Abstract
Background & aims:
Both liver disease (LD) and pancreatitis pose substantial burdens. There have been no general population-based studies on frequency of LD after an episode of pancreatitis. The aim of this study was to investigate the occurrence of LD in a population-based cohort of patients following pancreatitis.
Methods:
Nationwide data on the general population of nearly 3 million people were used to identify retrospectively diagnoses of acute pancreatitis, chronic pancreatitis (CP), LD and cirrhosis from 1998 to 2016. Acute pancreatitis was categorised as first (FAP) or recurrent (RAP) episode. Number of pancreatitis recurrences prior to LD diagnosis was determined.
Results:
A total of 20,931 pancreatitis patients were included, of which 874 developed LD following pancreatitis. The incidence of LD in FAP was 115.59 (95% confidence interval 102.19–128.98), in RAP – 217.63 (95% confidence interval 173.31–261.94), and in CP – 539.43 (95% confidence interval 494.72–584.13) patients per 100,000 pancreatitis patients per year. There was a significant increase in the probability of LD with increasing number of pancreatitis recurrences and, for the same number of pancreatitis recurrences, LD was significantly more frequent after CP than RAP (hazard ratio 1.666 (95% confidence interval 1.322–2.098; p = <0.001)).
Conclusions:
The frequency of LD increases from FAP to RAP to CP. While number of pancreatitis recurrences is a significant risk factor for development of LD, there is a higher probability of LD following CP than RAP even for the same number of recurrences. Interventions preventing pancreatitis and its progression may lower the burden of LD.
Keywords: Acute pancreatitis, Chronic pancreatitis, Cirrhosis, Liver disease, Population-based study
1. Introduction
Liver disease (LD) is a leading cause of morbidity and mortality worldwide, affecting individuals from all backgrounds and posing a considerable cost on health care systems [1,2]. Despite the major causes (such as chronic alcohol consumption, viral hepatitis B and C, and metabolic syndrome) being preventable and treatable, the burden remains high – nearly 30 million people are affected in Europe [3] and 300 million people – in China [4]. Due to such a high frequency, as well as poor long-term clinical outcomes (including considerably decreased quality of life and premature mortality), LD is a serious health care problem [2,4]. In the United States, LD is the second leading cause of mortality among all digestive diseases [5] and in China there are up to 400,000 deaths each year due to LD [4]. Unsurprisingly, treatment of LD is costly, with an estimated indirect cost of $10.6 billion in 2004 in the United States alone [6]. Cirrhosis represents the end-stage of LD, accounting for 1.2% of global disability-adjusted life years and representing the 12th leading cause of mortality globally, with over one million deaths annually [1,3,7]. Liver transplantation is the only effective treatment for cirrhosis [4,6]. Approximately 3000–5500 liver transplants are performed annually in the United States, Europe, and China each [3,4]. However, the outcomes are still sub-optimal as evidenced by findings of a large follow-up cohort study of 4000 patients that found the graft survival rate of 70% at 1 year and 41% at 18 years, while the overall survival rate was 59% at 18 years [8]. Numerous factors affect graft function and loss [8], one of which has been recently identified as pancreatitis [9].
There is only a limited body of knowledge on the association between LD and pancreatitis. A clinical study constrained to U.S. veterans found that 15.6% of patients with alcohol-induced LD and cirrhosis had pancreatitis [10]. A large autopsy study found 28% of patients had histological evidence of pancreatitis among 1022 patients with alcohol-induced LD [11]. Another study investigated the opposite question — occurrence of LD among patients with alcohol-induced chronic pancreatitis (CP), and found a frequency of 56% [12]. Strikingly, studies have shown that the presence of LD among patients with CP is more common than the presence of CP among patients with LD. A clinical study found that the presence of alcohol-induced LD was 3.5 times more common [13] and an autopsy study found that the presence of cirrhosis was more than 4 times more common among CP than the presence of CP among LD and cirrhosis, respectively [14]. Similar to LD, pancreatitis – another major disease of a parenchymatous organ – poses a substantial burden. Globally, acute pancreatitis (AP) is the most common pancreatic disease with an incidence of 33.74 cases per 100,000 person-years, which is significantly higher in North America than Europe, whereas CP has a global incidence of 9.62 cases per 100,000 person-years that is significantly higher in Europe [15]. While AP and CP have been traditionally considered separate diseases, an emerging paradigm suggests that AP and CP are a continuum of disease [16]. Specifically, a meta-analysis found that 10% of patients with a first episode of AP (FAP) and 36% of patients with recurrent AP (RAP) develop CP [16]. The majority of studies on the relationship between LD and pancreatitis (described above) did not evaluate the frequency of LD after AP, focusing predominantly on CP. Furthermore, no study has investigated incident cases of LD and there have been no general population-based studies addressing occurrence of LD after pancreatitis, which is the best study design to minimise the risk of selection bias. Based on the arguments above, there is a need to determine the frequency and predictors of LD in patients with pancreatitis.
The aim of this study was to investigate the occurrence of LD in a population-based cohort of patients with pancreatitis and risk factors associated with it.
2. Materials and methods
2.1. Data source
The New Zealand Ministry of Health Analytical Services (National Health Board, Ministry of Health, New Zealand) provided the data for this study. The retrospective dataset included information on age, sex, and the ninth (for patients admitted from 1995 to 1998) or tenth (for patients admitted from 1999 to 2016) revision International Classification of Diseases (ICD) codes. For the purpose of this study, all patients were anonymised by the Analytical Services. As per the Ministry of Health guidelines, ethical review was not required for this study. No contact was made with the study population.
2.2. Study population
A unique alpha-numeric code, the National Health Index, is assigned to all New Zealand residents at their very first contact with the health-care system. The population for this study (n = 20,931) constituted registered patients admitted to all 20 New Zealand District Health Boards from 1st January 1998 to 31st December 2016 with diagnosis of AP (ICD-9: 577.0 or ICD-10: K85.0–K85.3; K85.8–K85.9) and CP (ICD-9: 577.1 or ICD-10: K86.0) [2,10,17–19]. Each patient who had a diagnosis of pancreatic cancer after inclusion in the cohort was excluded.
