Abstract
Human genetic variation in the nicotinic receptor gene cluster CHRNA5/A3/B4, in particular the non-synonymous and frequent CHRNA5 variant rs16969968 (α5SNP), has an important consequence on smoking behavior in humans. A number of genetic association studies have additionally implicated the CHRNA5 gene in addictions to other drugs, and also body mass index (BMI). Here, we model the α5SNP, in a transgenic rat line, and establish its role in alcohol dependence, and feeding behavior. Rats expressing the α5SNP consume more alcohol, and exhibit increased relapse to alcohol seeking after abstinence. This high-relapsing phenotype is reflected in altered activity in the insula, linked to interoception, as established using c-Fos immunostaining. Similarly, relapse to food seeking is increased in the transgenic group, while a nicotine treatment reduces relapse in both transgenic and control rats. These findings point to a general role of this human polymorphism in reward processing, and multiple addictions other than smoking. This could pave the way for the use of medication targeting the nicotinic receptor in the treatment of alcohol use and eating disorders, and comorbid conditions in smokers.
Subject terms: Reward, Motivation
Introduction
Addiction is a psychiatric disorder defined by a loss of control over drug taking and seeking, characterized by chronic relapsing following attempts to quit [1]. Therapies and treatments to alleviate withdrawal symptoms can assist patients in maintaining abstinence, yet there is a critical need for more effective medication addressing relapse prevention [1]. Tobacco and alcohol addictions are the two leading causes of premature death among all main causes of excess mortality [2]. They show strikingly high rates of comorbidity and individuals with these two addictions represent the largest group of polysubstance abusers, although there is a strong lack in both the understanding of the biological basis and therapeutic approaches of such comorbidity [3, 4]. Nicotine, the main psychoactive substance of tobacco smoke responsible for its addictive properties, acts on the nicotinic acetylcholine receptors (nAChRs). They are pentameric ligand-gated ion channels widely expressed in the brain, where they are composed of α (α2–α6, α7, α9, α10) and β (β2–β4) subunits that co-assemble according to various combinations exhibiting distinct brain localizations and functional properties [5]. Over the past 10 years, a plethora of human genetic studies, including Genome-Wide Association Studies (GWAS), have identified a consistent association between a single nucleotide polymorphism (rs16969968) of the CHRNA5 gene encoding the α5 nAChR subunit (α5SNP) and the risk for higher scores for nicotine dependence [6–10]. The α5SNP, very frequent in the general population, is non-synonymous, changing an aspartic acid into an asparagine, and doubles the risk to develop heavy smoking in homozygous carriers [11–13]. We have recently created transgenic rats constitutively expressing the α5SNP, and notably identified increased relapse to nicotine seeking after abstinence in these rats using self-administration (SA) procedures [14]. Because of the strong co-occurrence of tobacco and alcohol dependence, several human candidate gene studies have attempted to identify possible links between the α5SNP and alcohol abuse but with discordant results [15–18]. Such incongruity in human studies can be attributed to several factors, such as varying linkage disequilibrium, population heterogeneity, cohort design, including patients with multiple substance addiction and criteria for phenotyping.
Here, we investigated the impact of the α5SNP on multiple behaviors related to alcohol abuse using drug-naive transgenic rats in complementary preclinical models of alcohol addiction. We notably assessed reinstatement of alcohol seeking after extinction of alcohol SA, a model of relapse with strong translational value [19], in combination with c-Fos immunostaining to correlate neuronal responses to relapse intensity. We also verified whether the addiction-like phenotypes observed in α5SNP rats could be due to impaired alcohol metabolism, locomotor activity or anxiety.
Drug addiction has been proposed to partially result from maladaptive motivation and reward processing, associated with dysfunctions in mechanisms important for the pursuit of natural reinforcers [1]. nAChRs are key players in reward-related mechanisms [20, 21], and in vitro studies have shown that the α5SNP causes a partial loss of nAChR function [7, 22, 23], notably within the reward pathway [24]. We further hypothesized that the α5SNP may impact reward-related mechanisms not only in the context of drug intake but also in physiological conditions, i.e. during natural reward processing. Interestingly, the α5SNP has been associated with higher body mass index (BMI) in never smokers, but lower BMI in current smokers, suggesting that this variant may cause alterations in food responding that could be counteracted by smoking [25]. Thus, we also examined the consequences of the α5SNP on appetence and motivation for food in a SA procedure, and assessed the effects of nicotine exposure on food seeking relapse behavior.
Materials and methods
Animals
Adult male wild-type (WT) rats and rats constitutively carrying the rs16969968 SNP (α5SNP rats) [14], on a Long-Evans background, were used. All experimental procedures were approved by the institutional Animal Care Committee (agreements N°0355.02 and 180021). All efforts were made to minimize animal suffering, and to reduce the number of animals. Details are given in Supplementary Methods.
Intermittent ethanol two-bottle choice paradigm
Drug-naïve rats were given one bottle with 20% ethanol (EtOH) v/v (Sigma Aldrich, Saint Quentin Fallavier, France) and one bottle with tap water in their home cages according to a weekly intermittent schedule. The acquisition was followed by a quinine adulteration phase (quinine hydrochloride, Sigma-Aldrich, Saint Quentin Fallavier, France) to test aversion-resistant alcohol intake (adapted from [26]). A subgroup of animals was then submitted to four months of withdrawal and re-exposed to EtOH for two choice sessions. Details are given in Supplementary Methods.
Ethanol operant oral self-administration procedure
Drug-naïve rats were submitted to operant oral SA of 12% EtOH v/v (Fisher Scientific, llkirch, France) in chambers equipped with two levers (Med Associates, St. Albans, Vt., USA). Habituation: Rats were exposed to progressively increased concentrations of EtOH for habituation. Acquisition: Rats acquired EtOH SA under fixed ratio (FR) schedules of reinforcement from FR1 to FR5. The unit dose was a 0.1 mL drop of 12% EtOH, associated with a 10 s presentation of a visual cue (light) above the active lever (AL). Progressive Ratio responding: Rats were switched to a progressive ratio (PR) schedule of reinforcement during three consecutive sessions wherein the response requirement increased with each successive EtOH reinforcement. Dose-response curve: Rats were switched back to FR5 for a few days before being tested for SA of different doses of EtOH (6, 12, 18, and 30%). Extinction: Rats were then submitted to an extinction phase where responses on levers were recorded, but did not result in EtOH or visual cue delivery. Reinstatement of EtOH seeking: A cue-induced reinstatement test was conducted. Rats were submitted to another extinction, and tested for “EtOH + cue”-induced reinstatement of EtOH seeking. Rats were then submitted to a last extinction, and a part of them, was tested again for “EtOH + cue”-induced reinstatement of EtOH seeking while the other part was submitted to an extinction session. Details are given in Supplementary Methods.
Immunofluorescence and c-Fos counting
Following the last “EtOH + cue”-induced reinstatement of EtOH seeking or extinction sessions, brains were extracted and processed for c-Fos immunofluorescence and quantification. Details are given in Supplementary Methods.
Blood ethanol concentration measurements
Drug naïve rats received an intraperitoneal injection of EtOH (2 g/kg) and were sacrificed at different time points post-injection (15, 30, 90, and 180 min) just before blood collection. Serum was assayed for EtOH content using an Ethanol Assay Kit (MAKO76, Sigma-Aldrich, Saint Quentin Fallavier, France). Details are given in Supplementary Methods.
