Abstract
The dopamine D3 receptor (D3R) has emerged as a promising pharmacotherapeutic target for the treatment of several diseases including schizophrenia, Parkinson’s disease, and substance use disorders. However, studies investigating the D3R’s precise role in dopamine neurotransmission or how it may be exploited to modulate responses to drugs of abuse have produced contrasting results, in part because most D3R-targeted compounds often also interact with D2 receptors (D2R). To resolve this issue, we set out to systematically characterize and compare the consequences of selective D2R or D3R antagonists on the behavioral-stimulant properties of cocaine in mice, and to identify putative neurobiological mechanisms underlying their behavior-modifying effects. Pretreatment with the selective D2R antagonist L-741,626 attenuated, while pretreatment with the selective D3R antagonist PG01037 enhanced, the locomotor-activating effects of both acute cocaine administration as well as sensitization following repeated cocaine dosing. While both antagonists potentiated cocaine-induced increases in presynaptic dopamine release, we report for the first time that D3R blockade uniquely facilitated dopamine-mediated excitation of D1-expressing medium spiny neurons in the nucleus accumbens. Collectively, our results demonstrate that selective D3R antagonism potentiates the behavioral-stimulant effects of cocaine in mice, an effect that is in direct opposition to that produced by selective D2R antagonism or nonselective D2-like receptor antagonists, and is likely mediated by facilitating D1-mediated excitation in the nucleus accumbens. These findings provide novel insights into the neuropharmacological actions of D3R antagonists on mesolimbic dopamine neurotransmission and their potential utility as pharmacotherapeutics.
Subject terms: Excitability, Addiction, Addiction
Introduction
Dopamine (DA) dysregulation is implicated in several neuropathological disorders including schizophrenia [1], Parkinson’s disease [2], depression [1, 3], and substance use disorders (SUD) [4, 5]. Five subtypes of DA receptors have been identified and are grouped into two families: the D1-like receptors (D1R and D5R) and D2-like receptors (D2R, D3R, and D4R) [6]. D2-like receptors serve as the target of clinically-available pharmacotherapeutics for conditions in which disrupted DA signaling has been identified, most notably Parkinson’s disease [6, 7] and schizophrenia [6, 8]. However, undesirable side effects limit the benefits and reduce compliance [9], and have contributed to the failure of nonselective compounds targeting D2-like receptors to show therapeutic potential for SUD [10, 11]. Consequently, there has been interest in identifying D2-like receptor subtype-selective compounds that may engender therapeutic benefits with minimal aversive side effect profiles [12–14], particularly for the treatment of psychostimulant use disorders which lack FDA-approved medications as an available treatment strategy [15].
The D3R exhibits several characteristics that have sparked investigations into its potential as an improved pharmacotherapeutic target for the treatment of DA-related diseases including SUDs [14, 16–18]. Among the D3R’s unique properties is a highly restricted pattern of distribution as compared to the D2R or D4R, with greatest abundance in limbic regions including the nucleus accumbens (NAc) [14, 19, 20]. Within the NAc, it functions both as a presynaptic inhibitory autoreceptor on DA terminals arising from the ventral tegmental area [6, 21, 22] and as a postsynaptic heteroreceptor on GABAergic medium spiny neurons (MSNs) [23]. While MSNs can be classified based on expression of either D1R (D1-MSNs) or D2R (D2-MSNs), both types co-express the D3R [24–26], with the D3R exhibiting the highest affinity for DA across all DA receptor subtypes [20, 27]. The D3R is thus well-suited to influence NAc DA neurotransmission and modulate the effects of psychostimulants and other drugs of abuse [13], but studies examining its modulatory impact on NAc DA signaling have produced conflicting results. For example, some animal studies indicate that genetic knockout or pharmacological antagonism of the D3R may facilitate basal or drug-induced increases in extracellular DA within the NAc [28–34], while others have reported no such effect [28, 31, 33, 35, 36] or even an attenuation of drug-induced DA increases [32]. Moreover, studies employing behavioral readouts of NAc DA neurotransmission using unconditioned behavioral responses (e.g., locomotor activity) in rodents have produced a similarly confusing picture. D3R knockout or pharmacological antagonism have been reported to increase [37–42], decrease [39, 43, 44], or have no effect [29, 35, 45, 46] on basal or drug-induced increases in locomotion. These discrepancies may be partially explained by several factors including the use of antagonists that exhibit only marginal selectivity for D3R vs. D2R, comparisons of acute pharmacological D3R antagonism vs. chronic genetic deletion, etc. Whatever the factors, a uniform mechanism to describe how selective D3R antagonism modulates NAc DA neurotransmission and associated behaviors has remained elusive, leaving unresolved the question as to how these potential medications may alter the symptomatology of DA-related disorders.
