Abstract
This study examined: (i) biocontaminant levels in flooded homes of New Orleans two years after the flooding; (ii) seasonal changes in biocontaminant levels, and (iii) correlations between biocontaminant levels obtained by different environmental monitoring methods. Endotoxin, (1→3)-β-d-glucan, fungal spores, and dust mite allergens were measured in 35 homes during summer and winter. A combination of dust sampling, aerosolization-based microbial source assessment, and long-term inhalable bioaerosol sampling aided in understanding exposure matrices. On average, endotoxin found in the aerosolized fraction accounted for <2% of that measured in the floor dust, suggesting that vacuuming could overestimate inhalation exposures. In contrast, the (1→3)-β-d-glucan levels in the floor dust and aerosolized fractions were mostly comparable, and 25% of the homes showed aerosolizable levels even higher than the dust-borne levels. The seasonal patterns for endotoxin in dust and the aerosolizable fraction were different from those found for (1→3)-β-d-glucan, reflecting the temperature and humidity effects on bacterial and fungal contamination. While the concentration of airborne endotoxin followed the same seasonal trend as endotoxin aerosolized from surfaces, no significant seasonal difference was identified for the concentrations of airborne (1→3)-β-d-glucan and fungal spores. This was attributed to the difference in the particle size; smaller endotoxin-containing particles can remain airborne for longer time than larger fungal spores or (1→3)-β-d-glucan-containing particles. It is also possible that fungal aerosolization in home environments did not reach its full potential. Detectable dust mite allergens were found only in dust samples, and more commonly in occupied homes. Levels of endotoxin, (1→3)-β-d-glucan, and fungi in air had decreased during the two-year period following the flooding as compared to immediate measurements; however, the dust-borne endotoxin and (1→3)-β-d-glucan levels remained elevated. No conclusive correlations were found between the three environmental monitoring methods. The findings support the use of multiple methods when assessing exposure to microbial contaminants.
Keywords: Flood-affected homes, Bioaerosols, Molds, Endotoxin, (1→3)-β-d-glucan, Allergen
1. Introduction
Human inhalation exposure to microbial contaminants and allergens in indoor environments can be determined by measuring the concentrations of these pollutants using various air sampling methods and collecting dusts with a conventional vacuuming method (HUD, 2004). In addition, dust aerosolizable from surfaces can be sampled using an unconventional microbial source testing method developed and validated by our group (Grinshpun et al., 2002; Sivasubramani et al., 2004a,b). To the authors’ knowledge, there is a lack of scientific data on exposure matrices for different microbial contaminants sampled by different environmental monitoring methods. We have used ‘exposure matrix’ here referring to simultaneous assessment of exposure to various microbial contaminants in air, dust, and from surfaces within which the real inhalation exposure originates and develops. The relationship between various bioaerosol exposure assessment methods is worthwhile to investigate. This would allow building an ideal exposure assessment strategy that can be adopted during a natural disaster, e.g., a major flood that can create profuse inhalation exposure risks to microbial contaminants, implicating significant public health concerns.
Hurricanes Katrina and Rita caused unprecedented flooding and disasters in New Orleans in August and September of 2005. Moisture damage in flood-affected homes provided ample ideal niches for the growth of fungi and bacteria, resulting in increased risk of exposure to some biocontaminants derived from these microorganisms. Due to the moisture damage and warm, damp conditions inside of the flood-affected homes, visible mold growth was detected in approximately 44% of homes (Riggs et al., 2008). Moisture damage could also promote bacterial growth in flood-affected materials. Fabian et al. (2000) found that total indoor bacterial counts exceeded outdoor counts by two orders of magnitude or more in water-damaged homes, whereas all samples from non-water damaged homes had lower bacterial counts indoors than outdoors. Flooded homes could be a significant source of airborne endotoxin (Rao et al., 2007).
Arlian et al. (1992) reported high average dust mite densities in New Orleans homes prior to Hurricanes Katrina and Rita. The elevated humidity, followed by major floods caused by these hurricanes, is expected to optimize the growth conditions for dust mites because previous researchers found significant increases in dust mite population in laboratory cultures at higher relative humidity levels (Voorhorst et al., 1969).
Airborne fungi, bacteria, their byproducts, and dust mite allergens are potential respiratory health hazards (Thorn, 2001; Douwes et al., 2003; Rylander et al., 1998; Rylander, 1999; IOM, 2000). Aerosolized particles can be inhaled by returning residents and renovation workers in flood-affected homes. Several studies have reported high exposure levels of the above listed contaminants (except dust mite allergens) in New Orleans homes. Chew et al. (2006) investigated three homes in three time periods: before, during and after renovation. It was observed that concentrations of airborne culturable fungi before renovation ranged from 22×103 to 515×103 colony-forming units (CFUs)/m3, airborne total fungal spore levels ranged from 82×103 to 630×103 spores/m3, and endotoxin levels in air ranged from 17 to 139 endotoxin units (EU)/m3. In a 20-home study, Rao et al. (2007) found the following geometric mean concentrations: 280×103 spores/m3 for airborne fungi, 1.6 μg/m3 for (1→3, 1→6)-β-d-glucans, and 23.3 EU/m3 for endotoxin. Schwab et al. (2007) collected air samples in six homes and found even higher airborne fungal spore concentrations (up to 735×103 spores/m3). It is to be noted that the air sampling time periods in the above-quoted three studies were very short: 1 to 20 min (Chew et al.); 36 to 144 min (Rao et al.); and 10 to 17 min (Schwab et al.). Solomon et al. (2006), who conducted air sampling during 6 h and 24 h in 8 homes, found indoor concentrations of airborne fungal spores ranging from 11×103 to 645×103 spores/m3. The investigators reported that the 6-h time-averaged concentration measured from 9 AM to 3 PM differed from the 24-h time-averaged one at each site, although the difference was not consistent in either magnitude or direction and was not statistically significant. Solomon et al. measured airborne endotoxin levels only inside two homes; they ranged from 4.5 to 7.3 EU/m3. There were several noteworthy limitations in the referred studies, such as: (a) the small sample size; (b) short air sampling period in most cases; (c) deployment of samplers with d50 cut-off size above the size of dry spores of common fungal species, including some Aspergillus and Penicillium (e.g., Solomon et al. used Burkard sampler with d50=2.52 μm; Schwab et al. used VersaTrap samplers with d50=2.3–2.7 μm); (d) lack of replicate measurements [except Chew et al.’s (2006) study]. Thus, the investigations performed up-to-date have limited applicability in assessing exposure levels of the returning occupants or renovation workers. The present study was initiated to address the above-listed limitations.
The main purpose was to examine the exposure matrices of endotoxin, (1→3)-β-d-glucan, fungi, and dust mite allergen (Der f 1, and Der p 1) levels in 35 unoccupied or partially occupied flood-affected homes of New Orleans from April to August, 2007 and from November 2007 to February, 2008. These exposure matrices were examined through long-term inhalable air sampling, sampling of contaminants aerosolizable from surfaces, and conventional dust sampling methods. In parallel to these measurements, environmental temperature, relative humidity, surface moisture, conditions of floor materials, and applications of bleach or other chemicals during the two seasons were recorded. The following research questions were addressed in the study:
What are the exposure levels of a few specific health-hazardous biocontaminants in the flood-affected homes two years after the flooding relative to the levels recorded within 2–6 months following the flooding?
Was there any seasonal change in these biocontaminant exposure levels between summer and winter in the flood-affected homes?
Do the exposure levels of the selected biocontaminants assessed by different environmental monitoring methods correlate?