Locomotor activity and anxiety-like behavior measurement
Locomotor activity and anxiety-like behavior of rats were recorded for 30 min in a square open-field. Anxiety-like behavior was further evaluated for 5 min in the dark–light box (DLB) test in the same drug-naïve rats. Details are given in Supplementary Methods.
Food operant self-administration procedure
Drug-naïve rats were submitted to operant SA of food (45 mg pellets, rodent purified diet F0021, Bio-Serv, Morangis, France) in chambers similar to those used for EtOH SA. Acquisition: Rats acquired food SA under FR schedules of reinforcement and the unit dose was one food pellet delivered into a magazine between the two levers associated with a 10 s presentation of a visual cue (light) above the AL. Progressive Ratio responding: Rats were switched to a PR schedule of reinforcement during three consecutive sessions wherein the response requirement increased with each successive reinforcement. Extinction: Rats were switched back to FR5 for a few days before being submitted to an extinction phase where responses on the levers were recorded, but did not result in food or visual cue delivery. Reinstatement of food seeking: rats were submitted to a food-induced reinstatement test, and to another extinction, before being tested for cue-induced reinstatement. Rats were then submitted to a last extinction phase before being tested again for food-induced reinstatement. For this last reinstatement session, half of the rats received a sub-cutaneous injection of nicotine ((-)Nicotine hydrogen tartrate, Sigma-Aldrich, St Louis, Mo., USA) dissolved in NaCl 0.9%, at the dose of 0.1 mg/kg (free base), while the other half received only NaCl 0.9%, 5 min before starting the session. Details are given in Supplementary Methods.
Statistics
All data were analyzed with Statistica (StatSoft, Inc., France). For two-group comparisons, data were analyzed with unpaired Student’s t or Mann–Whitney U tests when normality and variance homogeneity conditions were not met for parametric test use. Two- and three-way repeated measures ANOVAs were used (results reported in Supplementary Tables 1–5). Significant main effects (p < 0.05) were further analyzed using Bonferroni for multiple comparisons post hoc tests. Details are given in Supplementary Methods.
Results
Ethanol intake and preference in an intermittent two-bottle choice procedure in α5SNP and WT rats
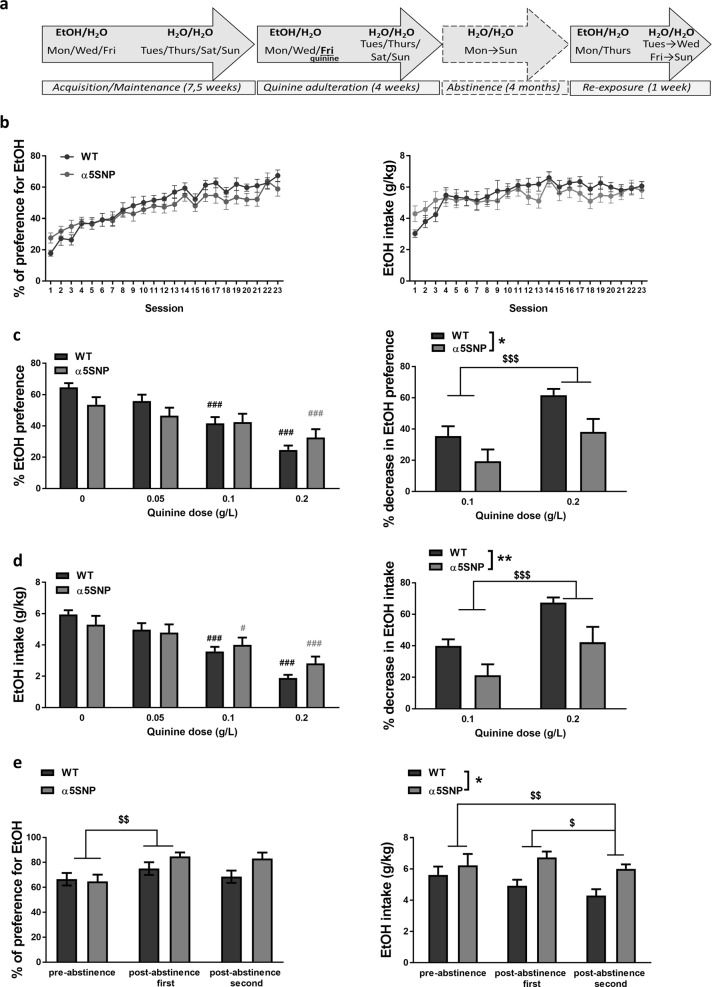
To examine the impact of the α5SNP on voluntary alcohol consumption, we first submitted rats carrying the α5SNP and WT rats to an intermittent two-bottle choice paradigm with limited access to EtOH (Fig. 1a). On the first day, EtOH preference was higher in α5SNP rats with a similar trend observed for EtOH intake (Fig. S1a). EtOH preference and intake progressively increased, reaching a plateau of ±60% for EtOH preference (Fig. 1b). Global EtOH preference and intake were not different between genotypes (see Table S1 for all ANOVA results). Yet, there was a significant groupXsession interaction for both parameters, indicating different patterns of EtOH consumption over time between groups. No evolution over time nor group differences were observed on the preference for the EtOH side on days where only water was available (Fig. S1b). Rats were then submitted to several sessions of adulterated EtOH consumption induced by concomitant exposure to quinine, a procedure proposed to model pathological consumption of alcohol [26] (Fig. 1c, d). Quinine dose-dependently decreased EtOH preference and intake. No global differences were obtained between genotypes, but α5SNP and WT rats differentially adapted their EtOH consumption according to increasing quinine doses. Post hoc tests showed that EtOH preference was decreased from the medium dose in WTs while this was only the case at the highest dose of quinine in α5SNP rats (Fig. 1c, left). The percentage of decrease in EtOH preference was higher at 0.2 g/L compared to 0.1 g/L, but lower in α5SNP rats compared to WTs (Fig. 1c, right). EtOH intake was decreased from the medium dose in both groups (Fig. 1d, left). Yet the percentage of decrease in EtOH intake, higher at 0.2 g/L compared to 0.1 g/L, was lower in α5SNP rats compared to WTs (Fig. 1d, right). A much stronger effect of quinine was observed on the preference for the EtOH side on days where only water was available, with no differences between groups, suggesting that the resistance to quinine adulteration observed in α5SNPs was EtOH-specific (Fig. S1c). Rats then underwent 4 months of abstinence before being re-exposed to EtOH during two choice sessions. Preference for EtOH was higher during the first re-exposure as compared to the last session before withdrawal in both groups (Fig. 1e, left). Such difference was not observed for EtOH intake (Fig. 1e, right), because of a parallel increase in animals’ weight (Fig. S1d). Finally, EtOH intake was lower during the second re-exposure session compared to both pre- and first post-abstinence sessions, and α5SNP rats consumed more EtOH than WTs overall (Fig. 1e, right).
Fig. 1.
