Abstract
The main psychedelic component of magic mushrooms is psilocybin, which shows promise as a treatment for depression and other mental disorders. Psychedelic effects are believed to emerge through stimulation of serotonin 2A receptors (5-HT2ARs) by psilocybin’s active metabolite, psilocin. We here report for the first time the relationship between intensity of psychedelic effects, cerebral 5-HT2AR occupancy and plasma levels of psilocin in humans. Eight healthy volunteers underwent positron emission tomography (PET) scans with the 5-HT2AR agonist radioligand [11C]Cimbi-36: one at baseline and one or two additional scans on the same day after a single oral intake of psilocybin (3–30 mg). 5-HT2AR occupancy was calculated as the percent change in cerebral 5-HT2AR binding relative to baseline. Subjective psychedelic intensity and plasma psilocin levels were measured during the scans. Relations between subjective intensity, 5-HT2AR occupancy, and plasma psilocin levels were modeled using non-linear regression. Psilocybin intake resulted in dose-related 5-HT2AR occupancies up to 72%; plasma psilocin levels and 5-HT2AR occupancy conformed to a single-site binding model. Subjective intensity was correlated with both 5-HT2AR occupancy and psilocin levels as well as questionnaire scores. We report for the first time that intake of psilocybin leads to significant 5-HT2AR occupancy in the human brain, and that both psilocin plasma levels and 5-HT2AR occupancy are closely associated with subjective intensity ratings, strongly supporting that stimulation of 5-HT2AR is a key determinant for the psychedelic experience. Important for clinical studies, psilocin time-concentration curves varied but psilocin levels were closely associated with psychedelic experience.
Subject terms: Medical research, Target validation, Drug development, Emotion
Introduction
Psilocybin is a classic serotonergic psychedelic drug and is the primary psychoactive compound in magic mushrooms [1]. Its effects are in many ways similar to those of LSD and mescaline [2]. Recent clinical trials have shown that psilocybin may be an effective treatment for neuropsychiatric disorders, including treatment-resistant major depressive disorder (MDD) [3], cancer-related anxiety and depression [4, 5], and for addiction to nicotine [6] and alcohol [7]. Thus, psilocybin is an emerging and promising drug for a range of mental disorders where existing drugs have shown shortcomings.
Preclinical findings [8], human blocking studies [9, 10] and preliminary data from a PET study [11] strongly suggest that serotonergic psychedelics exert their psychoactive effects through the serotonin 2A receptor (5-HT2AR). However, 5-HT2AR target engagement of psilocybin’s active metabolite, psilocin, as well as the pharmacodynamics, i.e., the relation between plasma psilocin levels and 5-HT2AR occupancy, still remain to be established. Importantly, the relationship between the subjective psychedelic experience, plasma psilocin levels and 5-HT2AR occupancy in the human brain is currently unknown.
Positron emission tomography (PET) is an imaging technique capable of quantifying receptor binding in vivo [12, 13]. Coupled with drug administration and appropriate radiotracer selection, PET-studies can provide valuable knowledge about relationships between drug levels, drug target occupancy, and associations with clinical response or side-effects [14]. In the present study we took advantage of the recent development of a 5-HT2R agonist radioligand, [11C]Cimbi-36 [15, 16], to elucidate the direct role of 5-HT2ARs in psilocybin’s psychedelic effects in humans. Here, we for the first time describe the relationships between subjective psychedelic effects, 5-HT2AR occupancy and psilocin plasma concentrations.
Methods and materials
Participants
Eight healthy participants (three females, mean age ± SD 33.0 ± 7.1 years) were recruited from a database of individuals interested in participating in a human neuroimaging study investigating psilocybin. After providing written informed consent, participants underwent a screening procedure including screening for present or previous psychiatric disorders using Mini-International Neuropsychiatric Interview, Danish translation version 6.0.0 [17], neurological illness or significant somatic illness. Participants were healthy, see Supplementary data for complete exclusion criteria and individual participant descriptive data. History of serotonergic psychedelic drug use was noted for the five subjects with such experience (number of times used: 1 [0–55] (median [range]), time since last intake: 42 [6–156] months; Supplementary data, Table 1). Participants were thoroughly informed about the study prior to inclusion, including effects of psilocybin, potential side-effects, and risks. On the day of information and screening (prior to intervention day), all participants attended a preparatory meeting with at least one of the psychologists present on intervention days to familiarize with the study setting and establish a rapport. The study was approved by the ethics committee for the capital region of Copenhagen (journal identifier: H-16028698, amendments: 56023, 56967, 57974, 59673, 60437, 62255) and Danish Medicines Agency (EudraCT identifier: 2016-004000-61, amendments: 2017014166, 2017082837, 2018023295).
Procedures
Participants underwent a physical exam, including ECG, blood screening for pathology, and a screening for psychopathology. Participants completed baseline [11C]Cimbi-36 PET (PET 0) and MR imaging prior to the psilocybin intervention day (mean ± SD: 49 ± 12 days). A screening procedure for amphetamines, opioids, benzodiazepines, barbiturates, tetrahydrocannabinol, cocaine, ketamine, phencyclidine, and gamma hydroxybutyrate was done using a urine test (Rapid Response, BTNX Inc., Markham, Canada). Participants were asked to be well-rested, refrain from alcohol the day before neuroimaging, have only a light breakfast and abstain from caffeine on study days. On the intervention day and before psilocybin administration, participants were informed again about potential psilocybin effects and safety precautions, as suggested previously [18]. Two psychologists providing interpersonal support were present on intervention days. During all PET scans (including baseline), a standardized list of music was played on a stereo system in the PET room. The playlist was adapted from one kindly provided by Prof. Roland Griffiths, Johns Hopkins Medicine.