All patients with AP were categorised as having either FAP or RAP during the study period. Patients with FAP were those who had a diagnosis of AP during the study period, had no prior diagnosis of AP or CP dating back to 1st January 1995, and no subsequent diagnosis of AP or CP during the study period. For patients with FAP, the date of first admission was considered the index date. Patients with RAP were those who had two or more admissions due to AP during the study period (admissions within 30 days were considered as the same admission), had no prior diagnosis of CP dating back to 1st January 1995, and no subsequent diagnosis of CP during the study period. For patients with RAP, the date of second admission was considered the index date. All patients with CP were categorised as CP and excluded from those designated as having AP. Patients with CP were those who had a diagnosis of CP during the study period and had no prior diagnosis of CP dating back to 1st January 1995. For patients with CP, the first diagnosis of CP during the study period was considered the index date.
Among patients with AP or CP, those with diagnosis of LD (ICD-9: 570–573 or ICD-10: K70.0–K70.4; K70.9; K71; K73; K74; K76.0) including cirrhosis (ICD-9: 571.0; 571.2; 571.5; 571.6 or ICD-10: E831; K70.3; K71.7; K74.3–K74.6) [2,10,20–22], were identified. Those with LD were categorised as LD after FAP, LD after RAP, and LD after CP. Patients with LD after FAP were those who did not have a LD diagnosis prior to FAP diagnosis. Patients with LD after RAP were those who did not have a LD diagnosis prior to RAP diagnosis. Patients with LD after RAP were excluded from the group of those designated as having LD after FAP. Patients with LD after CP were those who did not have a LD diagnosis prior to CP diagnosis. Patients with LD after FAP, RAP and CP had no diagnosis of LD back to 1st January 1995. The number of pancreatitis recurrences for those with RAP and CP was determined by the total number of hospital admissions with a diagnosis of pancreatitis that were at least 30 days apart, until first LD diagnosis [17].
Chronic alcohol consumption was determined based on the following ICD codes: 30390; 30500; 291.81; 9800 for ICD-9 or F10.20; F10.10; F10.239; T51.0 for ICD-10 [10,17,18] and tobacco-use was determined based on the following ICD codes: 3051; V1582; V1583 for ICD-9 or F17.200; Z87.891 for ICD-10 [17].
2.3. Statistical analyses
Statistical analyses were conducted using Excel (Microsoft, United States, 2016) and SPSS for Windows Version 24 (IBM Corporation, United States, 2015) in three steps.
First, all discrete variables were presented as counts. Percentage incidence and incidence rate of LD per 100,000 pancreatitis patients per year were determined using patients with LD as the numerator and number of patients with FAP, RAP, and CP as the denominator. Sensitivity analysis was conducted using patients with cirrhosis as the numerator and number of patients with FAP, RAP, and CP as the denominator. 95% confidence intervals (CIs) were calculated using Excel.
Second, having met all assumptions, binary logistic regression was conducted to investigate the associations between LD and pancreatitis groups (FAP was set as the reference) in the main analysis. A subgroup analysis based on chronic alcohol consumption and sensitivity analysis constrained to cirrhosis was conducted using binary logistic regression. The analysis was conducted using 3 models. Model 1 was an unadjusted model; model 2 was adjusted for age, and sex; and model 3 was adjusted for age, sex, and tobacco-use. Further, binary logistic regression was used to investigate the effect of chronic alcohol consumption and tobacco-use on LD and pancreatitis, compared with pancreatitis alone. All data were presented as odds ratio (OR) with corresponding 95% CI and p-value.
Third, having met all assumptions, univariable Cox regression was conducted to investigate the odds of incident LD in individuals with CP (RAP was set as the reference). Multivariable Cox regression was used to determine predictors of LD using sex, age, chronic alcohol consumption and tobacco-use as covariates. All data were presented as hazard ratio with corresponding 95% CI and p-value. For all statistical analyses, p-values <0.05 were deemed statistically significant.
3. Results
3.1. Overall characteristics of patients
The overall cohort encompassed 20,931 pancreatitis patients, including 13,458 with FAP, 2272 — with RAP, and 5201 — with CP. In the CP group, 3362 (65%) of patients did not have antecedent AP whereas 1839 (35%) — had it. In the overall cohort, there were a total of 874 patients with LD, of which 336 (38%) were patients with cirrhosis. The median (interquartile range) age of patients with LD was 57 (45–68) years and of patients with cirrhosis was 53.5 (43–63) years. In the LD group, 555 (64%) patients were males, 532 (61%) patients had chronic alcohol consumption, and 573 (66%) patients used tobacco. In the cirrhosis group, 225 (67%) patients were males, 291 (87%) patients had chronic alcohol consumption, and 247 (74%) patients used tobacco. The majority of patients (80%) had the ICD code for chronic liver disease and cirrhosis (ICD-9: 571). Among these patients, 26% had other chronic non-alcohol induced liver disease (ICD-9: 571.8), 12% had alcohol-induced cirrhosis of the liver (ICD-9: 571.2), 12% had acute alcohol-induced hepatitis (ICD-9: 571.1), and 11% had alcohol-induced liver damage, unspecified (ICD-9: 571.3). A total of 12% of patients had alcohol-induced cirrhosis of the liver (ICD-10: K70.3). A total of 15% of patients with cirrhosis had common bile duct pathologies or obstruction of the duodenum. Excluding patients who were diagnosed with LD and cirrhosis within 1 year after pancreatitis diagnosis, LD was diagnosed at a median (interquartile range) of 46 (24–75) months and cirrhosis was diagnosed at a median (interquartile range) of 42 (25–69) months after pancreatitis diagnosis.
