Abstract
Background:
Deficits in fluent language production are a hallmark of aphasia and may arise from impairments at different levels in the language system. It has been proposed that difficulty resolving lexical competition contributes to fluency deficits.
Aims:
The present study tested this hypothesis in a novel way: by examining whether narrative speech production fluency is associated with difficulty resolving lexical competition in spoken word recognition as measured by sensitivity to phonological neighborhood density.
Methods & Procedures:
Nineteen participants with aphasia and 15 neurologically intact older adults identified spoken words that varied in phonological neighborhood density and were presented in moderate noise.
Outcomes & Results:
Neurologically intact participants exhibited the standard inhibitory effect of phonological neighborhood density on response times: slower recognition of spoken words from denser neighborhoods. Among participants with aphasia, the inhibitory effect of phonological neighborhood density (less accurate recognition of spoken words from denser neighborhoods) was smaller for participants with greater fluency. The neighborhood effect was larger for participants with greater receptive vocabulary knowledge, indicating that the fluency effect was not a result of general lexical deficits.
Conclusions:
These results are consistent with the hypothesis that impaired lexical selection is a contributing factor in fluency deficits in post-stroke aphasia.
Keywords: spoken word recognition, phonological neighborhood density, fluency, lexical selection, aphasia
Introduction
Fluency is central to how we think about language impairment. In aphasia, deficits in fluent language production are characterized by sparse and halting speech that is uttered with great effort and poor articulation and can be independent of deficits in language comprehension (e.g., Geschwind, 1971). Fluency deficits may arise from impairments at different levels in the language production system, from impaired articulatory planning and execution (e.g., speech apraxia or dysarthria) to impaired syntactic or sequencing processes necessary for producing multi-word utterances (e.g., agrammatism or dynamic aphasia; e.g., Robinson, Blair & Cipolotti, 1998; Schwartz, 1987). Importantly, they can also arise from impaired lexical selection if the halting and sparse speech is a result of difficulty selecting specific lexical items for production from among their competitors (e.g., Mirman, Yee, Blumstein & Magnuson, 2011; Novick, Trueswell & Thompson-Schill, 2005; 2010; for reviews of the word retrieval impairment in aphasia and its treatment see Schwartz, Middleton & Hamilton, 2015; Wilshire & Coslett, 2000). The current study used a case series approach to evaluate whether the fluency deficit in aphasia was associated with a selection deficit that affected both language production and comprehension domains. The study extends beyond the laboratory by combining real-world measures of fluent language production with a fine-grained experimental measure of resolving competition during spoken word recognition (the phonological neighborhood density effect).
Efficient lexical retrieval is at the core of fluent language production (e.g., Sandoval, Gollan, Ferreira & Salmon, 2010) and is dependent upon the successful selection of a single lexical option from among a set of co-activated candidates – critical intermediate steps between conceptual preparation and phonological/articulatory encoding, planning, and execution (e.g., Dell & Chang, 2014; Dell, Schwartz, Martin, Saffran & Gagnon, 1997; Levelt, 1999; Levelt, Roelofs & Meyer, 1999; Roelofs, 1996; 1997; 2003; 2014; Schwartz, 2014). The number of available lexical candidates (i.e., vocabulary size) and selection play complementary roles in fluent language production: vocabulary is the primary requirement of verbal fluency, given that activation is limited to the available lexical candidates, while selection is the critical step in the resolution of lexical competition. Moreover, when vocabulary size is held constant, more skill in resolving lexical competition has been linked to more fluent language production (Bialystok, Craik & Luk, 2008; Luo, Luk & Bialystok, 2010).
Selecting a single lexical option from among a set of activated candidates is not unique to speech production; it is also a critical aspect of word comprehension, despite differences in the primary source of input to lexical processing – semantic for production, phonological for comprehension (see Roelofs, 2003 for a review of models of word production and recognition). Spoken word recognition requires listeners to map phonological representations onto stored lexical candidates. The inherent noisiness, transience, and sequential nature of speech signal makes the input open to multiple different lexical interpretations, producing a constant need for resolution of lexical competition (for recent reviews see Magnuson, Mirman, & Myers, 2013; Mirman, 2016). This process is more difficult when the unfolding speech input partially matches many phonologically similar lexical candidates, a property known as phonological neighborhood density (Luce, 1986). Spoken words with many phonologically-similar words (“neighbors”) are recognized more slowly and less accurately than words with few phonological neighbors (e.g., Luce & Large, 2001; Luce & Pisoni, 1998; Luce, Pisoni & Goldinger, 1990; Magnuson, Dixon, Tanenhaus & Aslin, 2007; Sommers, 1996). Phonological neighborhood density is one of the strongest predictors of ease of spoken word recognition across a variety of participant populations, including children (De Cara & Goswami, 2003), younger and older adults (Botezatu, Landrigan, Chen, & Mirman, 2015; Taler, Aaron, Steinmetz, & Pisoni, 2010), second language speakers (Marian, Blumenfeld, & Boukrina, 2008), and individuals with language and cognitive impairments (Sommers, 1998). Effects of phonological neighborhood density on spoken word recognition are consistent and robust, yet reflect individual differences in the ability to resolve lexical competition (Bartolotti & Marian, 2012; Marian et al., 2008; Sommers, 1998; Taler et al., 2010).
In the present study, we evaluated the hypothesis that difficulty resolving lexical competition contributes to the fluency deficit in aphasia by examining whether variations in fluency in a group of individuals with aphasia were associated with variations in sensitivity to phonological neighborhood density effects in spoken word comprehension. If fluency deficits are entirely due to either sub-lexical (speech motor planning and execution) deficits or supra-lexical (syntactic or word sequencing) deficits, then there should be no association between fluency and lexical selection in spoken word recognition. However, insofar as fluent production involves rapid lexical selection, then there should be an association between the fluency deficit in aphasia and sensitivity to phonological neighborhood effects. Overall, we expected the standard inhibitory effect of phonological neighborhood density: participants with aphasia and neurologically intact older control participants should perform more poorly when recognizing spoken words from high-density phonological neighborhoods compared to words from low-density neighborhoods (Luce, 1986; Luce & Large, 2001; Luce & Pisoni, 1998; Sommers, 1996), either in terms of slower response times or lower accuracy rates. Using a case series design (e.g., Schwartz & Dell, 2010), we made two contrasting predictions regarding how participants with aphasia would vary in sensitivity to neighborhood density: (1) individuals with more fluent production were expected to be better able to resolve lexical competition and therefore exhibit smaller effects of density. In contrast, (2) individuals with a larger receptive vocabulary were expected to have more potential lexical competitors and therefore exhibit larger effects of density. This second prediction follows intuitively from developmental data (e.g., Charles-Luce & Luce, 1990; De Cara & Goswami, 2003), which show that effects of density correlate positively with vocabulary size. This prediction is important for ruling out generic effects of lexical deficits (i.e., generic lexical deficits would produce lower fluency, reduced receptive vocabulary, and poorer recognition of more difficult high-density words).
Method
Participants
Nineteen participants with aphasia secondary to left hemisphere stroke (11 male; mean age = 58.4; age range = 35–76 years) and 15 neurologically-intact older adults (eight male; mean age = 67.9; age range = 61–79 years) were recruited from the Moss Rehabilitation Research Institute (MRRI) Cognitive Rehabilitation Research Registry (Schwartz, Brecher, Whyte, & Klein, 2005) and completed the study for payment. All participants were native speakers of English who passed a hearing test at 25 dBHL or better at 500, 1000, 2000 and 4000 Hz and reported normal or corrected-to-normal vision, and no history of drug abuse. Ethical approval was obtained from the Albert Einstein Healthcare Network Institutional Review Broad. All procedures were carried out in accordance with the approved guidelines and regulations.
Older adults showed no signs of cognitive impairment based on the Mini Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975; mean MMSE score = 28.1, range of MMSE scores: 26–30; MMSE scores >25 are considered to be in the normal range). The group of older adults was tested in order to verify that the materials and procedure used in this study produce the standard inhibitory effect of phonological neighborhood density on spoken word recognition in neurologically intact older adults.
Individuals with aphasia were in the chronic phase (mean time post onset = 6.2 years; range: 1.2 – 13.7 years post onset) and scored below 10 on the standardized fluency measure of the Western Aphasia Battery (WAB; Kertesz, 1982). The fluency measure evaluates the ability to produce spontaneous speech in unstructured (i.e., conversation) and semi-structured (i.e., picture description) contexts. The unstructured contexts required participants to respond to six personal questions (e.g., name, address, occupation, and reason for being in the clinic). In the semi-structured contexts, participants were encouraged to use full sentences to describe a picture depicting a picnic scene. Performance is scored on a scale from 0 (i.e., no words or short, meaningless utterances) to 10 (i.e., sentences of normal length and complexity, without slowing, halting or speech errors). Table A1 in the Appendix summarizes the expressive abilities associated with WAB fluency scores for the participants in this study (2 – 9). The examples provided in Table A1 come from our dataset.
Table A1.