Psilocybin interventions
On the intervention day, participants ingested between 3 and 30 mg psilocybin (3 mg capsules) approximately one hour prior (mean ± SD: 58 min ± 13) to the first [11C]Cimbi-36 post-drug PET scan (PET 1). Subjects 1–5 underwent a second post-drug PET scan (PET 2) later the same day (344 min ± 41 after psilocybin ingestion), while subjects 6, 7, and 8 underwent only PET 1 on the intervention day. Participants were blind to the dose of psilocybin they were given. Each scan lasted 120 min, descriptive data pertaining to PET scans are available in supplementary data (Supplementary Table 2). For assessment of plasma psilocin levels, venous blood samples were taken simultaneously with the [11C]Cimbi-36 injection and at 20-min intervals throughout each scan session. Subjective psychedelic intensity ratings (0–10 Likert scale, 0 = not intense at all, 10 = very intense) were assessed at 20-min intervals throughout the day until effects had waned. Between the two intervention scans, participants listened to music in the scanner room with staff support as appropriate. This three-scan protocol enabled the determination of 5-HT2AR occupancy during high and low plasma psilocin levels in five individuals. At the end of the intervention day (mean ± SD: 468 ± 80 min after psilocybin), participants filled out questionnaires capturing aspects of psychedelic experiences: 11-dimension altered states of consciousness questionnaire (11D-ASC) [19, 20], the 30-item mystical experiences questionnaire (MEQ30) [21] and the ego-dissolution inventory (EDI) [22]. All questionnaires were administered in Danish, having been translated and back-translated to English by native Danish, English, and bilingual speakers.
Psilocin plasma concentrations
Plasma psilocin concentrations were determined using ultra performance liquid chromatography and tandem mass spectrometry. Analysis was performed in units of μg/kg, although data are here presented in units of μg/L. For detailed description of analysis, see supplementary data.
Magnetic resonance imaging
High resolution 3D T1-weighted and T2-weighted images were acquired on a 3T Prisma scanner (Siemens, Erlangen, Germany) using a 64-channel head coil for the purpose of PET-image coregistration and segmentation (T1-weighted images: inversion time = 900 ms, echo time = 2.58 ms, repetition time = 1900ms, flip angle = 9°, in-plane matrix = 256 × 256, in-plane resolution = 0.9 × 0.9 mm, 224 slices and a slice thickness of 0.9 mm, no gap; T2-weighted images: echo time = 408 ms, repetition time = 3200 ms, in-plane matrix = 256 × 256, in-plane resolution = 0.9 × 0.9 mm, 208 slices and a slice thickness of 0.9 mm, no gap).
[11C]Cimbi-36 PET data acquisition, processing, and kinetic modeling
Acquisition and processing of [11C]Cimbi-36 PET data has been described previously [15, 16], a similar pipeline was used here. PET images were acquired for 120-min on a high-resolution research tomography PET-scanner (CTI/Siemens, Knoxville, USA) after a bolus injection of [11C]Cimbi-36 (Supplementary data, Table 2). Regions of interest were defined using Pvelab, a fully automated regional delineation procedure, and regional time-activity curves were extracted for kinetic modeling [15, 23].
Kinetic modeling was performed using the simplified reference tissue model (SRTM) [13, 15] with neocortex (a volume-weighted average of all cortical regions) chosen a priori as the region of interest due to the high expression of 5-HT2ARs and the consequent beneficial signal-to-noise ratio within this region [24]. Cerebellum was chosen as the reference region [15]. Non-displaceable binding potential (BPND) was the primary outcome measure [12].
[11C]Cimbi-36 metabolism
Analysis of [11C]Cimbi-36 radiometabolites was described in recent publications by our lab [15, 25]. We did not observe effects of the psilocybin intervention on [11C]Cimbi-36 radiometabolism or protein binding (see Supplementary data for details).
Data analysis
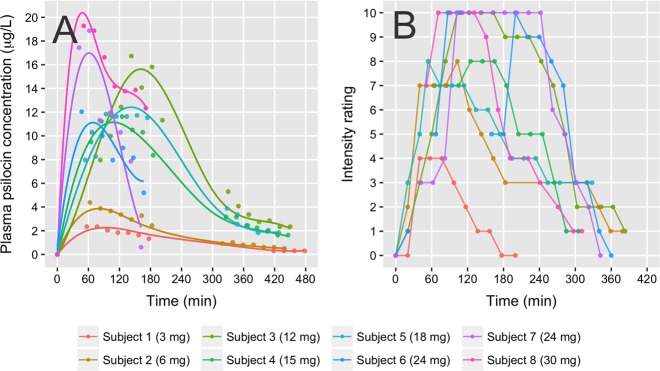
Within-scan plasma psilocin area under curve (psilocinAUC) was calculated from psilocin plasma concentration time curves (Fig. 1), using the trapezoid method in GraphPad Prism (version 7.01, GraphPad Software, Inc., CA, USA) and normalized by 120 min (duration of blood sampling and PET scan) to yield a mean psilocin concentration, which was used for statistical analyses and figures (Table 1).