3.2. Frequency of liver disease by pancreatitis group
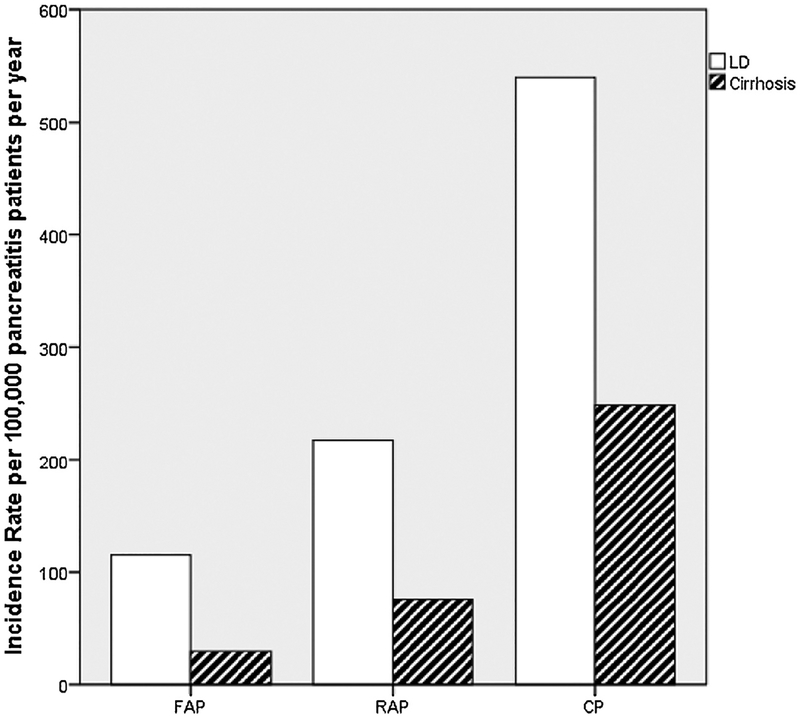
There were 280 patients with LD in the FAP group, of which 72 (26%) were patients with cirrhosis. In the FAP group, the incidence rate (95% CI) of LD was 115.59 (102.19, 128.98) and the one of cirrhosis was 29.72 (22.88, 36.57) patients per 100,000 pancreatitis patients per year (Table 1). There were 72 patients with LD in the RAP group, of which 31 (43%) were patients with cirrhosis. In the RAP group, the incidence rate (95% CI) of LD was 217.63 (173.31, 261.94) and the one of cirrhosis was 75.80 (49.30, 102.30) patients per 100,000 pancreatitis patients per year. There were 505 patients with LD in the CP group, of which 233 (46%) were patients with cirrhosis. In the CP group, the incidence rate (95% CI) of LD was 539.43 (494.72, 584.13) and the one of cirrhosis was 248.88 (217.65, 280.12) patients per 100,000 pancreatitis patients per year (Fig. 1).
Table 1.
Frequency of liver disease and cirrhosis by pancreatitis group.
| Patients with liverdisease | Total patients with pancreatitis | Crude incidence, % | Incidence rate per 100,000 pancreatitis patients per year (95% confidence interval) | |
|---|---|---|---|---|
| Liverdisease | ||||
| First episode of acute pancreatitis | 280 | 13458 | 2.1 | 115.59 (102.19, 128.98) |
| Recurrent acute pancreatitis | 89 | 2272 | 3.9 | 217.63(173.31,261.94) |
| Chronic pancreatitis | 505 | 5201 | 9.7 | 539.43 (494.72, 584.13) |
| Cirrhosis | ||||
| First episode ofacute pancreatitis | 72 | 13458 | 0.5 | 29.72(22.88,36.57) |
| Recurrent acute pancreatitis | 31 | 2272 | 1.4 | 75.80 (49.30, 102.30) |
| Chronic pancreatitis | 233 | 5201 | 4.5 | 248.88 (217.65, 280.12) |
Fig. 1.
Incidence of LD and cirrhosis by pancreatitis group.
Abbreviations: CP, chronic pancreatitis; FAP, first acute pancreatitis; LD, liver disease; RAP, recurrent acute pancreatitis.
3.3. Associations between liver disease and recurrence of acute pancreatitis
In the main analysis, compared with FAP, RAP was significantly associated with increased odds of LD in all three models (Table 2). Patients who had RAP had significantly higher odds of LD with an OR (95% CI; p-value) of 1.920 (1.506, 2.448; p = <0.001) in model 2, 1.919 (1.505, 2.446; p = <0.001) in model 1, and 1.837 (1.440, 2.344; p = <0.001) in model 3.
Table 2.
Odds of liver disease following recurrent acute pancreatitis stratified by chronic alcohol consumption.
| Chronic alcohol consumption | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | No | Yes | |||||||||||||
| Model | OR | 95% CI | P | Model | OR | 95% CI | P | Model | OR | 95% CI | P | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||
| Liver disease | 1 | 1.919 | 1.505 | 2.446 | <0.001 | 1 | 1.530 | 1.017 | 2.303 | 0.042 | 1 | 1.962 | 1.438 | 2.676 | <0.001 |
| 2 | 1.920 | 1.506 | 2.448 | <0.001 | 2 | 1.548 | 1.028 | 2.330 | 0.036 | 2 | 1.944 | 1.425 | 3.490 | <0.001 | |
| 3 | 1.837 | 1.440 | 2.344 | <0.001 | 3 | 1.534 | 1.019 | 2.312 | 0.041 | 3 | 1.839 | 1.346 | 2.513 | <0.001 | |
| Cirrhosis | 1 | 2.572 | 1.684 | 3.928 | <0.001 | 1 | 2.488 | 0.810 | 7.643 | 0.112 | 1 | 1.901 | 1.201 | 3.011 | 0.006 |
| 2 | 2.586 | 1.693 | 3.951 | <0.001 | 2 | 2.548 | 0.829 | 7.833 | 0.103 | 2 | 1.894 | 1.196 | 3.001 | 0.007 | |
| 3 | 2.375 | 1.552 | 3.632 | <0.001 | 3 | 2.586 | 0.840 | 7.961 | 0.098 | 3 | 1.754 | 1.105 | 2.783 | 0.017 | |
Abbreviations: CI, confidence interval; OR, odds ratio.