WAB Fluency scores in the participant sample.
| WAB Fluency Score | Characteristics and Examples |
|---|---|
| 2 | Single words, often speech errors, effortful and hesitant. Example: Picnic….oh pi- uh…fruit…book…uh sh—strawberry uh…fishing…uh s- s- sanding…s- uh kiting…uh boating….uh boy…s- radio…that’s it…uh……s- s- strawberries…uh soda…picnic basket…that’s it…uh flag…um s- dog…uh that’s it |
| 4 | Halting, telegraphic speech; mostly single words; speech errors; occasional prepositional phrases; severe word finding difficulty. No more than two complete sentences with the exception of stereotypic (automatic) or cliché sentences. Example: Pictures…uh…reading…uh uh…kite….dog….boy reading…uh…fly a kite…..sentences…I don’t sentences….I wish….flying uh….finshing uh lake….playing…playing….down lake….nice home….tiny car….think girl…I think….don’t know that…trees |
| 5 | Telegraphic, hesitant and effortful speech with some grammatical organization; marked word-finding difficulty. Speech errors may be prominent; few, but more than two propositional sentences. Example: he is- he is r- um…(pause)..…um…(pause)..…he is…(pause)… (pause)…this is- I can’t do that…(pause)… li- like /a—/ kite kite he’s on a, he is on um…(pause)…they are on…(pause)… a boat…I don’t know what they are…(pause)…what she is drinking…(pause)…she is drinking something…(pause)…he is reading a book…(pause)...I can tell what he i::s (stretched out the vowel in “is”) doing…(pause)...dog… [stops] |
| 6 | More propositional sentences with normal syntactic patterns; may have speech errors; significant word-finding difficulty and hesitations may be present. Example: The boy and girl was going in the picnic area for a blanket… (pause)… the boy was reading…(pause)..…the girl was drinking… (pause)..…the flag was flowing…(long pause)…the..(short pause)..boy was going for a kite…(pause)..…the ..( pause)..the sail was… (pause)...cruising down… (pause).…the boy…(pause)….the…(pause)..…house was… (pause)... awesome…(pause)..…the…(pause)..…boy was drinking in the cup… (pause)…the trees were beautiful |
| 8 | Circumlocutory, fluent speech; moderate word-finding difficulty; with or without speech errors; may have semantic jargon. The sentences are often complete, but may be irrelevant. Example: Seems to be a pink, a pink, um um it’s a new, n-, uh, someone is living nearby. They have trees, um a flag, there is saving a fish, um there’s a uh dog, it has a um k- /kflnt/, I can’t say it well, um person the girl is is making a w-, next to the water, a beach, not sure…they have music, a basket, some kind of liquid, um um under sitting um- it’s um a cloth or something. That’s phones that’s what I was saying, um it’s a walk to the car |
| 9 | Mostly complete, relevant sentences; occasional hesitations and speech errors; some word-finding difficulty; near normal, but still perceptibly aphasic. Example: Ok. There are…a man and a woman. Sitting in a…sitting outside and having a picnic...and he’s playing a book, he’s reading a book…and she’s /r-/ and she’s drinking some…something to drink. And…they’re sitting outside…in this in this area that has a bunch of...grass and...stuff outside and there’s a man outside and he’s /f-/ doing a kite. And...he’s he’s got his kite out there, he’s playing his kite. And there’s a dog sitting next to him. And there’s...a little boy that’s outside. He’s down toward the end...of...the the grassy area. Just sitting down there probably playing. Because there’s um...there’s uh...(I don’t know what this is called)...(I don’t know) and then there’s some people up here...in um...they’re in uh...I don’t know what that is either. I don’t know, sorry. |
Notes: Participants do not have to show all characteristics associated with a given fluency score. Examples are from descriptions of the picnic scene picture used in the structured condition of the WAB.
To ensure that participants with aphasia had sufficient speech perception and auditory word recognition ability to perform the experimental task and to control for potential confounds, only participants with at least 80% accuracy on phoneme discrimination, rhyme discrimination, and auditory lexical decision tasks were recruited. These tests, along with the WAB, had been previously administered as part of a large psycholinguistic battery and were obtained through the Moss Aphasia Psycholinguistics Project Database (Mirman et al., 2010; www.mappd.org). Phoneme discrimination was measured using a 40-item auditory-phonologic discrimination task (Martin, Schwartz, & Kohen, 2006), in which participants heard two words or non-words and were required to indicate whether the two were the “same” or “different”. Non-identical pairs differed by a single onset or final phoneme. The task was presented in two versions: a no-delay condition and a filled 5-second delay (audible counting to five) between pair items, which added a short-term memory component. In the rhyme discrimination test participants indicated whether each pair of 30 spoken words rhymed (adapted from Freedman & Martin, 2001). Auditory lexical decision scores were taken from the auditory lexical decision subtest of the Psycholinguistic Assessment of Language Processing in Aphasia (Kay, Lesser, & Coltheart, 1992). Participants had also scored in the 7–9 range (out of 10) on the Auditory Verbal Comprehension subsection of the WAB. Furthermore, to ensure that participants’ visual word recognition abilities would not interfere with their performance on the experimental task, participants completed the visual and auditory subtests of the Reading Comprehension Battery for Aphasia, Second Edition (LaPointe & Horner, 1998). Only participants with at least 80% accuracy on visual word recognition in the presence of visual and auditory distractors were recruited. These inclusion criteria ensured that auditory comprehension and reading abilities were excluded as potential confounds in the study.
The resulting sample varied on the WAB measures of fluency and severity (Aphasia Quotient, AQ), as well as on the Peabody Picture Vocabulary Test (PPVT, Dunn & Dunn, 1997), a measure of receptive vocabulary. Participants with aphasia included both the traditional “fluent” (i.e., Anomic, Conduction) and “nonfluent” (i.e., Broca’s, Transcortical motor) aphasia subtypes. However, those broad categories obscure substantial variability in fluency of speech production and the graded WAB fluency score provides a finer-grained measure than the dichotomous fluent/nonfluent aphasia distinction (for more discussion of the challenges in aphasia classification see Caplan, 2011). Detailed information about the participants with aphasia is shown in Table 1. Data from three additional participants were excluded from the analyses due to equipment malfunction.
Table 1.
Demographic and psycholinguistic data for participants with aphasia.
| Subject ID |
Sex | Age (yrs) |
Months Post Onset |
Educ (yrs) |
Aphasia Subtype |
WAB Fluency |
WAB AQ |
WAB COMP |
PPVT Stand Scores |
Phoneme Discrim*. |
Rhyme Discrim*. |
Auditory LD* |
Lesion Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR1392 | M | 69 | 82 | 19 | Anomic | 9 | 90.2 | 8.7 | 97 | 93 | 93 | 93 | 84.92 |
| MR2091 | M | 61 | 82 | 15 | Anomic | 9 | 87.8 | 9.2 | 65 | 95 | 98 | 96 | 195.29 |
| MR0281 | F | 53 | 164 | 16 | Conduction | 9 | 82.9 | 9.15 | 85 | 93 | 98 | 91 | 151.32 |
| MR2453 | M | 62 | 31 | 13 | Anomic | 9 | 90.4 | 9.1 | 82 | 85 | 90 | 87 | NA |
| MR2340 | F | 71 | 37 | 12 | Anomic | 9 | 93.5 | 9.95 | 76 | 90 | 98 | 87 | 12.4 |
| MR2221 | F | 35 | 51 | 18 | Conduction | 8 | 78.7 | 8.25 | 81 | 80 | 95 | 88 | 63.92 |
| MR2464 | M | 64 | 25 | 19 | Anomic | 8 | 84.7 | 9.75 | 110 | 98 | 98 | 91 | 61.02 |
| MR1088 | M | 50 | 99 | 18 | Anomic | 8 | 78.8 | 8.8 | 82 | 93 | 93 | 96 | 89.07 |
| MR2350 | M | 50 | 57 | 14 | Anomic | 6 | 73.7 | 7.15 | 66 | 88 | 93 | 91 | 55.69 |
| MR2011 | F | 49 | 111 | 13 | Anomic | 6 | 81.5 | 8.55 | 82 | 85 | 100 | 90 | 46.35 |
| XO2540 | M | 56 | 71 | 12 | Conduction | 6 | 65.3 | 7.05 | 90 | 95 | 98 | 89 | 57.64 |
| MR2538 | M | 57 | 25 | 16 | Anomic | 6 | 76.7 | 8.75 | 85 | 85 | 95 | 83 | 140.43 |
| MR4343 | M | 76 | 73 | 20 | Anomic | 5 | 74.5 | 7.75 | 104 | 90 | 93 | 88 | 40.95 |
| MR1846 | F | 54 | 66 | 14 | Conduction | 5 | 68 | 9.4 | 93 | 100 | 100 | 96 | 31.43 |
| MR2767 | F | 36 | 14 | 16 | TCM | 4 | 71.6 | NA | 92 | 93 | 88 | 90 | NA |
| MR2710 | F | 63 | 126 | 15 | Broca’s | 4 | 74.3 | 9.5 | 85 | 88 | 90 | 91 | 209.8 |
| MR0865 | M | 64 | 125 | 12 | Broca’s | 4 | 66 | 9 | 79 | 93 | 95 | 93 | 71.75 |
| MR2172 | F | 69 | 114 | 16 | Broca’s | 2 | 35.4 | 9.3 | 93 | 88 | 85 | 92 | 73.1 |
| MR2481 | M | 71 | 52 | 19 | Broca’s | 2 | 58.8 | 7.1 | 99 | 98 | 98 | 100 | 81.61 |
yrs = years; Educ = Education; WAB Fluency = Fluency measure from the Western Aphasia Battery; WAB AQ = Aphasia Quotient from the Western Aphasia Battery; WAB COMP = Auditory Verbal Comprehension Score from the Western Aphasia Battery; PPVT Stand. Scores = Peabody Picture Vocabulary Test Standard Scores; Phoneme Discrim. = Phoneme Discrimination (no delay); Rhyme Discrim. = Rhyme Discrimination (no delay); LD = Lexical Decision.