Fig. 1.
Psilocin and intensity rating time course. a Plasma psilocin levels. Individual data points are measured plasma psilocin concentrations, fitted with spline fits. b Time course of subjective intensity ratings. Time = 0 indicates time of psilocybin ingestion
Table 1.
Descriptive data related to psilocybin interventions and corresponding 5-HT2AR occupancy estimates
| ID | Dose (mg) | Weight-adjusted dose (mg/kg) | Cmax (μg/L) | Mean psilocin PET 1 (μg/L) | Mean psilocin PET 2 (μg/L) | Occupancy PET 1 (%) | Occupancy PET 2 (%) |
|---|---|---|---|---|---|---|---|
| Subject 1 | 3 | 0.05 | 2.3 | 1.9 | <LOQ* | 42.9 | 1.8 |
| Subject 2 | 6 | 0.07 | 4.4 | 3.5 | 0.7 | 56.2 | 26.7 |
| Subject 3 | 12 | 0.14 | 16.7 | 12.6 | 3.4 | 66.4 | 42.9 |
| Subject 4 | 15 | 0.2 | 11.7 | 10.5 | 2.3 | 63.2 | 30.9 |
| Subject 5 | 18 | 0.2 | 11.8 | 10.6 | 2.6 | 72.4 | 47.0 |
| Subject 6 | 24 | 0.27 | 12.0 | 9.0 | NA | 60 | NA |
| Subject 7 | 24 | 0.3 | 18.9 | 11.5 | NA | 66 | NA |
| Subject 8 | 30 | 0.3 | 19.3 | 15.6 | NA | 65.2 | NA |
*Below level of quantification
Neocortical [11C]Cimbi-36 BPND was plotted against mean psilocin concentration and the relationship modeled using the following equation:
where Occmax denotes the predicted highest attainable occupancy, CP is plasma psilocin concentration and EC50 is the plasma psilocin concentration at 50% Occmax [26] Modeling and curve fitting was performed in GraphPad Prism.
Subject 1 psilocin concentrations were below limit of quantification (LOQ, 0.5 µg/kg) but above limit of detection (LOD, 0.1 µg/kg) during all second scan time points. We evaluated psilocin-occupancy relations considering LOQ and LOD. Model parameters were similar (Occmax = 75.5% vs. 77.9%, EC50 = 1.81 μg/L vs. 2.12 μg/L, respectively). Due to the minor difference in outcomes, we set plasma psilocin concentrations for all time points to the mean value (0.3 μg/kg).
We calculated the EC50 [27] corresponding to PET 1 and PET 2 for each participant (mean EC50 ± SD: PET 1 = 4.5 ± 1.9 μg/L, PET 2 = 6.2 ± 6.0 μg/L). The determined EC50 did not differ between the two intervention scans (paired t-test, mean difference = −1.7, 95%CI [−10.2, 6.7], p = 0.6).
All statistical tests apart from non-linear modeling were performed in the statistical software package R (version 3.3.1).
We chose to assess associations between occupancy, plasma psilocin levels and subjective intensity ratings because the latter single, compound measure of drug-intensity was acquired simultaneously with PET 1 and PET 2, which was not the case with the MEQ-30, 11D-ASC and EDI questionnaires. Intensity ratings have previously been used in psychedelics research [28]. The questionnaires were not obtained until the end of the last scan session as we did not want to induce suggestive experiences by such a detailed questionnaire. Further, we believed that the intensity ratings would (1) be less sensitive to non-pharmacological modulators of psilocybin-induced altered states of consciousness (i.e., the context in which the drug is administrated [29]), (2) be feasible to administer during scans, and (3) yield a better temporal resolution. Intensity rating was stopped before the end of PET 2 for all participants (n = 5). Thus, for the purpose of calculating mean within-PET 2 intensity, participants were asked if intensity had changed during PET 2 compared to the last recorded rating. All participants responded that intensity had not changed during PET 2, and thus the last recorded score was extrapolated and used to calculate mean PET 2 intensity. For the purpose of modeling the association between occupancy and intensity, a quadratic function was used ), and for the purpose of modeling the association between psilocin levels and intensity, a non-linear stimulus-response function similar to the occupancy model was used: . 95% Wald-type confidence intervals were computed for β1 and β2 using quantiles of the Student’s t-distribution.
Post-hoc linear regression analyses of the association between mean PET 1 intensity ratings and three questionnaire responses (MEQ30, 11-D ASC, EDI) were performed. Our main hypothesis was that the outcome of the questionnaires would correlate with intensity ratings during PET 1. For these analyses, we report the unadjusted (punc.) and Bonferroni-adjusted (pFWE) p-values. Further exploratory post hoc linear regression analyses are available in Supplementary data. The coefficient of determination (R2) is reported as a measure of data variance explained by the respective model.
Voxel-level [11C]Cimbi-36 BPND maps were estimated using the PETSurfer tool within Freesurfer [30] as described previously [24] and used for visualization purposes only.
