Abstract
Candida auris is an invasive and multidrug-resistant ascomycetous yeast that is under global surveillance. All clinical cases of C. auris infection diagnosed from 1997 to 2019 in Japan were non-invasive and sporadic otitis media cases. In the present study, we performed whole-genome sequencing of seven C. auris strains isolated from patients with otitis media in Japan, all of which belonged to clade II. Comparative genome analysis using the high-quality draft genome sequences JCM 15448T revealed that single nucleotide variations (SNVs), clade-specific accessory genes, and copy number variations (CNVs) were identified in each C. auris clade. A total of 61 genes involved in cell wall and stress response-related functions was absent in clade II, and the pattern of conserved CNVs in each clade was more stable in clade II than in other clades. Our data suggest that the genomic structural diversity is stable in C. auris isolated from each biogeographic location, and Japanese strains isolated from patients with otitis media might belong to an ancestral type of C. auris. One Japanese strain, TWCC 58362, with reduced susceptibility to fluconazole, exhibited no mutation in ergosterol biosynthesis-related genes (ERG). However, TWCC 58362-specific variations, including SNVs, indels, and CNVs were detected, suggesting that gene duplication events in C. auris might contribute to antifungal drug resistance. Taken together, we demonstrated that genomic structural variations in C. auris could correlate to geographical dissemination, epidemiology, lesions in the host, and antifungal resistance.
Introduction
Candida albicans is one of the most pathogenic ascomycetous yeasts and remains the most common cause of Candida bloodstream infections (BSI). As with other pathogenic Candida species, the Center for Disease Control and Prevention (CDC) has alerted the global emergence of invasive infections caused by multidrug-resistant C. auris since 2016 (https://www.cdc.gov/fungal/candida-auris/candida-auris-alert.html), with large-scale nosocomial invasive infection cases caused by C. auris from at least five continents [1–12].
Although C. auris is associated with a high mortality rate, treatment is limited because of resistance to antifungal drugs [13]. It is reported that 93% and 35% of C. auris isolates were resistant to fluconazole (FLC) and amphotericin B, respectively, and that mutations in ERG11 and biofilm formation were associated with FLC resistance [13,14]. To make matters worse, C. auris can survive environmental cleaning with chorine-based reagents and hydrogen peroxide vapor [12]. Previous reports indicate that C. auris in nosocomial cases was transmitted by reusable axillary temperature probes, and could persist in health care settings, suggesting that reusable patient equipment might serve as a source of outbreaks of infection with C. auris [15]. Thus, monitoring of C. auris for the prevention of outbreaks should take place in both patients and in the environment.
In Japan, two large-scale fungal infection surveys have been conducted: (i) 1,486 clinical strains were isolated from clinical specimens from 2001 to 2005 [16]; and (ii) 316 strains were isolated from candidemia from 2002 to 2013 [17]. In total, 1,787 strains were identified, and C. auris was first isolated from the external ear canal of a patient in Japan in 2005 [18]. The second Japanese C. auris strain was identified from aural discharge [19]. Intriguingly, these two Japanese C. auris strains were isolated from patients with non-invasive sporadic cases of otitis media. In 2009, C. auris was also identified in patients with chronic otitis media in South Korea [20]. Kwon et al. described 57 clinical isolates of C. auris isolated from the ear obtained from 13 hospitals in Korea [21], with BSI caused by C. auris reported in South Korea in 1996, 2009, and 2017. All Korean isolates in these cases belonged to clade II [1,20,21]. Although Japan and South Korea are relatively close geographically, invasive infections caused by C. auris have not been reported in Japan as of 2019 [22]. The two previously reported Japanese C. auris strains were susceptible to antifungal agents [18,19]. The Japanese strains might therefore differ significantly from those isolated in other countries, which are resistant to one or multiple antifungal drugs and are highly pathogenic.
Phylogenomic analysis of C. auris isolates from several countries has been previously reported [13,15,23,24], indicating that the genotypic clade of C. auris is closely related to its geographical location, such as Venezuela, Japan, South Africa, India, and Pakistan. Thus far, the available genomic sequences of C. auris in the NCBI database were obtained from mainly nosocomial invasive infection cases in Australia, Colombia, Germany, India, Iran, the Netherlands, Pakistan, Russia, South Africa, the United Kingdom, the United States and Venezuela. One genomic sequence of a Japanese isolate (strain B11220) from a sporadic case was reported, possibly because of the lack of outbreak cases in Japan [25]. However, the phylogeny of the Japanese isolate is clearly unique compared to that of isolates from other countries.
In the present study, we aimed to reveal the unique characters of C. auris strains isolated in Japan. We obtained the whole genome sequence of seven C. auris strains isolated from otitis media patients in Japan and constructed a high-quality draft genome sequence of the first reported isolate, type strain JCM 15448T. Moreover, we performed phylogenomic and comparative genomic analysis among Japanese and global strains, to reveal invasive multidrug-resistant features of different C. auris strains. These results contribute to the global effort to manage C. auris infections, particularly in drug-resistant strains.
Results
Isolation and antifungal susceptibility of C. auris strains obtained from patients in Japan
In Japan, the first and second cases of C. auris infection were in 2005 and 2017, respectively [18,19] (Table 1). From 1997 to 2008, five ascomycetous yeast strains, which could not be classified using a conventional fungal identification kit, were isolated from patients with otitis media in Japan. The classification of these strains indicated that all strains belong to C. auris (Table 1). Two of these five isolates, TWCC 13846 and TWCC 13847, were isolated from the left and right ear of the same patient with otitis media on same date, respectively. An antifungal susceptibility test revealed that one of the seven strains, TWCC 58362, was not susceptible to FLC (Table 1).
Table 1. Metadata information and biological characterization of Candida auris strains isolated in Japan.
| Strain name | JCM 15448T | TWCC 13846 | TWCC 13847 | TWCC 13878 | TWCC 50952 | TWCC 58191 | TWCC 58362 |
|---|---|---|---|---|---|---|---|
| Country | Japan | Japan | Japan | Japan | Japan | Japan | Japan |
| Prefecture | Tokyo | Tokyo | Tokyo | Tokyo | Iwate | Tokyo | Tokyo |
| Year | 2005 | 2005 | 2005 | 1997 | 2008 | 2017 | 2008 |
| Host | Human | Human | Human | Human | Human | Human | Human |
| Disease | Otitis media | Otitis media | Otitis media | Otitis media | Otitis media | Otitis media | Otitis media |
| Source | Auditory canal | Otorrhea | Otorrhea | Otorrhea | Otorrhea | Otorrhea | Otorrhea |
| MIC (mg/L) | |||||||
| Micafungin | 0.03 | 0.03 | 0.03 | 0.06 | 0.03 | 0.06 | 0.125 |
| Caspofungin | N.A. | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 | 0.125 |
| Amphotericin B | N.A. | 0.25 | 0.25 | 0.25 | ≤0.03 | 0.25 | 0.25 |
| 5-Fluorocytosine | ≤0.031 | ≤0.125 | ≤0.125 | 0.5 | ≤0.125 | 0.25 | 0.5 |
| Fluconazole | 1 | 4 | 1 | 16 | 2 | 4 | >64 |
| Itraconazole | ≤0.015 | 0.03 | ≤0.015 | 0.125 | 0.03 | 0.06 | 0.25 |
| Voriconazole | ≤0.015 | ≤0.015 | ≤0.015 | 0.125 | 0.03 | 0.03 | 2 |
| Miconazole | N.A. | ≤0.03 | ≤0.03 | 0.06 | ≤0.03 | 0.125 | 1 |
| Reference (PubMed ID) | 19161556 | This study | This study | This study | This study | 29491246 | This study |
| BioSample ID | SAMD00117055 | SAMD00117056 | SAMD00117057 | SAMD00117058 | SAMD00117059 | SAMD00117060 | SAMD00117061 |
| Run ID | DRR129819, DRR129826 | DRR129820 | DRR129821 | DRR129822 | DRR129823 | DRR129824 | DRR129825 |
| Whole genome sequencing | NextSeq, Sequel | MiSeq | MiSeq | MiSeq | MiSeq | MiSeq | MiSeq |
| ST | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Clade | II | II | II | II | II | II | II |
N.A., Not applicable; ST, sequence type; orange shading, not susceptible.