2. Materials and methods
2.1. Selection of flood-affected homes in New Orleans area
We selected 35 moisture-problem homes from a pool of approximately 200 households affected by the flooding during Hurricanes Katrina and Rita, identified with help from local community organizations and agencies. The initial home inclusion criteria were: (1) at least 3 ft in height of flooding in the homes, which represented homes with heavy mold contamination, and (2) residents were dwelling in the home overnight, which allowed using exposure data in other prospective epidemiological studies involving the affected residents, as well as repeat exposure analysis of the same home. We anticipated that most floors in the selected homes would represent untreated hardwood or carpet floor materials. In a few instances, we found that flooded hardwood or carpet floor materials had been removed, and only concrete slabs remained. Moreover, we found that floors were sometimes treated with bleach and other chemicals. We expected that residents would mostly stay overnight in their homes; however, in most cases, they were staying during the day time (primarily doing renovation work), but not necessarily overnight. The characteristics of the selected 35 homes are summarized in Table 1. As shown in the table, most of the homes had hardwood floors, showed signs of water damage and visible mold, and remained partially occupied or unoccupied during the sample collection. Among chemicals used for remediation in homes, bleach was the most common. All 35 homes were tested in the summer campaign, but only 31 were available for testing in the winter campaign because the home owners were not willing to continue their participation in the study.
Table 1.
Characteristics of 35 flood-affected homes investigated in the present study.
| Home characteristics | Categories | Number of homes (n) | Percentage (%) |
|---|---|---|---|
| Floor materials | Carpet | 4 | 11 |
| Hardwood | 20 | 57 | |
| Concrete slab | 6 | 17 | |
| Tile | 3 | 9 | |
| Linoleum | 1 | 3 | |
| Plywood | 1 | 3 | |
| Signs of water damage | Yes | 31 | 89 |
| No | 4 | 11 | |
| Signs of visible mold | Yes | 34 | 97 |
| No | 1 | 3 | |
| Occupation status | Unoccupied | 27 | 77 |
| Occupied | 8 | 23 | |
| Bleach and other chemicals treatment status | Treated | 19 | 54 |
| Untreated | 16 | 46 | |
| Chemicals treatment type | Microban | 2 | 11 |
| Boracare | 3 | 16 | |
| Jomax | 1 | 5 | |
| Sterifab and Virex | 1 | 5 | |
| Zinase | 1 | 5 | |
| Bleach | 8 | 42 | |
| Unknown chemicals | 3 | 16 |
2.2. Survey and sampling methods
2.2.1. Walkthrough
A home walkthrough checklist was developed utilizing existing checklists (HUD, 2004; Cho et al., 2006). It included the size of the home, presence of pets, moisture problems and/or visible mold, as well as the measured temperature and humidity. In homes with visible mold and moisture problems, the moisture content of the test surface was measured with a Protimeter (Protimeter Surveymaster, GE Sensing & Inspection Technologies, Billerica, MA). The air temperature and relative humidity were determined with a thermohygrometer pen (Fisher Scientific, Pittsburgh, PA).
2.2.2. Sampling strategy
Conventional dust and air sampling methods, vacuuming and filtration-based inhalable aerosol sampling, were employed. We also utilized a source assessment method aiming at determining the aerosolization potential of specific biocontaminants from surfaces. This was done using a novel microbial source tester validated and used in our earlier studies (Grinshpun et al., 2002; Górny et al., 2002; Sivasubramani et al., 2004a,b; Niemeier et al., 2006; Seo et al., 2008; Adhikari et al., 2009), in which it was referred to as the Fungal Spore Source Strength Tester or FSSST. In each home, a bedroom with a floor area of 50–300 ft2 was selected for the field sampling. Our original plan was to collect five samples from each home: two samples by vacuuming the floor and the mattress, two samples with the source tester applied to the floor and the mattress, and one air sample. However, we found that the flood-affected mattresses were already discarded in most of the homes. In homes where no mattress was available, only three samples were collected. In most cases, the residents were not present during sample collection; however, if present, they continued their normal activities during measurements.
All the collected samples were immediately brought to the laboratory, extracted following standard methods, and divided into aliquots and preserved at −20 °C. The samples were then analyzed for endotoxin, (1→3)-β-d-glucan, total fungal spores, and dust mite allergens, as described below.
The measurements were conducted in two campaigns — “summer” (late spring through summer) and “winter” (late fall through winter). As the flood-affected homes were mostly devoid of electricity, we assumed that seasonal changes of temperature and humidity could have significantly affected the microbial growth in these environments.
2.2.3. Sample collection
Dust samples were collected from the floor into a small filter bag, by vacuuming with a Filter Queen Majestic® vacuum cleaner (Health-Mor, HMI Industries Inc., Seven Hills, OH) for 5 min from 1 m2 of the floor area. The HUD Standard Dust Sampling Protocol (HUD, 2004) was followed. For mattresses, 2-m2 areas were vacuumed for 5 min. Collected dust samples were sieved (355 μm sieve), and the resulting fine dust was divided into sub-samples and stored at −20 °C, until the analysis.
When assessing the source aerosolization potential, the aerosolization chamber of the FSSST was tightly held against the floor or mattress surface, covering an area of 0.012 m2. A push vacuum pump produced an airflow that passed through a HEPA filter (PALL Gelman Laboratory, Ann Arbor, MI). The incoming air flow directed through the 112-orifice stage at the bottom of the device created air jets towards the floor or mattress surface, and the particles containing above-mentioned microbiological contaminants were aerosolized by these air jets and collected into a BioSampler (SKC, Inc., Eighty Four, PA). The sampler was located at the outlet of the aerosolization chamber and operated by a pull vacuum pump at a flow rate of 12.5 L/min. The collection vessel in the BioSampler was filled with 20 mL suspension of pyrogen free sterile water mixed with 0.05% Tween 80. The flow rate balance was adjusted during sampling so that the incoming air flow was always slightly (by 0.5–1.0 L/min) lower than the sampling air flow. This prevented contaminating the indoor air by the FSSST operation. The device was thoroughly cleaned between the tests with 70% ethyl alcohol and air dried in a biosafety hood; a separate sterile BioSampler was used for each sample collection. After 15 min sampling (this duration was selected following our laboratory study; see Adhikari et al., 2009), the collection fluid of each BioSampler was divided into sub-samples and stored at −20 °C until analyzed. The results achieved by the aggressive sampling with the FSSST represent the aerosolization potential of microbial sources, which allows predicting maximum aerosol concentration for each microbial contaminant in each flood-affected home.
Air samples were collected in the middle of the bedroom, at a height of 1.1 m, using a Button Inhalable Aerosol Sampler (SKC Inc., Eighty Four, PA) equipped with 25-mm polycarbonate filters (pore size=2 μm). The Button Sampler was operated at a flow rate of 4 L/min for approximately 24 h. The flow rates of all pumps were calibrated before and after each sampling. The Button Sampler was thoroughly cleaned and sterilized by autoclaving between the measurements. After the sampling, the sample filters were stored at −20 °C, until the extraction.
2.3. Analysis of samples collected by vacuuming, microbial source tester, and air sampling
2.3.1. Endotoxin and (1→3)-β-d-glucan
For the analysis of endotoxin and (1→3)-β-d-glucan in dust samples, 25 mg of fine dust was used for extraction of each. Endotoxin was extracted in 1.0 mL of pyrogen free sterile water for 1 h by sonication, whereas (1→3)-β-d-glucan was extracted in 1.0 mL of 0.6 M NaOH solution by vigorously shaking. Both types of extracts were then centrifuged at 7000 rpm (5204×g) for 1 min. Supernatants were collected for the analysis. Button Sampler filters were previously extracted into 5 mL of pyrogen-free water containing 0.05% Tween 80 by vortexing and sonication and stored at −20 °C for several days until analysis. FSSST’s Biosampler suspensions were directly stored at −20 °C. Immediately before analysis, these extracts and previously stored suspensions from FSSST’s BioSampler (0.5 mL aliquots) were again either sonicated for endotoxin or vigorously shaken for (1→3)-β-d-glucan as described previously.
For endotoxin measurement, the supernatants from dust samples and sonicated extracts from the Button Sampler and Biosampler were analyzed with the endotoxin-specific kinetic chromogenic Limulus Amebocyte Lysate (LAL) assay (Pyrochrome, Associates of Cape Cod, East Falmouth, MA). Endotoxin concentrations in liquid extracts were converted into EU/m2 for dust and FSSST samples and in EU/m3 for air samples derived from the weight of total sieved dust collected and dust used for extraction, area sampled, air flow rates, and sampling duration. The lower limit of detection (LLOD) for endotoxin in suspension was 0.05 EU/mL.