Results
Psilocin occupancy at neocortical 5-HT2ARs
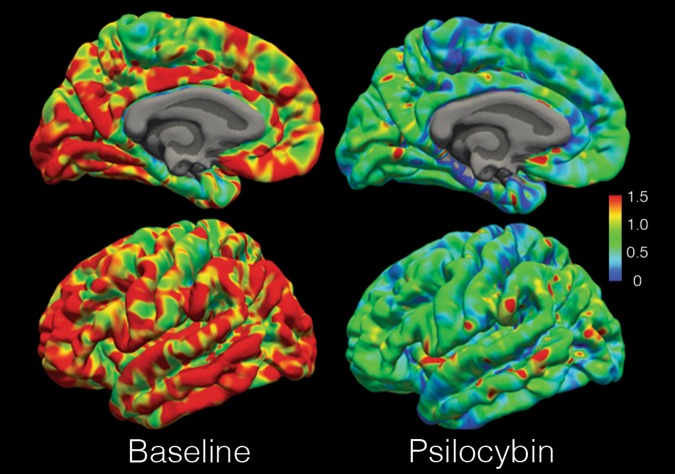
Psilocybin intake was in all PET scans associated with considerable dose-related 5-HT2AR occupancies (PET 1 range 43–72%). Occupancies at PET 2 were also substantial (range 27–47%) with the exception of Subject 1 for which occupancy was 2% (Table 1; Fig. 2).
Fig. 2.
Psilocybin occupancy of 5-HT2AR. [11C]Cimbi-36 BPND map of the cortical surface of the left hemisphere of Subject 5 at baseline and at the first post-psilocybin intervention scan. Color bar in units BPND
Psilocin levels and receptor occupancy relations
We found a high inter-individual variability in the dose response curves (e.g., maximum concentration (Cmax) median [range]: 11.9 [2.3–19.3] μg/L) Fig. 1). The relation between plasma psilocin levels and neocortex 5-HT2AR occupancy conformed well to the non-linear regression model. Occmax [95% CI] determined from this model was 76.6 [67.3; 88.0]%, EC50 [95% CI] was 1.95 [1.17; 3.15] μg/L, and R2 was 0.92 (Fig. 3).
Fig. 3.
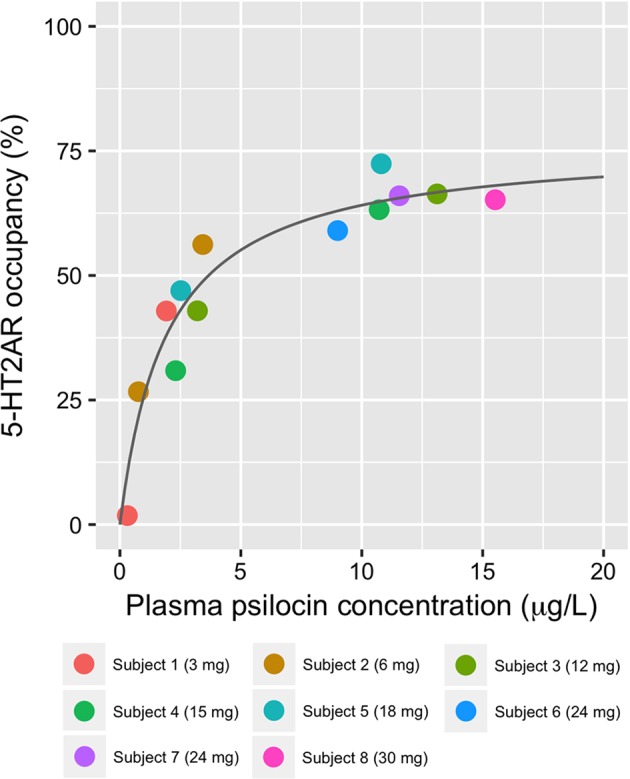
Relationship between mean within-scan plasma psilocin levels and neocortical 5-HT2AR occupancy. Estimated EC50 [95% CI]: 1.95 [1.16; 3.15] μg/L and Occmax [95% CI]: 76.6 [67.3; 88.0]%
Subjective intensity ratings correlate with occupancy and psilocin levels
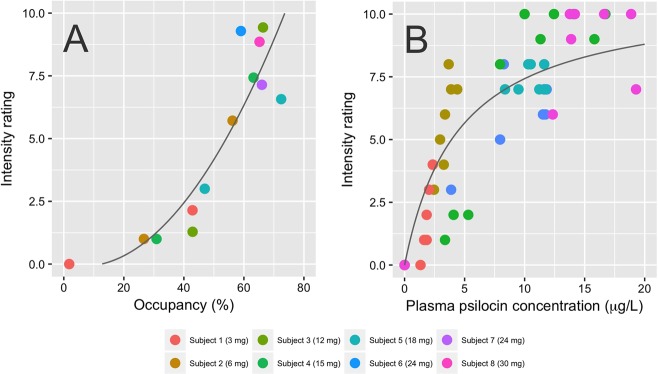
Subjective intensity ratings had a qualitatively similar time course compared to plasma psilocin levels (Fig. 1). We found a positive nonlinear association between mean within-scan intensity ratings and psilocin levels. Intensitymax [95% CI] was 10.8 [8.6; 14.7] and EC50 [95% CI] was 4.5 [2.1; 9.8] μg/L, and R2 was 0.35 (Fig. 4). We also observed a positive association between intensity ratings and occupancy that was well described by a quadratic relationship (β1 [95% CI]: −0.02 [−0.13; 0.1], β2 [95% CI]: 0.002 [0.0006; 0.003], R2: 0.81, Fig. 4).