High-quality draft-chromosomal and complete mitochondrial DNA sequences of C. auris JCM 15448
The draft-genome sequencing of C. auris JCM 15448 was obtained using a long-read library in PacBio Sequel, followed by error correction with paired-end short-read libraries using NextSeq. In addition, de novo assembly with only short reads was also performed. The statistics of NGS read and assembly data is summarized in S1 Fig. Twelve contigs were confirmed by circularization, with eleven and one contigs representing the draft chromosomal linear DNA and complete mitochondrial circular DNA (mtDNA), respectively (Table 2). The total contig size of the chromosomal and mtDNA were 12,098,315 and 27,071 bp, respectively, and the number of predicted genes (CDS, rRNA, and tRNA) are shown in Table 2. The total genome length of C. auris JCM 15448 is 229–615 kb shorter than those of C. auris B8441, B11221, and B11243 and is almost the same as that of C. auris B11220 (S1 Table). The average read depth of contig_7 and mtDNA was higher than that of the other contigs (Table 2). The nucleotide sequence structure of contig_7 includes 4 tandem repeats of an rRNA gene cluster (18S, 5.8S, 25S, and 5S ribosomal RNA gene). Contig_7 has 4 times the average coverage ratio against chromosome coverage, suggesting that C. auris JCM15448 possesses more than 4 rRNA gene clusters. The copy number of mtDNA is approximately 9 copies (Table 2). The comparison of mtDNA sequences among C. albicans SC5314, Clavispora lusitaniae CBS 6936, C. auris JCM 15448, C. duobushaemulonii B09383, and C. haemulonii B11899 suggests a diversity of synteny and structure in Saccharomycetales, and the existence of introns in NAD5 (NADH dehydrogenase subunit 5) and COB (cytochrome b) in the mtDNA of JCM 15448 (S2 Fig).
Table 2. Genetic features of chromosomal DNA draft sequences and complete mitochondrial DNA in C. auris JCM 15448.
| Type | Status | SeqID | CDS | rRNA | tRNA | Length (bp) | Average read deptha | Putative copy numberb | Accession number |
|---|---|---|---|---|---|---|---|---|---|
| Chromosomal DNA | High quality draft sequence | contig_1 | 1,677 | 0 | 54 | 3,850,246 | 604 | 1 | BGOX01000001 |
| High quality draft sequence | contig_2 | 1,385 | 0 | 42 | 3,155,023 | 626 | 1 | BGOX01000002 | |
| High quality draft sequence | contig_3 | 1,020 | 0 | 27 | 2,346,960 | 619 | 1 | BGOX01000003 | |
| High quality draft sequence | contig_4 | 389 | 3 | 4 | 943,590 | 629 | 1 | BGOX01000004 | |
| High quality draft sequence | contig_5 | 348 | 0 | 13 | 865,637 | 590 | 1 | BGOX01000005 | |
| High quality draft sequence | contig_6 | 387 | 0 | 11 | 861,415 | 617 | 1 | BGOX01000006 | |
| High quality draft sequence | contig_7 | 3 | 16 | 0 | 31,689 | 2,701 | 4 | BGOX01000007 | |
| High quality draft sequence | contig_8 | 10 | 0 | 0 | 27,068 | 676 | 1 | BGOX01000008 | |
| High quality draft sequence | contig_9 | 2 | 0 | 0 | 8,103 | 614 | 1 | BGOX01000009 | |
| High quality draft sequence | contig_10 | 1 | 0 | 1 | 6,957 | 723 | 1 | BGOX01000010 | |
| High quality draft sequence | contig_11 | 1 | 0 | 0 | 1,627 | 620 | 1 | BGOX01000011 | |
| Mitochondrial DNA | complete sequence | mtDNA | 15 | 2 | 32 | 27,071 | 5,561 | 9 | AP018713 |
aThe average read depth was calculated by illumina short-read mapping analysis using the bwa-mem program.
bThe copy number was calculated by the number of each contig read depth divided by that of average read depth of total chromosomal DNA excluding contig_7.
Comparative core genome and phylogenetic analysis of 133 C. auris strains
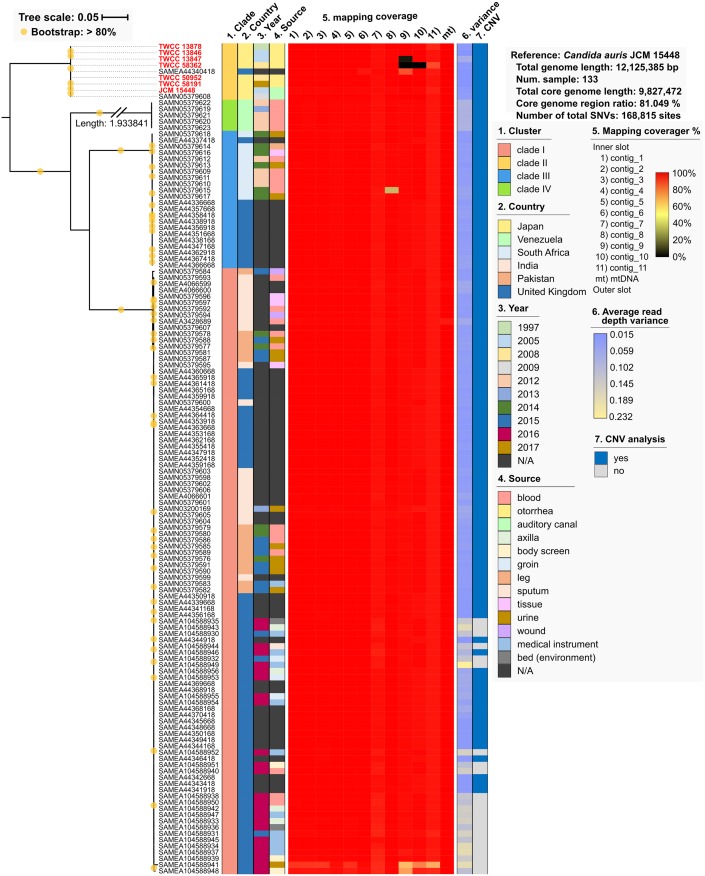
The SNVs on core genome regions were extracted using read-mapping analysis with the JCM 15448 genome as a reference. In this study, the core genome represents the genome sequences excluding repeat regions conserved in all strains by read mapping analysis. A total of 81.05% of the region was assigned as the core genome sequence among 133 C. auris strains, which included 7 Japanese strains that were characterized in the present study, and 126 available genome sequences in the SRA database (Fig 1 and S1 Dataset). The total number of SNVs on core genome regions was 168,815 among the 133 C. auris strains, and phylogenetic analysis using these SNVs indicated the existence of 4 distinct clades (Fig 1), which closely relate to the geographic location, and are consistent with a previous report [13,23,24]. The number of average pairwise SNVs between clade II and other clades ranged from 48,463 to 133,423. All Japanese strains, which were isolated from otorrhea or the auditory canal, and one strain (BioSample ID: SAMEA44340418) from the UK belonged to clade II (Fig 1), and the MLST allele profile of “a-a-a-a”, i.e., ST cluster 2 (Table 1 and S1 Dataset), was the same as that of C. auris strains isolated in Korea [21]. Phylogenetic analysis using mtDNA SNVs revealed the same phylogenetic relationship as that using the core genome sequence (S3 Fig), and mtDNA structures were highly conserved among strains in each clade (S4 Fig). Structural mtDNA variation was detected in COX1 of strains in clade I, III, and IV, and these genes were divided into two regions by a putative intron. These results demonstrate that nucleotide substitution diversity is related to the geographic location in both chromosomal DNA and mtDNA.
Fig 1. Comparative whole genome analysis of C. auris among seven Japanese strains and 126 strains deposited in SRA database.
Phylogenetic analysis with SNVs on core genome regions. 168,815 SNVs were detected in 133 strains. The maximum-likelihood phylogenetic tree was constructed using RAxML version 8.2.0. The clade, metadata, and mapping analysis results are described in the color schemes on the right of the phylogenetic tree.
The median pairwise SNV count for clade I, II, III, and IV was 43, 88, 27, and 7, respectively (S5 Fig). Mapping analysis indicated complete loss of contig_9 in two Japanese strains, TWCC 58362 and TWCC 13847, and loss of contig_10 in TWCC 58362 (Fig 1 and S2 Fig). The sequence of these contigs (9 and 10) could be acquired via horizontal gene transfer from other strains. These results suggest that the genetic variation within clade II is higher than that of other clades.