For (1→3)-β-d-glucan measurement, the supernatants from dust samples and sonicated extracts from the Button Sampler and Biosampler were analyzed with the (1→3)-β-d-glucan-specific kinetic chromogenic LAL assay (Glucatell, Associates of Cape Cod), as previously described by Lee et al. (2006) and Iossifova et al. (2007). The (1→3)-β-d-glucan concentration values were presented in μg/m2 for the dust and FSSST samples and in μg/m3 for air samples derived similarly as described above for endotoxin. The LLOD of (1→3)-β-d-glucan in suspension was 2.53 pg/mL.
2.3.2. Microscopic analysis of total fungal spores
Total fungal spore enumeration was conducted in air and FSSST samples collected from 10 randomly selected flood-affected homes. Total fungal spores in dust samples were not analyzed, because dust particles obstructed microscopic visualization. The filters from Button Samplers were extracted into 5 mL of pyrogen-free water containing 0.05% Tween 80, and suspensions from FSSST’s BioSampler were directly used for analysis. One milliliter aliquot of the Button Sampler filter extract (5 mL) and the 5 mL aliquot of the FSSST’s BioSampler suspension (20 mL) were filtered through a 13-mm mixed cellulose esterase filter. Each filter was placed onto a slide and allowed to completely dry in a clean biosafety hood. The dry filter was made clear by treating with acetone vapor, following our previously developed protocols (Adhikari et al., 2003, 2004). Fungal spores were identified and counted in 40 microscopic fields (7.4% area of the filter) using a bright light microscope (Labophot 2, Nikon Corp., Japan) at a magnification of 400× (in addition, a higher magnification of 1000× was sometimes used for confirming the identification of smaller spores). Fungal spores were identified morphologically to genus/group level. Results were expressed in spores/m2 for FSSST samples and in spores/m3 for air samples. The LLOD of the fungal spore enumeration (derived from the limit of one spore per 40 microscopic fields and calculated accounting for the sampled area, air flow rate, and sampling time) was 5587 spores/m2 for FSSST samples and 6 spores/m3 for air samples.
2.3.3. Dust mite allergens
For dust mite allergen extraction, 100 mg of fine dust was extracted in 2 mL of phosphate-buffered saline with 0.05% Tween 20 and was shaken on a platform shaker for 1 h at 30 °C. These extracts were serially diluted in phosphate-buffered saline with 1% bovine serum albumin and 0.05% Tween 20 (BSA-PBS-T) solution at pH 7.4. Button Sampler filters were previously extracted into 5 mL of pyrogen-free water containing 0.05% Tween 80 by vortexing and sonication and stored at −20 °C. FSSST’s Biosampler suspensions were directly stored at −20 °C. For these air and FSSST samples, undiluted or concentrated (using Amicon columns) filter extracts and suspensions were treated with BSA solution because Tween 80 had been already used for the extraction. In accordance to Luczynska et al. (1989), two-site monoclonal antibody (MAB) sandwich ELISAs (Indoor Biotechnologies, Inc., Charlottesville, VA) were used to analyze for Der p 1 and Der f 1. The analytical LLOD of the ELISA was 1.25 ng of allergen per mL of buffer. The sampling LLOD for dust samples was 0.025 ng/mg of dust collected (= 0.025 μg/g). Concentrating the FSSST extracts using Amicon columns allowed us to achieve a LLOD of 1.4–2.2 ng per air sample collected; the actual protein concentration varied by FSSST sample.
2.4. Statistical analysis
Prior to performing statistical analysis, the data were tested for distributions of the variables. The results of replicate measurements were averaged, and the standard deviations (SD) were calculated. Normality of the data distributions was tested by quantile–quantile or Q–Q plots. Pearson correlation (if normal distribution of data was achieved) or non-parametric Spearman’s correlation coefficients (if normal distribution of data was not achieved) were calculated to characterize the association between the levels of endotoxin, (1→3)-β-d-glucan, fungal spores, dust mite allergens, and three measured environmental variables: temperature, relative humidity, and surface moisture. Statistical significance in the differences in seasonal variations of endotoxin, (1→3)-β-d-glucan, fungal spores, and dust mite allergens were calculated by paired t-test. The level of statistical significance was considered at p-values below 0.05. All statistical tests were performed using the SPSS 11.0 for Windows (SPSS Inc., Chicago, IL) software.
3. Results and discussion
3.1. Exposure matrices of endotoxin
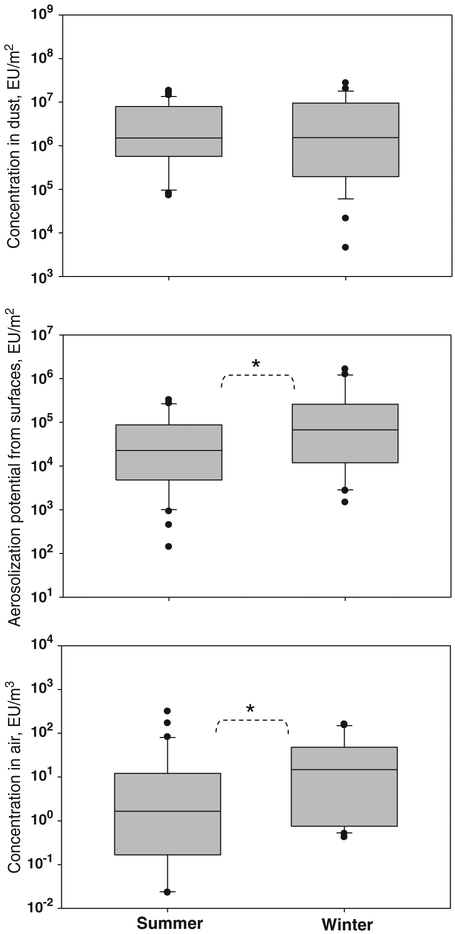
Fig. 1 presents the median values and quartiles for endotoxin concentration in dust, its aerosolizable concentration (from FSSST), and indoor air concentration. In addition, the following mean values and standard deviations were determined for the tested homes: (4.30±5.22)×106 EU/m2 (dust), (6.87±9.63)×104 EU/m2 (aerosolization potential) and 22.85±60.95 EU/m3 (indoor air) in summer, and(4.15±5.77)×106 EU/m2 (dust), (2.78±4.73)×105 EU/m2 (aerosolization potential) and 36.91±50.94 EU/m3 (indoor air) in winter.
Fig. 1.
Exposure matrices of endotoxin in flood-affected homes (n=35 in summer and 31 in winter). The lower and upper boundaries of the box specify the 25th and 75th percentiles, respectively. The line within the box indicates the median and the whiskers above and below the box indicate the 95th and 5th percentiles, respectively. * indicates statistically significant difference (paired t-test: p<0.05).
No statistically significant difference in endotoxin levels measured in the two seasons was observed for dust samples (p>0.05). The total surface concentrations of the sieved fine dust collected by vacuuming during the two seasons were approximately the same (mean±SD): 4.26±2.18 g/m2 (summer) and 4.02±2.39 g/m2 (winter). In contrast, statistically significant increases from summer to winter were observed for aerosolizable endotoxin (analyzed from the FSSST samples) and for the airborne endotoxin (analyzed from air samples). The aerosolization of endotoxin, as concluded from the FSSST results, was significantly higher in winter than in summer (p<0.05; paired t-test). This trend was observed in 71% of the homes and can be attributed to the seasonal differences in air temperature and humidity; e.g., less humid air in winter (see Section 3.5) is likely to be associated with higher arosolization rates. Lower humidity might have facilitated desiccation of endotoxin-containing particles on surfaces, thus enhancing aerosolization. The concentration of airborne endotoxin followed the trend obtained for its aerosolization potential. As a component of the bacterial cell membrane, endotoxin is expected to be present primarily in the fine particle size fraction, including considerable amount in the submicrometer range. Recently published data on the endotoxin analysis of materials collected in flood-affected New Orleans homes (Adhikari et al., 2009) support the above expectation. Being very small, the endotoxin-containing particles, once aerosolized, are likely to remain airborne for a long time.