Percent correct
Materials
Critical stimuli were 120 target words that varied systematically in phonological neighborhood density in two conditions of interest with 60 trials each: words with many phonological neighbors (high density condition: e.g., BAG) versus words with few phonological neighbors (low density condition: e.g., FOX). Phonological neighborhood density (high versus low) was operationalized as the number, t(59) = 5.6, p < 0.001, and summed log frequency, t(59) = 5.8, p < 0.001, of phonological neighbors, (i.e., words that differed from the target by no more than one phoneme through deletion, addition, or substitution following the “one phoneme” or DAS rule). Neighborhood density co-varied with measures of cohort density (word onset) and rhyme density (word offset), such that targets in the high-density condition also had a larger number, t(59) = 2.7, p < 0.01, and summed log frequency, t(59) = 5.8, p < 0.001, of onset (cohort) neighbors, as well as a larger number, t(59) = 6.6, p < 0.001, and summed log frequency, t(59) = 5.3, p < 0.001, of offset (rhyme) neighbors, relative to targets in the low-density condition. Based on prior work that cohort and rhyme competition might be dissociable in aphasia (e.g., Mirman et al., 2011) and that neighborhood effects can be specific to word position (e.g., Yates, Friend, & Ploetz, 2008), variation in both cohort and rhyme density was included to make sure that the high density words had both many cohorts and many rhymes, and that the low density words had few of both, thus ensuring that the overall neighborhood density effects would not be concentrated in particular word positions. The high density and low-density target words were matched on number of phonemes and lexical frequency using the American National Corpus (Ide & Suderman, 2004), as well as on the length of the auditory file (all p > 0.05). It should be noted that neighborhood density tends to be correlated with word length and frequency. To manipulate neighborhood density while matching the conditions on word length and frequency, it was necessary to identify words with relatively high (or low) neighborhood density for their length and frequency. For example, CLASS (one of our high-density words) has a relatively high neighborhood density for a four-phoneme word, even though it has lower density than WALL (one of our low-density words), which has relatively low density for a three-phoneme word. As a result, the raw ranges for each variable are misleading in that they appear to overlap. Similar issues apply to matching for frequency. Table 2 summarizes the lexical properties of the target words. Stimuli primarily classified as verbs were equally distributed across the two conditions: high density = 39, low density = 41, p > 0.05. All auditory targets were recorded by a female native speaker of American English in a quiet room and normalized at 60 dB prior to adding 62 dB of white noise to make word recognition more difficult.
Table 2.
Mean (Standard Deviation) and range of target word properties for phonological neighborhood density manipulation.
| Measure | High Density | Low Density | t | p |
|---|---|---|---|---|
| N | 60 | 60 | - | - |
| Number of Phonemes | 3.4 (0.56) 3 – 5 |
3.4 (0.59) 3 – 5 |
−0.7 | ns |
| Log Frequency | 1.2 (0.54) 0.42 – 3.55 |
1.2 (0.45) 0.42 – 2.46 |
−0.03 | ns |
| Number of Neighbors | 19.7 (9.3) 3 – 40 |
12.9 (7.3) 2 – 32 |
5.6 | < 0.0001 |
| Summed Log Frequency of Neighbors | 23.1 (12.8) 1.9 – 49.7 |
14.5 (9.9) 1.1 – 41.1 |
5.8 | < 0.0001 |
| Number of Cohort Neighbors | 72.5 (61.3) 6 – 237 |
46.4 (34) 4 – 144 |
2.7 | < 0.01 |
| Summed Log Frequency of Cohort Neighbors | 49.5 (40.8) 3.67 – 157.3 |
32.7 (22.5) 2.6 – 103.6 |
2.8 | < 0.01 |
| Number of Rhyme Neighbors | 21.7 (11.3) 3 – 56 |
9.7 (7.96) 1 – 34 |
6.6 | < 0.0001 |
| Summed Log Frequency of Rhyme Neighbors | 27.9 (22.4) 2.36 – 116.6 |
11 (11.1) 0.98 – 54.8 |
5.3 | < 0.0001 |
| Length of auditory file (ms) | 620 (96.3) 409 – 838 |
612 (96.8) 363 – 811 |
0.54 | ns |
In addition to the 120 target words, 480 words were selected to serve as visually-presented distractors in the spoken-to-written word matching task (see below). Distractors were of three types: cohort neighbors, rhyme neighbors and unrelated (i.e., onset neighbors of rhyme distractors and offset neighbors of cohort distractors), and did not differ in number of phonemes, frequency, phonological neighborhood density and cohort density from targets (all p > 0.05). See Appendix Table A2 for lexical properties of the distractor words and Appendix Table A3 for the complete list of experimental stimuli.
Table A2.
Mean (Standard Deviation) characteristics of items in the response array.
| Stimulus Characteristics | Target | Cohort distractor | Rhyme distractor | Onset neighbor of rhyme distractor | Offset neighbor of cohort distractor |
|---|---|---|---|---|---|
| High Density Condition | e.g., BAG | e.g., BAT | e.g., TAG | e.g., TAB | e.g., CHAT |
| N | 60 | 60 | 60 | 60 | 60 |
| Number of Phonemes | 3.4 (0.56) | 3.5 (0.62) | 3.4 (0.69) | 3.6 (0.57) | 3.2 (0.46) |
| Log Frequency | 1.2 (0.54) | 1.2 (0.67) | 1.2 (0.7) | 1.2 (0.72) | 1.2 (0.68) |
| Number of Neighbors | 19.7 (9.3) | 17.7 (9.5) | 18.9 (8.1) | 15.0 (8.0) | 18.9 (7.3) |
| Summed Log Frequency of Neighbors | 23.1 (12.8) | 19.7 (12.8) | 21.9 (13.3) | 16.3 (10.4) | 20.7 (9.2) |
| Low Density Condition | e.g., FOX | e.g., FOG | e.g., BOX | e.g., BOSS | e.g., JOG |
| N | 60 | 60 | 60 | 60 | 60 |
| Number of Phonemes | 3.4 (0.59) | 3.4 (0.58) | 3.6 (0.61) | 3.5 (0.57) | 3.5 (0.65) |
| Log Frequency | 1.2 (0.45) | 1.1 (0.67) | 1.2 (0.72) | 1.1 (0.63) | 1.1 (0.68) |
| Number of Neighbors | 12.9 (7.3) | 16.2 (8.3) | 12.8 (7.4) | 14.4 (7.9) | 16.3 (8.0) |
| Summed Log Frequency of Neighbors | 14.5 (9.9) | 18.8 (10.7) | 13.6 (10.1) | 15.4 (10.0) | 18.2 (11.1) |
Table A3.
Experimental materials.