Fig. 4.
Subjective intensity of the psychedelic experience at the time of the PET scan, neocortical 5-HT2AR occupancy and plasma psilocin concentration. a Relationship between intensity ratings and neocortical 5-HT2AR occupancy. The fitted line was obtained using a quadratic function. b Relationship between intensity and psilocin concentration, fitted to a single site receptor binding model
Psychedelic questionnaire responses
As expected, psilocybin had profound effects on the mental state of the participants (MEQ30 total score median [range]: 2.9 [1.6–4.5], 11D-ASC global score (sum of all dimensions) median [range]: 428.1 [35.1–772.1], EDI median [range]: 52 [4.0–97.9]) (see Figs. S1–2 and Table S3 for detailed responses). Post hoc linear regressions showed positive associations between mean PET 1 intensity ratings and total MEQ30 score (β-estimate [95% CI]: 0.34 [0.044; 0.64], punc. = 0.03, pFWE = 0.09, R2: 0.57), global 11-D ASC score (β-estimate [95% CI]: 76.4 [27.8; 125], punc. = 0.008, pFWE = 0.024, R2: 0.71) and EDI score (β-estimate [95% CI]: 11.1 [2.23; 20], punc. = 0.02, pFWE = 0.06, R2: 0.61). For further information, see Figure S3.
Discussion
We here show that psilocybin ingestion of between 3 and 30 mg is associated with dose-dependent occupancy of cerebral 5-HT2ARs. Further, plasma psilocin concentration and 5-HT2AR occupancy are positively associated and the relationship conforms with a single-site binding model. Lastly, subjective intensity ratings are positively correlated with both neocortical 5-HT2AR occupancy and plasma psilocin levels, strongly supporting that stimulation of cerebral 5-HT2ARs is paramount for the psychedelic effects of psilocybin.
Similar to previous 5-HT2AR PET-imaging occupancy studies with other 5-HT2AR drugs [31, 32], we found that the single-site binding model provided a good fit of the relation between drug blood levels and 5-HT2AR occupancy, and predicted maximum occupancies were similar. Here, it is important to emphasize that the occupancies detected with an agonist radioligand (such as [11C]Cimbi-36) may differ from that of antagonist radioligands because an agonist radioligand may bind preferentially to receptors in the high-affinity state [33, 34]. Thus, given that high-affinity receptors are believed to be most important for neurotransmission, an agonist radioligand may yield a more relevant estimate of receptor levels.
We found the EC50 of psilocin to be 1.95 μg/L. This corresponds to 10 nM, which is in the same range of Ki values from in vitro studies (rat cortex) performed with another 5-HT2AR agonist, [125I]DOI: 6 nM [35] or 25 nM [36].
The post hoc linear regressions showed positive associations between mean PET 1 intensity ratings and MEQ30, global 11-D ASC score, and EDI score, and intensity ratings correlated also with both occupancy and with psilocin levels (Fig. 4). Thus, although the participants scored their overall intensity of the psychedelic experience based on a number of different components (e.g., imagery, changes in perception, stimulation of mood, feeling of enhanced meaning, somatic sensations, etc.), and probably also as a function of previous drug experience and psychological make-up (“set”), including personal coping style, our results show that intensity ratings constitute a meaningful global measure of psychedelic experience that is feasible to obtain with high temporal resolution.
Previous studies in humans reported that antagonists at 5-HT2A and 2C receptors can prevent perceptual effects after subsequent ingestion of psilocybin [9, 10]. Our data show that psilocin plasma levels correlate with occupancy (Fig. 3), that psilocin levels and occupancy correlate with intensity (Fig. 4), and that intensity correlates with scores of MEQ30, 11D-ASC and EDI. Thus, our findings strongly support that 5-HT2AR stimulation is central for psychedelic experiences in humans, and adding our findings to the existing literature, the evidence is by now strong that the 5-HT2AR is indeed the critical molecular mediator of psychedelic effects of psilocybin.
Our model can in future studies assist to estimate psilocin brain 5-HT2AR receptor occupancy without the use of PET-imaging, by determining plasma psilocin levels. For example, Brown and colleagues recently reported that ingestion of 25 mg psilocybin results in a mean Cmax of about 15 ng/mL [37]. Assuming analysis methods of similar quality, similar stability of psilocin samples and a plasma density of 1.02 g/mL [38], this plasma psilocin level corresponds to 69% occupancy. There is considerable inter-individual variability in psilocybin pharmacokinetics [37, 39, 40]. Consistent with this, Cmax for Subject 3 (12 mg, 0.14 mg/kg) was higher than Cmax values for Subjects 4, 5, and 6 (15, 18, and 24 mg, respectively; 0.2, 0.2, and 0.3 mg/kg). Importantly, our data convincingly demonstrate that plasma psilocin levels correlate closely with the overall psychedelic experience, and it is possible that future clinical trials may benefit from relating psilocin levels and/or estimated occupancies to clinical effects, rather than absolute doses.