Clade-specific accessory genes in C. auris
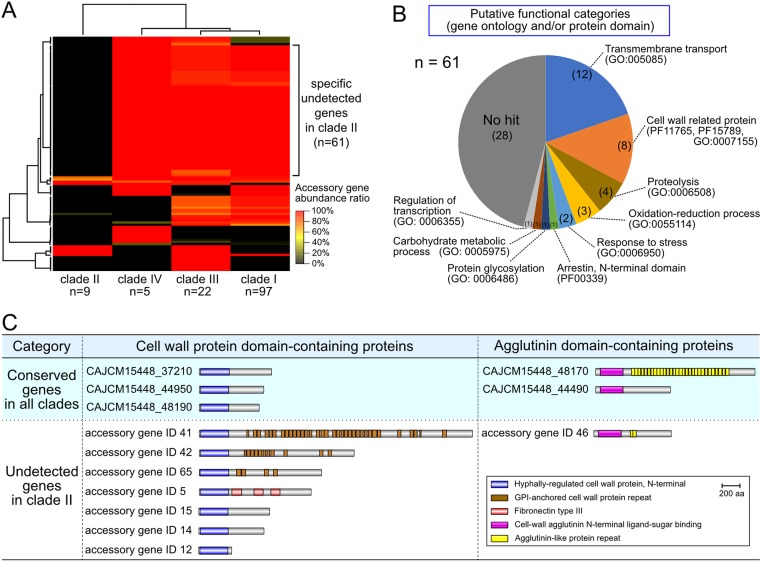
Mapping analysis indicated a partial gene loss in C. auris. To further investigate the genomic variation of C. auris, a pan-genomic approach was adopted, because read-mapping analysis as described above could bias the apparent gene composition based on the reference sequences. The unmapped reads from the read-mapping analysis were extracted and assembled, followed by BLASTN search with the nucleotide database and read depth checking. In each sample, excluding clade II strains, there was a strong correlation of the average read depth between mapping data of reference sequences and contigs that were related to other C. auris genomes assembled from unmapped reads (S1 Dataset). On the other hand, the read depth of contigs that seemed to originate from other organisms (i.e. bacteria, other fungi, etc.) are four times lower than the those of the C. auris genome (S1 Dataset). If these sequences are integrated in the C. auris genome by recombination, mating events, or HGT, these read depths must also be also the same as those of the C. auris genome (S6 Fig). The total length of contigs originating from other organisms is over than 5 Mb in three samples (SAMEA104588948, SAMN05379605, and SAMN05379606). However, these read depths are similar to or higher than those of C. auris. In this case, it is suggested that these sequences are generated from the contamination of original samples. Therefore, these results strongly suggested that 13 of the 133 deposited SRA samples could be contaminated with the genomic DNA of other organisms, including C. glabrata and Cyberlindnera jadinii (S1 Dataset). All nine strains in clade II had no unmapped reads, suggesting that there might be no additional gene acquisition by horizontal gene transfer (HGT) (S1 Dataset). The pan-genomic analysis revealed that the number of clusters against the total, core, and accessory genes was 5,336, 5,214, and 109, respectively. A heatmap analysis revealed that 61 of 109 accessory genes were not detected in clade II (Fig 2A and S2 Dataset). These 61 unidentified genes in only clade II were characterized using gene ontology and domains predicted from protein sequences as shown in Fig 2B. A total of 33 out of 61 genes were assigned to some functional category, which was predicted by InterProScan. Out of these, 12, 8, 3, and 2 genes belonged to transmembrane transporter, cell wall, oxidation-reduction, and stress response related proteins, respectively. The pan-genomic analysis showed that the cell wall proteins were classified into the two groups, i.e., hyphally regulated cell wall protein domain (HD) and agglutinin domain (AD)-containing proteins. Three HD and two AD- containing protein genes were conserved in all strains, while seven HD and one AD-containing protein genes were unidentified in clade II (Fig 2C). Intriguingly, these seven HD-containing proteins possessed unique domain structures (differences in the GPI-anchored cell wall protein repeat, and the existence of a fibronectin type III domain), suggesting that these predicted cell wall proteins might have different functions related to adhesion against various environmental surfaces.
Fig 2. Pangenomic analysis of C. auris strains.
(A) The abundance ratio of the accessory gene among the C. auris clades determined using hierarchical cluster analysis. The relative abundance is shown using color gradients. Sixty-one genes were determined to be specific unidentified genes in clade II. (B) Functional prediction analysis in specific unidentified genes of clade II. A pie chart showing the ratio of the 61 genes categorized by protein domain information and/or gene ontology terms. (C) List of specific unidentified genes, which are related to cell wall proteins, in clade II. The predicted functional domains of the amino acid sequences are described in schematic illustrations. In the pangenome of C. auris, ten and three proteins were predicted to be cell wall protein domain-containing proteins and agglutinin domain-containing proteins, respectively. Eight genes coding cell wall-related proteins remain unidentified in only clade II.
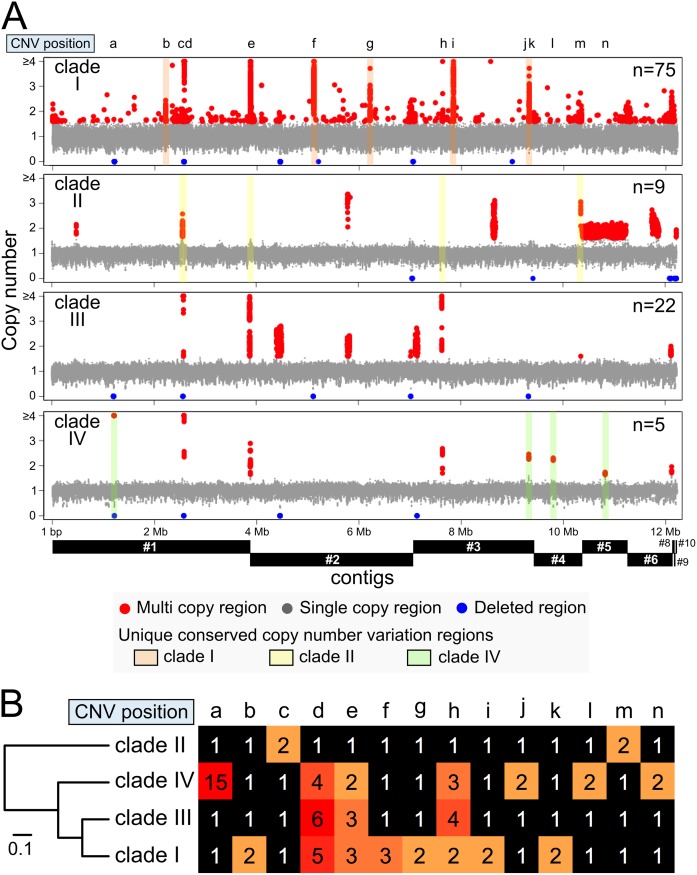
Clade-specific copy number variation among C. auris strains
To characterize copy number variation (CNV), all samples were verified using the variance of read depth. The statistical analysis of variance of read depth indicated that 22 of 133 samples contained high variance, i.e., abnormal distribution of read mapping depth (Fig 1 and S7 Fig). These 22 samples belonged to the same BioProject, ID PRJE20230, and were excluded from further CNV analysis because of the potential existence of artificial biases in these DNA-Seq libraries. Out of the remaining 111 samples, two Japanese strains, TWCC 58362 and TWCC 13847, had a complete loss of contig_9, and TWCC 58362 also had a complete loss of contig_10 (Fig 1). However, the distribution of read mapping depth was stable in other contigs of these two samples. As contig_7 and mtDNA include multi-copy regions, these sequences were excluded from CNV analysis. An average copy number of more than 1.6 copies was denoted as a high copy region and was distinguished from single copy regions (S8 Fig). Comparative CNV analysis indicated the existence of unique conserved copy number regions in clade I, II, and IV (Fig 3A and S3 Dataset). In particular, two conserved high copy and three conserved single copy regions were observed in clade II vs. the other clades (Fig 3A and Table 3). The phylogenetic analysis with conserved CNVs also revealed that the number and frequency of CNVs in clade II were the most stable among the four clades (Fig 3B). Comparison of the phylogenetic tree using chromosomal SNVs, mitochondrial DNA SNVs, accessory gene patterns, and conserved CNV patterns indicated that the four trees had a similar topology pattern, and that clade II belongs to an out-group (S9 Fig). These results suggest that the genetic variation, which includes SNVs, CNVs and accessory gene patterns, is conserved within each clade, and that the genomic structures of clade II might be closely related to those of a common ancestor.
Fig 3. Comparison of copy number variations (CNVs) among four C. auris clades.
(A) A graphic representing the CNV data excluding 22 high variance samples is merged in each clade. A high copy region was defined as having more than 1.6 copies. The conserved CNV regions in each clade are labeled with colored boxes as follows: orange: clade I, yellow: clade II, green: clade IV. Alphabets of small letter indicate the positions of conserved CNVs. CNVs greater than four copies are shown as four copies for ease of interpretation. (B) Phylogenetic tree analysis with conserved CNVs among four clades. The positions of CNV are displayed by alphabets as described above. The copy numbers are showed by a color gradient with black, orange and red. The detailed information of conserved CNVs are described in Table 3.