Additionally, the endotoxin aerosolization ratio was determined as its concentration in air divided by the concentration in vacuumed dust. This aerosolization ratio was higher in winter than in summer in 76% homes. This finding is consistent with the aerosolization potential data obtained using the FSSST.
Overall, we found that endotoxin levels in the vacuumed dust samples were mostly two orders of magnitudes higher than in the FSSST samples, indicating that only a small fraction of endotoxin (on average, <2%) was aerosolizable from the floor dust. This suggests that vacuuming could considerably overestimate the inhalation exposure risks associated with endotoxin released from the floors of the flood-affected homes. Similar findings were obtained in our recently published laboratory study (Adhikari et al., 2009), where the dust-borne endotoxin collected from flood-affected materials was 102- to 103-fold higher compared to its aerosolizable level (determined from the FSSST samples). It is acknowledged that vacuumed floor dust samples have a limited utilization for assessing the seasonal changes in exposure because contaminant levels in dust are less influenced by short-term variability in indoor activities than the corresponding levels in the air.
The dust-borne endotoxin levels obtained in the present study are approximately 102 to 104 times higher than those found in dust samples collected from homes in New York (3892 EU/m2; Perzanowski et al., 2006) and Cincinnati (24 EU/m2; Iossifova et al., 2007). This indicates that major flooding, such as the one which occurred in New Orleans, produces sustainable elevation of endotoxin in dust in flood-affected homes that remains high (as measured from dust samples), even two years after the flood. At the same time, the concentration of endotoxin measured in indoor air was only moderately elevated. Other investigators, who conducted indoor air monitoring in a more immediate aftermath of Hurricane Katrina, reported higher airborne endotoxin levels in flood-affected homes: a range of 17–139 EU/m3 (Chew et al., 2006) and a geometric mean of 22.3 EU/m3 (Rao et al., 2007). Recent measurements in non-moldy Cincinnati homes revealed a geometric mean of 8.7 EU/m3 for airborne concentration of endotoxin (Reponen et al., 2009). For comparison, the geometric mean of airborne endotoxin obtained by integrating the summer and winter campaigns in our study was 3.03 EU/m3 with a geometric standard deviation of 14.24 EU/m3. This suggests that the airborne endotoxin levels in the flood-affected homes had decreased in two years after the floods, down to typical levels measured in non-damaged homes.
Although mattresses were not available in most homes, we had three pairs of samples (two from the summer and one from the winter campaign) that allowed comparing the endotoxin levels from the floor samples to those detected in mattresses. A proper statistical comparison is not possible with this limited amount of data. However, based on the mean values calculated from the vacuumed dust sample data, the endotoxin level on the floors was found to be approximately 5-fold higher than in mattresses: (8.39±5.13)×105 EU/m2 versus (1.70±0.64)×105 EU/m2. The FSSST samples demonstrated less pronounced difference: (1.09±1.85)×105 EU/m2 (floors) versus (0.68±1.14)×105 EU/m2 (mattresses).
We also compared the levels of endotoxin in chemically treated homes in summer versus winter using paired t-test and did not find any statistically significant difference of endotoxin in dust (n=11), FSSST (n=15), and air samples (n=9).
3.2. Exposure matrices of (1→3)-β-d-glucan
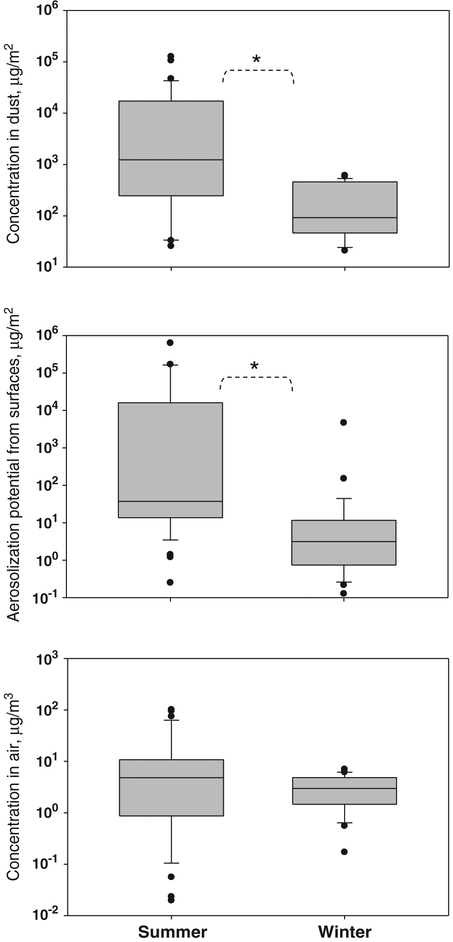
The measured (1→3)-β-d-glucan levels in dust, FSSST, and air samples are presented in a box plot format in Fig. 2 where data quartiles and medians are presented. In addition, for the summer dataset, the following mean values and standard deviations were calculated for the dust, FSSST, and air samples: (15.50±28.89)×103 μg/m2, (39.20±110.96)×103 μg/m2, and 15.92±27.04 ng/m3, respectively. The corresponding values in winter were lower: (0.22±0.20)×103 μg/m2, (0.17±0.87)×103 μg/m2, and 3.14±2.05 ng/m3.
Fig. 2.
Exposure matrices of (1→3)-β-d-glucan in flood-affected homes (n=35 in summer and 31 in winter). The lower and upper boundaries of the box specify the 25th and 75th percentiles, respectively. The line within the box indicates the median and the whiskers above and below the box indicate the 95th and 5th percentiles, respectively. * indicates statistically significant difference (paired t-test: p<0.05).
The (1→3)-β-d-glucan measured on floor surfaces were compared to those obtained in mattresses in three pairs. No consistent differences were observed with respect to the (1→3)-β-d-glucan concentration in either vacuumed dust or FSSST samples. This is likely because too few samples were available.
In contrast to endotoxin, the concentration of (1→3)-β-d-glucan in dust and its maximum plausible aerosolizable fraction were lower in winter than in summer, perhaps reflecting inhibited fungal growth at lower temperature and humidity levels. Lower availability of moisture in the winter season could have inhibited the fungal growth on surfaces, which translated to lower levels of (1→3)-β-d-glucan found in dust and consequently in the aerosolizable fraction. Unlike the dust samples and aerosolizable samples, the air samples did not reveal statistically significant seasonal difference with respect to (1→3)-β-d-glucan. As a polyglucose molecule comprises a large amount of cell wall of most fungal spores, the presence of (1→3)-β-d-glucan is linked to fungal spores or their fragments. The aerosolization rate of (1→3)-β-d-glucan (by mass) is usually higher for larger particles (Adhikari et al., 2009). According to Seo et al. (2009), the concentration of (1→3)-β-d-glucan in supermicrometer airborne fungal spores may be ~101- to 102-fold greater than that in submicrometer fragments. The aerodynamic sizes of single fungal spores and spore aggregates present in indoor air are usually above 2 μm and may even exceed 10 μm. These relatively large particles are characterized by significant gravitational settling velocities, which make most of them settle in homes within hours after aerosolization. This may explain why the concentration of (1→3)-β-d-glucan in air did not follow the trends observed for the dust and aerosolizable samples. In addition, the aerosolization of (1→3)-β-d-glucan in the tested home environments might not have reached the full potential quantified by the aerosolization-based microbial source assessment.
At the same time, the aerosolization ratio of (1→3)-β-d-glucan calculated by relating the air to the dust concentration was higher in winter in most of the tested homes (71%), which can be explained by lower humidity in winter. In contrast to endotoxin, the (1→3)-β-d-glucan levels in the floor dust and aerosolized fractions were mostly comparable, and 25% homes showed the aerosolizable level even higher than the dust-borne one. There were 9% of such homes in winter and 35% in summer. Similar to endotoxin, the lower humidity observed in winter has likely facilitated desiccation of (1→3)-β-d-glucan-containing particles on surfaces, which enhanced aerosolization.