The purpose of this study was to definitively determine the impact of selective D3R antagonism on NAc DA neurotransmission when administered either alone or under conditions of enhanced DA tone, achieved via administration of the monoamine transporter inhibitor and psychostimulant, cocaine. To achieve specific pharmacological D3R blockade, we used the highly-selective D3R antagonist PG01037, which exhibits ~133-fold selectivity for the D3R vs. D2R [47, 48]. The effects of PG01037 on multiple aspects of NAc DA neurotransmission were assessed and compared to those produced by the selective D2R antagonist, L-741,626, which exhibits ~15–42-fold selectivity for the D2R vs. D3R [49, 50]. We first measured changes in basal and cocaine-increased locomotor activity in mice following pretreatment with either of these two selective antagonists and found that they exerted opposing influences on cocaine-induced locomotion. We then investigated potential underlying presynaptic and postsynaptic mechanisms, first by examining each antagonist’s effect on stimulated presynaptic DA release and DA clearance in the NAc via ex vivo fast-scan cyclic voltammetry (FSCV), and then by assessing for the first time the effects of selective D2R or D3R antagonism on the activity of the distinct D1-expressing vs. D2-expressing subpopulations of NAc MSNs using ex vivo whole cell electrophysiology.
Materials and methods
Animals
All procedures were performed in strict accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Emory University or University of Texas at San Antonio. C57BL/6 mice were used for all studies. Additional details are provided in the Supplementary Materials and Methods.
Effects of selective D3R or D2R antagonism on locomotor behavior
Behavioral testing
Locomotor activity was assessed using methods adapted from those previously described [51]. For acute cocaine-induced locomotion studies, mice were introduced to the locomotor chamber for a 90 min habituation period, then injected with either L-741,626 (vehicle, 3.0–10.0 mg/kg i.p.) or PG01037 (vehicle, 1.0–10.0 mg/kg i.p.) and immediately returned to the chamber for 30 min of locomotor activity measurement, then administered cocaine (1.0–30.0 mg/kg i.p.) and immediately returned to the chamber for 120 min of locomotor activity measurement. In a separate group of mice, a drug mixture containing one dose of SCH23390 (vehicle, 0.03, 0.3 mg/kg) and one dose of PG01037 (vehicle, 10.0 mg/kg) served as the pretreatment, followed by 3.0 mg/kg cocaine.
Sensitization testing took place over 5 consecutive days and was performed in 8 separate groups of mice. Mice were first introduced to the locomotor chamber for 90 min, injected with L-741,626 (vehicle or 10.0 mg/kg i.p.) or PG01037 (vehicle or 10.0 mg/kg i.p.) and immediately returned to the chamber for 30 min, and then administered either saline or cocaine (15.0 mg/kg i.p. in L-741,626-pretreated subjects; 5.0 mg/kg i.p. in PG01037-pretreated subjects) and immediately returned to the chamber for an additional 60 min. Each dose combination was tested in separate groups of mice, and each mouse received the same dose combination across the 5 days.
Drugs
All drugs were administered i.p. at a volume of 10.0 ml/kg. Sources and vehicles for all compounds are provided in the Supplementary Materials and Methods.
Intra-NAc PG01037 microinfusion
Mice were first habituated to the locomotor chambers for 120 min. Injectors projecting 2 mm beyond the bottom of implanted cannulae were inserted to facilitate bilateral intra-NAc infusions of artificial cerebrospinal fluid (aCSF; 0.3 µL) or aCSF containing PG01037 (3 µg/0.3 µL). Infusions were immediately followed by administration of cocaine (10 mg/kg i.p.) and replacement to the locomotor chamber for 120 min. Mice received each treatment in a counter-balanced order, with test sessions separated by 1 week.
Effects of selective D3R or D2R antagonism on presynaptic DA release in NAc
Ex vivo slice FSCV of the NAc core (Supplementary Figure S1) was performed as described previously [52]. Stable baseline measures were acquired in aCSF followed by ascending concentrations of drug (PG01037 or L-741,626: 1 pM, 100 pM, 1 nM) or in aCSF, then 1 µM cocaine, then 1 µM cocaine + aforementioned ascending concentrations of either PG01037 or L-741,626. Maximal release and rate of clearance were determined using Demon Voltammetry and Analysis Software (Wake Forest Innovations, Winston-Salem, NC) and were averaged in each drug condition. The kinetic constant tau was used to characterize the rate of DA clearance as described previously [53]. Additional methodological details are provided in the Supplementary Materials and Methods.