Recently, it has been argued that psychedelic “microdosing”, entailing a dose small enough to avoid noticeable perceptual effects [41], comes with benefits such as enhanced creativity, social interaction and mood. Although a dose range of 0.5–2 mg psilocybin has been suggested as a psilocybin microdose (Dr. James Fadiman, Institute of Transpersonal Psychology, personal communication), there are currently no data available to identify such a cut-off. Subject 1 received 3 mg (0.05 mg/kg), had noticeable perceptual effects and an occupancy of 43%. This indicates that a smaller dose/lower occupancy would be needed for microdosing studies. Based on our data, a dose range of 0.5–2 mg is a reasonable suggestion for potential psilocybin microdose studies.
A few limitations of the study should be noted. When fitted to a single-site binding model without constraining Occmax = 100%, we found Occmax = 77%. Possible explanations for this include violations of kinetic modeling assumptions [13, 42], rapid internalization of 5-HT2AR or psilocybin-associated lowering of brain 5-HT levels. Although weaker than for 5-HT2AR, psilocin has also affinity to 5-HT 2B, 5-HT 2C, and 5-HT 1A receptors [36, 43]; the affinity for the serotonin transporter (SERT) is about 100 times lower [43]. A net decrease in cerebral 5-HT levels due to psilocin agonist activity at 5-HT1A autoreceptors could lead to an underestimation of occupancy due to decreased competition at 5-HT2ARs during intervention scans [44]. In vitro studies reported that 5-HT2AR stimulation led to 5-HT2AR internalization [45–48]. We cannot exclude that [11C]- Cimbi-36, being an agonist radioligand, has different affinity to internalized 5-HT2AR, leading to an underestimation of occupancy. We did not observe a difference between EC50 values of PET 1 and 2, suggesting that if internalization occurred, it occurred either very rapidly (within a few minutes) or very slowly (days after). For Subject 1 who received only 3 mg, occupancy was 43% at PET 1 and 2% at PET 2, speaking against 5-HT2AR internalization. Nevertheless, it would be interesting to investigate long-term effects of a single psilocybin dose on cerebral 5-HT2AR levels, as a potential molecular mediator of the long-term effects on personality and mood [3–5, 49]. Such a study is currently ongoing in our lab.
We did not observe statistically significant median head motion during PET 1 or PET 2 compared to baseline scans (Supplementary Methods and Materials). Participants 7 and 8 exhibited maximum motion of up to 35 and 20 mm during PET 1, respectively. Although this could affect the kinetic modeling, model fits were acceptable and comparable to baseline scans. Our conclusions are based on only eight participants, but five were investigated three times which generated two occupancy measures for each of these participants. The majority of male participants, that participants were recruited as specifically interested in a neuroimaging study investigating psilocybin, and the narrow age range decreases generalizability of our findings to the extent there are sex-dependent or age-dependent differences in psilocybin [50] or radioligand kinetics and differences in psilocin levels, occupancy or intensity ratings as a function of propensity to seek study participation in a psychedelics research study. PET-environment was positively correlated with anxiety during a psilocybin intervention [51] and we cannot exclude that the PET-environment influenced the psychedelic experience [29], making experiences less comparable to therapeutic or naturalistic settings. Yet, our participants experienced anxiety only to a very limited extent (11-D ASC anxiety subscale (median [range]: 4.25 [0; 17.3]). The study was not placebo-controlled and it is possible that this may have ultimately affected intensity ratings. Also, we cannot rule out that metabolites of psilocin or expectation-induced changes in 5-HT levels could affect the occupancy estimates, although we are unaware of evidence suggesting this.
In summary, we find that in humans, psychedelic effects of psilocybin are closely correlated with psilocin stimulation of the 5-HT2AR, and our data allows for an objective assessment of psilocybin effects on 5-HT2AR in future studies, by measuring plasma psilocin levels.
Funding and disclosure
The study was supported by Innovation Fund Denmark (grant ID 4108-00004B), Independent Research Fund Denmark (grant ID 6110-00518B), and Ester M. og Konrad Kristian Sigurdssons Dyreværnsfond (grant ID 850-22-55166-17-LNG). M.K.M. was supported through a scholarship stipend from Rigshospitalet’s Research Council (grant ID R130-A5324). D.B. was supported by a scholarship stipend from the Lundbeck Foundation. B.O. was supported by the Lundbeck foundation (grant ID R231-2016-3236) and Marie-Curie-NEUROMODEL (Grant ID 746850).
Supplementary information
Acknowledgements
We gratefully acknowledge the assistance of Lone Freyr, Gerda Thomsen, Svitlana Olsen, Josephine Torp, Annette Johansen, Camilla Larsen, Hanne Hansen, Vibeke Dam, Simone Pleinert, Sophia Armand, Dorthe Givard, and Peter Jensen. The John and Birthe Meyer Foundation is gratefully acknowledged for the donation of the Cyclotron and PET-scanner. We also gratefully acknowledge the University of Chemistry and Technology and the National Institute of Mental Health (Prague, Czech Republic) for production of psilocybin and Glostrup Apotek (Glostrup, Denmark) for encapsulation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/8/2019
The original version of this article contained an error in the labelling of Figures 2 and 3. While the captions and figures themselves are correct, in order to correspond with the in-text references, they have now been re-numbered in both the PDF and HTML versions of the article.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-019-0324-9).