First episode of acute pancreatitis was used as the reference. Model 1 was an unadjusted model; model 2 was adjusted for age, and sex, and model 3 was adjusted for age, sex, and tobacco-use. Significant p-values (<0.05) are shown in bold.
In the subgroup analysis based on chronic alcohol consumption, compared with FAP, RAP was significantly associated with increased odds of LD in all three models of both groups (Table 2). Patients who had chronic alcohol consumption compared with those who did not had an OR (95% CI; p-value) of 1.962 (1.438, 2.676; p = <0.001) and 1.530 (1.017, 2.303; p =0.042) in model 1, 1.944 (1.425, 3.490; p = <0.001) and 1.548 (1.028, 2.330; p = 0.036) in model 2, and 1.839 (1.346, 2.513; p = <0.001) and 1.534 (1.019, 2.312; p = 0.041) in model 3, respectively.
In the sensitivity analysis constrained to cirrhosis, compared with FAP, patients who had RAP had significantly higher odds of cirrhosis with an OR (95% CI; p-value) of 2.586 (1.693, 3.951; p = <0.001) in model 2, 2.572 (1.684, 3.928; p = <0.001) in model 1, and 2.375 (1.552, 3.632; p = <0.001) in model 3.
3.4. Associations between liver disease and chronic pancreatitis
In the main analysis, compared with FAP, CP was significantly associated with increased odds of LD in all three models (Table 2). Patients who had CP had significantly higher odds of LD with an OR (95% CI; p-value) of 5.061 (4.357, 5.879; p = <0.001) in model 1, 4.853 (4.175, 5.641; p = <0.001) in model 2, and 4.586 (3.939, 5.340; p = <0.001) in model 3.
In the subgroup analysis based on chronic alcohol consumption, compared with FAP, CP was significantly associated with increased odds of LD in all three models of both groups (Table 2). However, there were significantly more patients with LD among patients who had chronic alcohol consumption compared with those who did not with an OR (95% CI; p-value) of 6.508 (5.285, 8.015; p = < 0.001) and 3.346 (2.670, 4.193; p = <0.001) in model 1, 6.118 (4.958, 7.551; p = <0.001) and 3.286 (2.620, 4.120; p = <0.001) in model 2, and 5.615 (4.534, 6.953; p = <0.001) and 3.252 (2.590, 4.083; p = <0.001) in model 3, respectively.
In the sensitivity analysis constrained to cirrhosis, compared with FAP, patients who had CP had significantly higher odds of cirrhosis with an OR (95% CI; p-value) of 8.720 (6.681, 11.380; p = <0.001) in model 1, 8.339 (6.382, 10.896; p = 0.007) in model 2, and 7.557 (5.770, 9.897; p = <0.001) in model 3.
3.5. Predictors of liver disease in patients with pancreatitis
In the overall cohort, sex and chronic alcohol consumption in the FAP group, sex in the RAP group, and chronic alcohol consumption in the CP group were significant predictors of LD. Compared with females, males had a hazard ratio (95% CI) of 1.783 (1.395, 2.278) in the FAP group, and 1.940 (1.221, 3.081) in the RAP group. Patients who had chronic alcohol consumption had a hazard ratio (95% CI) of 1.482 (1.171, 1.875) in the FAP group, and 1.818 (1.494, 2.214) in the CP group, compared with those who did not (Table 3). Further, patients who had chronic alcohol consumption and tobacco-use had significantly higher odds of both pancreatitis and LD than pancreatitis alone with an OR (95% CI; p-value) of 2.313 (2.013, 2.657; p = <0.001) and 1.914 (1.660, 2.206; p = <0.001), respectively, and 2.895 (2.523, 3.322; p = <0.001) with both chronic alcohol consumption and tobacco-use (Tables 4 and 5).
Table 3.
Odds of liver disease following chronic pancreatitis stratified by chronic alcohol consumption.
| Chronic alcohol consumption | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | No | Yes | |||||||||||||
| Model | OR | 95% CI | P | Model | OR | 95% CI | P | Model | OR | 95% CI | P | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||
| Liver disease | 1 | 5.061 | 4.357 | 5.879 | <0.001 | 1 | 3.346 | 2.670 | 4.193 | <0.001 | 1 | 6.508 | 5.285 | 8.015 | <0.001 |
| 2 | 4.853 | 4.175 | 5.641 | <0.001 | 2 | 3.286 | 2.620 | 4.120 | <0.001 | 2 | 6.118 | 4.958 | 7.551 | <0.001 | |
| 3 | 4.586 | 3.939 | 5.340 | <0.001 | 3 | 3.252 | 2.590 | 4.083 | <0.001 | 3 | 5.615 | 4.534 | 6.953 | <0.001 | |
| Cirrhosis | 1 | 8.720 | 6.681 | 11.380 | <0.001 | 1 | 6.299 | 3.258 | 12.176 | <0.001 | 1 | 8.272 | 6.165 | 11.100 | <0.001 |
| 2 | 8.339 | 6.382 | 10.896 | <0.001 | 2 | 6.223 | 3.215 | 12.045 | <0.001 | 2 | 7.743 | 5.753 | 10.423 | <0.001 | |
| 3 | 7.557 | 5.770 | 9.897 | <0.001 | 3 | 6.336 | 3.264 | 12.301 | <0.001 | 3 | 6.919 | 5.118 | 9.353 | <0.001 | |
Abbreviations: CI, confidence interval; OR, odds ratio.
First episode of acute pancreatitis was used as the reference. Model 1 was an unadjusted model; model 2 was adjusted for age, and sex, and model 3 was adjusted for age, sex, and tobacco-use. Significant p-values (<0.05) are shown in bold.