Table 3. Unique conserved copy number variations on chromosomal DNA among each C. auris cluster.
| Description | Contig name | Region | Position (See Fig 3) | Average copy numbersa | Main CDS on CNV regions | |||
|---|---|---|---|---|---|---|---|---|
| clade I | clade II | clade III | clade VI | |||||
| clade IV unique CNV | contig_1 | 1205001..1208000 | a | 1 | 1 | 1 | 15 | CAJCM15448_05240 (hypothetical protein) |
| clade I unique CNV | contig_1 | 2211001..2212000 | b | 2 | 1 | 1 | 1 | CAJCM15448_09730 (hypothetical protein) |
| clade II unique CNV | contig_1 | 2556001..2561000 | c | 1 | 2 | 1 | 1 | CAJCM15448_11290 (hypothetical protein) |
| CAJCM15448_11300 (putative ZIP zinc/iron transporter) | ||||||||
| clade II unique CNV | contig_1 | 2573001..2578000 | d | 5 | 1 | 6 | 4 | non coding region |
| clade II unique CNV | contig_2 | 1..4000 | e | 3 | 1 | 3 | 2 | CAJCM15448_16780 (hypothetical protein) |
| clade I unique CNV | contig_2 | 1239001..1246000 | f | 3 | 1 | 1 | 1 | CAJCM15448_22010 (putative group II intron reverse transcriptase/maturase) |
| clade I unique CNV | contig_2 | 2339001..2340000 | g | 2 | 1 | 1 | 1 | CAJCM15448_26910 (hypothetical protein) |
| clade II unique CNV | contig_3 | 583001..584000 | h | 2 | 1 | 4 | 3 | CAJCM15448_33140 (hypothetical protein) |
| clade I unique CNV | contig_3 | 794001..798000 | i | 2 | 1 | 1 | 1 | CAJCM15448_34040 (putative group II intron reverse transcriptase/maturase) |
| clade IV unique CNV | contig_3 | 2273001..2274000 | j | 1 | 1 | 1 | 2 | CAJCM15448_40520 (putative group II intron reverse transcriptase/maturase) |
| clade I unique CNV | contig_3 | 2277001..2279000 | k | 2 | 1 | 1 | 1 | CAJCM15448_40520 (putative group II intron reverse transcriptase/maturase) |
| clade IV unique CNV | contig_4 | 384001..385000 | l | 1 | 1 | 1 | 2 | non coding region |
| clade II unique CNV | contig_4 | 935001..936000 | m | 1 | 2 | 1 | 1 | non coding region |
| clade IV unique CNV | contig_5 | 443001..445000 | n | 1 | 1 | 1 | 2 | CAJCM15448_46580 (hypothetical protein) |
aNumber of copy numbers is indicated using rounding up digits after the decimal point to form an integer.
Comparative genome analysis of clade II C. auris
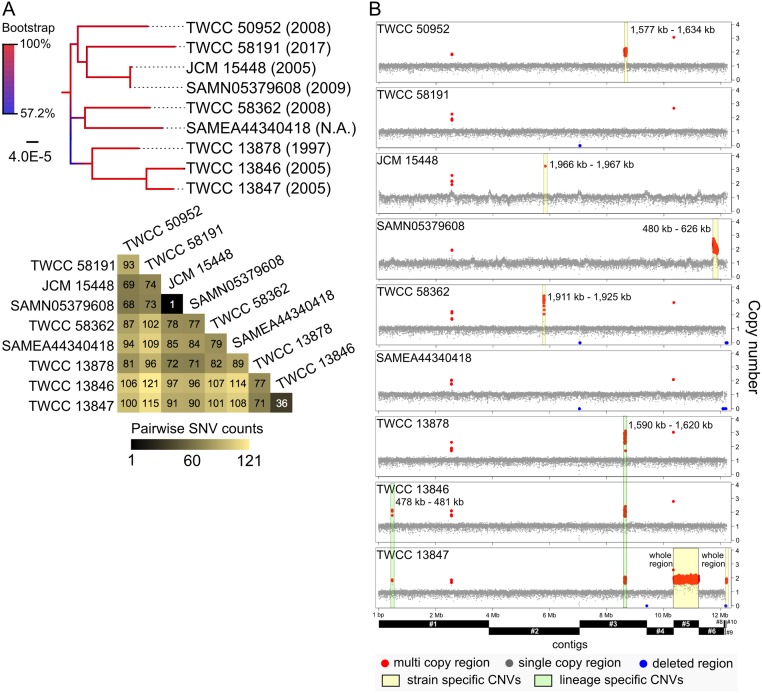
More detailed comparative analysis was carried out between seven Japanese C. auris strains and two samples deposited in the SRA database. In this comparison, 313 SNVs were detected in clade II in total. Only one SNV was detected between JCM 15448 and B11220 (BioSample ID: SAMN05379608; geographic location: Japan) (Fig 4A), and this SNV was a nonsynonymous mutation (L244I) in putative O-succinyl benzoate-CoA ligase gene (CAJCM15448_24570). However, CNV differences were detected between these two strains (Fig 4B), suggesting that they may have originated from the same clonal lineage, but not the same strain. Our results and the results of previous reports indicate that the MICs of FLC in JCM 15448 and B11220 are 1 μg/ml and 4 μg/ml, respectively [14,25]. Although both strains were susceptible to FLC, the MIC of FLC in B11220 was four times higher than that of JCM 15448. The duplication of approximately 146 kb, comprising 63 genes, was detected in B11220, and two of these genes, CAJCM15448_50480 and CAJCM15448_50500, encode putative cytochrome P450 and putative phospholipid-translocating P-type ATPase (flippase), respectively. These data suggest that the genetic variations between JCM 15448 and B11220 might correlate to slight differences in susceptibility to FLC.
Fig 4. Comparative whole genome analysis of nine strains of clade II C. auris.
(A) Core genome SNV phylogenetic tree of strains belonging to clade II. All-against-all comparisons of SNV counts are shown by a lower triangular matrix using a color gradient with black and yellow. (B) Comparative CNV analysis among strains in clade II. Unique CNV regions are labeled by yellow and light green boxes, and base range is noted on each contig.
Mapping analysis revealed that there are several deleted regions and CNVs in clade II. These structural variations highlight strain- and lineage-specific features (Fig 4B). Notably, TWCC 13878, 13846, and 13847 belong to the same lineage and contain the CNV region from 1,590 to 1,620 kb on contig_3 (Fig 4B). Only two strains, TWCC 13846 and 13847, contain the CNV region from 478 to 481 kb on contig_1. However, TWCC 13847 possess other unique CNVs in contig_5, contig_9, and contig_10. These results indicate that some CNVs were conserved during the divergence of this lineage.
Isolates TWCC 13846 and 13847 were obtained from the left and right ear of a patient with chronic middle otitis on same date, respectively. However, 36 sites of SNVs and three regions of CNVs were detected between these two strains, suggesting that the common ancestral clone might have acquired different mutations with a slight variation in the environment over a long time or a population of different genotypes might have colonized in each ear.
In the present study, only one isolate (TWCC 58362) was resistant to FLC (Table 1). It was previously reported that mutation or upregulation of ERG (ERG3 and ERG11) were associated with azole resistance in C. albicans and C. auris [13,24,26,27]. All ergosterol biosynthesis-related genes, including ERG3 and ERG11, are present in the JCM 15448 strain (S2 Table). Somewhat paradoxically, nucleotide mutations were not detected in these ergosterol biosynthesis genes and the adjacent regions in the TWCC 58362 strain. Comparative analysis of clade II strains identified 86 unique mutation sites in TWCC 58362, which are therefore novel resistance genetic markers for FLC (S4 Dataset). In particular, nonsynonymous mutations in 20 genes, multi copy regions in 10 genes, and gene loss of two genes was identified in TWCC 58362. The multi copy genes were included in one region of approximately 14 kb in contig_2, and the number of duplications in this CNV were approximately three times that of single copy regions (Fig 4B and S3 Dataset). These results suggest that specific mutation sites, including CNVs in TWCC 58362, might relate to an uncharacterized antifungal resistance mechanism.
Discussion
In the present study, we obtained high-quality draft genome sequences, including complete mtDNA sequences, for C. auris isolated in Japan. Comparative analysis revealed that the synteny and structure of mtDNA dramatically changed among Candida and related species and that C. auris mtDNA might contain introns in NAD5 and COB genes (S2 Fig). Mitochondrial DNA structures have great evolutionary variability, even in closely related fungal species [28], and therefore, the mtDNA of C. auris may have also evolved in a unique manner in the genus Candida. The mtDNA sequences except for the COX1 region are highly conserved in C. auris strains, and the phylogenetic relationship of mtDNA is similar to that of the chromosomal DNA (Fig 1 and S3 Fig). This suggests that the nuclear and mitochondrial DNA of C. auris might have followed parallel evolution, albeit at different evolutionary rates.