The geometric mean of (1→3)-β-d-glucan concentration obtained from the dust samples in this study (winter and summer data combined) was 116 μg/m2, which is more than 6-fold higher than the value obtained from the vacuum-collected floor dust samples in a 574-home study conducted in Cincinnati (geometric mean=18 μg/m2; Iossifova et al., 2007). Thus, similar to endotoxin, dust-borne (1→3)-β-d-glucan levels in flood-affected New Orleans homes remained considerably high, even though two years had passed since the flood.
The (1→3)-β-d-glucan concentrations in air samples had a geometric mean of 2.71 ng/m3 for the entire data set (the summer and winter campaigns together). This level is lower than airborne concentrations of (1→3)-β-d-glucan reported in previous studies conducted in flood-affected homes and close to the typical levels measured in non-flood-damaged homes. For example, Rao et al. (2007) found a geometric mean of the airborne (1→3)-β-d-glucan and (1→6)-β-d-glucan to be 1600 ng/m3 when sampling in flood-affected homes of New Orleans within three months after the floods; approximately one year after the floods, Reponen et al. (2007) found a geometric mean of (1→3)-β-d-glucan to be much lower: 6.57 ng/m3. The geometric mean of airborne (1→3)-β-d-glucan concentrations measured in non-moldy homes in Cincinnati were even lower: 0.92 ng/m3 (Lee et al., 2006), 1.0 ng/m3 (Crawford et al., 2009) and 2.0 ng/m3 (Reponen et al., 2009).
The levels of (1→3)-β-d-glucan obtained in the summer and winter campaigns were also compared separately for homes treated with bleach and other chemicals versus non-treated homes using paired t-test. The strong statistically significant decrease from summer to winter was confirmed for each of the two sub-groups of homes based on both the dust (n=8) and FSSST (n=9) samples. Air samples (n=7), in contrast, showed no significant seasonal differences in (1→3)-β-d-glucan levels in either sub-group.
3.3. Exposure matrices of fungi
Levels (mean±SD) of total fungi in FSSST and air samples collected in summer were (9.34±16.11)×105 spores/m2 and (3.03±2.55)×103 spores/m3, respectively. The corresponding values in winter were: (4.38 ± 8.86)×105 spores/m2 and (4.44 ± 2.55)×103 spores/m3. The spore type specific data are presented in Table 2. No significant seasonal difference was determined for airborne total fungal spores similar to (1→3)-β-d-glucan. This suggests that the fungal aerosolization in home environments did not reach the full potential and/or the aerosolized fungal spores (including agglomerates) were sufficiently large to be subjected to significant gravitational settling. However, most of the FSSST samples showed lower spore concentration during winter with the p-value obtained in paired t-test close to significance level (p=0.063). Mattress versus floor and chemically treated versus non-treated homes were not compared with respect to fungal spores because of the small sample size (only ~30% of samples were chosen for fungal spore analysis).
Table 2.
Concentration of total fungal spores in FSSST and air samples during summer and winter.
| Summer | Winter | |||||||
|---|---|---|---|---|---|---|---|---|
| Fungal spores in FSSST samples (n = 10) | Fungal spores in air samples (n = 10) | Fungal spores in FSSST samples (n = 8a) | Fungal spores in air samples (n = 10) | |||||
| Range (spores/m2) | Percentage of occurrence (%) | Range (spores/m3) | Percentage of occurrence (%) | Range (spores/m2) | Percentage of occurrence (%) | Range (spores/m3) | Percentage of occurrence (%) | |
| Spore types | ||||||||
| Alternaria | <LLOD-14,899 | 10 | <LLOD-17 | 10 | <LLOD-59,597 | 25 | 0 | |
| Aspergillus/Penicillium | 39,110–1,013,144 | 100 | 100–3504 | 100 | <LLOD-953,547 | 88 | 131–12,519 | 100 |
| Arthrinium | <LLOD-104,294 | 10 | <LLOD-130 | 20 | <LLOD-119,193 | 13 | 0 | |
| Ascospores | 11,174–208,589 | 100 | <LLOD-1105 | 90 | <LLOD-119,193 | 63 | <LLOD-3130 | 80 |
| Basidiospores | <LLOD-208,589 | 50 | <LLOD-765 | 80 | 0 | <LLOD-709 | 30 | |
| Bispora | <LLOD-5587 | 10 | <LLOD-14 | 10 | 0 | 0 | ||
| Botrytis | <LLOD-5587 | 10 | <LLOD-8 | 10 | 0 | <LLOD-14 | 10 | |
| Botryodiplodia | 0 | <LLOD-95 | 10 | 0 | 0 | |||
| Cercospora | 0 | <LLOD-41 | 20 | 0 | <LLOD-14 | 10 | ||
| Coprinus | 0 | <LLOD-52 | 20 | 0 | 0 | |||
| Chaetomium | <LLOD-2,771,247 | 50 | <LLOD-945 | 70 | <LLOD-595,967 | 25 | 0 | |
| Cladosporium | <LLOD-59,597 | 50 | <LLOD-408 | 80 | <LLOD-11,174 | 33 | 38–6203 | 60 |
| Curvularia | <LLOD-23,349 | 20 | <LLOD-8 | 10 | 0 | 0 | ||
| Drechslera | 0 | <LLOD-95 | 30 | 0 | 0 | |||
| Epicoccum | <LLOD-14,899 | 10 | < LLOD-7 | 10 | 0 | 0 | ||
| Ganoderma | 0 | <LLOD-389 | 80 | <LLOD-59,597 | 13 | <LLOD-39 | 10 | |
| Nigrospora | <LLOD-44,698 | 10 | 0 | 0 | <LLOD-165 | 10 | ||
| Periconia | 0 | <LLOD-378 | 10 | 0 | 0 | |||
| Peronospora | 0 | <LLOD-95 | 20 | 0 | 0 | |||
| Pithomyces | <LLOD-14,899 | 10 | 0 | 0 | 0 | |||
| Polythrincium | 0 | <LLOD-8 | 10 | 0 | 0 | |||
| Psathyrella | 0 | <LLOD-8 | 10 | 0 | 0 | |||
| Rusts | 0 | <LLOD-25 | 20 | 0 | 0 | |||
| Smuts/Myxomycetes | 0 | <LLOD-544 | 60 | <LLOD-238,387 | 13 | 0 | ||
| Sporidesmium | 0 | <LLOD-23 | 20 | 0 | <LLOD-14 | 10 | ||
| Stachybotrys | 0 | < LLOD-136 | 20 | <LLOD-5587 | 13 | 0 | ||
| Tetraploa | 0 | <LLOD-6 | 10 | 0 | 0 | |||
| Unknown spores | 5587–134,093 | 100 | <LLOD-816 | 60 | <LLOD-297,984 | 50 | <LLOD-1521 | 40 |
| Total spores | 55,872–4,559,149 | 200–8128 | 27,936–2,443,465 | 232–15,649 | ||||
Abbreviation: LLOD: Lower limit of detection.
n=8 for winter FSSST samples because spores in two samples were not visible under the microscope due to excess of dust particles.
Researchers who measured airborne fungal spore levels within 2–6 months after the floods reported much higher concentration levels, ranging from 280×103 to 735×103 spores/m3 (Chew et al., 2006; Solomon et al., 2006; Rao et al., 2007; Schwab et al., 2007). Approximately one year after the floods, the indoor concentration of airborne fungi decreased, as evident from the study of Reponen et al. (2007) that reported a geometric mean of 12×103 spores/m3 (from three flood-affected homes). Previous studies in non-moldy homes in Cincinnati demonstrated the following geometric means of airborne spore concentrations: 573 spores/m3 (Lee et al., 2006), 214 spores/m3 (Crawford et al., 2009) and 120 spores/m3 (Reponen et al., 2009). Another study that included both non-moldy and moldy Cincinnati homes revealed a geometric mean of 145 spores/m3 (Osborne et al., 2006). We concluded that while the airborne spore concentrations in the flood-affected homes seem to have decreased after the initial water damage (the geometric mean of both the summer and winter data obtained in this study was 1.9 ×103 spores/m3), the levels were still slightly higher than those typically measured in homes not affected by major flooding.