| Condition | Target | Cohort distractor | Rhyme distractor | Onset neighbor of rhyme distractor | Offset neighbor of cohort distractor |
|---|---|---|---|---|---|
| High Density | BAG | BAT | TAG | TAB | CHAT |
| TRAIN | TRUNK | DRAIN | DRAFT | CHUNK | |
| PATCH | PAD | LATCH | LAMB | DAD | |
| CAP | CAT | LAP | LAG | MAT | |
| LIP | LID | HIP | HINT | KID | |
| CALF | CASH | HALF | HANG | SASH | |
| MOP | MOB | COP | COT | JOB | |
| WITCH | WINK | DITCH | DISK | THINK | |
| BELL | BET | CELL | CENT | JET | |
| CONE | COMB | TONE | TOTE | DOME | |
| HAT | HAM | RAT | RAG | CLAM | |
| BEAR | BELT | WEAR | WEDGE | FELT | |
| PAN | PASS | TAN | TAX | MASS | |
| HAIR | HAND | PAIR | PAL | LAND | |
| CAN | CAMP | FAN | FACT | LAMP | |
| MAN | MASH | CLAN | CLERK | DASH | |
| TREE | TRAY | SPREE | SPEECH | PLAY | |
| FORK | FOLD | PORK | POLE | MOLD | |
| SKULL | SKY | LULL | LUST | CRY | |
| BAND | BACK | STAND | STICK | RACK | |
| FROG | FREAK | LOG | LOFT | PEAK | |
| PLUG | PLOT | RUG | RUT | DOT | |
| BRIDGE | BRICK | FRIDGE | FRIEND | KICK | |
| CLUB | CLOG | TUB | TUG | HOG | |
| GROOM | GRADE | BOOM | BOOK | SHADE | |
| CLASS | CLOCK | GRASS | GRIEF | ROCK | |
| PEAR | PEG | SWEAR | SWAN | LEG | |
| BRAIN | BREW | GAIN | GANG | CREW | |
| FRAME | FROST | BLAME | BLESS | COST | |
| MATCH | MAP | BATCH | BATH | GAP | |
| RAKE | RAIL | SHAKE | SHAME | ||
| TIN | TICK | GRIN | GRIP | CLICK | |
| BEAN | BEACH | DEAN | DEAL | PEACH | |
| WIRE | WIDE | FIRE | FIND | HIDE | |
| HEAD | HEM | SPREAD | SPICE | GEM | |
| TIDE | TILE | BRIDE | BRAKE | FILE | |
| HAIL | HAZE | JAIL | JADE | MAZE | |
| SHORE | SHAWL | SORE | SAUCE | CRAWL | |
| PINE | PILE | LINE | LIME | MILE | |
| CHOP | CHOCK | STOP | STEAM | DOCK | |
| TAP | TACT | STRAP | STEP | PACT | |
| JAR | JAW | CAR | CART | PAW | |
| KING | KISS | STRING | STALL | BLISS | |
| ROSE | ROPE | NOSE | NOTE | HOPE | |
| STAIN | STEEL | SPRAIN | SPOON | WHEEL | |
| SEAL | SEAT | MEAL | MEAT | CHEAT | |
| CLAY | CLIP | DAY | DESK | SHIP | |
| WING | WISH | STING | STEW | FISH | |
| FLEA | FLASK | SEA | SEAM | TASK | |
| DECK | DENT | PECK | PEP | TENT | |
| COKE | COPE | JOKE | JOLT | POPE | |
| TRACK | TREAT | SLACK | SLICE | WHEAT | |
| PLATE | PLAN | GATE | GAUGE | VAN | |
| GUEST | GUESS | TEST | TELL | MESS | |
| SPOT | SPEAK | SHOT | SHOP | LEAK | |
| SLIT | SLAP | WIT | WIN | WRAP | |
| BREEZE | BREAK | SNEEZE | SNOB | STEAK | |
| BLADE | BLOOM | TRADE | TRICK | ROOM | |
| DRINK | DRUG | SINK | SIT | BUG | |
| SKUNK | SKIN | FUNK | FUSS | GIN | |
| Low Density | JUDGE | JUNK | NUDGE | NUT | BUNK |
| DOG | DOLL | SMOG | SMILE | POLL | |
| BULL | BUSH | PULL | PUT | PUSH | |
| CHICK | CHIN | PICK | PILL | BIN | |
| WALL | WALK | HALL | HAWK | CHALK | |
| BOAT | BOLT | STOAT | STORE | COLT | |
| SHEEP | SHEET | JEEP | JEANS | FEET | |
| CHAIN | CHEST | MAIN | MAID | PEST | |
| KNIFE | NIGHT | WIFE | WINE | FIGHT | |
| HOOK | HOOD | LOOK | LOOM | MOOD | |
| BONE | BOW | STONE | STAMP | ROW | |
| CAKE | CANE | LAKE | LACE | LANE | |
| GUN | GUM | NUN | NUMB | DRUM | |
| THRONE | THROW | PHONE | FOLK | CROW | |
| NET | NECK | PET | PEN | TREK | |
| CHAIR | CHECK | STAIR | STAGE | WRECK | |
| YARN | YARD | BARN | BARK | CARD | |
| PUMP | PUN | LUMP | LUNCH | SUN | |
| SALT | SOLD | HALT | HORN | COLD | |
| FOX | FOG | BOX | BOSS | JOG | |
| HORSE | HAUL | FORCE | FORM | MAUL | |
| GIFT | GIG | LIFT | LINK | WIG | |
| CHILD | CHIME | WILD | WIPE | TIME | |
| FENCE | FETCH | SENSE | SECT | STRETCH | |
| VEST | VENT | REST | REALM | RENT | |
| SWITCH | SWIM | PITCH | PIN | TRIM | |
| SNAKE | SNOW | FLAKE | FLOOR | GROW | |
| THREAD | THREAT | BREAD | BROW | SWEAT | |
| GOAT | GOLD | FLOAT | FLAW | HOLD | |
| BALL | BORE | CALL | CORE | CHORE | |
| CAPE | CAVE | SHAPE | SHAVE | SLAVE | |
| LODGE | LOCK | DODGE | DON | FLOCK | |
| SONG | SOCK | GONG | GOLF | MOCK | |
| TOOTH | TOOL | BOOTH | BOOST | POOL | |
| TUBE | TUNE | CUBE | CUTE | DUNE | |
| PUP | PUFF | CUP | CUB | CUFF | |
| FUDGE | FUN | SMUDGE | SMOKE | BUN | |
| COUCH | COUNT | VOUCH | VOW | MOUNT | |
| DUKE | DUDE | FLUKE | FLUSH | NUDE | |
| WOOL | WOOD | SCHOOL | SCOOP | STOOD | |
| MOUTH | MOUSE | SOUTH | SOUND | HOUSE | |
| RIB | RINK | CRIB | CRATE | BLINK | |
| PIG | PIT | DIG | DISH | KIT | |
| ROOF | ROOT | HOOF | HOOP | BOOT | |
| DOVE | DUST | GLOVE | GLOBE | RUST | |
| HEDGE | HEN | LEDGE | LENS | MEN | |
| COACH | COAST | POACH | POKE | TOAST | |
| TEAR | TEACH | GEAR | GILLS | REACH | |
| PATH | PACK | MATH | MASK | SNACK | |
| SPONGE | SPARK | PLUNGE | PLUM | SHARK | |
| MILK | MINT | SILK | SING | LINT | |
| DISC | DILL | WHISK | WIND | GRILL | |
| PIPE | PIKE | STRIPE | STOCK | BIKE | |
| RANCH | RAFT | BRANCH | BRAT | SHAFT | |
| CLIFF | CLASP | SNIFF | SNIP | GRASP | |
| TRIBE | TRASH | BRIBE | BRUSH | FLASH | |
| CLOTH | CLUE | MOTH | MOSS | GLUE | |
| BENCH | BEND | TRENCH | TRAIL | SPEND | |
| QUILT | QUIZ | GUILT | GIVE | WHIZ | |
| BLONDE | BLOCK | POND | POT | SHOCK |
Procedure
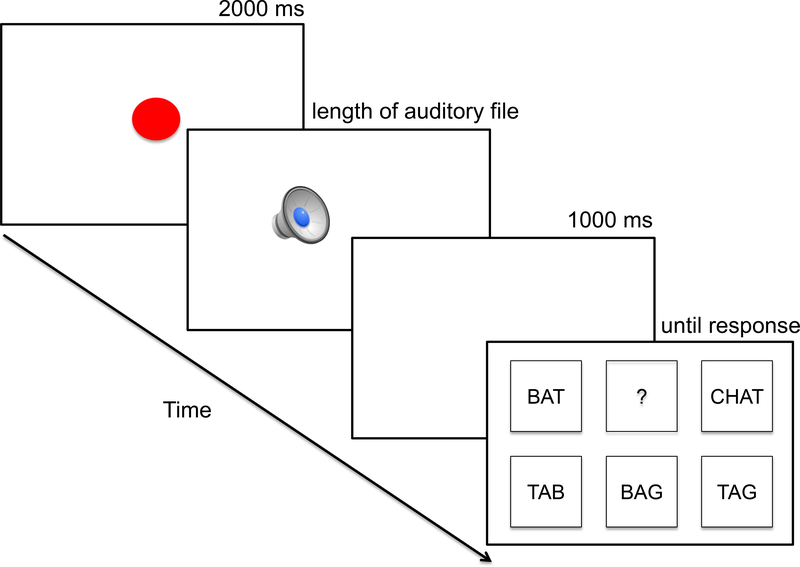
Participants were seated at a comfortable distance (about 15 inches) from a touch-sensitive computer monitor and asked to perform a spoken-to-written word matching task, consisting of 120 trials with a mid-way break. The task was modeled on the identification of words in noise task (Luce & Pisoni, 1998), in which participants were asked to listen to stimuli played in noise over headphones and type the words that they thought they heard. Because stroke survivors often experience hemiparesis, the experimental procedure was simplified so that it would not depend on the fine-motor skills required for typing. The task was presented electronically using the E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA). The trial event sequence is shown in Figure 1. Each trial began with a trial preparation screen presented for 2000 ms with a red circle in the center that decreased in size until it disappeared, at which point the auditory stimulus was presented over headphones. This was followed by a 1000 ms blank screen to allow auditory word recognition without bias from visually-presented response options (for discussion of how response options modulate neighborhood effects see Chen & Mirman, 2015). Finally, a 2 × 3 array of six response options was presented and remained on the screen until a response was made. Each cell of the array was approximately 2 × 2 inches.