Although the monophyletic clades of C. auris have disseminated in specific biogeographic locations, including Japan (Fig 1), the median of pairwise SNV counts of clade II is significantly higher than that of other clades (S5 Fig). All Japanese strains used in this study were isolated from patients with non-invasive, sporadic cases of otitis media, suggesting that the sporadic nature of these cases might be explained by SNV genomic analysis. The CDC issued a warning related to the global emergence of invasive infections caused by multidrug-resistant C. auris in 2016 (https://www.cdc.gov/fungal/candida-auris/candida-auris-alert.html), and cases of large-scale nosocomial invasion have been reported in more than 30 countries on six continents [22,29,30]. On the other hand, all Japanese C. auris strains were isolated from ear specimen and not from invasive mycoses [22], highlighting the differences in virulence and/or environmental viability might exist between C. auris clinical isolates from Japan and other countries. However, the comparative genomic analysis revealed several unidentified genes in the Japanese strains (Fig 2A), including transmembrane transport proteins, oxidation-reduction related proteins, and stress response proteins, which might be important for coping with environmental stress (Fig 2B). Pangenomic analysis also suggested that the features of clinical isolates that caused outbreaks or invasive infections might be related to the number of and/or variations to genes encoding cell wall proteins, which were classified into two groups: 1) hyphally-regulated cell wall protein domain (HD) and 2) agglutinin domain (AD)-containing proteins. It was previously reported that HD-containing protein in C. albicans, named Hyr1, is an important virulence factor mediating resistance to neutrophil killing, and is required for pathogenesis during Candida mucosal biofilm infections [31–33]. HD-containing proteins are comprised of various domain structures and are different lengths, suggesting that these cell wall proteins could serve different functions, including mediating virulence. Eight agglutinin-like sequence (Als) family proteins are made up of an agglutinin domain and agglutinin-like protein repeats [34,35], and one of these eight proteins, Als3, is linked to epithelial cell interaction [36]. Thus, we speculate that the AD-containing proteins of C. auris might be also related to cell adhesion. All C. auris strains from Japan, which lack multiple cell wall proteins, were detected from ear specimens but not invasive mycoses, leading us to speculate that these eight cell wall proteins are not essential proteins and might act as virulence factors, including cell adhesion ability.
Clinical information and our comparative genomic data suggest that the Japanese strains isolated from patients with otitis media might be closely related to the most common ancestral type rather than other strains isolated elsewhere. HGT, gene duplications, gain and loss are an important evolutionary process in prokaryotes [37]. It was suggested that HGT is also important evolutional mechanisms in unicellular eukaryote, particularly in fungi [38]. Some strains that caused outbreaks or invasive infections might therefore have evolved through the acquisition of function with HGT against environmental stress and host cell interactions. To demonstrate this hypothesis, further genomic comparisons between invasive and non-invasive isolates belonging to clade II may identify the genetic factors underlying invasive and multidrug-resistant phenotypes.
Previous reports have described the relationship between SNV patterns and geographic locations [13,23,24]. In the present study, we revealed that the geographic location of C. auris strains closely related to genetic features, including nucleotide substitutions, accessory gene patterns, and CNVs (Figs 1, 2A and 3B). CNV patterns were also conserved in a small lineage of clade II (Fig 4B). We therefore speculate that nucleotide substitutions, and gene acquisition and duplication events might also be related to the evolutionary steps associated with geographic segregation of C. auris. In addition, our data suggests that clade II might be an ancestral type because of the low frequency of CNVs observed in clade II compared to other clades.
In the present study, one of the seven Japanese strains, TWCC 58362, was resistant to FLC (Table 1). This strain had no mutations detected in ERG, which have previously been associated with azole resistance [13,24,26,27]. Although biofilm formation is associated with FLC resistance [14], it has been reported that C. auris isolates from clade II from ear specimens did not form biofilms [39]. Comparative genomic analysis revealed that TWCC 58362 has unique mutation sites, which included SNVs, indels, and CNVs (S4 Dataset), suggesting that other mechanisms for drug resistance might exist in this strain. In C. albicans, chromosome 5 monosomy, which is related to CNVs, confers reduced susceptibility to several antifungals and shifts the ratio such that a diminished dose of genes for negative regulation determines tolerance to caspofungin [40,41]. The same phenomenon might not be applicable to C. auris, because C. auris is haploid while C. albicans is diploid. Comparative analysis between JCM 15448 and B11220 revealed that duplication of putative cytochrome P450 (CAJCM15448_50480) and putative phospholipid-translocating P-type ATPase (CAJCM15448_50500) in B11220 might be related to the degree of susceptibility to FLC. Ergosterol is one of the important components of the fungal cell membrane, and FLC bind to Erg11, which is a member of the cytochrome P450 family, to inhibit ergosterol biosynthesis [42–44]. A family of P-type ATPases has previously been associated with transport or flipping of phospholipids across cell membranes [45]. Therefore, duplications of these genes (CAJCM15448_50480 and CAJCM15448_50500) through CNVs might be related to the degree of susceptibility to FLC via homeostasis of ergosterol biosynthesis and/or stability of the cell membrane structure. Our data hints that the variability of CNVs in C. auris might represent a new mechanism of antifungal drug resistance.
FLC resistance in C. auris has been reported in several countries, and our data represent the first case of FLC resistance in a clinical strain in Japan. Azole antifungals are used for both human and animal pharmacotherapy and as an agricultural pesticide, antifouling coating, and timber preservative [46]. Spatiotemporal data from the United States Geological Survey (USGS) shows that the consumption of agricultural triazole is increasing in the USA (https://water.usgs.gov/nawqa/pnsp/usage/maps/compound_listing.php). Consumption of triazoles and diazoles, which are used as pesticides, is reported by the Food and Agriculture Organization of the United Nations (FAO), has increased in Japan, Germany, France, the UK, Poland, Spain, and Italy, every year (FAOSTAT: http://www.fao.org/faostat/en/?#home). Although the trend of azole usage in Japan is similar to those of Spain and Italy, the total consumption of azoles in Japan is 2 to 8.7 times lower than those of Germany, France, the UK, and Poland. The number of reports of azole-resistant fungal species has increased in many counties [46], although the direct relationship between increased antifungal drug resistance in fungi and the consumption of antifungal drugs is not currently known. The percentage of isolates that are not susceptible to FLC, or with decreased susceptibility to FLC, is positively correlated with the usage of FLC at individual hospitals in Korea [47]. We speculate that the frequency of usage of antifungal drugs in humans and environments might be related to the appearance of FLC resistance in C. auris in a biogeographic location-dependent manner.
In conclusion, we present a high-quality draft chromosomal and complete mitochondrial DNA sequences of C. auris JCM 15448 strain, and performed a large-scale comparative genomic analysis among C. auris strains isolated from several countries across three continents. Our data suggest that C. auris with stable genomic structures, which conserved gene acquisition and copy number variations, have been expanding from Asia to each biogeographic location, followed by an increasing epidemic in the world, with the exception of Japan. We speculate that Japanese isolates belonging to clade II might have evolved to adapt to the host, and have a propensity for the ear, resulting in a non-invasive clinical phenotype, which is supported by our genomic and clinical data. Our comparative analysis among Japanese isolates revealed that antifungal drug resistance in C. auris might correlate to nonsynonymous substitution by point mutation and pseudogenization by indels, as well as gene duplication by CNVs. Finally, we demonstrated that the specific genetic background of C. auris in East Asia, including Japan, is obviously distinct from those of other countries, and that FLC resistant strains belonging to the East Asian clade, clade II, possess marked genetic variations related to resistance mechanisms against antifungal drugs.
Materials and methods
Fungal growth conditions and identification
Two strains of C. auris, JCM 15448 and TWCC 58191, were previously isolated in Japanese hospitals [18,19]. Ascomycetous yeast strains, isolated from otorrhea of patients with chronic otitis media in Japan from 1997 to 2017 (Table 1), were cultured using Sabouraud dextrose agar (Becton Dickinson, Baltimore, MD, USA) for 2 days at 35°C. For identification of these clinical isolates, DNA extraction was performed using Isoplant II DNA extraction kit (Nippon Gene inc., Tokyo, Japan), direct-sequencing of the 26S D1/D2 domain and ITS region, and a BLASTN homology search against known sequences.