References
- 1.Hofmann A, Heim R, Brack A, Kobel H. Psilocybin, ein psychotroper Wirkstoff aus dem mexikanischen Rauschpilz. Experientia. 1958;14:107–9. doi: 10.1007/BF02159243. [DOI] [PubMed] [Google Scholar]
- 2.Wolbach AB, Miner EJ, Isbell H. Comparison of psilocin with psilocybin, mescaline and LSD-25. Psychopharmacologia. 1962;3:219–23. doi: 10.1007/BF00412109. [DOI] [PubMed] [Google Scholar]
- 3.Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016;0366:11–13. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30:1181–97. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30:1165–80. doi: 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson Matthew W, Garcia-Romeu Albert, Cosimano Mary P, Griffiths Roland R. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of Psychopharmacology. 2014;28(11):983–992. doi: 10.1177/0269881114548296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa P, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29:289–99. doi: 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- 8.González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012;72:898–906. doi: 10.1016/j.biopsych.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 11.Quednow BB, Geyer M a, Halberstadt AL (Elsevier B.V.: 2010). Serotonin and Schizophrenia. Handb Behav Neurobiol Serotonin 21:1–19.
- 12.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 13.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 14.Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Differential effects of aripiprazole on D 2, 5-HT 2, and 5-HT 1A receptor occupancy in patients with schizophrenia: A Triple Tracer PET Study. Am J Psychiatry. 2007;164:1411–7. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- 15.Ettrup A, Cunha-Bang S, da, McMahon B, Lehel S, Dyssegaard A, Skibsted AW, et al. Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36. J Cereb Blood Flow Metab. 2014;34:1188–96. doi: 10.1038/jcbfm.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ettrup A, Svarer C, McMahon B, Cunha-Bang S, da, Lehel S, Møller K, et al. Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36: test–retest reproducibility and head-to-head comparison with the antagonist [18F]altanserin. Neuroimage. 2016;130:167–74. doi: 10.1016/j.neuroimage.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 18.Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22:603–20. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dittrich A, Lamparter D, Maurer M. 5D-ABZ: Fragebogen zur Erfassung Aussergewöhnlicher Bewusstseinszustände. Eine kurze Einführung. Zürich: PSIN Plus Publications; 2006. [Google Scholar]
- 20.Studerus Erich, Gamma Alex, Vollenweider Franz X. Psychometric Evaluation of the Altered States of Consciousness Rating Scale (OAV) PLoS ONE. 2010;5(8):e12412. doi: 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett Frederick S, Johnson Matthew W, Griffiths Roland R. Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. Journal of Psychopharmacology. 2015;29(11):1182–1190. doi: 10.1177/0269881115609019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nour MM, Evans L, Nutt D, Carhart-Harris RL (2016). Ego-dissolution and psychedelics: validation of the ego-dissolution inventory (EDI). Front Hum Neurosci. 2016;10:1–13. [DOI] [PMC free article] [PubMed]
- 23.Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbøl S, Frøkjaer VG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–79. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Beliveau V, Ganz M, Feng L, Ozenne B, Højgaard L, Fisher PM, et al. A high-resolution in vivo atlas of the human brain’s serotonin system. J Neurosci. 2017;37:120–8. doi: 10.1523/JNEUROSCI.2830-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen Annette, Hansen Hanne D, Svarer Claus, Lehel Szabolcs, Leth-Petersen Sebastian, Kristensen Jesper L, Gillings Nic, Knudsen Gitte M. The importance of small polar radiometabolites in molecular neuroimaging: A PET study with [11C]Cimbi-36 labeled in two positions. Journal of Cerebral Blood Flow & Metabolism. 2017;38(4):659–668. doi: 10.1177/0271678X17746179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunn RN, Rabiner EA. Imaging in central nervous system drug discovery. Semin Nucl Med. 2017;47:89–98. doi: 10.1053/j.semnuclmed.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Kenakin T. The mass action equation in pharmacology. Br J Clin Pharmacol. 2016;81:41–51. doi: 10.1111/bcp.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carhart-Harris RL, Erritzoe D, Williams TM, Stone JM, Reed LJ, Colasanti A, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA. 2012;109:2138–43. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carhart-Harris Robin L, Roseman Leor, Haijen Eline, Erritzoe David, Watts Rosalind, Branchi Igor, Kaelen Mendel. Psychedelics and the essential importance of context. Journal of Psychopharmacology. 2018;32(7):725–731. doi: 10.1177/0269881118754710. [DOI] [PubMed] [Google Scholar]
- 30.Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, et al. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage. 2014;92:225–36. doi: 10.1016/j.neuroimage.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gründer MDG, Grunder G, Yokoi F, Offord SJ, Ravert HT, Dannals RF, et al. Time course of 5-HT2A receptor occupancy in the human brain after a single oral dose of the putative antipsychotic drug MDL 100,907 measured by positron emission tomography. Neuropsychopharmacol. 1997;17:175–85. doi: 10.1016/S0893-133X(97)00044-4. [DOI] [PubMed] [Google Scholar]