Figure 1.
The trial event sequence started with a 2000 ms preparation screen, followed by an auditory stimulus presented over headphones, a 1000 ms blank screen and ended with a 2 × 3 array of response options that remained on the screen until a response was selected.
The response array contained the target (e.g., BAG), a cohort distractor (e.g., BAT), a rhyme distractor (e.g., TAG), an onset neighbor of the rhyme distractor (e.g., TAB), an offset neighbor of the cohort distractor (e.g., CHAT), and an undecided response option (i.e., ?). This made it difficult to guess the target simply from the structure of the response array (i.e., the target was not the only option with phonologically related distractors; there were two other response options that also had cohort-related and rhyme-related distractors). These response options were carefully chosen to resemble lexical competition in the spoken domain, which involves competition from both close phonological neighbors (e.g., cohort and rhyme distractors) and more distant phonological neighbors (e.g., the onset neighbor of the rhyme distractor; the offset neighbor of the cohort distractor). Also, to ensure that the task is not forced choice, the undecided response option provide participants a response alternative when none of the available response choices seemed to be a good fit. The response options were presented in Courier New font black capital letters against a white background and their locations were randomized on each trial.
Participants were instructed to select the cell containing the word they heard using their left hand on a touch-sensitive monitor in order to control for right side hemiparesis present in some individuals with left hemisphere stroke. All participants were encouraged to select the “?” response option whenever they were unable to recognize the target from among the other response options, analogous to responding “I don’t know” in the free-response version of the task. Both speed and accuracy were stressed. A set of 20 practice trials preceded the experimental set. In 5 practice trials, the target word was not available as a response option in order to encourage participants to choose the “?” response option when the auditory target did not match any of the other response options on the screen (i.e., to avoid guessing). None of the practice stimuli appeared in the experimental sets. During the practice trials, participants were given feedback about their response speed and accuracy.
Since the spoken-to-written word matching task requires visual word recognition, task performance may be confounded by deficits in visual word recognition. However, deficits in visual word recognition would be expected to have general effects on overall accuracy or response times without differential effects of phonological neighborhood density. Visual word recognition deficits that differentially affected recognition of high and low phonological neighborhood words would suggest difficulty resolving lexical competition, so they would also be measuring the construct of interest in this study.
Data Analysis
Correct-response latencies were analyzed using linear mixed-effects models with the fixed effect of neighborhood density (high versus low) and random effects of participants and items (Baayen, Davidson & Bates, 2008; Barr, Levy, Scheepers & Tily, 2013). Response accuracy was analyzed using mixed-effects logistic regression. For participants with aphasia, trials on which the response was more than 3 standard deviations above participant or item means were removed from the analyses (7.8% of trials), as these very slow responses likely reflected a distinct response strategy and would be confounded by decay of the target word from working memory.
The analyses were implemented in R version 3.2.3 (R Development Core Team, 2016; http://cran.us.r-project.org/) using the lme4 package version 1.1–11 (Bates et al., 2016). For both the response latency and accuracy analyses, the base model included the fixed effect of neighborhood density (high versus low), and a maximal random effect structure defined by the experiment design (Barr et al., 2013), consisting of random effects of items and participants and by-participant random slopes of phonological neighborhood density and phonological neighborhood type (cohort versus rhyme). For analyses of data from participants with aphasia, fixed effects of fluency (WAB Fluency scores), receptive vocabulary (PPVT standard scores) and overall severity of language impairment (residualized WAB-AQs) were evaluated individually, first in terms of their overall effect on response latencies and accuracy (i.e., as a main effect), then in terms of their interaction with density. This interaction term was the critical test of whether differences in fluency, receptive vocabulary or overall severity of language impairment modulated effects of phonological neighborhood density. Improvement in model fit for each of these steps was evaluated using the likelihood ratio test (χ2 test with degrees of freedom equal to the number of parameters added). For the models, parameter-specific p-values were computed using the normal approximation (i.e., treating the t-value as a z-value; for discussion see Barr et al., 2013). To ease interpretation of the models, continuous predictors were centered before being entered in the analyses. Uncentered predictors are shown in all figures.
Separate models were used for each critical predictor (WAB Fluency, PPVT scores, and residualized WAB-AQs) because the predictors had very different roles that corresponded to separate hypotheses: Our primary hypothesis was about WAB Fluency, and the PPVT and residualized WAB-AQ analyses were included to rule out an alternative explanation that an observed relationship between Fluency and neighborhood density effects could arise from severity of lexical-semantic deficit (discussed above).
Results
Table 3 shows participants’ average response latencies and accuracy rates. Neurologically intact older adults exhibited the standard inhibitory effect of phonological neighborhood density on spoken word recognition: they responded faster for words from low-density phonological neighborhoods than high-density phonological neighborhoods, Estimate = 102.3, SE = 42.03, p = 0.015. Their accuracy patterned in the same direction, slightly more accurate for words from low density neighborhoods, though this difference was not statistically significant, Estimate = −0.17, SE = 0.15, p = 0.259. These results confirm that the materials and procedure used in this study produce the standard inhibitory effect of phonological neighborhood density on spoken word recognition in older, neurologically intact adults. This finding justifies subsequent use of these materials and procedures for the experimental protocol for the participants with aphasia.
Table 3.
Mean (Standard Error) response latencies and accuracy rates.
| Group | Response Latencies |
Accuracy Rates |
||
|---|---|---|---|---|
| Low-Density Neighborhood | High-Density Neighborhood | Low-Density Neighborhood | High-Density Neighborhood | |
| Healthy older adults | 2000 (130) | 2205 (155) | 87.3 (25.9) | 82.9 (24.9) |
| Individuals with aphasia | 3057 (234) | 3271 (239) | 77.3 (24.2) | 69.6 (23.3) |
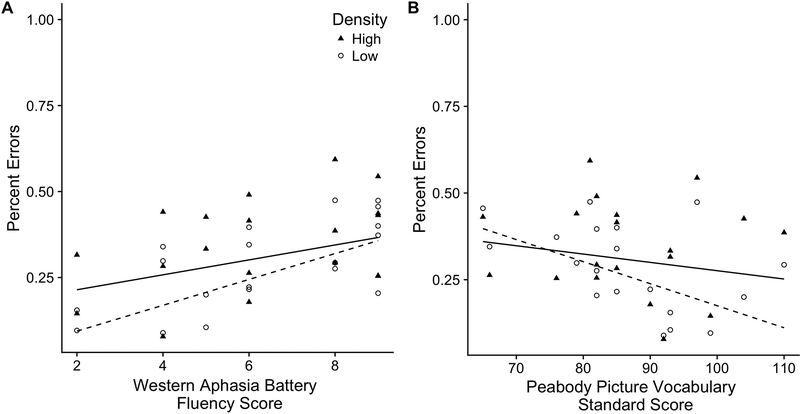
Individuals with aphasia did not differ on response latencies, Estimate = 100, SE = 66.6, p = 0.13, and accuracy rates, Estimate = −0.2, SE = 0.13, p = 0.12, in their identification of low density versus high density words. However, their performance patterned in the expected direction: slower and less accurate responses for high-density words than low-density words (see Table 3). The effect of phonological neighborhood density on response accuracy (i.e., higher accuracy for words from low rather than high density phonological neighborhoods) was significantly modulated by WAB Fluency scores, χ2(1) = 4.8, p = 0.03. Lower-fluency participants with aphasia showed a larger effect of phonological density relative to higher-fluency participants with aphasia (Figure 2A). There was also a main effect of fluency, Estimate = −0.173, SE = 0.06, p = 0.005: lower-fluency participants had higher overall accuracy. Although it did not reach significance, χ2(1) = 2.43, p = 0.12; Estimate = −25, SE = 15.9, p = 0.12, the effect of WAB Fluency scores on response latencies patterned in the same direction: larger effect of phonological density in the lower-fluency participants with aphasia than in the higher-fluency individuals with aphasia.
Figure 2.
Continuous model fits of percent errors for words from high-density relative to low-density phonological neighborhoods in participants with aphasia as a function of WAB Fluency Scores (panel A) and PPVT standard scores (panel B).