Antifungal susceptibility test
The antifungal susceptibility test was performed according to the Clinical and Laboratory Standards Institute (CLSI) broth microdilution methods M27-A3 [48]. We used tentative breakpoints for C. auris proposed by the CDC (https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html) and Lockhart et al. [13]. Briefly, interpretive breakpoints for C. auris were defined based on the breakpoints established for other closely related Candida species as follows: micafungin (MCFG) at ≥ 4 μg/mL, caspofungin (CPFG) at ≥ 2 μg/mL, amphotericin B (AMB) at ≥ 2 μg/mL, 5-fluorocytosine (5FC) at ≥ 128 μg/mL, fluconazole (FLC) at ≥ 32 μg/mL, and voriconazole (VRC) at ≥ 2 μg/mL.
Whole genome sequencing
To prepare long-chain genomic DNA isolated from JCM 15448, DNA extraction was performed as described previously [49]. Briefly, fungal cells were cultured in 10 ml RPMI1640 medium for 2 days, suspended in 450 μl Tris-EDTA (TE) buffer. The cell suspension was supplemented with 50 μl SDS and 500 μl phenol/chloroform, followed by bead-beating for 10 min by vortexing. After centrifugation at 17,400 x g for 5 min, the upper aqueous phase was subjected to electrophoresis on a 1% TAE agarose gel, followed by purification of long size DNA (15-40kb) using a Zymoclean large-fragment DNA recovery kit (Zymoresearch, Irvine, CA, USA). Short read and long read DNA libraries with purified DNA were constructed using QIAseq FX DNA Library Kit (QIAGEN, Hilden, Germany) and the SMRTbell template prep kit 1.0 (PacBio, Menlo Park, CA, USA) according to the manufacturer instructions, followed by whole-genome sequencing using NextSeq (Illumina, San Diego, CA, USA), MiSeq (Illumina), and Sequel (PacBio).
De novo assembly and annotation
Polishing of pre-assembled long reads was performed using Falcon version 0.3.0, followed by de novo assembly with polished long reads using Canu version 1.4 [50], Minimap version 0.2-r124 [51], Racon version 1.1.0 [52] and Circulator version 1.5.3 [53]. Error correction of the tentative complete or draft sequence was performed using Pilon version 1.18 [54] with Illumina short reads excluding adapter and low-quality sequences, which were trimmed using Fastq-mcf version 1.04.636 [55] and Sickle version 1.33 (https://github.com/najoshi/sickle). De novo assembly with short reads was performed using A5-miseq version 20140604 [56]. Gene prediction of complete or high-quality draft sequences was performed as follows: GeneMark-ES version 4.21 [57] using the fungal genome mode parameter against protein coding sequence (CDS); Aragorn version 1.2.38 [58] with default against tRNA; BLASTN search with rRNA sequences of C. albicans SC5314 (assembly accession number: GCA_000182965.3) against rRNA. Annotation of CDS was performed using Hmmscan in the HMMer software package version 3.1b2 [59] with TIGRFAMs database [60], RPS-BLAST [61] with the COG database [62] and InterProScan v49.0 [63]. The multi locus sequence typing (MLST) analysis was performed using a BLASTN similarity search using 4 allele sequences and allele profiles, which were reported by Kwon et al [21].
Single nucleotide variation (SNV) detection and core-genome phylogenetic analysis
Publicly available short read sequencing data for C. auris was retrieved from the SRA database hosted by the NCBI in August 2018. A total of 133 samples (7 from the present study and 126 from the public database) were used in SNV detection analysis. Publicly available data of strains isolated from six countries (India, Japan, Pakistan, South Africa, the UK, and Venezuela) were included in seven projects {BioProject ID: PRJEB14717 (not published), PRJEB20230 [24], PRJEB21518 (not published), PRJEB9463 [64], PRJNA267757 [65] and PRJNA328792 [13,25]}. Read mapping was performed using bwa-MEM [66] with default parameters against the JCM 15448 genome sequence as a reference, followed by variant calling using VarScan v2.3.4 [67]. The repeat sequences in reference sequences were detected using NUCmer [68] with default parameters. The "core genome" was defined as the conserved region of the JCM 15448 genome among all strains and excluding repeat regions. Variant sites on repeat regions and low read depth regions, i.e., deleted regions, were excluded from the final SNV dataset. The concatenated sequences constructed from SNVs were used to construct a maximum-likelihood phylogenetic tree using RAxML version 8.2.0 with GTRCAT model and 1,000 bootstrap replicates [69]. A pairwise SNV distance matrix was created using Snp-dists (https://github.com/tseemann/snp-dists). The phylogenetic tree was visualized using Interactive Tree Of Life (iTOL) version 3 [70].
Copy number variation (CNV) analysis
The mapping method with Illumina short reads was performed as described in the above subsection. The average coverage read depth with a window size of 1,000 bp (non-overlapping windows) was analyzed using igvtools version 2.3.98 [71] with the following parameters: z7 and w1000. The copy number ratio in 1,000 bp was calculated with the following formula: copy number ratio in 1,000 bp = read depth in 1,000 bp/average read depth of chromosomal contigs, excluding contig_7 which had tandem repeats of rRNA gene clusters. The scatterplot of CNV data was visualized using the plot function of R program.
Accessory gene analysis
Unmapped reads in each sample were collected from mapping data followed by de novo assembly using A5-miseq. These contigs were searched using Megablast with the nucleotide database, and the taxonomic classification of contigs was validated using MEGAN version 6 [72], to eliminate putative contamination contig data. Gene prediction of the validated contigs was performed using GeneMark-ES as previously described. To generate orthologous gene clusters among genes predicted from unmapped contigs and JCM 15448 contigs, clustering analysis based on amino acid sequences was performed using USEARCH version 8.1.1812 [73] with the following parameters after sorting by sequence length: id = 0.9, minqt = 0.5, maxqt = 2, minsl = 0.5, query_cov = 0.5, and target_cov = 0.5. The database for each sample was constructed with consensus sequences, which were converted from mapping data, and from contigs, which were assembled from unmapped reads. The nucleotide sequences of these orthologous gene clusters were searched using Megablast against database of each sample. For hierarchical clustering analysis, the abundance ratio of accessory genes was calculated for each C. auris clade, and clustered using the amap package in R with the distance matrix computation using a Pearson distribution and hierarchical clustering using Ward’s method. The heatmap with dendrograms was drawn using heatmap.2 in the R package gplots. In this pan-genomic analysis, we defined a conserved gene as shared in ≥ 129 strains, and an accessory gene as shared in ≥ 4 strains, because all sequences are draft genomes and several contain DNA contamination.
Data deposition
The sequences of JCM 15448 are available in DDBJ/EMBL/GenBank with the following accession numbers: chromosomal DNA of the draft genome, BGOX01000001-BGOX01000011; complete mtDNA, AP018713. Raw read sequence data have been deposited in DRA database under the following accession numbers: JCM 15448, DRR129819 and DRR129826; TWCC 13846, DRR129820; TWCC 13847, DRR129821; TWCC 13878, DRR129822; TWCC 50952, DRR129823; TWCC 58191, DRR129824; and TWCC 58362, DRR129825 (as shown in Table 1).
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
The summary of long- and short-read information is described in the left panel. The histogram indicates read length distribution of subreads and polished long reads. The summary of de novo assembly information is described in the right panel.
(TIFF)
Schematic representation and comparative analysis of complete or draft mitochondrial DNA sequences among Candida albicans SC5314, Clavispora lusitaniae CBS 6936, C. auris JCM 15448T, C. duobushaemulonii B09383 and C. haemulonii B11899. Pairwise alignment was performed using TBLASTX, followed by visualization using Easyfig version 2.1 with following parameters: minimum length of blast hits, 30; maximum e-value, 1e-10; minimum identity value, 60. The red bars between mtDNA sequences represent individual amino acid sequences matches translated from nucleotide sequences. Light blue fragmented arrows represent the coding sequences (CDS) including intron predicted by comparative analysis.
(TIFF)
Twenty-eight SNVs were detected in 133 strains. The maximum-likelihood phylogenetic tree was constructed using FastTree version 2.1.10. Information of cluster type, metadata is described in color schemes on outside slots of the phylogenetic tree. The constructed clusters are closely similar to SNV trees with whole genome.
(TIFF)
Pairwise alignment was performed using BLASTN, followed by visualization using Easyfig version 2.1 with parameters described in S1 Fig. The mtDNA structures was conserved in each cluster.
(TIFF)
All-against-all comparisons of SNV counts were performed among each clade. Statistical analysis was performed using Wilcoxon rank sum test; the difference was statistically significant (p < 0.001) among all clusters. The boxplot indicates that median of pairwise SNV of clade I, III and IV is lower than those of clade II because of including outbreak case.
(TIFF)
The contigs assembled with unmapped reads against reference sequences were analyzed by BLASTN, followed by taxonomic classification of contigs. These data reveal that the average read depth of non-C. auris contigs show different distributions from those of C. auris contigs.