The composition of predominant spore types had remained the same as observed by other investigators within 6 months of the floods. Similar to the observation of Solomon et al. (2006), we also found that the highest concentrations were of Aspergillus/Penicillium, Cladosporium, ascopsores among the fungal types identified in air samples. Chaetomium and Stachybotrys were identified in air samples occasionally in summer (see Table 2).
3.4. Exposure matrices of dust mite allergens
As stated in the Introduction section, we anticipated high levels of dust mite allergens in the flood-affected homes. However, detectable levels of at least one of the two allergens (Der p1 or Der f1) were found in only a few floor or mattress dust samples (from six homes sampled in summer and three homes sampled in winter). Overall, detectable levels of these allergens were found in dust samples collected in seven out of eight occupied homes and in two out of 27 unoccupied homes. All FSSST and air samples had the dust mite allergen values below the LLOD, even after concentrating the samples. The concentrations of Der p1 and Der f1 in dust samples collected from floors and mattresses are presented in Table 3.
Table 3.
Dust mite allergen levels in dust samples collected during summer and winter. Only homes that had detectable levels of dust mite allergens are included.
| Home ID | Floor material | Signs of water damage | Signs of visible mold | Occupation status | Bleach and other chemicals treatment status | Der p1 (μg/g) | Der f1 (μg/g) | ||
|---|---|---|---|---|---|---|---|---|---|
| Floor | Mattress | Floor | Mattress | ||||||
| Summer | |||||||||
| 2234S | Carpet | Yes | Yes | Unoccupied | Treated | <LLOD | N/A | 0.08 | N/A |
| 3605M | Hardwood | Yes | Yes | Occupied | Untreated | <LLOD | 1.64 | 11.78 | >ULOD |
| 834J | Carpet | Yes | No | Occupied | Treated | 1.78 | 1.65 | 13.51 | >ULOD |
| 1350SA | Linoleum | Yes | Yes | Occupied | Untreated | <LLOD | N/A | 0.06 | N/A |
| 7141R | Hardwood | Yes | Yes | Unoccupied | Untreated | <LLOD | N/A | 0.08 | N/A |
| 11011H | Hardwood | Yes | Yes | Occupied | Treated | <LLOD | N/A | 0.07 | N/A |
| Winter | |||||||||
| 926M | Carpet | Yes | Yes | Occupied | Untreated | <LLOD | N/A | 2.21 | N/A |
| 3605M | Hardwood | Yes | Yes | Occupied | Untreated | 4.07 | 18.86 | 0.31 | 5.90 |
| 1350StA | Linoleum | Yes | Yes | Occupied | Untreated | <LLOD | N/A | 0.145 | N/A |
Abbreviations: LLOD: Lower limit of detection; ULOD: Upper limit of detection.
Note: All other dust samples, FSSST samples, and air samples showed<LLOD levels for both Der p1 and Der f1.
In general, it appeared that moisture damage did not promote dust mite population growth after the flood. In all three homes where dust mite allergen levels were measured both in the floor and mattress dusts, significantly higher levels of Der p1 and Der f 1 were found in mattresses (see Table 3), indicating that moisture-damaged mattresses could provide better niches for the dust mite populations. As shown in Table 3, these three homes were occupied and were not treated with bleach and other chemicals. Previously, we also found that the amounts of dust mite allergens aerosolized from different flood-affected materials collected in New Orleans homes were below the detection limit (Adhikari et al., 2009).
While relative humidity of 70–80% can promote the dust mite population growth, further increase in the humidity (above 85%) does not provide favorable growth conditions (Voorhorst et al., 1969; Spieksma, 1990). These “extreme” humidity conditions likely occurred immediately after the flood and probably diminished the existing dust mite populations. However, it is not clear whether this disappearance directly reflects the humidity effect or was caused by indirect ecological effects that enhanced growth of other microorganisms (e.g., fungi), which could have inhibited the growth of mite population. We also anticipate that after the floods, mite re-infestation did not occur because the homes were unoccupied for a long time. Pike (1998) previously found that houses with a low number of occupants take a long time to become infested with dust mites and by inference, people must be occupying the house for infestation to occur, which was mostly lacking in the flood-affected homes. There is also the possibility that the allergens in the flood-affected materials were altered in some way that precluded measurement by ELISA. For example, dust mite allergens can be affected by tannic acid and other chemicals (Woodfolk et al., 1995; Chew et al., 1999).
3.5. Effects of temperature, relative humidity, and moisture levels on endotoxin and (1→3)-β-d-glucan levels
The temperature levels in the investigated homes ranged from 26.40 to 42.75 °C (mean±SD=30.60±3.03 °C) during summer and 13.8 to 34.7 °C (mean±SD=20.62±4.82 °C) during winter. The relative humidity levels were 47.00 to 69.50% (mean±SD=61.97±5.66%) during summer and 35.00 to 78.50% (mean±SD=49.98±11.75%) during winter. Surface moisture was measured during sampling by a protimeter, and readings were qualitatively classified into ‘dry’, ‘at risk’, and ‘wet’ categories, corresponding to color changing LEDs in the protimeter showing green (dry), yellow (at risk) and red (wet) lights. According to the manufacturer, surface materials in the green zone are in safe air-dry condition; in the yellow zone, moisture levels are higher than normal, but not critical; further investigation is recommended; and the red zone represents excessive moisture levels. If sustained, red zone moisture levels can lead to decay in organic materials. A total of 11, 17, and 7 homes during summer, and 17, 11, and 1 home during winter were classified into ‘dry’, ‘at risk’, and ‘wet’ categories, respectively (no data were recorded in two homes due to an instrument problem). This suggests that moisture levels on the surfaces had decreased in the tested homes between summer and winter, but a considerable number of homes were still under ‘at risk’ category, even two years after the floods.
We calculated Pearson correlation coefficients between the log-transformed concentration levels of endotoxin, (1→3)-β-d-glucan, and fungi, in one comparison, and the levels of temperature and relative humidity, as another comparison. The following statistically significant correlations were observed: (a) significant negative correlation between temperature and (1→3)-β-d-glucan in dust (r=−0.532, p<0.05) and FSSST (r=−0.576, p<0.05) during summer only; (b) significant negative correlation between temperature and endotoxin in dust (r=−0.644, p<0.05) during summer only; and (c) significant negative correlation between relative humidity and (1→3)-β-d-glucan in FSSST only during winter (r=−0.457, p<0.05). No significant associations were observed between the protimeter moisture level readings and the levels of endotoxin and (1→3)-β-d-glucan. Negative correlations with temperature indicate that higher temperature did not facilitate the growth of specific fungal and bacterial species, which were prevalent in the flood-affected homes and contributed to a major portion of the endotoxin and (1→3)-β-d-glucan levels.
3.6. Correlation between different exposure matrices
Because endotoxin and (1→3)-β-d-glucan are potential immunomodulators for various atopic respiratory disorders, it is worthwhile to examine their correlation in indoor home environments. Their separate effects or combined synergistic effects can aggravate respiratory allergy and other pulmonary diseases in different ways, and little is known about this emerging research area. As the endotoxin level was mostly higher in winter months than in summer but (1→3)-β-d-glucan demonstrated the opposite trend (higher in summer), we did not anticipate a correlation between the datasets combined for both seasonal campaigns. When seasonal data were separately considered, we found a significant positive correlation between the endotoxin and (1→3)-β-d-glucan in dust samples (Pearson correlation coefficient, r=0.535; p<0.05) and air samples (r=0.699; p<0.05) during summer, but not in winter. While assessing the aerosolization potential of sources through the FSSST sampling, we determined no correlation between endotoxin and (1→3)-β-d-glucan. No significant positive correlations were observed between the levels of (1→3)-β-d-glucan and fungal spores detected in FSSST and air samples. This lack of correlation can be attributed to various organic sources for (1→3)-β-d-glucan in the sediments of flood water, other than fungi.