Table 4.
Predictors of liver disease in pancreatitis.
| Variable | HR | 95% CI | P | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| FAP | Sex (Female vs. Male) | 1.783 | 1.395 | 2.278 | <0.001 |
| Age | 1.006 | 1.000 | 1.012 | 0.043 | |
| Chronic alcohol consumption (No vs. Yes) | 1.482 | 1.171 | 1.875 | 0.001 | |
| Tobacco-use (No vs. Yes) | 1.136 | 0.896 | 1.440 | 0.292 | |
| RAP | Sex (Female vs. Male) | 1.940 | 1.221 | 3.081 | 0.005 |
| Age | 0.987 | 0.974 | 0.999 | 0.035 | |
| Chronic alcohol consumption (No vs. Yes) | 1.519 | 0.968 | 2.385 | 0.069 | |
| Tobacco-use (No vs. Yes) | 1.389 | 0.876 | 2.201 | 0.162 | |
| CP | Sex (Female vs. Male) | 1.146 | 0.952 | 1.379 | 0.149 |
| Age | 1.004 | 0.999 | 1.010 | 0.127 | |
| Chronic alcohol consumption (No vs. Yes) | 1.818 | 1.494 | 2.214 | <0.001 | |
| Tobacco-use (No vs. Yes) | 1.157 | 0.944 | 1.420 | 0.161 | |
Abbreviations: CI, confidence interval; CP, chronic pancreatitis; FAP, first acute pancreatitis; HR, hazard ratio; RAP, recurrent acute pancreatitis. Significant p-values (<0.05) are shown in bold.
Table 5.
Odds of developing both liver disease and pancreatitis compared with pancreatitis alone based on chronic alcohol consumption and tobacco-use.
| Variable | OR | 95% CI | P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Chronic alcohol consumption | 2.313 | 2.013 | 2.657 | <0.001 |
| Tobacco-use | 1.914 | 1.660 | 2.206 | <0.001 |
| Chronic alcohol consumption and tobacco-use | 2.895 | 2.523 | 3.322 | <0.001 |
Abbreviations: CI, confidence interval; OR, odds ratio. Significant p-values (<0.05) are shown in bold.
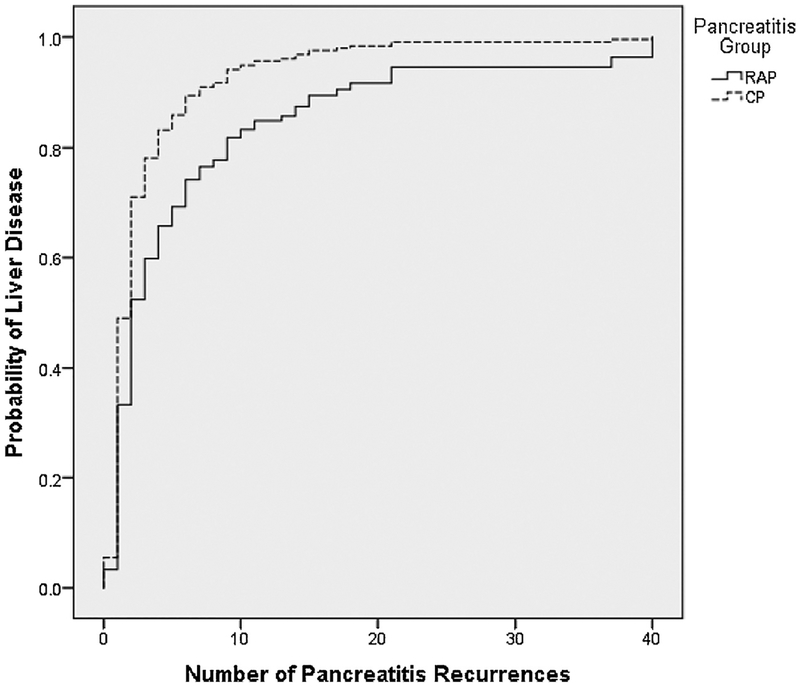
In the sub-cohort of patients who had recurrent episodes of pancreatitis, the number of recurrences was associated with an increased probability of LD (Fig. 2). For patients with the same number of pancreatitis recurrences, there was a significantly higher hazard of LD in CP with a hazard ratio (95% CI; p-value) of 1.666 (1.322, 2.098; p = <0.0001), compared with RAP. For example, for patients with 5 pancreatitis recurrences, the probability of LD in individuals with CP was 0.83 compared with 0.66 in RAP who did not have CP (Fig. 2).
Fig. 2.
Association between probability of liver disease and number of pancreatitis recurrences in patients with recurrent acute pancreatitis (RAP) and chronic pancreatitis (CP).
4. Discussion
This is the first general population-based cohort study investigating the burden of LD following pancreatitis and factors associated with it. Unlike previous autopsy and clinical studies studying the same question, a distinct strength of this study is the large sample size and minimisation of selection bias due to population-based nature of the study. Findings from this research show, for the first time, that the frequency of LD after RAP (that did not have CP) was 1.9 times higher than FAP and LD after CP was 4.7 times higher than FAP, demonstrating a stepwise increase. Second, the number of pancreatitis recurrences was a significant factor associated with LD and, for the same number of pancreatitis recurrences, the frequency of LD was 1.7 times significantly higher in patients with CP than RAP (that did not have CP). Third, findings of this study show that chronic alcohol consumption was a significant predictor of LD, with the odds of LD being 3.2 times significantly higher in patients who had chronic alcohol consumption, compared with those who did not, in the CP group. Further, chronic alcohol abusers had 2.3 times higher statistically significant odds of having both pancreatitis and LD than pancreatitis alone.