Ethanol intake and preference in a two-bottle choice procedure. a Scheme of the procedure. b Percentage of EtOH preference (left) and total intake (right) during acquisition in WT (n = 15) and α5SNP (n = 14) rats [two-way repeated measure ANOVAs]. c Percentage of EtOH preference (left) [two-way repeated measure ANOVAs and Bonferroni post hoc. WTs: 0 vs. 0.1 g/L: p < 0.0001, 0 vs. 0.2 g/L: p < 0.0001; α5SNPs: 0 vs. 0.2 g/L: p < 0.001] and percentage of decrease in EtOH preference (right) [two-way repeated measure ANOVAs] during quinine adulteration in WT (n = 15) and α5SNP (n = 13) rats. d Total EtOH intake (left) [two-way repeated measure ANOVAs and Bonferroni post hoc. WTs: 0 vs. 0.1 g/L: p < 0.0001, 0 vs. 0.2 g/L: p < 0.0001; α5SNPs: 0 vs. 0.1 g/L: p < 0.05, 0 vs. 0.2 g/L: p < 0.0001] and percentage of decrease in EtOH intake (right) [two-way repeated measure ANOVAs] during quinine adulteration in WT (n = 15) and α5SNP (n = 13) rats. e Percentage of EtOH preference (left) [two-way repeated measure ANOVAs and Bonferroni post hoc. pre- vs. 1st post-abstinence: p < 0.01] and total intake (right) [two-way repeated measure ANOVAs and Bonferroni post hoc. pre- vs. 2nd post-abstinence: p < 0.01, 1st post- vs. 2nd post-abstinence: p < 0.05] during pre-, 1st and 2nd post-abstinence re-exposure sessions in WT (n = 8) and α5SNP (n = 6) rats. Data are mean + s.e.m. Group effect (WT vs. α5SNP): *p < 0.05, **p < 0.01. Session effect (quinine vs. no quinine for each dose): #p < 0.05, ###p < 0.001 in WT (black) or α5SNP rats (gray). Quinine dose or session effect (pre- vs. post-abstinence, or post-abstinence 1st vs. 2nd) in both groups: $p < 0.05, $$p < 0.01, $$$p < 0.001
Ethanol operant self-administration in α5SNP and WT rats
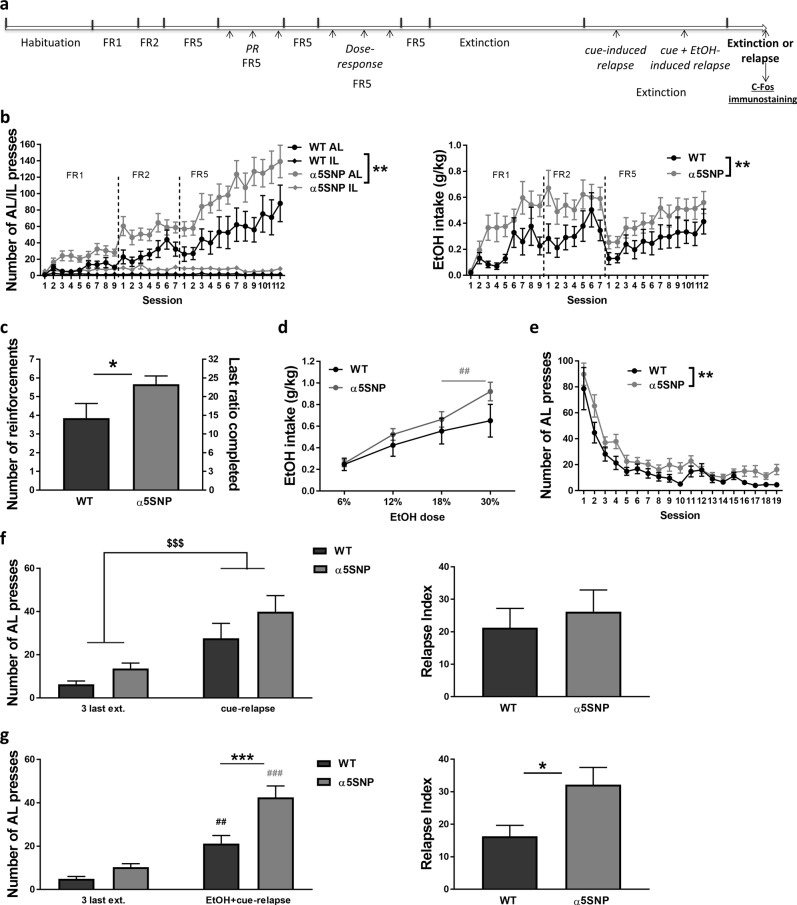
To further characterize the impact of the α5SNP on EtOH addiction-like behaviors, we next submitted drug-naïve α5SNP and WT rats to a chronic EtOH oral SA procedure in operant chambers, which examines multiple aspects of EtOH abuse with good face validity [27] (Fig. 2a). Both groups acquired EtOH SA with a progressive increase in AL presses to obtain a drop of a 12% EtOH solution (0.1 mL) (see Table S2 for all ANOVA results). However, α5SNP rats self-administered more EtOH than WTs, with increased number of lever presses (Fig. 2b, left), and higher EtOH intake (Fig. 2b, right). When tested under a PR schedule of reinforcement to further measure EtOH motivational effects, α5SNP rats reached a higher break point than WTs (Fig. 2c). Rats were then tested for SA of multiple doses of EtOH. α5SNP and WT rats both increased but differentially adapted their EtOH intake with an increase in dose (Fig. 2d). Post hoc tests showed that EtOH intake was still increased from the next-to-last to the last doses in α5SNP rats while it was stabilized in WTs. An extinction was then conducted where AL presses no longer resulted in EtOH or visual cue delivery, inducing a progressive decrease of AL responding in both groups, with the global number of lever presses remaining higher in α5SNP rats as compared to WTs (Fig. 2e). After stabilization of lever responding, rats were submitted to several sessions of reinstatement of EtOH seeking. WTs and α5SNPs exhibited a similar cue-induced reinstatement of EtOH seeking as observed on AL presses (Fig. 2f, left), and on relapse index (calculated as the subtraction of the number of AL presses averaged for the three last extinction sessions from the number of AL presses during the relapse session) (Fig. 2f, right). In contrast, α5SNPs had a significantly higher level of reinstatement than WTs when re-exposed to EtOH additionally to the cue, as observed on both AL presses (Fig. 2g, left) and relapse index (Fig. 2g, right). Post hoc confirmed increased number of AL presses in both groups after EtOH + cue re-exposure and higher AL responding in α5SNP rats compared to WTs in relapse conditions. These data confirm that the rs16969968 enhances addiction-like behaviors for EtOH, and particularly the intensity of relapse to EtOH seeking after extinction, similarly to what we previously observed for nicotine [14].
Fig. 2.