(TIFF)
This graph shows a histogram of read mapping variances. Twenty-two samples were rejected in CNV analysis because of abnormal distribution of read mapping depth. These all samples are included in same BioProject ID (PRJEB20230).
(TIFF)
This graph shows a histogram of average copy number in 111 samples which are collected by mapping variance analysis. Error bars represent standard deviation. The statistical data shows that lower than approximately 1.5 and more than 1.6 copy numbers are single and high copy, respectively.
(TIFF)
These trees indicate similar topology pattern, and the clade II belongs out of group against clade I, III and IV.
(TIFF)
(PDF)
(PDF)
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Data Availability
All sequence files are available from the DDBJ/EMBL/Genbank database (accession numbers BGOX01000001-BGOX01000011 and AP018713). All raw read sequence files are available from the DRA/SRA database (accession numbers DRR129819-DRR129826).
Funding Statement
This work was supported by grants from the Japan Agency for Medical Research and Development, AMED (JP19fk0108048 and JP19fk0108103 to MK, and JP18fk0108045 to YM and KK). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, et al. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol. 2011;49: 3139–3142. 10.1128/JCM.00319-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash A, Singh PK, et al. New clonal strain of Candida auris, Delhi, India. Emerging Infect Dis. 2013;19: 1670–1673. 10.3201/eid1910.130393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarma S, Kumar N, Sharma S, Govil D, Ali T, Mehta Y, et al. Candidemia caused by amphotericin B and fluconazole resistant Candida auris. Indian J Med Microbiol. 2013;31: 90–91. 10.4103/0255-0857.108746 [DOI] [PubMed] [Google Scholar]
- 4.Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis. 2014;33: 919–926. 10.1007/s10096-013-2027-1 [DOI] [PubMed] [Google Scholar]
- 5.Magobo RE, Corcoran C, Seetharam S, Govender NP. Candida auris-associated candidemia, South Africa. Emerging Infect Dis. 2014;20: 1250–1251. 10.3201/eid2007.131765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo B, Melo ASA, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, et al. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J Infect. 2016;73: 369–374. 10.1016/j.jinf.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 7.Borman AM, Szekely A, Johnson EM. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. mSphere. 2016;1 10.1128/mSphere.00189-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borman AM, Szekely A, Johnson EM. Isolates of the emerging pathogen Candida auris present in the UK have several geographic origins. Med Mycol. 2017;55: 563–567. 10.1093/mmy/myw147 [DOI] [PubMed] [Google Scholar]
- 9.Ben-Ami R, Berman J, Novikov A, Bash E, Shachor-Meyouhas Y, Zakin S, et al. Multidrug-Resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerging Infect Dis. 2017;23 10.3201/eid2302.161486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, et al. Investigation of the First Seven Reported Cases of Candida auris, a Globally Emerging Invasive, Multidrug-Resistant Fungus-United States, May 2013-August 2016. Am J Transplant. 2017;17: 296–299. 10.1111/ajt.14121 [DOI] [PubMed] [Google Scholar]
- 11.Morales-López SE, Parra-Giraldo CM, Ceballos-Garzón A, Martínez HP, Rodríguez GJ, Álvarez-Moreno CA, et al. Invasive Infections with Multidrug-Resistant Yeast Candida auris, Colombia. Emerging Infect Dis. 2017;23: 162–164. 10.3201/eid2301.161497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5: 35 10.1186/s13756-016-0132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin Infect Dis. 2017;64: 134–140. 10.1093/cid/ciw691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominguez EG, Zarnowski R, Choy HL, Zhao M, Sanchez H, Nett JE, et al. Conserved Role for Biofilm Matrix Polysaccharides in Candida auris Drug Resistance. mSphere. 2019;4 10.1128/mSphereDirect.00680-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyre DW, Sheppard AE, Madder H, Moir I, Moroney R, Quan TP, et al. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N Engl J Med. 2018;379: 1322–1331. 10.1056/NEJMoa1714373 [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi H, Uchida K, Nishiyama Y, Group TJASP. Species distribution and in vitro susceptibility to three antifungal triazoles of clinical Candida isolates from a five-year nation-wide survey in Japan. Medical Mycology Research. 2012;3: 17–26. [Google Scholar]
- 17.Ishikane M, Hayakawa K, Kutsuna S, Takeshita N, Ohmagari N. Epidemiology of Blood Stream Infection due to Candida Species in a Tertiary Care Hospital in Japan over 12 Years: Importance of Peripheral Line-Associated Candidemia. PLoS ONE. 2016;11: e0165346 10.1371/journal.pone.0165346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53: 41–44. 10.1111/j.1348-0421.2008.00083.x [DOI] [PubMed] [Google Scholar]
- 19.Iguchi S, Mizushima R, Kamada K, Itakura Y, Yoshida A, Uzawa Y, et al. The Second Candida auris Isolate from Aural Discharge in Japan. Jpn J Infect Dis. 2018;71: 174–175. 10.7883/yoken.JJID.2017.466 [DOI] [PubMed] [Google Scholar]
- 20.Kim M-N, Shin JH, Sung H, Lee K, Kim E-C, Ryoo N, et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin Infect Dis. 2009;48: e57–61. 10.1086/597108 [DOI] [PubMed] [Google Scholar]
- 21.Kwon YJ, Shin JH, Byun SA, Choi MJ, Won EJ, Lee D, et al. Candida auris Clinical Isolates from South Korea: Identification, Antifungal Susceptibility, and Genotyping. Land GA, editor. J Clin Microbiol. American Society for Microbiology Journals; 2019;57: 3139 10.1128/JCM.01624-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iguchi S, Itakura Y, Yoshida A, Kamada K, Mizushima R, Arai Y, et al. Candida auris: A pathogen difficult to identify, treat, and eradicate and its characteristics in Japanese strains. J Infect Chemother. 2019;25: 743–749. 10.1016/j.jiac.2019.05.034 [DOI] [PubMed] [Google Scholar]
- 23.Escandón P, Chow NA, Caceres DH, Gade L, Berkow EL, Armstrong P, et al. Molecular epidemiology of Candida auris in Colombia reveals a highly-related, country-wide colonization with regional patterns in Amphotericin B resistance. Clin Infect Dis. 2018. 10.1093/cid/ciy411 [DOI] [PubMed] [Google Scholar]
- 24.Rhodes J, Abdolrasouli A, Farrer RA, Cuomo CA, Aanensen DM, Armstrong-James D, et al. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg Microbes Infect. 2018;7: 43 10.1038/s41426-018-0045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muñoz JF, Gade L, Chow NA, Loparev VN, Juieng P, Berkow EL, et al. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun. Nature Publishing Group; 2018;9: 5346 10.1038/s41467-018-07779-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarma S, Upadhyay S. Current perspective on emergence, diagnosis and drug resistance in Candida auris. Infect Drug Resist. 2017;10: 155–165. 10.2147/IDR.S116229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry KW, Nickels JT, Edlind TD. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob Agents Chemother. 2000;44: 2693–2700. 10.1128/aac.44.10.2693-2700.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolondra A, Labedzka-Dmoch K, Wenda JM, Drzewicka K, Golik P. The transcriptome of Candida albicans mitochondria and the evolution of organellar transcription units in yeasts. BMC Genomics. 2015;16: 827 10.1186/s12864-015-2078-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saris K, Meis JF, Voss A. Candida auris. Curr Opin Infect Dis. 2018;31: 334–340. 10.1097/QCO.0000000000000469 [DOI] [PubMed] [Google Scholar]
- 30.Chowdhary A, Sharma C, Meis JF. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. Hogan DA, editor. PLoS Pathog. Public Library of Science; 2017;13: e1006290 10.1371/journal.ppat.1006290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis. 2010;201: 1718–1728. 10.1086/652407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo G, Ibrahim AS, French SW, Edwards JE, Fu Y. Active and passive immunization with rHyr1p-N protects mice against hematogenously disseminated candidiasis. PLoS ONE. 2011;6: e25909 10.1371/journal.pone.0025909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dwivedi P, Thompson A, Xie Z, Kashleva H, Ganguly S, Mitchell AP, et al. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLoS ONE. 2011;6: e16218 10.1371/journal.pone.0016218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyer LL, Green CB, Oh S-H, Zhao X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family—a sticky pursuit. Med Mycol. 2008;46: 1–15. 10.1080/13693780701435317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoyer LL. The ALS gene family of Candida albicans. Trends Microbiol. 2001;9: 176–180. 10.1016/s0966-842x(01)01984-9 [DOI] [PubMed] [Google Scholar]
- 36.Murciano C, Moyes DL, Runglall M, Tobouti P, Islam A, Hoyer LL, et al. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS ONE. 2012;7: e33362 10.1371/journal.pone.0033362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koskiniemi S, Sun S, Berg OG, Andersson DI. Selection-driven gene loss in bacteria. PLoS Genet. 2012;8: e1002787 10.1371/journal.pgen.1002787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzpatrick DA. Horizontal gene transfer in fungi. FEMS Microbiol Lett. 2012;329: 1–8. 10.1111/j.1574-6968.2011.02465.x [DOI] [PubMed] [Google Scholar]