The liver and pancreas are closely related parenchymatous organs [23]. In both organs, diseases are commonly characterised by a cascade of damage to parenchymal cells, stellate cell activation, and fibrosis [23]. It is believed that stellate cells found in the liver and pancreas have similar morphology and function [23,24]. The hepatic and pancreatic stellate cells play a key role in the development of fibrosis in the liver and pancreas, suggesting that similar pathogenetic mechanisms may result in the development of disease in these organs [14,25]. These cells can change from a quiescent to an activated phenotype in response to parenchymal cell damage (which may be the result of chronic alcohol consumption and/or tobacco-use) [23]. For example, ethanol has been found to inhibit cystic fibrosis transmembrane conductance regulator function in pancreatic epithelial cells and impair ductal function [26]. Innate immune signalling (lipopolysaccharide), paracrine cytokines (interleukin-1, −6 and −8, and tumour necrosis factor-α), and oxidative stress are signalling molecules and pathways involved in stellate cell activation [23,27,28]. Consequently, activated stellate cells produce autocrine cytokines and reactive oxygen radicals, promoting inflammation and further perpetuating activation [23,29]. The Sentinel Acute Pancreatitis Event hypothesis proposes that FAP, or the sentinel episode of AP, recruits and activates the pancreatic stellate cells [16]. This first hit to the pancreas triggers an inflammatory cascade towards the development of CP, with the persistence of chronic low-grade inflammation even once the acute inflammation has resolved [30–33]. Sustained pancreatic injury via oxidative stress or recurrent episodes of inflammation, which may or not be clinically evident, results in the development of CP. This inflammatory cascade may trigger and/or contribute to the activation of hepatic stellate cells which can lead to LD [34].
Given that 15% of patients with cirrhosis had common bile duct pathologies or obstruction of the duodenum in our study cohort, it is pertinent to discuss the possibility of involvement of the common bile duct as a mechanistic cause leading to LD. In pancreatitis, strictures of the common bile duct are the consequences of compression of the duct by fibrosis and inflammation in the head of the pancreas, through which the common bile duct transverses [35]. The incidence of common bile duct obstruction in patients with CP ranges from 3 to 23%, with an average of 6% [36]. Repeated insult to the common bile duct may lead to secondary biliary cirrhosis as a cause of LD. Therefore, biliary diversion surgery for high-risk patients with pancreatitis and common bile duct pathology may, in theory, prevent LD and help in reducing the occurrence of cirrhosis [35]. It is plausible that pancreatitis and LD have intertwined pathogenetic pathways, explaining the development of LD following pancreatitis and its different frequencies in the FAP, RAP and CP groups demonstrated in this study.
A novel finding of this study is that the number of pancreatitis recurrences was a significant factor associated with LD. Moreover, for the first time, we have shown that for the same number of pancreatitis recurrences, the frequency of LD was 1.7 times significantly higher in patients with CP than RAP (who did not have CP). This may be because increasing recurrent hits to the pancreas perpetuates the inflammatory cascade leading to continued parenchymal cell damage and fibrosis [23,37]. Furthermore, injury to the intestine in AP results in loss of barrier function, increased permeability, and subsequent translocation of bacterial toxins into the portal venous and lymphatic systems [38]. Recurrent injury may increase gut-derived lipopolysaccharide, which stimulates expression of proinflammatory cytokines, such as interleukin-6 and tumour necrosis factor-α, causing the manifestation of chronic inflammation [39–41]. This increased inflammation in the pancreas, alongside repeated oxidative stress, may contribute to the activation of hepatic stellate cells and/or perpetuate their activation which may cause damage to the liver [42]. With increasing number of pancreatitis recurrences, there may also be continued exposure to stimuli that cause parenchymal cell damage in the liver. One of the clinical implications of the above finding is that, while it is acknowledged that chronic alcohol consumption and recurrence of pancreatitis are co-dependent variables that may be associated with liver disease (i.e., alcohol recidivism leads to more pancreatitis, and more cirrhosis), some patients with pancreatitis in the absence of chronic alcohol consumption also develop cirrhosis and, hence, may need to be screened for cirrhosis. Future purposefully designed studies are needed to investigate this question.
Another notable finding of this study is that chronic alcohol consumption was a significant predictor of LD. In the CP group, the occurrence of LD was 3.2 times significantly higher in patients who had chronic alcohol consumption and there were consistently more patients with LD among patients who had chronic alcohol consumption, compared with those who did not. There was a consistent statistical interaction effect evident, demonstrating the detrimental effect of chronic alcohol consumption. For example, in the overall cohort, there was an increasing trend in the odds of LD and cirrhosis from RAP to CP, but this was much more prominent in patients who had chronic alcohol consumption, compared with those who did not. Further, patients who had chronic alcohol consumption had 2.3 times higher statistically significant odds of having both pancreatitis and LD than pancreatitis alone. Studies have shown that alcohol is a significant modifiable risk factor affecting the development of AP, RAP, and CP as it sensitises the pancreas to injury and promotes disease progression following initiation of pancreatic injury [16,17,43–46]. Therefore, interventions targeting alcohol use will likely result in prevention of LD by reducing pancreatitis, its progression to CP in those with FAP and RAP, and by reducing injury for those with CP.