Ethanol (EtOH) operant self-administration. a Scheme of the procedure. b Number of active (AL) and inactive (IL) lever presses (left) [three-way repeated measure ANOVAs] and total EtOH intake (right) [two-way repeated measure ANOVAs] during acquisition in WT (n = 15) and α5SNP (n = 16) rats. c Number of reinforcements and last ratio completed in WT (n = 15) and α5SNP (n = 16) rats under progressive ratio [Mann–Whitney z = −2.095, p < 0.05]. d Dose-response curve of the amount of EtOH consumed under FR5 schedule in WT (n = 15) and α5SNP (n = 16) rats [two-way repeated measure ANOVAs and Bonferroni post hoc α5SNPs: 18% vs. 30%, p < 0.01]. e Number of AL presses during extinction in WT (n = 15) and α5SNP (n = 16) rats [two-way repeated measure ANOVAs]. f Number of AL presses (left) [two-way repeated measure ANOVAs] and relapse index (right) [Unpaired Student’s t-test. t28 = 0.555, NS] in WT (n = 15) and α5SNP (n = 15) rats during cue-induced EtOH seeking reinstatement. g Number of AL presses (left) [two-way repeated measure ANOVAs and Bonferroni post hoc, WT: ext. vs. rel., p < 0.001; α5SNPs: ext. vs. rel., p < 0.0001; Relapse: WT vs. α5SNP, p < 0.001] and relapse index (right) [Unpaired Student’s t-test. t25 = −2.566, p < 0.05] in WT (n = 14) and α5SNP (n = 13) rats during EtOH + cue-induced EtOH seeking reinstatement. Data are mean + s.e.m. Group effect (WT vs. α5SNP): *p < 0.05, **p < 0.01, ***p < 0.001. Session (ext. vs. rel.) or dose effect: $$$p < 0.001 in both groups, ##p < 0.01, ###p < 0.001 in WT (black) or α5SNP rats (gray)
Neuronal activation associated with EtOH + cue-induced reinstatement of EtOH seeking in α5SNP and WT rats
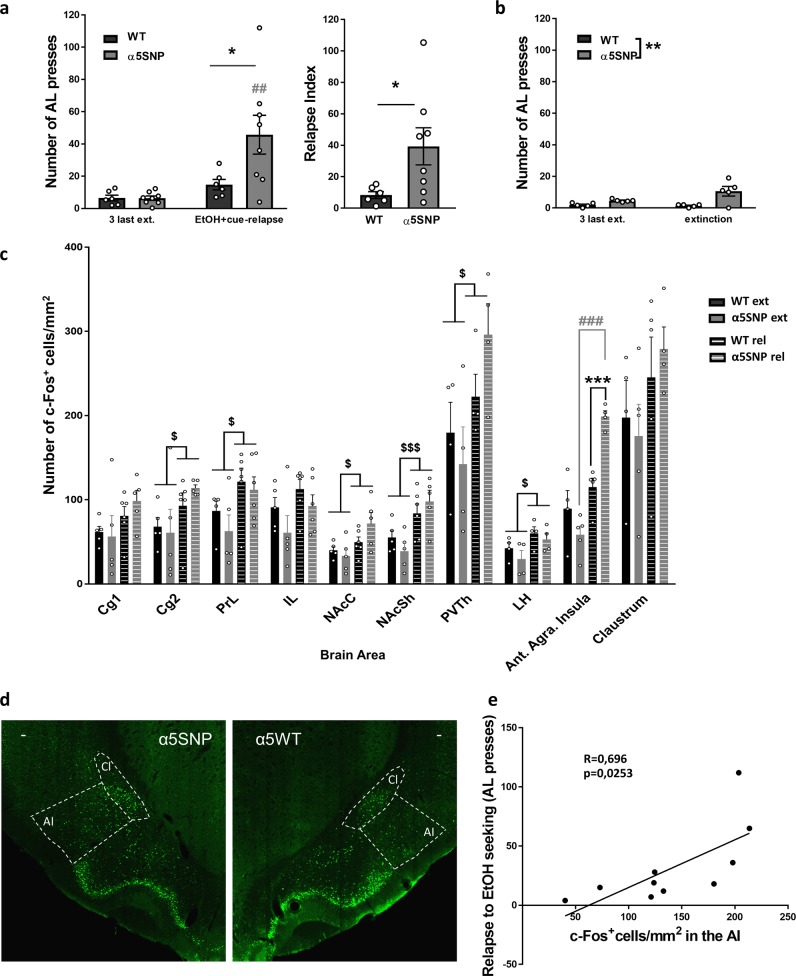
To identify the brain structures implicated in the impact of the rs16969968 on relapse to EtOH seeking, we examined the expression of the c-Fos immediate early gene product, a marker of neuronal activation [28], associated with EtOH + cue-induced reinstatement of EtOH seeking in multiple areas. Rats underwent a new phase of extinction before being submitted to either a session of relapse or a session of extinction for subsequent c-Fos quantification. We confirmed higher level of EtOH + cue-induced reinstatement in α5SNP rats compared to WTs (Fig. 3a) (see Table S3 for all ANOVA results). In rats submitted to an extinction session, AL responding was still different between groups, but there was no session effect (Fig. 3b). EtOH + cue-induced reinstatement of EtOH seeking was associated with an increase of c-Fos expression in several areas in both groups compared to extinction, namely the cingulate cortex, area 2 (Cg2), the prelimbic cortex (PrL), the nucleus accumbens core (NAcbC) and shell (NAcbS), the paraventricular thalamus (PVTh), and the lateral hypothalamus (LH) (Fig. 3c). Moreover, a strong increase in c-Fos expression was observed in the anterior agranular insula (AI) of α5SNP rats during EtOH seeking relapse compared to extinction, while this area was not activated in relapsing WT rats (Fig. 3c, d). Furthermore, the number of c-Fos-positive cells in the AI was correlated with the level of reinstatement of EtOH seeking (Fig. 3e).
Fig. 3.
Neuronal activation associated with Ethanol (EtOH) + cue-induced reinstatement of EtOH. a Number of active lever (AL) presses (left) [two-way repeated measure ANOVAs and Bonferroni post hoc α5SNPs: ext. vs. rel., p < 0.01; Relapse: WT vs. α5SNP, p < 0.05] and relapse index (right) [Mann–Whitney z = −2.130, p < 0.05] in WT (n = 6) and α5SNP (n = 8) rats during EtOH + cue-induced EtOH seeking reinstatement in rats used for c-Fos quantification. b Number of AL presses in WT (n = 5) and α5SNP (n = 5) rats during extinction in rats used for c-Fos quantification [two-way repeated measure ANOVAs]. c Levels of expression of c-Fos during EtOH + cue-induced reinstatement of EtOH seeking or extinction in WT [n = 4–6 (rel.) and 4–5 (ext.)] and α5SNP [n = 4-6 (rel.) and 4–5 (ext.)] rats [two-way repeated measure ANOVAs, and Bonferroni post hoc for Ant. Insula. α5SNPs: ext. vs. rel., p < 0.0001; Relapse: WT vs. α5SNP, p < 0.001]. d Representative c-Fos immunofluorescence (X20) in the anterior insula (AI) and Claustrum (Cl) performed on brain slices after reinstatement of EtOH seeking. White bar represents 50 μm. e Correlation between the number of c-Fos-positive cells in the AI and the level of reinstatement [Two-tailed Spearman R = 0.696; p < 0.05], WT rats (n = 5) and α5SNP (n = 4) are shown. Data are mean + s.e.m. Group effect (WT vs. α5SNP): *p < 0.05, **p < 0.01, ***p < 0.001. Session (ext. vs. rel.) effect: $p < 0.05 and $$$p < 0.001 in both groups, ##p < 0.01 and ###p < 0.001 in α5SNP rats (gray)
EtOH metabolism, locomotor activity and anxiety-like behaviors in α5SNP and WT rats
Since possible direct interactions between EtOH and nACRs have been reported [29, 30], we verified in drug-naïve animals that the EtOH addiction-like phenotypes observed in α5SNP rats were not due to differences in EtOH metabolism. Blood EtOH concentrations were similar between groups at all time-points following a 2 g/kg EtOH injection (Fig. 4a) (see Table S4 for ANOVA results). High response to novelty and trait-anxiety have been shown to confer vulnerability to drug SA and addiction, including to alcohol abuse where alcohol may be used as a form of emotional self-medication [31]. Thus we next examined whether locomotor reactivity to novelty and anxiety-like behaviors were altered in drug naïve α5SNP rats. The open-field distance travelled and velocity, reflecting locomotor activity, and % of time spent in the center, more related to anxiety-like behavior, were similar between genotypes (Fig. 4b). Anxiety-like behaviors in the DLB were not altered in α5SNP rats as suggested by similar percentage of time spent in the light side, number of transitions and latency to first entry into the light side (Fig. 4c). Thus, the EtOH addiction-like profile observed in α5SNP rats does not seem due to an impact of the rs16969968 on EtOH metabolism or on predisposing behavioral endophenotypes.
