. doi: 10.1016/j.stem.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 14.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nature biotechnology 2014; 32(3): 252–260. doi: 10.1038/nbt.2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruenloh W, Kambal A, Sondergaard C, McGee J, Nacey C, Kalomoiris S et al. Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells. Tissue engineering. Part A 2011; 17(11-12): 1517–1525. e-pub ahead of print 2011/02/01; doi: 10.1089/ten.TEA.2010.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai CC, Yew TL, Yang DC, Huang WH, Hung SC. Benefits of hypoxic culture on bone marrow multipotent stromal cells. American journal of blood research 2012; 2(3): 148–159. [PMC free article] [PubMed] [Google Scholar]
- 17.Liew A, O'Brien T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther 2012; 3(4): 28. doi: 10.1186/scrt119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qadura M, Terenzi DC, Verma S, Al-Omran M, Hess DA. Concise Review: Cell Therapy for Critical Limb Ischemia: An Integrated Review of Preclinical and Clinical Studies. Stem Cells 2018; 36(2): 161–171. e-pub ahead of print 2017/12/12; doi: 10.1002/stem.2751 [DOI] [PubMed] [Google Scholar]
- 19.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005; 7(5): 393–395. doi: 10.1080/14653240500319234 [DOI] [PubMed] [Google Scholar]
- 20.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nature medicine 2013; 19(1): 35–42. doi: 10.1038/nm.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bortolotti F, Ukovich L, Razban V, Martinelli V, Ruozi G, Pelos B et al. In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem cell reports 2015; 4(3): 332–339. doi: 10.1016/j.sterner.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black AB, Dahlenburg H, Pepper K, Nacey C, Pontow S, Kuhn MA et al. Human Myoblast and Mesenchymal Stem Cell Interactions Visualized by Videomicroscopy. Human gene therapy methods 2015. doi: 10.1089/hgtb.2015.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. Journal of virology 1998; 72(11): 8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Candotti F, Shaw KL, Muul L, Carbonaro D, Sokolic R, Choi C et al. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans. Blood 2012; 120(18): 3635–3646. doi: 10.1182/blood-2012-02-400937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. Journal of virology 1999; 73(4): 2886–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells 2011; 29(11): 1727–1737. e-pub ahead of print 2011/09/08; doi: 10.1002/stem.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beegle JR, Magner NL, Kalomoiris S, Harding A, Zhou P, Nacey C et al. Preclinical evaluation of mesenchymal stem cells overexpressing VEGF to treat critical limb ischemia. Molecular therapy. Methods & clinical development 2016; 3: 16053. doi: 10.1038/mtm.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francois M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy 2012; 14(2): 147–152. doi: 10.3109/14653249.2011.623691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moll G, Aim JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 2014; 32(9): 2430–2442. doi: 10.1002/stem.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinnadurai R, Garcia MA, Sakurai Y, Lam WA, Kirk AD, Galipeau J et al. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem cell reports 2014; 3(1): 60–72. doi: 10.1016/j.stemcr.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 2015; 33(6): 1818–1828. doi: 10.1002/stem.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moya A, Paquet J, Deschepper M, Larochette N, Oudina K, Denoeud C et al. Human Mesenchymal Stem Cell Failure to Adapt to Glucose Shortage and Rapidly Use Intracellular Energy Reserves Through Glycolysis Explains Poor Cell Survival After Implantation. Stem Cells 2018; 36(3): 363–376. e-pub ahead of print 2017/12/22; doi: 10.1002/stem.2763 [DOI] [PubMed] [Google Scholar]
- 33.Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA et al. Hypoxic Preconditioning of Mesenchymal Stromal Cells Induces Metabolic Changes, Enhances Survival and Promotes Cell Retention in Vivo. Stem Cells 2015. doi: 10.1002/stem.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fury B, Hackett T, Fierro F, Nolta J, Bauer G. Clinically Relevant Formulation and Conditions for Transportation of Genetically Modified Bone Marrow Mesenchymal Stem Cells Engineered to Overexpress Vascular Endothelial Growth Factor. Cytotherapy 2015; 17(6): S42–S42. [Google Scholar]