This population-based study has several limitations related to the use of administrative data [17]. First, only public hospital data were used to determine the incidence rates of LD and cirrhosis after pancreatitis. Therefore, the incidence rates might have been underestimated as data on patients diagnosed in the community and private hospitals (who were not transferred to public hospitals) were not recorded in the database [17,47]. However, this was a nationwide study that included a respectable sample size of data from all 20 New Zealand District Health Boards. Therefore, the risk of selection bias was minimal, unlike previous clinical and anatomical autopsy studies in this setting. Second, diagnoses of AP, CP, LD and cirrhosis, and definitions of chronic alcohol consumption and tobacco-use were determined from ICD-9 and −10 codes only, without clinical, laboratory or pathological data [47]. It is unclear how the diagnoses of pancreatitis and liver disease were reached and whether these were based on clinical judgement, non-invasive testing, and/or biopsy. However, a recent systematic review and meta-analysis showed that approximately two out of ten patients are misidentified as having either AP or CP with the indiscriminate use of ICD codes and limiting the use of codes to adult patients with incident episode of AP may improve identification of patients with pancreatitis in administrative databases [48]. Furthermore, use of ICD codes is the standard approach to nationwide studies and has been widely used [48–51]. Third, we did not subclassify pancreatitis based on aetiology. However, codes for aetiology are more inaccurate and less reliable than pancreatitis overall [37,48]. Fourth, behavioural aspects of chronic alcohol consumption (e.g. type of alcohol, quantity of daily intake, duration of use), tobacco-use (e.g. method, duration and frequency of use) and dietary intake were unable to be determined [14]. Future studies should investigate how behavioural aspects of chronic alcohol consumption, tobacco-use, and dietary intake affect the risk of LD development. Fifth, we did not adjust for the comorbid diseases that affect both the pancreas and liver such as viral hepatitis and IgG-4 disease. However, the codes for viral hepatitis were not included, and IgG-4 disease is rare (with a prevalence of approximately 60 individuals per million population) and is likely to be under-diagnosed and under-reported in our study population [52]. Sixth, the RAP group that developed LD was the smallest with only 89 patients. Given the limitation of differentiating RAP from CP based on ICD codes and taking into account the relatively small number of patients, the finding on the number of recurrences associated with LD should be interpreted with caution. Last, complications of pancreatitis, such as portal vein thrombosis, may have been incorrectly coded as LD. However, our sensitivity analyses constrained to cirrhosis only yielded estimates similar to all LD.
In conclusion, this is the first robust nationwide population-based study to investigate and quantify the burden of LD following pancreatitis. Findings of this study indicate a stepwise increase in the frequency of LD in patients who progress from FAP to RAP to CP, an increase in the probability of LD with increasing number of pancreatitis recurrences, and a higher probability of LD in CP than RAP (that did not have CP) in patients with the same number of pancreatitis recurrences. Interventions preventing the progression of pancreatitis may result in a decreased burden of LD.
Acknowledgment
This study was part of the Clinical and epidemiological investigations in Metabolism, nutrition, and pancreatic diseases (COSMOS) program. COSMOS is supported in part by the Royal Society of New Zealand (Rutherford Discovery Fellowship to Associate Professor Petrov), which played no role in the study design; collection, analysis, or interpretation of data; or writing of the manuscript.
Footnotes
Conflict of interest
None declared.
References
- [1].Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC Med 2014;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peery AF, Crockett SD, Barritt AS, Lund JL, Dellon ES, Williams JL, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015;149:1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 2013;58:593–608. [DOI] [PubMed] [Google Scholar]
- [4].Wang F, Fan J, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology 2014;60:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part III: liver, biliary tract, and pancreas. Gastroenterology 2009;136:1134–44. [DOI] [PubMed] [Google Scholar]
- [6].Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterol Hepatol 2011;7:661–71. [PMC free article] [PubMed] [Google Scholar]
- [7].Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of diseasestudy 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- [8].Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, Ruppert K, et al. Long-term survival after liver transplantation in 4000 consecutive patients at a single center. Ann Surg 2000;232:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wehmeyer MH, Dammermann W, Seiz O, Zinser ME, Galante A, Lohse AW, et al. Chronic pancreatitis in patients with liver cirrhosis negatively affects graft survival after liver transplantation. Pancreatology 2017;17:898–904. [DOI] [PubMed] [Google Scholar]
- [10].Buchner AM, Sonnenberg A. Comorbid occurrence of liver and pancreas disease in United States military veterans. Am J Gastroenterol 2001;96:2231–7. [DOI] [PubMed] [Google Scholar]
- [11].Renner IG, Savage WT, Stace NH, Pantoja JL, Schultheis WM, Peters RL. Pancreatitis associated with alcoholic liver disease: a review of 1022 autopsy cases. Dig Dis Sci 1984;29:593–9. [DOI] [PubMed] [Google Scholar]
- [12].Gullo L, Casadei R, Campione O, Grigioni W, Marrano D. Alcoholic liver disease in alcoholic chronic pancreatitis: a prospective study. Ital J Gastroenterol 1995;27:69–72. [PubMed] [Google Scholar]
- [13].Sofia C, Cadime A, Cotrim I, Souto P, Freitas D, Monteiro G. Coexistence of hepatic and pancreatic disease in the chronic alcoholic. Unusual or frequent situation? Acta Med Port 1992;5:235–8. [PubMed] [Google Scholar]
- [14].Pace A, de Weerth A, Berna M, Hillbricht K, Tsokos M, Bläker M, et al. Pancreas and liver injury are associated in individuals with increased alcohol consumption. Clin Gastroenterol Hepatol 2009;7:1241–6. [DOI] [PubMed] [Google Scholar]
- [15].Xiao AY, Tan MLY, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016;1:45–55. [DOI] [PubMed] [Google Scholar]
- [16].Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS, et al. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015;149:1490–500. [DOI] [PubMed] [Google Scholar]