When the association between the levels of endotoxin and (1→3)-β-d-glucan collected by three different methods were examined, we found a statistically significant positive correlation for (1→3)-β-d-glucan between dust and FSSST samples only (r=0.28; p<0.05). This points to a similarity between (1→3)-β-d-glucan on floor surfaces (available to become airborne by air movement) and inside floor materials (not readily aerosolizable). No similar correlation was observed for endotoxin. It is probably because endotoxin associated with bacterial growth remained primarily inside the floor material. The lack of correlation further underscores that using a single exposure assessment method may not be adequate for understanding the inhalation exposure risks associated with microbial contaminants.
3.7. Benefit of using different matrices of exposure: the present study versus other studies in flood-affected homes New Orleans
Unlike many similar investigations, our study design allowed combining long-term inhalable air sampling and conventional dust sampling with the assessment of potential aerosolization of biocontaminants from surfaces using a unique microbial source strength tester. Levels of airborne microorganisms determined by conventional air sampling generally serve as the direct and most reasonable way to assess the inhalation exposure. During specific time intervals, however, the air sampling data may not be a perfect representative of true exposures because of an inability to detect microbial colonization and successive microbial aerosolization from surfaces (Horner, 2003). The release of microbial contaminants from moisture-damaged surfaces may not occur during the collection of air samples. Furthermore, it may be sporadic, even with little or no disturbance of the surfaces. Previous researchers have used vacuumed floor dust as a surrogate for inhalation exposure because it is believed to be less influenced by short-term variability in indoor activities and ventilation and, therefore, more representative of long-term exposure than short-term air samples (Wickman et al., 1992). On the other hand, vacuuming can overestimate the inhalation exposure risks for the aerosolized microbial biocontaminants from flood-affected materials, as was concluded in this study as well as in our recently published study (Adhikari et al., 2009). To summarize, all currently available exposure assessment methods for airborne microorganisms have some affirmative features and some limitations. Although resource-wise it is not always feasible, assessing exposure matrices from different sampling methods (as conducted in this study) may provide an imperative benefit, particularly when environmental monitoring is performed in response to a major disaster with significant public health implications.
Previous bioaerosol exposure assessments in the flood-affected homes of New Orleans, conducted 2–6 months after Hurricanes Katrina and Rita, were primarily performed by one-time short-term air sampling and conventional dust sampling. Seasonal variation in concentrations of endotoxin and (1→3)-β-d-glucan after the flood have never been investigated, although the data could be critical in determining the suitability of the affected homes for re-occupation. The present study utilized three different methods to quantify the microbial exposures through assessing the settled dust by vacuuming (representing the reservoir for biocontaminants in homes) and by forceful aerosolization (representing maximum potential contamination of indoor air), as well as inhalable aerosol (representing inhalation exposure). As the field campaign included 35 homes during summer and 31 homes during winter, the sample size was significantly greater than in most of the previously published papers on microbial contaminants in homes that flooded during Hurricanes Katrina and Rita. The achieved results supplement the previous findings of Chew et al. (2006) and Rao et al. (2007), which used short-term sampling in investigating airborne concentration levels of endotoxin, glucans, and fungi in flood-affected homes of New Orleans. Long-term (24 h) and inhalable bioaerosol sampling generated in the present study using the Button Inhalable Sampler produced novel and significant information, given that inhalable aerosol sampling has not been conducted in other studies in flood-affected homes of New Orleans. Finally, we believe that, beyond providing information about biocontamination resulted from flooding events, the findings of this study will benefit relevant epidemiological investigations on biocontaminants and associated health effects.
3.8. Future potential research strategies based on our study findings
In this study, no conclusive relationships were found between the inhalable airborne levels of endotoxin, (1→3)-β-d-glucan, and total fungal spores and their respective levels in dusts and FSSST samples. At the same time, biocontaminants that may be potentially aerosolized from floor and other surfaces can play an important role in influencing the inhalation exposure doses. Our findings suggest that a complex relationship exists between microbial contaminants, environmental factors and different activities. Further research on microbial ecology and diversity in moisture-damaged homes is necessary to understand this relationship, which is critical for the development of public health policies. Until this relationship is well characterized, investigating multiple exposure matrices has clear advantages over employing a single exposure assessment method. Additional scientific information can be gathered if microbial species in collected samples are identified in future prospective studies in flood-affected tropical homes and their ideal growth temperature conditions are determined. It appears that the effects of temperature, relative humidity, and moisture levels on endotoxin and (1→3)-β-d-glucan are not straightforward in the complex niches of flood-affected homes. Follow-up studies on the ecological aspects of fungal and bacterial species in moisture-damaged homes will help interpret the associations between environmental factors and these biocontaminants. Further research is also needed to understand the effects of flood-water damage on mite population growth and allergen levels. Additional field monitoring can also allow evaluation of the time needed for the dust mite population to replenish to the pre-flood levels.
4. Conclusions
Elevated levels of endotoxin and (1→3)-β-d-glucan were detected in the dust samples collected from the flood-affected homes in New Orleans about two years after the floods caused by Hurricanes Katrina and Rita. The concentrations of airborne fungal spores were also higher than in “normal” home environments, but exhibited considerable decreases during a two-year period after the initial water damage. Our findings indicate that collection of dust samples by vacuuming could considerably overestimate the inhalation exposure risks associated with endotoxin. While no significant difference was observed in the dust-borne endotoxin levels measured in summer and winter, the aerosolization of endotoxin was higher in winter than in summer in 71% homes, which was supported by the source aerosolization and air sampling data. The concentration of airborne endotoxin followed the trend determined for its aerosolization potential. In contrast to endotoxin, the (1→3)-β-d-glucan levels in the floor dust and aerosolized fractions were mostly comparable, and 25% of the homes showed the aerosolizable level even higher than the dust-borne one. The seasonal patterns for endotoxin in dust and the aerosolizable fraction were different from those found for (1→3)-β-d-glucan. This reflects the difference in effects of the air temperature and humidity on bacterial and fungal contamination. While the concentration of airborne endotoxin followed the same seasonal trend as its aerosolization potential, no significant seasonal difference was identified for the concentrations of airborne (1→3)-β-d-glucan, as well as for airborne fungal spores. Detectable dust mite allergens were only found in a few dust samples and not in FSSST and air samples, indicating negligible chance of exposures to Der p1 and Der f1 allergens in flood-affected homes two years after a major flood. No conclusive correlations were found between the three environmental monitoring methods. This disparity indicates that investigating multiple exposure matrices is more advantageous than employing a single exposure assessment method for microbial contaminants in moisture-damaged homes.
Acknowledgements
This study has been supported by the US Department of Housing and Urban Development (Office of Healthy Homes and Lead Hazard Control) through Grant No. OHLHH 0155-06 and the National Institutes of Health through Grant No. P30 ES009089. We greatly appreciate the support provided by these agencies.