Fig. 4.
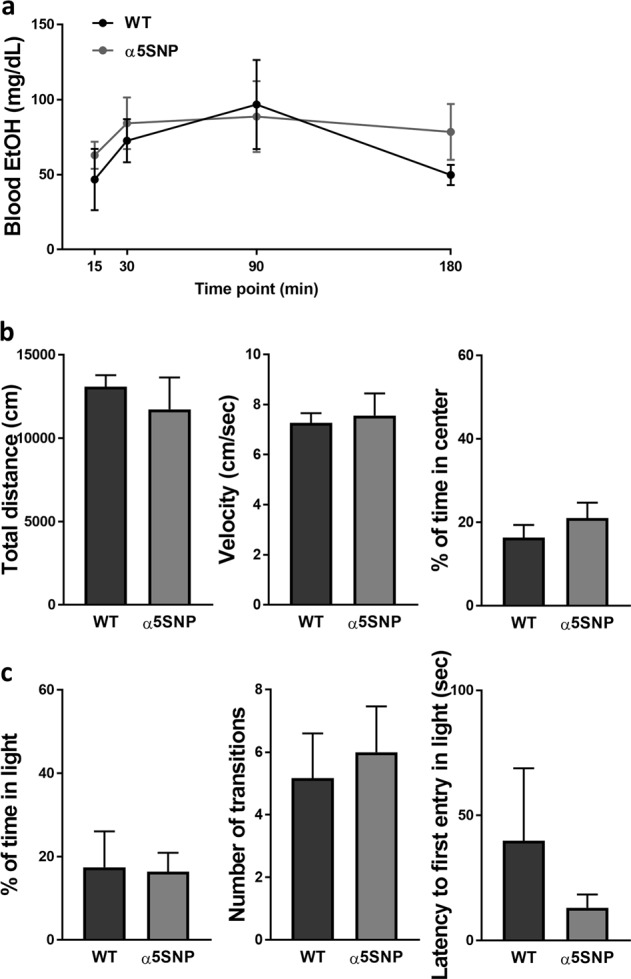
Ethanol (EtOH) metabolism, locomotor activity and anxiety-like behavior. a Blood EtOH concentrations in WT and α5SNP rats, at 15 (WT n = 4, α5SNP n = 4), 30 (WT n = 5, α5SNP n = 5), 90 (WT n = 3, α5SNP n = 4) or 180 (WT n = 5, α5SNP n = 4) minutes following intraperitoneal EtOH injection (2 g/kg) [two-way repeated measure ANOVAs]. b Total distance moved (left) [Unpaired Student’s t-test. t16 = −0.670, NS], mean velocity (middle) [t16 = 0.304, NS] and % time spent in the center (right) [t15 = −1.000, NS] in a novel open-field in WT (n = 9) and α5SNP (n = 8 – 9) rats. c Percentage time spent in the light side (left) [Unpaired Student’s t-test. t18 = −0.093, NS], number of transitions (middle) [t18 = 0.391, NS] and latency to the first entry into the light side (right) [t15 = −0.859, NS] in a dark–light box in WT (n = 12) and α5SNP (n = 8) rats. Data are mean + s.e.m
Operant self-administration of food in α5SNP and WT rats
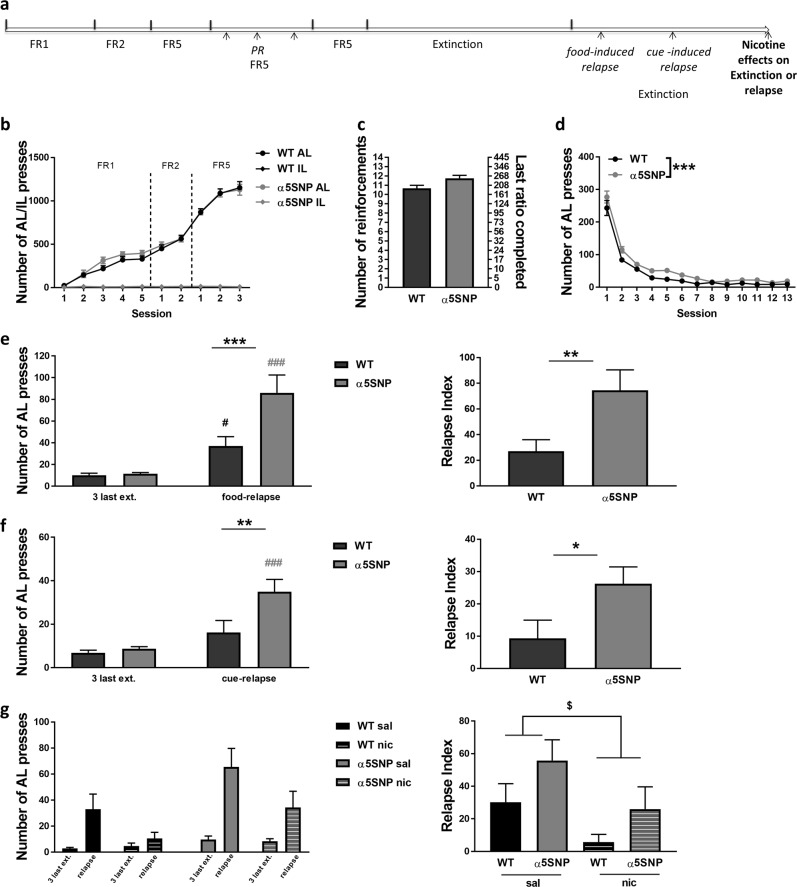
Our data indicate that the rs16969968 impacts addiction-like processes not only for nicotine, as previously observed [14], but also for alcohol. We therefore wanted to establish whether this variant may alter reward processing in general and examined if the increased appetitive behavior observed for both nicotine and EtOH in α5SNP rats may be extended to natural reward. Drug-naïve rats were submitted to a chronic food SA procedure in operant chambers (Fig. 5a). Both groups similarly acquired food SA (Fig. 5b) (see Table S5 for all ANOVA results). Rats were next tested under a PR schedule of reinforcement as another measurement of food reinforcing efficacy. α5SNP rats showed a trend towards higher break point (Fig. 5c). Food SA behavior was then extinguished during a phase where AL presses no longer resulted in food or visual cue delivery, inducing a progressive decrease of AL responding in both groups. However, the number of AL presses was globally higher in α5SNP rats as compared to WTs suggesting a resistance to extinction in α5SNP rats (Fig. 5d). Rats were next submitted to several sessions of reinstatement of food seeking. α5SNPs had a significantly higher level of reinstatement than WTs in response to food priming, as observed on both AL presses (Fig. 5e, left) and relapse index (Fig. 5e, right). Post hoc confirmed increased AL pressing in both groups after food priming and higher AL responding in α5SNP rats compared to WTs in relapse conditions. Re-exposure to the visual cue previously associated with food delivery also differentially affected AL pressing in WT and α5SNP rats (Fig. 5f, left), with increased AL pressing observed only in α5SNPs. Cue-induced relapse index was also higher in α5SNPs compared to WTs (Fig. 5f, right). Nicotine was shown to regulate appetite and food intake [32]. To test whether nicotine could regulate food-seeking relapse and “rescue” the high-relapsing phenotype observed in α5SNPs, we exposed rats to nicotine (or saline) before submitting them to a last food priming-induced reinstatement of food seeking session (Fig. 5g). Again, we found that food priming significantly induced relapsing only in α5SNPs, as observed on both AL press number and relapse index. Nicotine decreased AL pressing in response to food priming in both groups. The relapse index was also found significantly decreased by nicotine in both groups. These data reveal that the rs16969968 not only influences behaviors oriented towards drugs of abuse but is also associated with impairments in food-reward processing, including increased relapse to food seeking after extinction, which can be regulated by nicotine intake.