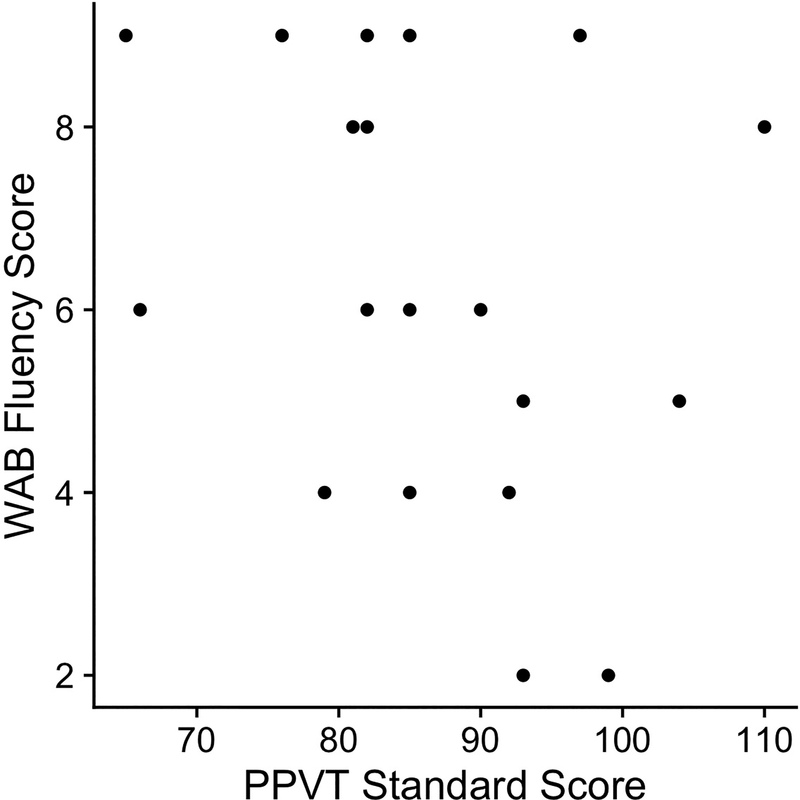
The effect of phonological neighborhood density on response accuracy was also significantly modulated by receptive vocabulary as measured by PPVT standard scores, χ2(1) = 4.8, p = 0.03. Participants with larger receptive vocabularies showed larger phonological neighborhood density effects than participants with smaller receptive vocabularies did (Figure 2B). There was also a marginal main effect of receptive vocabulary, Estimate = −0.024, SE = 0.012, p = 0.06: participants with larger receptive vocabularies were slightly more accurate in identifying spoken words. Parameter estimates for the full models are presented in Table 4. There was also no significant correlation between PPVT standard scores and WAB Fluency, r = −0.302, p = 0.209, indicating that the opposite effects of receptive vocabulary and fluency were not the same underlying pattern. The scatterplot showing the weak relationship between WAB Fluency scores and PPVT standard scores is presented in Figure 3.
Table 4.
Parameter estimates (Standard Error) for three predictor models evaluating response accuracy.
| Term | Predictor |
||
|---|---|---|---|
| WAB Fluency Score | PPVT Standard Score | Residualized WAB-AQ Score | |
| Intercept | 1.04 (0.17)*** | 1.02 (0.19)*** | 1.02 (0.19)*** |
| Density | −0.2 (0.13) | −0.20 (0.12) | −0.20 (0.13) |
| Predictor | −0.17 (0.06)** | 0.02 (0.01). | −0.00 (0.02) |
| Density-x-Predictor | 0.07 (0.03)* | −0.01 (0.005)* | 0.01 (0.01) |
p < 0.1
p < 0.05
p < 0.01
p < 0.001.
Figure 3.
Correlation scatterplot of WAB fluency scores and PPVT standard scores.
Fluency was significantly correlated with overall severity of language impairment (WAB-AQs), r = 0.865, p < 0.001, which is not surprising given that the fluency score contributes toward the calculation of the overall severity score along with the scores for auditory verbal comprehension, repetition and naming and word finding. To evaluate whether overall aphasia severity modulates the effect of phonological neighborhood density on response accuracy, we regressed WAB Fluency scores out of WAB-AQs and used the regression residuals to predict the effect of phonological neighborhood density. Without the fluency component, the measure of overall aphasia severity did not modulate the phonological neighborhood density effect, χ2(1) = 0.33, p = 0.565. There was no significant correlation between PPVT standard scores and WAB AQs, r = −0.252, p = 0.299, indicating that individuals with a more severe form of aphasia did not also have more limited vocabulary.
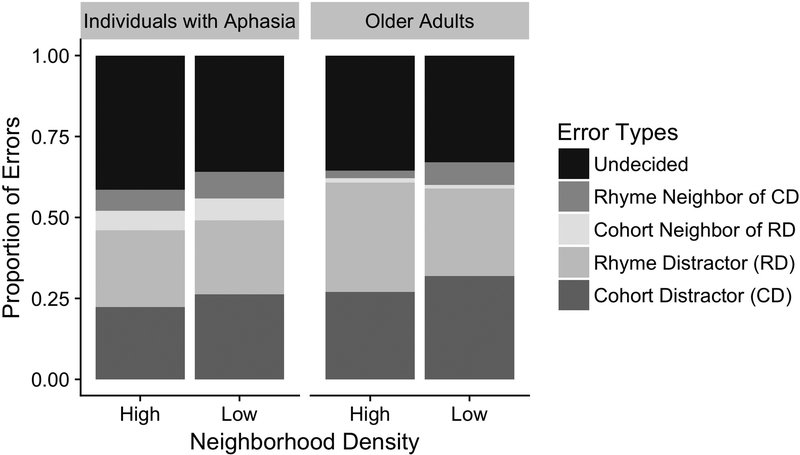
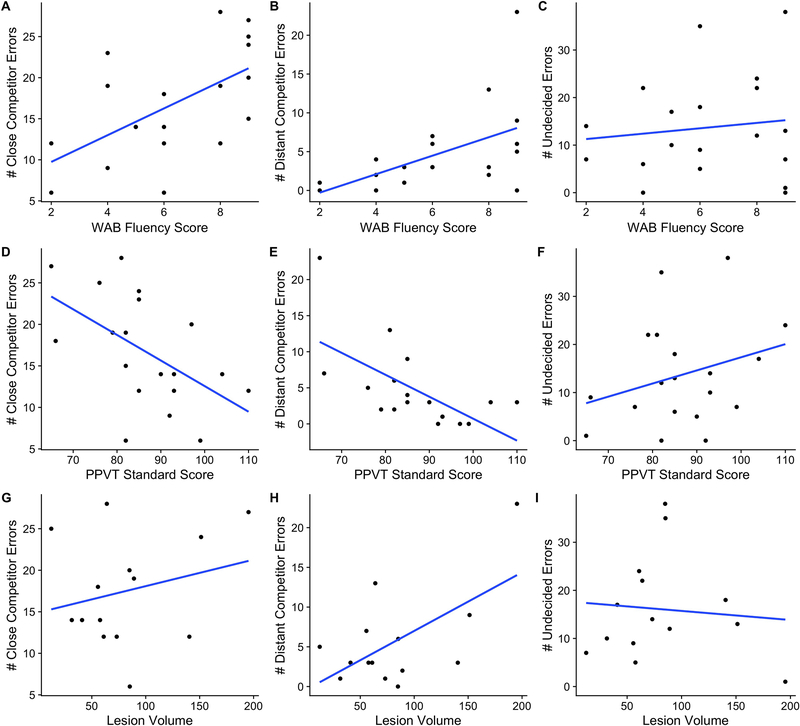
Individuals with aphasia made overall more errors in spoken word recognition than older adults, Estimate = 0.54, SE = 0.24, p = 0.02. The error patterns made by the two groups are largely similar, as shown in Figure 4. When making an incorrect response, participants in both groups were more likely to select a close phonological competitor (i.e., cohort distractor or rhyme distractor) than a more distant phonological competitor (i.e., cohort neighbor of the rhyme distractor or rhyme neighbor of the cohort distractor), t(33) = 13.3, p < 0.001. Older adults were more likely to incorrectly select a rhyme distractor than individuals with aphasia were, Estimate = 0.53, SE = 0.0, p < 0.0001. Individuals with aphasia were more likely than neurologically healthy older adults to incorrectly select a response from among more distant neighbors (i.e., cohort neighbor of the rhyme distractor: Estimate = −1.6, SE = 0.57, p = 0.005, or the rhyme neighbor of the cohort distractor: Estimate = −0.88, SE = 0.45, p = 0.05). Among individuals with aphasia, higher fluency was positively associated with incorrectly selecting a close phonological competitor, r = 0.58, p = 0.009, or distant phonological competitor, r = 0.51, p = 0.025 (see Figure 5 Panels A and B), while higher vocabulary scores were negatively correlated with phonological competitor errors, close competitor: r = −0.53, p = 0.018, distant competitor: r = −0.63, p = 0.0035, (see Figure 5 Panels D and E). Lesion volume was positively correlated with distant competitor errors, r = 0.6, p = 0.02 (see Figure 5H), but not close phonological competitor errors, r = 0.24, p = 0.41 (see Figure 5G). Critically, undecided errors where not predicted by fluency, r = 0.12, p = 0.6, vocabulary size, r = 0.29, p = 0.23, or lesion volume, r = −0.09, p = 0.77, respectively (see Figure 5 Panels C, F and I).
Figure 4.
Proportion of errors by type for individuals with aphasia (left panel) and neurologically healthy older adults (right panel).
Figure 5.
Correlation scatterplots of error type and WAB fluency score (Panels A, B and C), PPVT standard score (Panels D, E and F) and lesion volume (Panels G, H and I).