References
- Adhikari A, Martuzevicius D, Reponen T, Grinshpun SA, Cho SH, Sivasubramani SK, et al. Performance of the Button Personal Inhalable Sampler for the measurement of outdoor aeroallergens. Atmos Environ 2003;37:4723–33. [Google Scholar]
- Adhikari A, Reponen T, Lee SA, Grinshpun SA. Assessment of human exposure to airborne fungi in agricultural confinements: personal inhalable sampling versus stationary sampling. Ann Agric Environ Med 2004;11:269–77. [PubMed] [Google Scholar]
- Adhikari A, Jung J, Reponen T, Lewis JS, DeGrasse EC, Grimsley LF, et al. Aerosolization of fungi, (1→3)-β-d-glucan, and endotoxin from flood-affected materials collected in New Orleans homes. Environ Res 2009;109:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlian LG, Bernstein D, Bernstein IL, Friedman S, Grant A, Lieberman P, et al. Prevalence of dust mites in the homes of people with asthma living in eight different geographic areas of the United States. J Allergy Clin Immunol 1992;90:292–300. [DOI] [PubMed] [Google Scholar]
- Chew GL, Higgins KM, Milton DK, Burge HA. The effects of carpet fresheners on the behaviour of indoor allergen assays. Clin Exp Allergy 1999;29:470–7. [DOI] [PubMed] [Google Scholar]
- Chew GL, Wilson J, Rabito FA, Grimsley F, Iqbal S, Reponen T, et al. Mold and endotoxin levels in the aftermath of Hurricane Katrina: a pilot project of homes in New Orleans undergoing renovation. Environ Health Perspect 2006;114:1883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Reponen T, Bernstein DI, Olds R, Levin L, Liu X, et al. The effect of home characteristics on dust antigen concentrations and loads in homes. Sci Total Environ 2006;371:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford C, Reponen T, Lee T, Iossifova Y, Levin L, Adhikari A, et al. Temporal and spatial variation of indoor and outdoor airborne fungal spores, pollen, and (1→3)-β-d-glucan. Aerobiologia 2009;25:147–58. [Google Scholar]
- Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg 2003;47:187–200. [DOI] [PubMed] [Google Scholar]
- Fabian MP, Reponen T, Miller SL, Hernandez MT. Total and culturable airborne bacteria and fungi in arid region flood-damaged residences. J Aerosol Sci 2000;31(suppl.1): 35–6. [Google Scholar]
- Górny RL, Reponen T, Willeke K, Robine E, Boissier M, Grinshpun SA. Fungal fragments as indoor biocontaminants. Appl Environ Microbiol 2002;68:3522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinshpun SA, Górny RL, Reponen T, Willeke K, Trakumas S, Hall P, et al. New method for assessment of potential spore aerosolization from contaminated surfaces. Proceedings of the sixth international aerosol conference, September 8–13, Taipei, Taiwan, 2 ; 2002. p. 767–8. [Google Scholar]
- Horner WE. Assessment of the indoor environment: evaluation of mold growth indoors. Immunol Allergy Clin North Am 2003;23:519–31. [DOI] [PubMed] [Google Scholar]
- HUD Office of Healthy Homes and Lead Hazard Control, 2004. Vacuum dust sample collection protocol for allergens. Updated (2008) version available: http://www.hud.gov/offices/lead/library/hhts/DustSampleCollectionProtocol_v2_05.08.pdf [accessed October 5, 2009].
- Institute of Medicine (IOM). Indoor biologic exposures. Clearing the air: asthma and indoor air exposures. Washington, DC: National Academy Press; 2000. p. 105–222. [Google Scholar]
- Iossifova Y, Reponen T, Bernstein D, Levin L, Zeigler H, Kalra H, et al. House dust (1→3)-β-d-glucan and wheezing in infants. Allergy 2007;62:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Grinshpun SA, Kim K-Y, Iossifova Y, Adhikari A, Reponen T. Relationship between indoor and outdoor airborne fungal spores, pollen, and (1→3)-β-d-glucan in homes without visible mold growth. Aerobiologia 2006;22:227–36. [Google Scholar]
- Luczynska CM, Arruda LK, Platts-Mills TA, Miller JD, Lopez M, Chapman MD. A two-site monoclonal antibody ELISA for the quantification of the major Dermatophagoides spp. allergens, Der p 1 and Der f 1. J Immunol Methods 1989;118:227–35. [DOI] [PubMed] [Google Scholar]
- Niemeier RT, Sivasubramani SK, Reponen T, Grinshpun SA. Assessment of fungal contamination in moldy homes: comparison of different methods. J Occup Environ Hyg 2006;3:262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne M, Reponen T, Adhikari A, Cho S-H, Grinshpun SA, Levin L, et al. Specific fungal exposures, allergic sensitization, and rhinitis in infants. Pediatric Allergy Immunol. 2006;17:450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 2006;117:1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike A. The colonisation of new and retrofitted houses by the house dust mite Dermatophagoides pteronyssinus. MSc dissertation. 1998; Victoria University of Wellington, Wellington, New Zealand. [Google Scholar]
- Rao CY, Riggs MA, Chew GL, Muilenberg ML, Thorne PS, Van Sickle D, et al. Characterization of airborne molds, endotoxins, and glucans in homes in New Orleans after Hurricanes Katrina and Rita. Appl Environ Microbiol 2007;73:1630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reponen T, Seo SC, Grimsley F, Lee T, Crawford C, Grinshpun SA. Fungal fragments in moldy houses: a field study in homes in New Orleans and southern Ohio. Atmos Environ 2007;41:8140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reponen T, Singh U, Vesper S, Schaffer C, Ryan P, Levin L, et al. Optimizing mold exposure assessment in asthma research. Paper: 140; Proceedings of healthy buildings, 2009. ISIAQ’s (International Society of Indoor Air Quality and Climate) ninth international healthy buildings conference and exhibition; September; 2009 Syracuse, NY, USA. [Google Scholar]
- Riggs MA, Rao CY, Brown CM, Van Sickle D, Cummings KJ, Dunn KH, et al. Resident cleanup activities, characteristics of flood-damaged homes and airborne microbial concentrations in New Orleans, Louisiana, October 2005. Environ Res 2008;106:401–9. [DOI] [PubMed] [Google Scholar]
- Rylander R. Indoor air-related effects and airborne (1→3)-β-d-glucan. Environ Health Perspect 1999;107(suppl. 3):501–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander R, Norrhall M, Engdahl U, Tunsater A, Holt PG. Airways inflammation, atopy, and (1→3)-beta-d-glucan exposures in two schools. Am J Respir Crit Care Med 1998;158:1685–7. [DOI] [PubMed] [Google Scholar]
- Schwab KJ, Gibson KE, Williams DL, Kulbicki KM, Lo CP, Mihalic JN, et al. Microbial and chemical assessment of regions within New Orleans, LA impacted by Hurricane Katrina. Environ Sci Technol 2007;41:2401–6. [DOI] [PubMed] [Google Scholar]
- Seo SC, Reponen T, Levin L, Borchelt T, Grinshpun SA. Aerosolization of particulate (1→3)-β-d-glucan from moldy materials. Appl Environ Microbiol 2008;74: 585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SC, Reponen T, Levin L, Grinshpun SA. Size-fractionated (1→3)-beta-d-glucan concentrations aerosolized from different moldy building materials. Sci Total Environ 2009;407:806–14. [DOI] [PubMed] [Google Scholar]
- Sivasubramani SK, Niemeier RT, Reponen T, Grinshpun SA. Fungal spore source strength tester: laboratory evaluation of a new concept. Sci Total Environ 2004a;329:75–86. [DOI] [PubMed] [Google Scholar]
- Sivasubramani SK, Niemeier RT, Reponen T, Grinshpun SA. Assessment of the aerosolization potential for fungal spores in moldy homes. Indoor Air 2004b;14: 405–12. [DOI] [PubMed] [Google Scholar]
- Solomon GM, Hjelmroos-Koski M, Rotkin-Ellman M, Hammond SK. Airborne mold and endotoxin concentrations in New Orleans, Louisiana, after flooding, October through November 2005. Environ Health Perspect 2006;114:1381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieksma FT. Mite biology. Clin Rev Allergy 1990;8:31–49. [DOI] [PubMed] [Google Scholar]
- Thorn J. The inflammatory response in humans after inhalation of bacterial endotoxin: a review. Inflamm Res 2001;50:254–61. [DOI] [PubMed] [Google Scholar]
- Voorhorst R, Spieksma FThM, Varekamp H. Housedust atopy and the house-dust mite. Leiden, The Netherlands: Stafleu; 1969. [Google Scholar]
- Wickman M, Gravesen S, Nordvall SL, Pershagen G, Sundell J. Indoor viable dust-bound microfungi in relation to residential characteristics, living habits, and symptoms in atopic and control children. J Allergy Clin Immunol 1992;89:752–9. [DOI] [PubMed] [Google Scholar]
- Woodfolk JA, Hayden ML, Couture N, Platts-Mills TAE. Chemical treatment of carpets to reduce allergen: comparison of the effects of tannic-acid and other treatments on proteins derived from dust mites and cats. J Allergy Clin Immunol 1995;96:325–33. [DOI] [PubMed] [Google Scholar]