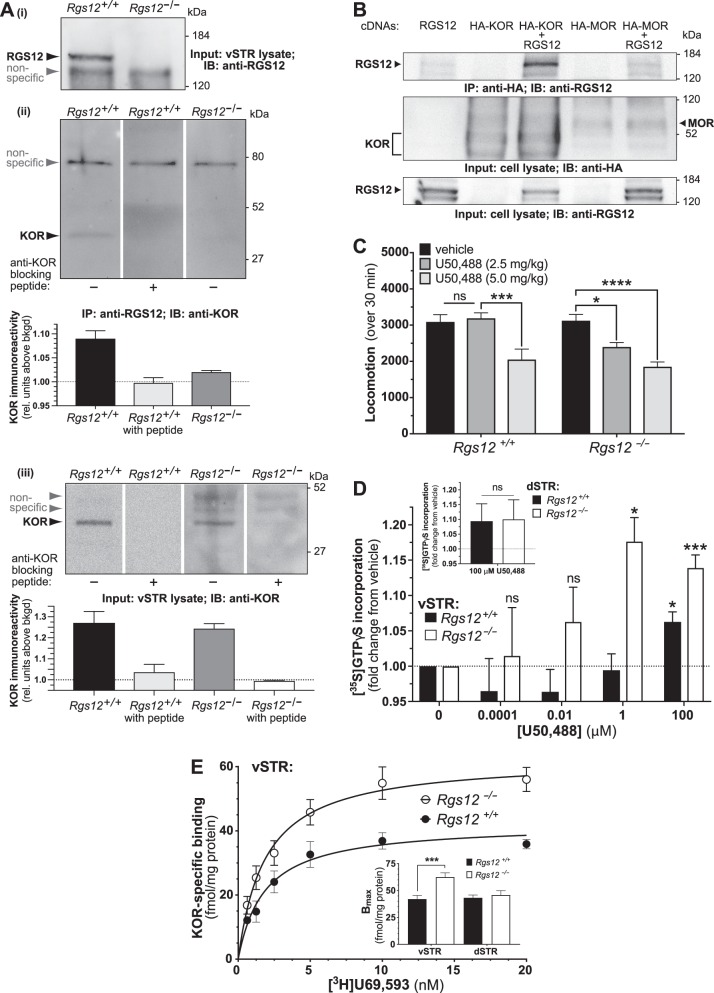
Fig. 2.
RGS12 interacts with KOR and RGS12 loss results in elevated KOR sensitivity and binding sites in the vSTR. a Representative immunoblots indicating that KOR and RGS12 co-immunoprecipitate in the vSTR of wildtype mice (N = 2 experiments). Subpanel (i) indicates loss of RGS12 immunoreactivity within whole lysates of ventral striatum from Rgs12-null mice. Subpanel (ii) demonstrates that endogenous KOR protein is co-immunoprecipitated from ventral striatal lysate upon immunoprecipitation of endogenous RGS12 protein. Subpanels (ii) and (iii) indicate that immunoblots probed with a mixture of anti-KOR and an anti-KOR blocking peptide yield no KOR immunoreactivity, confirming antibody specificity. Immunoprecipitation of RGS12 in RGS12-null vSTR results in greatly reduced signal of KOR immunoreactivity (see densitometric quantitation below immunoblot of subpanel (ii)), supporting that KOR immunoreactivity in wildtype IP samples is not due to non-specific pull down. b Co-immunoprecipitation analyses of HEK293T cells transiently transfected with human opioid receptor (with N-terminal HA-tag) and/or full-length RGS12 vector DNA. Robust content of RGS12 is seen in the anti-HA antibody immunoprecipitate when RGS12 is co-expressed with KOR, whereas co-expression of the related opioid receptor MOR yields only non-specific binding signal (i.e., compare first vs last lanes). Parallel immunoblots containing whole cell lysates resolved by SDS-PAGE demonstrate the presence or absence of RGS12, KOR, and/or MOR expression in appropriate conditions. c Total locomotion (over 30 min) by RGS12-null mice and wildtype littermate controls following administration of U50,488. RGS12-null, but not wildtype, mice exhibit a hypolocomotor effect to 2.5 mg/kg U50,488 relative to vehicle controls. At 5 mg/kg U50,488, RGS12-null and wildtype controls both display reduced locomotor activity relative to vehicle controls. Data are the mean ± SEM and tested by two-way ANOVA with Dunnet’s post hoc test (n = 7–13 mice per group) (ns, p > 0.05; *p < 0.05; ***p < 0.001; ****p < 0.0001). d [35S]GTPγS incorporation into vSTR membranes from RGS12-null mice and wildtype littermate controls upon activation with KOR agonist U50,488. Data are normalized to vehicle (saline) control (expressed as fold change). vSTR membranes from RGS12-null mice exhibit increased sensitivity to U50,488 relative to wildtype controls. Inset, [35S]GTPγS binding (at 100 μM U50,488) by dSTR membranes from RGS12-null mice and wildtype littermate controls. All data are the mean ± SEM and tested by two-way ANOVA with Sidak’s post hoc test (n = 13–19 mice per group) (*p < 0.05; ***p < 0.001). e [3H]U69,593 saturation binding analysis of vSTR membranes from RGS12-null (Rgs12−/−) mice and wildtype (Rgs12+/+) littermate controls. Non-specific binding was determined in the presence of 10 μM nor-BNI (KOR antagonist). Inset, As derived from data in panel B and parallel binding data from dSTR membrane samples, Bmax was quantified and seen to be increased in vSTR (but not dSTR) of RGS12-null mice (RGS12-null vSTR Bmax = 62.5 ± 3.9 fmol/mg protein vs wildtype vSTR Bmax = 42.1 ± 3.3 fmol/mg protein). The KD for [3H]U69,593 did not differ across genotypes (RGS12-null vSTR: 1.8 ± 0.4 nM; WT vSTR: 1.8 ± 0.4 nM). Data are the mean ± SEM and tested by two-way ANOVA with Sidak’s post hoc test (n = 9–12 mice per group) (***p < 0.001)