Discussion
We evaluated whether the fluency deficit in aphasia is associated with lexical selection difficulties across language production and comprehension. The key prediction was that, if impaired ability to resolve lexical competition contributes to the fluency deficit in aphasia, then individuals whose language production is less fluent should have more difficulty resolving lexical competition, even in a task that requires no language production. To test this prediction, we examined effects of phonological neighborhood density on spoken word recognition in individuals with aphasia with varied degrees of fluency deficit. The results were consistent with this prediction: among 19 participants with aphasia, fluency of language production modulated the size of the phonological neighborhood density effect on spoken word recognition, with less fluent individuals exhibiting larger effects. Further analyses demonstrated that the observed effect of fluency was not due to general lexical deficits. Receptive vocabulary had the opposite relation to neighborhood density effects: individuals with larger receptive vocabularies exhibited larger inhibitory effects of phonological neighborhood density in spoken word recognition. This finding converges with data from children (e.g., Charles-Luce & Luce, 1990; De Cara & Goswami, 2003) that shows a positive relation between vocabulary size and sensitivity to neighborhood density. It also rules out the possibility that general lexical deficits produced the relation between lower fluency and increased sensitivity to neighborhood density.
Fluent speech production requires cooperation among the lexical, syntactic, and articulatory levels of the language production system. In order to fluently produce language, multi-word utterance frames must be constructed, lexical items selected from among competitors to fill those frames, and articulated following a motor plan. Resolution of lexical neighborhood competition in word recognition has no plausible relation to the syntactic and articulatory stages of language production (such as articulatory motor control or syntactic knowledge), so they cannot explain the observed relationship between fluency and sensitivity to lexical neighborhood density in word recognition. Problems with selecting a lexical item from a set of competitors (including preceding and anticipated words) could produce halting speech, omission of closed class words (if they fail to be selected), and blends or other speech errors if incomplete selection results in activation of multiple lexical candidates cascading to articulatory planning (e.g., Goldrick & Chu, 2014; McMillan & Corley, 2010). All of these would be regarded as disfluent speech, highlighting lexical selection as a key step in fluent language production, and the only one that might be related to neighborhood effects in spoken word recognition. Recent evidence also indicates that lexical selection deficits contribute to omission errors in object naming (Chen, Middleton, & Mirman, in press), suggesting that even single word production can be impaired by lexical selection deficits. More broadly, there is growing evidence that impaired ability to select lexical items for production is one cause of non-fluent aphasia (e.g., Mirman, Yee, Blumstein & Magnuson, 2011; Novick et al., 2005; 2010; Robinson et al., 1998; Schnur, Schwartz, Brecher & Hodgson, 2006) and the present data indicate that this selection mechanism is related to the mechanisms involved in resolving lexical competition during spoken word recognition.
One direction for future research is more refined assessment of the components of fluent language production. The present study relied on a relatively coarse measure of fluency based on qualitative assessment of spontaneous speech in unstructured (i.e., conversational) and semi-structured (i.e., picture description) contexts. We chose the WAB Fluency measure due to its historical and clinical prominence in aphasia research. However, more fine-grained measures of fluency exist, including those that specifically measure grammatical complexity (Berndt, Wayland, Rochon, Saffran & Schwartz, 2000) or the informativeness and efficiency of connected speech (Nicholas & Brookshire, 1993) or the motor planning process (Dabul, 2000; Strand, Duffy, Clark & Josephs, 2014). Finer-grained, ecologically valid assessment of lexical selection for production would provide a further test of this claim and it remains an open question to what extent the syntactic and motoric aspects of language production are involved in or interact with language comprehension (for some perspectives see Dell & Chang, 2014; Hickok & Poeppel, 2007; Pickering & Garrod, 2013; Segaert, Menenti, Weber, Petersson & Hagoort, 2012).
Conclusions
Our results reveal an association between impaired lexical selection in spoken word recognition and deficits in fluent speech production. This suggests that impaired lexical selection is one contributing factor in fluency deficits. These findings have implications for aphasia rehabilitation, suggesting that fluency deficits may be targeted in therapy through selection training from both production and comprehension perspectives. Since both production and comprehension require lexical selection and the selection mechanism appears to be shared, comprehension tasks that emphasize selection may train the same selection mechanism that is involved in choosing among lexical competitors for fluent language production.
Acknowledgements
This research was supported by NIH grant R01DC010805 to DM. We thank Qi Chen, Adelyn Brecher, Myrna Schwartz and Gary Dell for helpful discussions, and Kristen Graziano and Casey Ferrara for help with participant recruitment. We also thank the individuals with aphasia for their participation.
APPENDIX
Footnotes
Disclosure of interest
The authors report no conflict of interest.
References
- Baayen RH, Davidson DJ, & Bates DM, (2008). Mixed-effects modeling with crossed random effects fur subjects and items. Journal of Memory and Language, 59(4), 390–412. 10.1016/j.jml.2007.12.005 [DOI] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, & Tily HJ (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 255–278. 10.1016/j.jml.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolotti J, & Marian V (2012). Language learning and control in monolinguals and bilinguals. Cognitive Science, 36(6), 1129–1147. 10.1111/j.1551-6709.2012.01243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Bojesen Christensen RH, Singmann H, Dai B, Grothendieck G, Green P (2016). lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–11. https://cran.r-project.org/web/packages/lme4
- Berndt R, Wayland S, Rochon E, Saffran E, & Schwartz M (2000). Quantitative production analysis: A training manual for the analysis of aphasic sentence production. Hove, UK: Psychology Press. [Google Scholar]
- Bialystok E, Craik FIM, & Luk G (2008). Lexical access in bilinguals: Effects of vocabulary size and executive control. Journal of Neurolinguistics, 21(6), 522–538. 10.1016/j.jneuroling.2007.07.001 [DOI] [Google Scholar]
- Botezatu MR, Landrigan J-F, Chen Q & Mirman D (2015). Phonological neighborhood density modulates errors in spoken word recognition. In Noelle DC, Dale R, Warlaumont AS, Yoshimi J, Matlock T, Jennings CD, & Maglio PP (Eds.), Proceedings of the 37th Annual Conference of the Cognitive Science Society (pp. 250–255). Austin, TX: Cognitive Science Society. [Google Scholar]
- Caplan D (2011). Aphasic Syndromes In Heilman KM & Valenstein E (Eds.), Clinical Neuropsychology (5th ed., pp. 22–41). New York, USA: Oxford University Press. [Google Scholar]
- Charles-Luce J, & Luce PA (1990). Similarity neighborhoods of words in young children’s lexicons. Journal of Child Language, 17(1), 205–215. 10.1017/S0305000900013180 [DOI] [PubMed] [Google Scholar]
- Chen Q, Middleton E, and Mirman D (in press). Words fail: Lesion-symptom mapping of errors of omission in post-stroke aphasia. Journal of Neuropsychology. DOI: 10.1111/jnp.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, & Mirman D (2015). Interaction between phonological and semantic representations: Time matters. Cognitive Science, 39(3), 538–558. 10.1111/cogs.12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabul BL (2000). Apraxia battery for adults. Second Edition. Austin, TX: Pro-Ed. [Google Scholar]
- De Cara B, & Goswami U (2003). Phonological neighborhood density: effects of a rhyme awareness task in five-year-old children. Journal of Child Language, 30(3), 695–710. 10.1017/S0305000903005725 [DOI] [PubMed] [Google Scholar]
- Dell G, & Chang F (2014). The P-chain: relating sentence production and its disorders to comprehension and acquisition. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(20120394), 1–9. 10.1098/rstb.2012.0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Martin N, Saffran EM, & Gagnon DA (1997). Lexical access in aphasic and nonaphasic speakers. Psychological Review, 104(4), 801–838. 10.1037/0033-295X.104.4.801 [DOI] [PubMed] [Google Scholar]
- Dunn LM, & Dunn LM (1997). Examiner’s manual for the PPVT-III: Peabody Picture Vocabulary Test Third Edition. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Folstein M, Folstein SE, & McHugh PR (1975). “Mini-Mental State” a practical method of grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Freedman ML, & Martin RC (2001). Dissociable components of short-term memory and their relation to long-term learning. Cognitive Neuropsychology, 18(3), 193–226. 10.1080/02643290126002 [DOI] [PubMed] [Google Scholar]
- Geschwind N (1971). Current concepts: Aphasia. New England Journal of Medicine, 284, 654–656. [DOI] [PubMed] [Google Scholar]