Effects of selective D3R or D2R antagonism on postsynaptic D1-MSN or D2-MSN activity in NAc
NAc D1-MSN and D2-MSN activity was assessed using ex vivo electrophysiology in slices (Supplementary Figure S1) derived from Drd1a-tdTomato and Drd2-EGFP mice. Following 60 μM DA HCl application, either 1 nM PG01037, 1 nM L-741,626, or 3 μM sulpiride were applied with 60 µM DA HCl in the same manner. In a separate experiment, the application of 1 nM PG01037 was tested alone. Primary indices of neuronal activity were spike frequency as a function of applied current, F–I curve slope values, and rheobase (defined as the amount of current necessary to elicit one action potential via a single 1-s injection of current). Additional methodological details are provided in the Supplementary Materials and Methods.
Statistics
Data were analyzed using paired t-test, one-way or two-way repeated measures analysis of variance (ANOVA), with post hoc tests as specified in the text using GraphPad Prism v. 7.04 (GraphPad Software, La Jolla, CA). Significance was set at p < 0.05 for all tests. See the Supplementary Materials and Methods for additional details.
Results
Pretreatment with selective D2R or D3R antagonists produces opposite effects on cocaine-induced locomotion
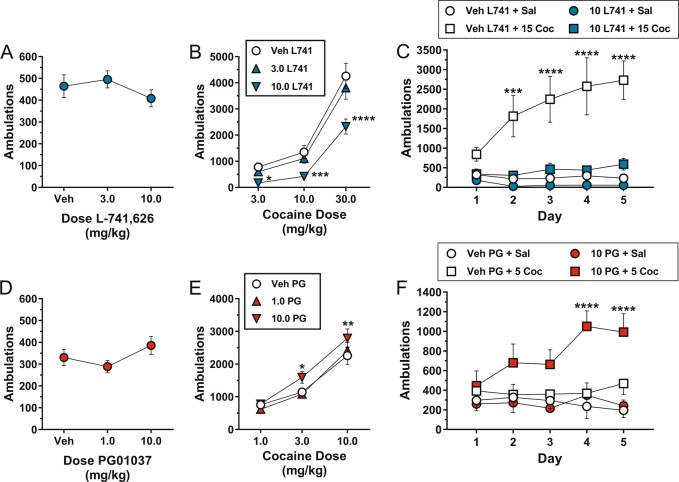
Neither of the active doses of L-741,626 (3.0 or 10.0 mg/kg) significantly affected locomotor activity compared to vehicle in the first 30 min following drug administration (one-way RM ANOVA: main effect of dose [F(2,28) = 4.90, p = 0.015; post hoc Dunnett’s tests comparing active doses to vehicle, p > 0.05]; Fig. 1a). By contrast, cocaine-induced increases in locomotor activity were robustly modulated by L-741,626 pretreatment (two-way RM ANOVA: main effect of cocaine dose [F(2,28) = 82.11, p < 0.0001], main effect of L-741,626 dose [F(2,28) = 36.17, p < 0.0001], interaction [F(4,56) = 5.17, p = 0.001]; Fig. 1b). Post hoc analyses revealed that 10.0 mg/kg L-741-626 significantly reduced the locomotor-activating effects of each cocaine dose, shifting the cocaine dose–response function rightward and downward (Fig. 1b). The effects of L-741,626 were evident immediately after cocaine administration and persisted throughout the observation period (Supplementary Figure S2A-C).
Fig. 1.