- 32.Nordstrom AL, Mansson M, Jovanovic H, Karlsson P, Halldin C, Farde L, et al. PET analysis of the 5-HT2A receptor inverse agonist ACP-103 in human brain. Int J Neuropsychopharmacol. 2008;11:163–71. doi: 10.1017/S1461145707007869. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald LW, Conklin DS, Krause CM, Marshall AP, Patterson JP, Tran DP, et al. High-affinity agonist binding correlates with efficacy (intrinsic activity) at the human serotonin 5-HT2A and 5-HT2C receptors: evidence favoring the ternary complex and two-state models of agonist action. J Neurochem. 1999;72:2127–34. doi: 10.1046/j.1471-4159.1999.0722127.x. [DOI] [PubMed] [Google Scholar]
- 34.López-Giménez JF, Villazón M, Brea J, Loza MI, Palacios JM, Mengod G, et al. Multiple conformations of native and recombinant human 5-hydroxytryptamine(2a) receptors are labeled by agonists and discriminated by antagonists. Mol Pharmacol. 2001;60:690–9. [PubMed] [Google Scholar]
- 35.McKenna DJ, Repke DB, Lo L, Peroutka SJ. Differential interactions of indolealkylamines with 5- hydroxytryptamine receptor subtypes. Neuropharmacology. 1990;29:193–198. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- 36.Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Gumbay MG, Watts VJ, Barker EL, et al. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem. 2000;43:4701–10. doi: 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- 37.Brown Randall T., Nicholas Christopher R., Cozzi Nicholas V., Gassman Michele C., Cooper Karen M., Muller Daniel, Thomas Chantelle D., Hetzel Scott J., Henriquez Kelsey M., Ribaudo Alexandra S., Hutson Paul R. Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults. Clinical Pharmacokinetics. 2017;56(12):1543–1554. doi: 10.1007/s40262-017-0540-6. [DOI] [PubMed] [Google Scholar]
- 38.Trudnowski RJ, Rico RC (1974). Specific gravity of blood and plasma at 4 and 37 °C. Clin Chem. 1974;20:615–6. [PubMed]
- 39.Hasler F, Bourquin D, Brenneisen R, Bär T, Vollenweider FX. Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm Acta Helv. 1997;72:175–84. doi: 10.1016/S0031-6865(97)00014-9. [DOI] [PubMed] [Google Scholar]
- 40.Lindenblatt H, Krämer E, Holzmann-Erens P, Gouzoulis-Mayfrank E, Kovar K. Quantitation of psilocin in human plasma by high-performance liquid chromatography and electrochemical detection: comparison of liquid–liquid extraction with automated on-line solid-phase extraction. J Chromatogr B Biomed Sci Appl. 1998;709:255–63. doi: 10.1016/S0378-4347(98)00067-X. [DOI] [PubMed] [Google Scholar]
- 41.Fadiman J, Korb S. microdosingpsychedelics.com. 2017; https://sites.google.com/view/microdosingpsychedelics/home.
- 42.Salinas CA, Searle GE, Gunn RN. The simplified reference tissue model: model assumption violations and their impact on binding potential. J Cereb Blood Flow Metab. 2015;35:304–11. doi: 10.1038/jcbfm.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rickli A, Moning OD, Hoener MC, Liechti ME. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol. 2016;26:1327–37. doi: 10.1016/j.euroneuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Jørgensen Louise M, Weikop Pia, Villadsen Jonas, Visnapuu Tanel, Ettrup Anders, Hansen Hanne D, Baandrup Anders O, Andersen Flemming L, Bjarkam Carsten R, Thomsen Carsten, Jespersen Bo, Knudsen Gitte M. Cerebral 5-HT release correlates with [11C]Cimbi36 PET measures of 5-HT2A receptor occupancy in the pig brain. Journal of Cerebral Blood Flow & Metabolism. 2016;37(2):425–434. doi: 10.1177/0271678X16629483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karaki S, Becamel C, Murat S, Mannoury la Cour C, Millan MJ, Prézeau L, et al. Quantitative phosphoproteomics unravels biased phosphorylation of serotonin 2A receptor at Ser 280 by hallucinogenic versus nonhallucinogenic agonists. Mol Cell Proteom. 2014;13:1273–85. doi: 10.1074/mcp.M113.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckholtz N, Zhou D, Freedman D, Potter W. Lysergic acid diethylamide (LSD) administration selectively downregulates serotonin2 receptors in rat brain. Neuropsychopharmacology. 1990;3:137–48. [PubMed] [Google Scholar]
- 47.Buckholtz Neil S., Zhou Dongfeng, Freedman Daniel X. Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sciences. 1988;42(24):2439–2445. doi: 10.1016/0024-3205(88)90342-6. [DOI] [PubMed] [Google Scholar]
- 48.Buckholtz NS, Freedman DX, Middaugh LD. Daily lsd administration selectively decreases serotonin2 receptor binding in rat brain. Eur J Pharmacol. 1985;109:421–5. doi: 10.1016/0014-2999(85)90407-8. [DOI] [PubMed] [Google Scholar]
- 49.MacLean Katherine A, Johnson Matthew W, Griffiths Roland R. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. Journal of Psychopharmacology. 2011;25(11):1453–1461. doi: 10.1177/0269881111420188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tylš F, Páleníček T, Kadeřábek L, Lipski M, Kubešová A, Horáček J. Sex differences and serotonergic mechanisms in the behavioural effects of psilocin. Behav Pharmacol. 2016;27:309–20. doi: 10.1097/FBP.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 51.Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of psilocybin response in healthy volunteers. PLoS One. 2012;7:e30800. doi: 10.1371/journal.pone.0030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.