- Goldrick M, & Chu K (2014). Gradient co-activation and speech error articulation: Comment on Pouplier and Goldstein (2010). Language, Cognition and Neuroscience, 29(4), 452–458. 10.1080/01690965.2013.807347 [DOI] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. 10.1038/nrn2113 [DOI] [PubMed] [Google Scholar]
- Ide N, & Suderman K (2004). The American National Corpus First Release. In Lino MT, Xavier MF, Ferreira F, Costa R, & Silva R (Eds.), Proceedings of the Fourth Language Resources and Evaluation Conference (LREC) (pp. 1681–1684). Lisbon: ELRA - European Language Resources Association. [Google Scholar]
- Kay J, Lesser R, & Coltheart M (1992). PALPA: Psycholinguistic Assessments of Language Processing in Aphasia. Hove, UK: Lawrence Erlbaum Associates Ltd. [Google Scholar]
- Kertesz A (1982). Western Aphasia Battery. New York: Grune & Stratton. [Google Scholar]
- Levelt WJM (1999). Models of word production. Trends in Cognitive Sciences, 3(6), 223–232. 10.1016/S1364-6613(99)01319-4 [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, & Meyer AS (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22(1), 1–38. 10.1017/S0140525X99001776 [DOI] [PubMed] [Google Scholar]
- Luce PA (1986). Neighborhoods of words in the mental lexicon. Retrieved from Bloomington: [Google Scholar]
- Luce PA, & Large N (2001). Phonotactics, neighborhood density, and entropy in spoken word recognition. Language and Cognitive Processes, 16(5/6), 565–581. 10.1080/01690960143000137 [DOI] [Google Scholar]
- Luce PA, & Pisoni DB (1998). Recognizing spoken words: the neighborhood activation model. Ear and Hearing, 19(1), 1–36. 10.1097/00003446-199802000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce PA, Pisoni DB, & Goldinger SD (1990). Similarity neighborhoods of spoken words In Altmann GTM (Ed.), Cognitive models of speech processing: Psycholinguistic and computational perspectives (pp. 122–147). Cambridge, MA: MIT. [Google Scholar]
- Luo L, Luk G, & Bialystok E (2010). Effect of language proficiency and executive control on verbal fluency performance in bilinguals. Cognition, 114(1), 29–41. 10.1016/j.cognition.2009.08.014 [DOI] [PubMed] [Google Scholar]
- Magnuson JS, Dixon JA, Tanenhaus MK, & Aslin RN (2007). The dynamics of lexical competition during spoken word recognition. Cognitive Science, 31(1), 1–24. 10.1080/03640210709336987 [DOI] [PubMed] [Google Scholar]
- Magnuson JS, Mirman D, & Myers E (2013). Spoken Word Recognition Oxford Handbooks Online: Oxford University Press. [Google Scholar]
- Marian V, Blumenfeld HK, & Boukrina OV (2008). Sensitivity to phonological similarity within and across languages. Journal of Psycholinguistic Research, 37(3), 141–170. 10.1007/s10936-007-9064-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Schwartz MF, & Kohen FP (2006). Assessment of the ability to process semantic and phonological aspects of words in aphasia: A multi-measurement approach. Aphasiology, 20(2–4), 154–166. 10.1080/02687030500472520 [DOI] [Google Scholar]
- McMillan CT, & Corley M (2010). Cascading influences on the production of speech: Evidence from articulation. Cognition, 117(3), 243–260. 10.1016/j.cognition.2010.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D (2016). Zones of proximal development for models of spoken word recognition In Mirkovic MGGJ (Ed.), Current issues in the psychology of language: Speech perception and spoken word recognition: Psychology Press. [Google Scholar]
- Mirman D, Strauss TJ, Brecher AR, Walker GM, Sobel P, Dell GS, et al. (2010). A large, searchable, web-based database of aphasic performance on picture naming and other tests of cognitive function. Cognitive Neuropsychology, 27(6), 495–504. 10.1080/02643294.2011.574112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Yee E, Blumstein SE, & Magnuson JS (2011). Theories of spoken word recognition in aphasia: Evidence from eye-tracking and computational modeling. Brain and Language, 117(2), 53–68. 10.1016/j.bandl.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas NE, & Brookshire RH (1993). A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. Journal of Speech and Hearing Research, 36(2), 338–350. 10.1044/jshr.3602.338 [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, & Thompson-Schill SL (2005). Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cognitive, Affective, & Behavioral Neuroscience, 5(3), 263–281. 10.3758/CABN.5.3.263 [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, & Thompson-Schill SL (2010). Broca’s area and language processing: Evidence for the cognitive control connection. Language and Linguistics Compass, 4(10), 906–924. 10.1111/j.1749-818x.2010.00244.x [DOI] [Google Scholar]
- Pickering MJ, & Garrod S (2013). An integrated theory of language production and comprehension. Behavioral and Brain Sciences, 36(4), 329–392. 10.1017/S0140525X12001495 [DOI] [PubMed] [Google Scholar]
- Psychology Software Tools, Inc. [E-Prime 2.0]. (2012). Retrieved from http://www.pstnet.com.
- R Development Core Team. (2016). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.r-project.org/ [Google Scholar]
- Robinson G, Blair J, & Cipolotti L (1998). Dynamic aphasia: an inability to select between competing verbal responses? Brain, 121(1), 77–89. 10.1093/brain/121.1.77 [DOI] [PubMed] [Google Scholar]
- Roelofs A (1996). Computational models of lemma retrieval In Dijkstra T & de Smedt K (Eds.), Computational psycholinguistics (pp. 308–327). London: Taylor & Francis. [Google Scholar]
- Roelofs A (1997). The WEAVER model of word-form encoding in speech production. Cognition, 64(3), 249–284. 10.1016/S0010-0277(97)00027-9 [DOI] [PubMed] [Google Scholar]
- Roelofs A (2003). Modeling the relation between the production and recognition of spoken word forms In Schiller NO & Meyer AS (Eds.), Phonetics and phonology in language comprehension and production: Differences and similarities (pp. 115–158). Berlin: Mouton de Gruyter. [Google Scholar]
- Roelofs A (2014). A dorsal-pathway account of aphasic language production: The WEAVER++/ARC model. Cortex, 59, 33–48. 10.1016/j.cortex.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Sadat J, Martin CD, Costa A, & Alario F-X (2014). Reconciling phonological neighborhood effects in speech production through single trial analysis. Cognitive Psychology, 68, 33–58. 10.1016/j.cogpsych.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Sandoval TC, Gollan TH, Ferreira VS, & Salmon DP (2010). What causes the bilingual disadvantage in verbal fluency? The dual-task analogy. Bilingualism: Language and Cognition, 13(2), 231–252. 10.1017/s1366728909990514 [DOI] [Google Scholar]
- Schnur TT, Schwartz MF, Brecher A, & Hodgson C (2006). Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language, 54, 199–227. 10.1016/j.jml.2005.10.002 [DOI] [Google Scholar]
- Schwartz MF (1987). Patterns of speech production deficit within and across aphasia syndromes: Application of a psycholinguistic model In Coltheart M, Sartori G, & Job R (Eds.), The cognitive neuropsychology of language (pp. 163–199). Hove: Lawrence Erlbaum Associates, Ltd. [Google Scholar]
- Schwartz MF (2014). Theoretical analysis of word production deficits in adult aphasia. Philosophical Transactions of the Royal Society of London.Series B, Biological Sciences, 369(1634), 20120390 10.1098/rstb.2012.0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, & Klein MG (2005). A patient registry for cognitive rehabilitation research: A strategy for balancing patients’ privacy rights with researchers’ need for access. Archives of Physical Medicine and Rehabilitation, 86(9), 1807–1814. 10.1016/j.apmr.2005.03.009 [DOI] [PubMed] [Google Scholar]
- Schwartz MF, & Dell GS (2010). Case series investigations in cognitive neuropsychology. Cognitive Neuropsychology, 27(6), 477–494. 10.1080/02643294.2011.574111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Middleton EL, & Hamilton R (2015). Word retrieval impairment in adult aphasia In Huntley Bahr R & Silliman ER, (Eds.), Routledge Handbook of Communication Disorders (pp. 278–287). New York, NY: Routledge. [Google Scholar]
- Segaert K, Menenti L, Weber K, Petersson KM, & Hagoort P (2012). Shared syntax in language production and language comprehension — An fMRI study. Cerebral Cortex, 22, 1662–1670. 10.1093/cercor/bhr249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers MS (1996). The structural organization of the mental lexicon and its contribution to age-related declines in spoken-word recognition. Psychology and Aging, 11(2), 333–341. 10.1037//0882-7974.11.2.333 [DOI] [PubMed] [Google Scholar]
- Sommers MS (1998). Spoken word recognition in individuals with dementia of the Alzheimer’s type: changes in talker normalization and lexical discrimination. Psychology and Aging, 13(4), 631–646. 10.1037//0882-7974.13.4.631 [DOI] [PubMed] [Google Scholar]
- Strand EA, Duffy JR, Clark HM, & Josephs K (2014). The apraxia of speech rating scale: A tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders, 51, 43–50. 10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taler V, Aaron GP, Steinmetz LG, & Pisoni DB (2010). Lexical neighborhood density effects on spoken word recognition and production in healthy aging. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences, 65(5), 551–560. 10.1093/geronb/gbq039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilshire CE, & Coslett HB (2000). Disorders of word retrieval in aphasia: Theories and potential applications In Nadeau SE, Gonzalez Rothi LJ & Crosson B, (Eds.), Aphasia and Language: Theory to Practice, (pp. 82–107). New York: The Guilford Press. [Google Scholar]
- Yates M, Friend J, & Ploetz DM (2008). Phonological neighbors influence word naming through the least supported phoneme. Journal of Experimental Psychology: Human Perception and Performance, 34(6), 1599–1608. 10.1037/a0011633 [DOI] [PubMed] [Google Scholar]