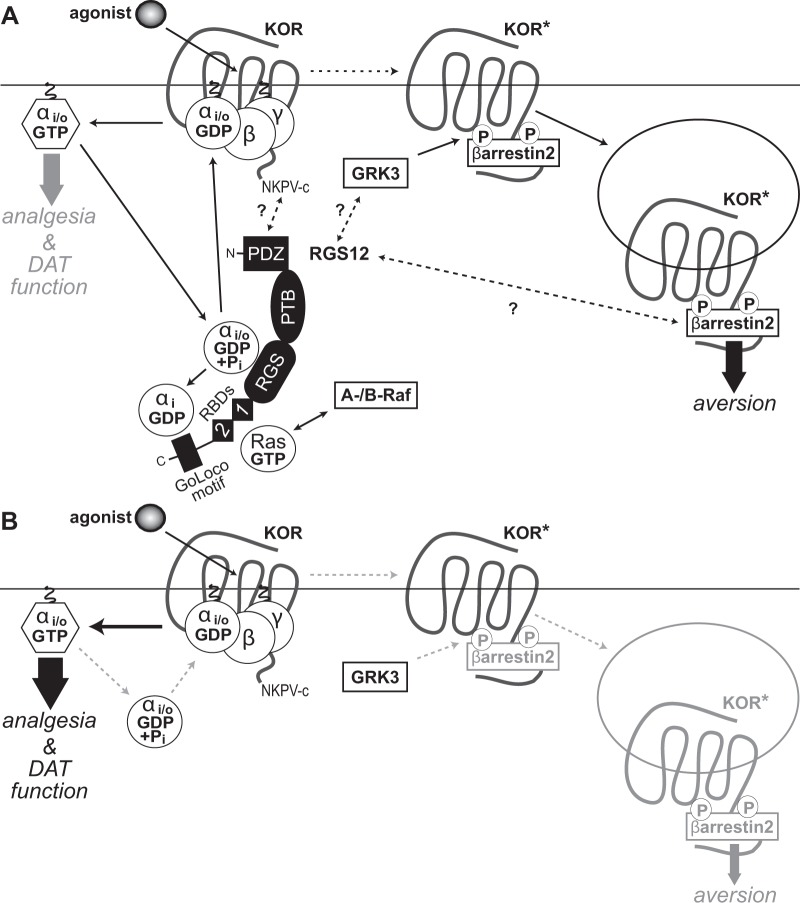
Fig. 5.
Proposed model of RGS12 action in the differential outputs of KOR-activated signaling. a Agonist-induced activation of KOR dissociates Gi/o-coupled heterotrimers into free G protein subunits, evokes G protein-dependent signal transduction (thick grey arrow) and, ultimately, produces analgesia and regulates DAT function. RGS12 expression is seen to reduce KOR-mediated G protein signaling via its central RGS domain (by accelerating GTP hydrolysis of Gαi/o·GTP) and its C-terminal GoLoco motif (by binding Gαi·GDP). RGS12 also contains an N-terminal PDZ domain that interacts with MEK2 [38] and findings from this study suggest interaction with the C-terminal PDZ domain docking site of KOR (NKPV-c). RGS12 also contains tandem Ras-binding domains (RBDs) that interact with activated H-Ras and A-/B-Raf to enable MAPK/ERK scaffolding properties [38]. Agonist-stimulated KOR activation is also known to drive GRK3-dependent β-arrestin-2 recruitment [103], resulting in receptor internalization, β-arrestin-dependent signal transduction (thick black arrow) and, ultimately, aversive behavior. RGS12 expression is seen to augment KOR agonist-stimulated β-arrestin recruitment independent of its GAP and GDI activity, suggesting that RGS12 may modulate GRK3 activity on activated KOR (KOR*) and/or enhance β-arrestin-2 recruitment and/or function by as yet undetermined molecular mechanisms (represented by question marks over dashed arrows). b Loss of RGS12 in mice results in concomitant increases in KOR-mediated G protein signaling and decreases in β-arrestin-2 recruitment, thus resulting in enhanced KOR agonist-stimulated analgesia and disrupted DAT function (thicker black arrow), as well as an attenuation of KOR-stimulated aversion (thinner grey arrow), respectively