Fig. 5.
Food operant self-administration. a Scheme of the procedure. b Number of active lever (AL) and inactive lever (IL) presses during acquisition in WT (n = 25) and α5SNP (n = 18) rats [two-way repeated measure ANOVAs]. c Number of reinforcements and last ratio completed in WT (n = 24) and α5SNP (n = 18) rats under progressive ratio [Mann–Whitney. z = 2.312, p = 0.068]. d Number of AL presses during extinction in WT (n = 25) and α5SNP (n = 18) rats [two-way repeated measure ANOVAs]. e Number of AL presses (left) [two-way repeated measure ANOVAs and Bonferroni post hoc WTs: ext. vs. rel., p < 0.05; α5SNPs: ext. vs. rel., p < 0.0001; Relapse: WT vs. α5SNP, p < 0.001] and relapse index (right) [Unpaired Student’s t-test. t41 = 2.849, p < 0.01] in WT (n = 25) and α5SNP (n = 18) rats during food-induced food seeking reinstatement. f Number of AL presses (left) [two-way repeated measure ANOVAs and Bonferroni post hoc α5SNPs: ext. vs. rel., p < 0.001; Relapse: WT vs. α5SNP, p < 0.01] and relapse index (right) [Unpaired Student’s t-test. t41 = 2.420, p < 0.05] in WT (n = 25) and α5SNP (n = 18) rats during cue-induced food seeking reinstatement. g Number of AL presses (left) [three-way repeated-measure ANOVAs and Bonferroni post hoc. α5SNPs: ext. vs. rel., p < 0.001; Relapse: sal. vs. nic., p < 0.01] and relapse index (right) [two-way repeated-measure ANOVAs] in saline-treated [WT (n = 13), α5SNP (n = 9)] and nicotine-treated [WT (n = 12), α5SNP (n = 9)] rats during food-induced food seeking reinstatement. Data are mean + s.e.m. Group effect (WT vs. α5SNP): *p < 0.05, **p < 0.01, ***p < 0.001. Session (ext. vs. rel.) effect: $p < 0.05 in both groups, #p < 0.05 in WT rats (black), ###p < 0.001 in α5SNP rats (gray)
Discussion
Evidence for a link between the α5SNP (rs16969968), a frequent coding variant at a highly conserved site in the nAChR second intracellular loop, and smoking risk is extremely robust. Here we show that this variant impacts responses to other reinforcers than nicotine, enhancing appetence for food and increasing alcohol addiction-like behaviors in rats. This polymorphism may have multiple phenotypic consequences contributing to several reward-related disorders and their comorbidity.
The α5SNP was previously shown to cause a partial loss of function of α5 containing nAChRs (α5*nAChRs) in response to nicotinic agonists [7, 22, 23, 33], including in human induced pluripotent stem cell (iPSC)-derived midbrain dopaminergic (DA) neurons [24], and was associated with consumption of increased amounts of high dose nicotine and increased relapse to nicotine seeking after extinction of nicotine SA in rodents [14, 34]. Here, we demonstrate that α5SNP-induced addiction-like phenotypes, in particular increased relapse after extinction, are not specific to nicotine but are also observed for alcohol and food. Alcohol and tobacco use are highly correlated. Alcoholics are three times more likely to smoke than the general population [3], and at higher risk to die from smoking-related illnesses than from alcohol-related causes [35]. Several mechanisms have been proposed to underlie alcohol and tobacco addiction comorbidity, including cross-cue conditioning, cross-tolerance and -reinforcement [36, 37] and nicotinic modulation of alcohol effects [30, 38, 39]. Both alcohol and nicotine can modulate signalling pathways implicated in addiction through both distinct and common molecular targets. In fact, EtOH has been shown to act as an allosteric modulator of nAChRs and suggested to alter the balance between activation and desensitization of nAChRs caused by nicotine [30]. Previous studies reported no alterations in EtOH intake in α5 knock-out (KO) mice in a drinking-in-the-dark paradigm [38, 40], although EtOH intake was actually decreased in these mice, in a similar paradigm, after a restraint stress [38]. The same study also reported decreased EtOH-induced conditioned place preference in α5 KO mice. We previously found that α5 KO mice display increased anxiety-like behaviors [41], a phenotype we did not observe in α5SNP rats. Differences in stress and anxiety levels may contribute to differences in the response to EtOH reward. It would be of interest to further study a possible impact of the variant on the link between stress response and drug intake. Importantly, the partial loss of function of α5*nAChRs resulting from the α5SNP [7, 22, 23, 33] is likely to have different consequences compared to the complete absence of such receptors in a KO animal. Here, we show that α5SNP rats exhibit greater appetence and motivation for EtOH and increased relapse to EtOH seeking after extinction, the latter being associated with increased activation of the insula. The insula was proposed to integrate internal and external stimuli into interoceptive states to control motivated behavior, including drug craving [42–44]. It was previously implicated in alcohol interoceptive effect, SA and seeking behavior [43, 45, 46]. The α5SNP may potentiate alcohol addiction-like behaviors through direct alteration of alcohol-nAChR interaction, or indirectly through the modulation of acetylcholine control of neuronal activity, notably within the insular cortex. Interestingly, the anterior agranular insula has also been implicated in relapse to nicotine seeking [47]. Since the agranular insula receives multi-modality sensory inputs, including from the primary olfactory cortex [48], sensitivity to EtOH may be altered in α5SNP rats through altered olfactory processing. It has also been established that the α5SNP decreases the sensitivity to nicotine aversive effects in humans [49], an effect further supported by preclinical studies [14, 34]. It may be the case that aversive effects of alcohol are also diminished in α5SNP carriers, which may contribute to the higher EtOH drinking observed during the first two-bottle choice session in α5SNP rats and to their propensity for EtOH self-administration, notably at high doses. Moreover, even though anxiety and locomotor activity in a novel environment were found unaltered in α5SNP rats in the present study, other behavioral traits previously associated with vulnerability to drug abuse, such as novelty preference [31, 50], may be altered in these rats and contribute to their initial preference for ethanol, which will need further investigation. It is well characterized that alcohol consumption is an important cause of relapse to smoking following smoking cessation [51, 52]. An impact of the α5SNP on alcohol drinking may thus also partly explain the strong influence of this polymorphism on smoking dependence, in addition to its direct consequences on nicotine effects. Further investigation for a possible association between the α5SNP and alcohol abuse, notably focusing on relapse rates and delays, may be of great importance.