- [17].Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol 2012;107:1096–103. [DOI] [PubMed] [Google Scholar]
- [18].Chung WS, Lin CL. Incidence and risk of acute coronary syndrome in patients with acute pancreatitis: a nationwide cohort study. Pancreatology 2017;17:675–80. [DOI] [PubMed] [Google Scholar]
- [19].Hsu MT, Lin CL, Chung WS. Increased risk of acute coronary syndrome in patients with chronic pancreatitis: a nationwide cohort analysis. Medicine (Baltimore) 2016;95:e3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].May FP, Rolston VS, Tapper EB, Lakshmanan A, Saab S, Sundaram V. The impact of race and ethnicity on mortality and healthcare utilization in alcoholic hepatitis: a cross-sectional study. BMC Gastroenterol 2016;16:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ratib S, West J, Crooks CJ, Fleming KM. Diagnosis of liver cirrhosis in England, a cohort study, 1998–2009: a comparison with cancer. Am J Gastroenterol 2014;109:190–8. [DOI] [PubMed] [Google Scholar]
- [22].Jepsen P, Andersen MW, Villadsen GE, Ott P, Vilstrup H. Time-trends in incidence and prognosis of hepatocellular carcinoma in Denmark: a nationwide register-based cohort study. Liver Int 2017;37:871–8. [DOI] [PubMed] [Google Scholar]
- [23].Clemens DL, Mahan KJ. Alcoholic pancreatitis: lessons from the liver. World J Gastroenterol 2010;16:1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 2007;117:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol 2012;3:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bharmal SH, Pendharkar SA, Singh RG, Petrov MS. Associations between gastrointestinal humoral factors and pancreatic proteolytic enzymes in alcohol-related versus non-alcohol-related pancreatitis. Alcohol 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [27].Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017;14:397–411. [DOI] [PubMed] [Google Scholar]
- [28].Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res 2011;35:787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004;34:9–19. [DOI] [PubMed] [Google Scholar]
- [30].Gillies N, Pendharkar SA, Asrani VM, Mathew J, Windsor JA, Petrov MS. Interleukin-6 is associated with chronic hyperglycemia and insulin resistance in patients after acute pancreatitis. Pancreatology 2016;16:748–55. [DOI] [PubMed] [Google Scholar]
- [31].Pendharkar SA, Singh RG, Petrov MS. Cross-talk between innate cytokines and the pancreatic polypeptide family in acute pancreatitis. Cytokine 2017;90:161–8. [DOI] [PubMed] [Google Scholar]
- [32].Singh RG, Pendharkar SA, Gillies NA, Miranda-Soberanis V, Plank LD, Petrov MS. Associations between circulating levels of adipocytokines and abdominal adiposity in patients after acute pancreatitis. Clin Exp Med 2017;17:477–87. [DOI] [PubMed] [Google Scholar]
- [33].Chand SK, Singh RG, Pendharkar SA, Bharmal SH, Petrov MS. Interplay between innate immunity and iron metabolism after acute pancreatitis. Cytokine 2018;103:90–8. [DOI] [PubMed] [Google Scholar]
- [34].Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Warshaw AL, Schapiro RH, Ferrucci JT, Galdabini JJ. Persistent obstructive jaundice, cholangitis, and biliary cirrhosis due to common bile duct stenosis in chronic pancreatitis. Gastroenterology 1976;70:562–7. [PubMed] [Google Scholar]
- [36].Vijungco JD, Prinz RA. Management of biliary and duodenal complications of chronic pancreatitis. World J Surg 2003;27:1258–70. [DOI] [PubMed] [Google Scholar]
- [37].Schneider A, Whitcomb DC. Hereditary pancreatitis: a model for inflammatory diseases of the pancreas. Best Pract Res Clin Gastroenterol 2002;16:347–63. [DOI] [PubMed] [Google Scholar]
- [38].Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. B J Surg 2014;101:1644–56. [DOI] [PubMed] [Google Scholar]
- [39].Singh RG, Pendharkar SA, Cervantes A, Cho J, Miranda-Soberanis V, Petrov MS. Abdominal obesity and insulin resistance after an episode of acute pancreatitis. Dig Liver Dis 2018;50:1081–7. [DOI] [PubMed] [Google Scholar]
- [40].Gomes JM, Costa JA, Alfenas RC. Metabolic endotoxemia and diabetes mellitus: a systematic review. Metabolism 2017;68:133–44. [DOI] [PubMed] [Google Scholar]
- [41].Andersson U, Matsuda T. Human interleukin 6 and tumor necrosis factor alpha production studied at a single-cell level. Eur J Immunol 1989;19:1157–60. [DOI] [PubMed] [Google Scholar]
- [42].Pandol SJ, Gorelick FS, Gerloff A, Lugea A. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig Dis 2011;28:776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nordback I, Pelli H, Lappalainen-Lehto R, Järvinen S, Räty S, Sand J. The recurrence of acute alcohol-associated pancreatitis can be reduced: a randomized controlled trial. Gastroenterology 2009;136:848–55. [DOI] [PubMed] [Google Scholar]
- [44].Alsamarrai A, Das SL, Windsor JA, Petrov MS. Factors that affect risk for pancreatic disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol 2014;12:1635–44. [DOI] [PubMed] [Google Scholar]
- [45].Singhvi A, Yadav D. Myths and realities about alcohol and smoking in chronic pancreatitis. Curr Opin Gastroenterol 2018;34:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Korc M, Jeon CY, Edderkaoui M, Pandol SJ, Petrov MS. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2017;31:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pendharkar SA, Mathew J, Zhao J, Windsor JA, Exeter DJ, Petrov MS. Ethnic and geographic variations in the incidence of pancreatitis and post-pancreatitis diabetes mellitus in New Zealand: a nationwide population-based study. N Z Med J 2017;130:55–68. [PubMed] [Google Scholar]
- [48].Xiao AY, Tan ML, Plana MN, Yadav D, Zamora J, Petrov MS. The use of international classification of diseases codes to identify patients with pancreatitis: a systematic review and meta-analysis of diagnostic accuracy studies. Clin Transl Gastroenterol 2018;9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cervantes A, Waymouth EK, Petrov MS. African-Americans and indigenous peoples have increased burden of diseases of the exocrine pancreas: a systematic review and meta-analysis. Dig Dis Sci 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [50].Rakoski MO, McCammon RJ, Piette JD, Iwashyna TJ, Marrero JA, Lok AS, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology 2012;55:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pendharkar SA, Mathew J, Petrov MS. Age- and sex-specific prevalence of diabetes associated with diseases of the exocrine pancreas: a population-based study. Dig Liver Dis 2017;49:540–4. [DOI] [PubMed] [Google Scholar]
- [52].Uchida K, Masamune A, Shimosegawa T, Okazaki K. Prevalence of IgG4-related disease in Japan based on nationwide survey in 2009. Int J Rheumatol 2012;2012:358371. [DOI] [PMC free article] [PubMed] [Google Scholar]