- 39.Oh BJ, Shin JH, Kim M-N, Sung H, Lee K, Joo MY, et al. Biofilm formation and genotyping of Candida haemulonii, Candida pseudohaemulonii, and a proposed new species (Candida auris) isolates from Korea. Med Mycol. 2011;49: 98–102. 10.3109/13693786.2010.493563 [DOI] [PubMed] [Google Scholar]
- 40.Yang F, Kravets A, Bethlendy G, Welle S, Rustchenko E. Chromosome 5 monosomy of Candida albicans controls susceptibility to various toxic agents, including major antifungals. Antimicrob Agents Chemother. 2013;57: 5026–5036. 10.1128/AAC.00516-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Zhang L, Wakabayashi H, Myers J, Jiang Y, Cao Y, et al. Tolerance to Caspofungin in Candida albicans Is Associated with at Least Three Distinctive Mechanisms That Govern Expression of FKS Genes and Cell Wall Remodeling. Antimicrob Agents Chemother. 2017;61 10.1128/AAC.00071-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. American Society for Microbiology (ASM); 1999;12: 501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perea S, Patterson TF. Antifungal resistance in pathogenic fungi. Clin Infect Dis. 2002;35: 1073–1080. 10.1086/344058 [DOI] [PubMed] [Google Scholar]
- 44.Albertson GD, Niimi M, Cannon RD, Jenkinson HF. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. American Society for Microbiology (ASM); 1996;40: 2835–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen JP, Vestergaard AL, Mikkelsen SA, Mogensen LS, Chalat M, Molday RS. P4-ATPases as Phospholipid Flippases-Structure, Function, and Enigmas. Front Physiol. Frontiers; 2016;7: 275 10.3389/fphys.2016.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360: 739–742. 10.1126/science.aap7999 [DOI] [PubMed] [Google Scholar]
- 47.Won EJ, Shin JH, Choi MJ, Lee WG, Park Y-J, Uh Y, et al. Antifungal susceptibilities of bloodstream isolates of Candida species from nine hospitals in Korea: application of new antifungal breakpoints and relationship to antifungal usage. Chaturvedi V, editor. PLoS ONE. 2015;10: e0118770 10.1371/journal.pone.0118770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinical and Laboratory Standards Institute. M27-A3: Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard 3rd ed. Wayne: Clinical and Laboratory Standard Institute.
- 49.Kato K, Hashino M, Ito T, Matsui M, Suzuki S, Kai K, et al. Rapid and affordable size-selected PacBio single-molecule real-time sequencing template library construction using the bead-beating DNA extraction method. Journal of Biological Methods. 2017;4: e79 10.14440/jbm.2017.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27: 722–736. 10.1101/gr.215087.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H. Minimap and miniasm: fast mapping and de novo assembly for noisy long sequences. Bioinformatics. 2016;32: 2103–2110. 10.1093/bioinformatics/btw152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaser R, Sović I, Nagarajan N, Šikić M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017;27: 737–746. 10.1101/gr.214270.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunt M, Silva ND, Otto TD, Parkhill J, Keane JA, Harris SR. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol. 2015;16: 294 10.1186/s13059-015-0849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9: e112963 10.1371/journal.pone.0112963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aronesty E. Comparison of Sequencing Utility Programs. TOBIOIJ. 2013;7: 1–8. 10.2174/1875036201307010001 [DOI] [Google Scholar]
- 56.Coil D, Jospin G, Darling AE. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics. 2015;31: 587–589. 10.1093/bioinformatics/btu661 [DOI] [PubMed] [Google Scholar]
- 57.Ter-Hovhannisyan V, Lomsadze A, Chernoff YO, Borodovsky M. Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome Res. 2008;18: 1979–1990. 10.1101/gr.081612.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laslett D, Canback B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004;32: 11–16. 10.1093/nar/gkh152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mistry J, Finn RD, Eddy SR, Bateman A, Punta M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013;41: e121 10.1093/nar/gkt263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haft DH, Selengut JD, Richter RA, Harkins D, Basu MK, Beck E. TIGRFAMs and Genome Properties in 2013. Nucleic Acids Res. 2013;41: D387–95. 10.1093/nar/gks1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marchler-Bauer A, Panchenko AR, Shoemaker BA, Thiessen PA, Geer LY, Bryant SH. CDD: a database of conserved domain alignments with links to domain three-dimensional structure. Nucleic Acids Res. 2002;30: 281–283. 10.1093/nar/30.1.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, et al. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45: D200–D203. 10.1093/nar/gkw1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30: 1236–1240. 10.1093/bioinformatics/btu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma C, Kumar N, Meis JF, Pandey R, Chowdhary A. Draft Genome Sequence of a Fluconazole-Resistant Candida auris Strain from a Candidemia Patient in India. Genome Announc. American Society for Microbiology; 2015;3: 41 10.1128/genomeA.00722-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chatterjee S, Alampalli SV, Nageshan RK, Chettiar ST, Joshi S, Tatu US. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genomics. 2015;16: 686 10.1186/s12864-015-1863-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26: 589–595. 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koboldt DC, Chen K, Wylie T, Larson DE, McLellan MD, Mardis ER, et al. VarScan: variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics. 2009;25: 2283–2285. 10.1093/bioinformatics/btp373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5: R12 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22: 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 70.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44: W242–5. 10.1093/nar/gkw290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinformatics. 2013;14: 178–192. 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huson DH, Beier S, Flade I, Górska A, El-Hadidi M, Mitra S, et al. MEGAN Community Edition—Interactive Exploration and Analysis of Large-Scale Microbiome Sequencing Data. PLoS Comput Biol. 2016;12: e1004957 10.1371/journal.pcbi.1004957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26: 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
The summary of long- and short-read information is described in the left panel. The histogram indicates read length distribution of subreads and polished long reads. The summary of de novo assembly information is described in the right panel.
(TIFF)
Schematic representation and comparative analysis of complete or draft mitochondrial DNA sequences among Candida albicans SC5314, Clavispora lusitaniae CBS 6936, C. auris JCM 15448T, C. duobushaemulonii B09383 and C. haemulonii B11899. Pairwise alignment was performed using TBLASTX, followed by visualization using Easyfig version 2.1 with following parameters: minimum length of blast hits, 30; maximum e-value, 1e-10; minimum identity value, 60. The red bars between mtDNA sequences represent individual amino acid sequences matches translated from nucleotide sequences. Light blue fragmented arrows represent the coding sequences (CDS) including intron predicted by comparative analysis.
(TIFF)
Twenty-eight SNVs were detected in 133 strains. The maximum-likelihood phylogenetic tree was constructed using FastTree version 2.1.10. Information of cluster type, metadata is described in color schemes on outside slots of the phylogenetic tree. The constructed clusters are closely similar to SNV trees with whole genome.
(TIFF)
Pairwise alignment was performed using BLASTN, followed by visualization using Easyfig version 2.1 with parameters described in S1 Fig. The mtDNA structures was conserved in each cluster.
(TIFF)
All-against-all comparisons of SNV counts were performed among each clade. Statistical analysis was performed using Wilcoxon rank sum test; the difference was statistically significant (p < 0.001) among all clusters. The boxplot indicates that median of pairwise SNV of clade I, III and IV is lower than those of clade II because of including outbreak case.
(TIFF)
The contigs assembled with unmapped reads against reference sequences were analyzed by BLASTN, followed by taxonomic classification of contigs. These data reveal that the average read depth of non-C. auris contigs show different distributions from those of C. auris contigs.
(TIFF)
This graph shows a histogram of read mapping variances. Twenty-two samples were rejected in CNV analysis because of abnormal distribution of read mapping depth. These all samples are included in same BioProject ID (PRJEB20230).
(TIFF)
This graph shows a histogram of average copy number in 111 samples which are collected by mapping variance analysis. Error bars represent standard deviation. The statistical data shows that lower than approximately 1.5 and more than 1.6 copy numbers are single and high copy, respectively.
(TIFF)
These trees indicate similar topology pattern, and the clade II belongs out of group against clade I, III and IV.
(TIFF)
(PDF)
(PDF)
Data Availability Statement
All sequence files are available from the DDBJ/EMBL/Genbank database (accession numbers BGOX01000001-BGOX01000011 and AP018713). All raw read sequence files are available from the DRA/SRA database (accession numbers DRR129819-DRR129826).