Our present study further reveals an impact of the α5SNP on the response to natural reward, beyond its influence on nicotine and alcohol addiction-like behaviors. This suggests that the nAChR dysfunction associated with this variant alters acetylcholine modulation of reward pathways, affecting reinforcer processing in general. This polymorphism may also contribute to aberrant learning processes and to stronger associative memories that underlie reinforcer seeking [53]. Here, we demonstrate that the α5SNP is associated with resistance to previously acquired operant behavior extinction and increased food seeking relapse. Many smokers report that they consume tobacco to control appetite [32, 54]. Weight control is cited as the primary reason to start smoking in teenage girls in the United States [55], and weight gain is perceived as a significant impediment for smoking cessation [32, 54, 56, 57]. Nicotine also decreases food intake and body weight in mice [58]. Accordingly, we further show that nicotine reduces the intensity of food seeking relapse after extinction. To our knowledge, there is no study to date assessing a possible link between the α5SNP and eating behavior or disorders in humans. However, an increased BMI in non-smoking α5SNP carriers has been reported, while the BMI was decreased in carriers who are smokers [25]. Higher BMI increases the risk for tobacco dependence [59]. Our present data raise the hypothesis of an additional operating mode of the α5SNP for increased risk for heavy smoking, by contributing to a sub-population of comorbid eating and tobacco use disorders [60].
The present study demonstrates that the rs16969968 impacts alcohol addiction-related processes and appetence for food in rats, in addition to direct consequences on the brain’s response to nicotine. These data call for new human genetics studies to refine our knowledge of the influence of this variant in psychiatric sub-populations including alcohol addicts and patients with eating disorders. Here we restricted our preclinical investigations on male subjects from one genetic background to limit the number of animals used. It would be important, in future human studies, to assess the effect of this variant according to gender and genetic ancestry. Finally, since this polymorphism decreases the response of α5*nAChRs to agonists, positive allosteric modulators (PAMs) of these receptors, by potentiating the effects of their primary ligands, may represent novel therapeutic strategy to address several psychiatric disorders. Such novel therapeutic approach could also lead to more favorable outcomes for comorbid issues in smokers.
Funding and disclosure
This work was supported by the Institut Pasteur, Centre National de la Recherche Scientifique UMR 3571, Fondation pour la recherche en alcoologie, Fondation de la Recherche Médicale (SPF20140129365 and DPA20140629803), Agence Nationale de la Recherche (ANR), Neuroscience and BLANC, LABEX BIO-PSY, FP7 ERANET program NICO-GENE grant agreement convention ANR n° 2010-NEUR-004-01, European Commission FP7 RTD Project HEALTH-2009-Neurocyp.08-202088 grant 242167, French National Cancer Institute grant CANCEROPOLE IDF 2016-1-TABAC-01-IP-1 MASKOS, ANR programme SNP-NIC, European ERANET programme iPS&BRAIN (all to U.M.). The laboratory of U.M. is part of the Ecole des Neurosciences de Paris Ile-de-France RTRA network. U.M. is a member of the LABEX BIO-PSY. This work was supported by French state funds managed by the ANR within the Investissements d’Avenir programme under reference ANR-11-IDEX-0004-02. The authors declare no competing interests.
Supplementary information
Acknowledgements
We thank Camille Ponthieu and Quentin Rodriguez for help with SA experiments, Romain Icick for helpful discussion, and the Institut de la Vision Platform for nanozoomer imaging.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Morgane Besson, Phone: +33140613777, Email: morgane.besson@pasteur.fr.
Uwe Maskos, Email: uwe.maskos@pasteur.fr.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0462-0).
References
- 1.Yücel Murat, Oldenhof Erin, Ahmed Serge H., Belin David, Billieux Joel, Bowden-Jones Henrietta, Carter Adrian, Chamberlain Samuel R., Clark Luke, Connor Jason, Daglish Mark, Dom Geert, Dannon Pinhas, Duka Theodora, Fernandez-Serrano Maria Jose, Field Matt, Franken Ingmar, Goldstein Rita Z., Gonzalez Raul, Goudriaan Anna E., Grant Jon E., Gullo Matthew J., Hester Robert, Hodgins David C., Le Foll Bernard, Lee Rico S. C., Lingford-Hughes Anne, Lorenzetti Valentina, Moeller Scott J., Munafò Marcus R., Odlaug Brian, Potenza Marc N., Segrave Rebecca, Sjoerds Zsuzsika, Solowij Nadia, van den Brink Wim, van Holst Ruth J., Voon Valerie, Wiers Reinout, Fontenelle Leonardo F., Verdejo-Garcia Antonio. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction. 2018;114(6):1095–1109. doi: 10.1111/add.14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziedonis DM, Guydish J, Williams J, Steinberg M, Foulds J. Barriers and solutions to addressing tobacco dependence in addiction treatment programs. Alcohol Res Health. 2006;29:228–35. [PMC free article] [PubMed] [Google Scholar]
- 3.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–15. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 4.Van Skike CE, Maggio SE, Reynolds AR, Casey EM, Bardo MT, Dwoskin LP, et al. Critical needs in drug discovery for cessation of alcohol and nicotine polysubstance abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:269–87. doi: 10.1016/j.pnpbp.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- 6.Berrettini WH, Doyle GA. The CHRNA5-A3-B4 gene cluster in nicotine addiction. Mol Psychiatry. 2012;17:856–66. doi: 10.1038/mp.2011.122. [DOI] [PubMed] [Google Scholar]
- 7.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–71. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, et al. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomark Prev. 2008;17:3517–25. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunzell DH, Stafford AM, Dixon CI. Nicotinic receptor contributions to smoking: insights from human studies and animal models. Curr Addict Rep. 2015;2:33–46. doi: 10.1007/s40429-015-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4:e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Improgo MR, Scofield MD, Tapper AR, Gardner PD. The nicotinic acetylcholine receptor CHRNA5/A3/B4 gene cluster: dual role in nicotine addiction and lung cancer. Prog Neurobiol. 2010;92:212–26. doi: 10.1016/j.pneurobio.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenbaum L, Lerer B. Differential contribution of genetic variation in multiple brain nicotinic cholinergic receptors to nicotine dependence: recent progress and emerging open questions. Mol Psychiatry. 2009;14:912–45. doi: 10.1038/mp.2009.59. [DOI] [PubMed] [Google Scholar]
- 14.Forget B, Scholze P, Langa F, Morel C, Pons S, Mondoloni S, et al. A human polymorphism in CHRNA5 is linked to relapse to nicotine seeking in transgenic rats. Curr Biol. 2018;28:3244–53 e7. doi: 10.1016/j.cub.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, et al. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:926–33. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallfors J, Loukola A, Pitkaniemi J, Broms U, Mannisto S, Salomaa V, et al. Scrutiny of the CHRNA5-CHRNA3-CHRNB4 smoking behavior locus reveals a novel association with alcohol use in a Finnish population based study. Int J Mol Epidemiol Genet. 2013;4:109–19. [PMC free article] [PubMed] [Google Scholar]
- 17.Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, Arias AJ, et al. Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology. 2010;35:1921–31. doi: 10.1038/npp.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–10. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacol (Berl) 2013;229:453–76. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Kloet SF, Mansvelder HD, De Vries TJ. Cholinergic modulation of dopamine pathways through nicotinic acetylcholine receptors. Biochem Pharm. 2015;97:425–38. doi: 10.1016/j.bcp.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Grasing K. A threshold model for opposing actions of acetylcholine on reward behavior: molecular mechanisms and implications for treatment of substance abuse disorders. Behav Brain Res. 2016;312:148–62. doi: 10.1016/j.bbr.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)(2)alpha5 AChR function. Mol Pharm. 2011;79:119–25. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, et al. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–35. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Deflorio C, Blanchard S, Carisi MC, Bohl D, Maskos U. Human polymorphisms in nicotinic receptors: a functional analysis in iPS-derived dopaminergic neurons. FASEB J. 2017;31:828–39. doi: 10.1096/fj.201600932R. [DOI] [PubMed] [Google Scholar]