Effects of pretreatment with the D2R antagonist L-741,626 or the D3R antagonist PG01037 on basal locomotor activity, acute cocaine-induced locomotor activity, and cocaine-induced sensitization in mice. a Total number of ambulations in the 30-min period following i.p. administration of L-741,626. b Effects of pretreatment with L-741,626 on locomotor activity in the 60-min period following i.p. administration of cocaine. c Effects of L-741,626 on cocaine-induced sensitization. Mice received one of the following four combinations of L-741,626 and cocaine daily for 5 days: vehicle + saline; vehicle + 15.0 mg/kg cocaine; 10.0 mg/kg L-741,626 + saline; 10.0 mg/kg L-741,626 + 15.0 mg/kg cocaine. Shown are the total number of ambulations in the 60-min period following administration of saline or 15.0 mg/kg cocaine, which was administered 30 min after pretreatment with either 10.0 mg/kg L-741,626 or its vehicle. d Total number of ambulations in the 30-min period following i.p. administration of PG01037. e Effects of pretreatment with PG01037 on locomotor activity in the 60-min period following i.p. administration of cocaine. f Effects of PG01037 on cocaine-induced sensitization. Mice received one of the following four combinations of PG01037 and cocaine daily for 5 days: vehicle + saline; vehicle + 5.0 mg/kg cocaine; 10.0 mg/kg PG01037 + saline; 10.0 mg/kg PG01037 + 5.0 mg/kg cocaine. Shown are the total number of ambulations in the 60-min period following administration of saline or 5.0 mg/kg cocaine, which was administered 30 min after pretreatment with either 10.0 mg/kg PG01037 or its vehicle. For panels (a), (b), (d), and (e), all mice (n = 15) received all treatments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, significant difference by Dunnett’s test as compared to vehicle dose of pretreatment drug at the same dose of cocaine. For panels (c) and (f), mice (n = 6–8 per group) received the same drug combination once per day for 5 consecutive days. ***p < 0.001, ****p < 0.0001, significant difference by Dunnett’s test compared to day 1 within the same drug combination group. For all panels, each data point represents mean ± SEM ambulations
Chronic exposure to cocaine produces neurobiological adaptations that cause sensitized behavioral and neurochemical responses to subsequent cocaine challenges [54]. We wondered whether blockade of D2Rs would modulate the induction of locomotor sensitization following repeated exposure to cocaine. Mice treated with the daily combination of vehicle + 15.0 mg/kg cocaine exhibited a robust sensitization of cocaine-induced locomotion, but the development of sensitization was abolished by daily pretreatment with 10.0 mg/kg L-741,626 prior to 15.0 mg/kg cocaine (two-way mixed-design ANOVA: main effect of group [F(3,24) = 12.14, p < 0.0001], main effect of day [F(4,96) = 4.66, p = 0.002], interaction [F(12,96) = 4.88, p < 0.0001]; Fig. 1c). Notably, administration of 10.0 mg/kg L-741,626 alone (i.e., prior to saline) did not produce significant changes in locomotor activity, although because the ambulations in the vehicle/saline group were already very low, it is possible that a floor effect prevented the detection of a measurable influence of L-741,626 (Fig. 1c).
We next assessed the effects of pretreatment with the selective D3R antagonist PG01037 on acute cocaine-induced increases in locomotion. Neither of the active doses of PG01037 (1.0 or 10.0 mg/kg) significantly affected locomotor activity compared to vehicle in the first 30 min following drug administration (one-way RM ANOVA: main effect of dose [F(2,28) = 5.87, p = 0.007; post hoc Dunnett’s tests comparing active doses to vehicle, p > 0.05]; Fig. 1d). In direct opposition to the effects of L-741,626, cocaine-induced increases in locomotor activity were enhanced following pretreatment with PG01037 (two-way RM ANOVA: main effect of cocaine dose [F(2,28) = 50.05, p < 0.0001], main effect of PG01037 dose [F(2,28) = 14.34, p < 0.0001], interaction [F(4,56) = 1.48, p = 0.221]; Fig. 1e). Post hoc analyses showed that pretreatment with 10.0 mg/kg PG01037 produced a leftward and upward shift of the cocaine dose–response function (Fig. 1e). The potentiation of cocaine-induced locomotion by PG01037 occurred immediately upon cocaine administration and was not attributable to a prolongation of cocaine’s time course of action (Supplementary Figure S3A-C). Based on these results, we hypothesized that PG01037 may similarly potentiate cocaine-induced sensitization. We optimized our sensitization protocol for the detection of enhanced sensitization by testing the effects of PG01037 in conjunction with a low dose of cocaine (5.0 mg/kg) that does not induce behavioral sensitization alone. Two-way mixed-design ANOVA revealed significant main effect of group [F(3,26) = 4.95, p = 0.008], main effect of day [F(4,104) = 2.01, p = 0.099], and their interaction [F(12,104) = 2.35, p = 0.010]. Post hoc analyses indicated that mice treated with the daily combination of vehicle + saline, vehicle + 5.0 mg/kg cocaine, or 10.0 mg/kg PG01037 + saline failed to exhibit sensitization. By contrast, sensitized locomotor responses developed in mice pretreated daily with 10.0 mg/kg PG01037 prior to 5.0 mg/kg cocaine (Fig. 1f).
Previous work has shown that intra-NAc administration of nonselective D2-like antagonists attenuates cocaine-induced locomotor activity [55, 56]. However, studies investigating the specific role of the D3R subtype in modulating cocaine-induced locomotion have produced mixed results [29, 35, 37–46]. We therefore tested whether intra-NAc infusion of PG01037 would recapitulate the effects of systemic D3R blockade described above. In a separate cohort of mice, we delivered bilateral intra-NAc infusions of PG01037 (3 µg/side) or vehicle (aCSF) via guide cannula followed immediately by i.p. administration of 10.0 mg/kg cocaine. Intra-NAc delivery of PG01037 enhanced cocaine-induced locomotor activity (t(8) = 2.10, p = 0.034; Supplementary Figure S4A-B), suggesting that the NAc is a critical site in which D3R antagonists act to potentiate the locomotor-activating effects of cocaine.