- 25.Taylor AE, Morris RW, Fluharty ME, Bjorngaard JH, Asvold BO, Gabrielsen ME, et al. Stratification by smoking status reveals an association of CHRNA5-A3-B4 genotype with body mass index in never smokers. Plos Genet. 2014;10:e1004799. doi: 10.1371/journal.pgen.1004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–73. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–52. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs KJ. Measurement of immediate-early gene activation-c-fos and beyond. J Neuroendocrinol. 2008;20:665–72. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 29.Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem Pharm. 2013;86:1181–93. doi: 10.1016/j.bcp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klenowski Paul M., Tapper Andrew R. The Neuropharmacology of Alcohol. Cham: Springer International Publishing; 2018. Molecular, Neuronal, and Behavioral Effects of Ethanol and Nicotine Interactions; pp. 187–212. [DOI] [PubMed] [Google Scholar]
- 31.Belin D, Belin-Rauscent A, Everitt BJ, Dalley JW. In search of predictive endophenotypes in addiction: insights from preclinical research. Genes Brain Behav. 2016;15:74–88. doi: 10.1111/gbb.12265. [DOI] [PubMed] [Google Scholar]
- 32.Picciotto MR, Mineur YS. Nicotine, food intake, and activation of POMC neurons. Neuropsychopharmacology. 2013;38:245–45. doi: 10.1038/npp.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sciaccaluga M, Moriconi C, Martinello K, Catalano M, Bermudez I, Stitzel JA, et al. Crucial role of nicotinic alpha5 subunit variants for Ca2+ fluxes in ventral midbrain neurons. FASEB J. 2015;29:3389–98. doi: 10.1096/fj.14-268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morel C, Fattore L, Pons S, Hay YA, Marti F, Lambolez B, et al. Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol Psychiatry. 2014;19:930–6. doi: 10.1038/mp.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–103. doi: 10.1001/jama.1996.03530380039029. [DOI] [PubMed] [Google Scholar]
- 36.Adams S. Psychopharmacology of tobacco and alcohol comorbidity: a review of current evidence. Curr Addict Rep. 2017;4:25–34. doi: 10.1007/s40429-017-0129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcohol Clin Exp Res. 2002;26:1928–9. doi: 10.1111/j.1530-0277.2002.tb02506.x. [DOI] [PubMed] [Google Scholar]
- 38.Dawson A, Wolstenholme JT, Roni MA, Campbell VC, Jackson A, Slater C, et al. Knockout of alpha 5 nicotinic acetylcholine receptors subunit alters ethanol-mediated behavioral effects and reward in mice. Neuropharmacology. 2018;138:341–48. doi: 10.1016/j.neuropharm.2018.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolu S, Marti F, Morel C, Perrier C, Torquet N, Pons S, et al. Nicotine enhances alcohol intake and dopaminergic responses through beta2* and beta4* nicotinic acetylcholine receptors. Sci Rep. 2017;7:45116. doi: 10.1038/srep45116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos N, Chatterjee S, Henry A, Holgate J, Bartlett SE. The alpha5 neuronal nicotinic acetylcholine receptor subunit plays an important role in the sedative effects of ethanol but does not modulate consumption in mice. Alcohol Clin Exp Res. 2013;37:655–62. doi: 10.1111/acer.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Besson M, Guiducci S, Granon S, Guilloux JP, Guiard B, Reperant C, et al. Alterations inalpha5* nicotinic acetylcholine receptors result in midbrain- and hippocampus-dependent behavioural and neural impairments. Psychopharmacol (Berl) 2016;233:3297–314. doi: 10.1007/s00213-016-4362-2. [DOI] [PubMed] [Google Scholar]
- 42.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 43.Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76 Pt B:342–50. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belin-Rauscent A, Daniel ML, Puaud M, Jupp B, Sawiak S, Howett D, et al. From impulses to maladaptive actions: the insula is a neurobiological gate for the development of compulsive behavior. Mol Psychiatry. 2016;21:491–9. doi: 10.1038/mp.2015.140. [DOI] [PubMed] [Google Scholar]
- 45.Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends Cogn Sci. 2015;19:414–20. doi: 10.1016/j.tics.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaramillo AA, Van Voorhies K, Randall PA, Besheer J. Silencing the insular-striatal circuit decreases alcohol self-administration and increases sensitivity to alcohol. Behav Brain Res. 2018;348:74–81. doi: 10.1016/j.bbr.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pushparaj A, Kim AS, Musiol M, Trigo JM, Le Foll B. Involvement of the rostral agranular insular cortex in nicotine self-administration in rats. Behav Brain Res. 2015;290:77–83. doi: 10.1016/j.bbr.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 48.Mori K, Manabe H, Narikiyo K, Onisawa N. Olfactory consciousness and gamma oscillation couplings across the olfactory bulb, olfactory cortex, and orbitofrontal cortex. Front Psychol. 2013;4:743. doi: 10.3389/fpsyg.2013.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen KP, DeVito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M. A CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology. 2015;40:2813–21. doi: 10.1038/npp.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez-Martinez IO, Acevedo-Roque CR, Montes-Angeles CD, Martinez M, Miranda F. Mental nerve injury induces novelty seeking behaviour leading to increasing ethanol intake in Wistar rats. Arch Oral Biol. 2019;99:66–72. doi: 10.1016/j.archoralbio.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Businelle MS, Ma P, Kendzor DE, Frank SG, Wetter DW, Vidrine DJ. Using Intensive longitudinal data collected via mobile phone to detect imminent lapse in smokers undergoing a scheduled quit attempt. J Med Internet Res. 2016;18:e275. doi: 10.2196/jmir.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lynch Krystal L, Twesten Jenny E, Stern Alexandra, Augustson Erik M. Level of Alcohol Consumption and Successful Smoking Cessation. Nicotine & Tobacco Research. 2018;21(8):1058–1064. doi: 10.1093/ntr/nty142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–39. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Zoli M, Picciotto MR. Nicotinic regulation of energy homeostasis. Nicotine Tob Res. 2012;14:1270–90. doi: 10.1093/ntr/nts159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fulkerson JA, French SA. Cigarette smoking for weight loss or control among adolescents: gender and racial/ethnic differences. J Adolesc Health. 2003;32:306–13. doi: 10.1016/S1054-139X(02)00566-9. [DOI] [PubMed] [Google Scholar]
- 56.Clark MM, Decker PA, Offord KP, Patten CA, Vickers KS, Croghan IT, et al. Weight concerns among male smokers. Addict Behav. 2004;29:1637–41. doi: 10.1016/j.addbeh.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 57.Pomerleau CS, Zucker AN, Stewart AJ. Characterizing concerns about post-cessation weight gain: results from a national survey of women smokers. Nicotine Tob Res. 2001;3:51–60. doi: 10.1080/14622200020032105. [DOI] [PubMed] [Google Scholar]
- 58.Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–2. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor Amy E, Richmond Rebecca C, Palviainen Teemu, Loukola Anu, Wootton Robyn E, Kaprio Jaakko, Relton Caroline L, Davey Smith George, Munafò Marcus R. The effect of body mass index on smoking behaviour and nicotine metabolism: a Mendelian randomization study. Human Molecular Genetics. 2018;28(8):1322–1330. doi: 10.1093/hmg/ddy434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solmi M, Veronese N, Sergi G, Luchini C, Favaro A, Santonastaso P, et al. The association between smoking prevalence and eating disorders: a systematic review and meta-analysis. Addiction. 2016;111:1914–22. doi: 10.1111/add.13457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.