Selective blockade of D2R or D3R facilitates cocaine-induced increases in NAc DA release
The results described above demonstrate that D2R antagonism attenuates, while D3R antagonism enhances, the locomotor stimulant effects of cocaine. Moreover, previous findings [55, 56] and our present work (Supplementary Figure S4) indicated that the NAc is a key neuroanatomical substrate for these effects. However, the neuropharmacological mechanisms underlying these disparate outcomes remained unresolved. Within the NAc, D2Rs and D3Rs function both as presynaptic autoreceptors on DAergic terminals and as postsynaptic heteroreceptors on MSNs [6], and are thus positioned to modulate presynaptic DA release and postsynaptic DA receptor-mediated changes in neuronal activity, respectively. We therefore sought to address whether D2R or D3R antagonism similarly or distinctly impact each of these measures.
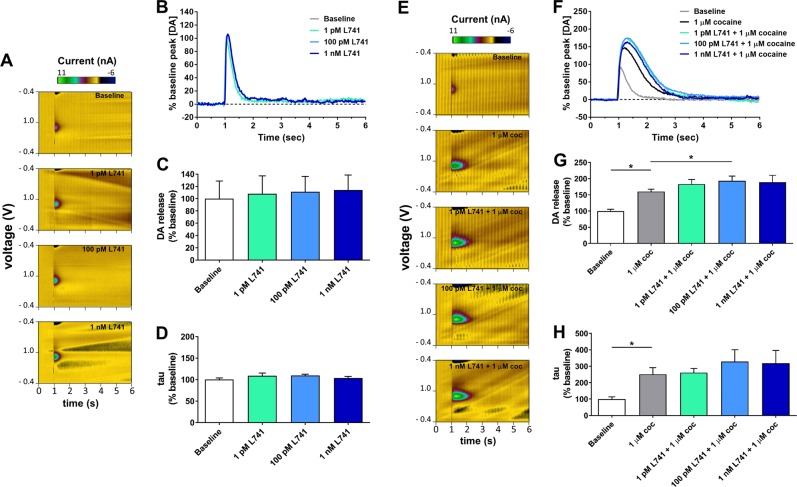
We first quantified electrically-evoked presynaptic DA release in the NAc in the presence of D2R or D3R antagonists alone and in combination with cocaine using ex vivo slice FSCV. Figure 2a, b show representative color plots and current traces for DA detection with increasing concentrations of L-741,626. Application of L-741,626 alone failed to alter peak stimulated DA release (one-way RM ANOVA: main effect of concentration [F(3,9) = 1.26, p = 0.346]; Fig. 2c) or the rate of DA clearance (one-way RM ANOVA: main effect of concentration [F(3,9) = 1.09, p = 0.403]; Fig. 2d). Figure 2e, f show representative color plots and current traces for DA detection following combined application of cocaine (1 µM) and increasing concentrations of L-741,626. One-way RM ANOVA (main effect of treatment condition [F(4,16) = 18.22, p < 0.001]) with post hoc Dunnett’s tests indicated that cocaine enhanced stimulated DA release compared to baseline as reported previously [57], and application of 100 pM L-741,626 potentiated the cocaine-induced increase in DA release (Fig. 2g). Application of the highest concentration of L-741,626 (1 nM) trended towards producing this effect as well. Statistical analysis also detected a significant main effect of condition on DA clearance (one-way RM ANOVA: main effect of treatment condition [F(4,12) = 6.362, p = 0.006]), however post hoc analyses revealed that cocaine produced an expected increase in tau that was seemingly unaffected by co-application of L-741,626 (Fig. 2h).
Fig. 2.
Effects of selective D2R antagonism alone or in combination with cocaine on stimulated DA release and DA clearance in the NAc as measured by ex vivo fast scan cyclic voltammetry. Representative a color plots and b current traces depict electrochemical detection of DA in the presence of varied concentrations of the selective D2R antagonist L-741,626. Quantification of c peak stimulated DA release and d DA clearance as a function of applied concentrations of L-741,626 (n = 4). Representative e color plots and f current traces depict electrochemical detection of DA in the presence of 1 µM cocaine alone and in combination with varied concentrations of L-741,626. Quantification of g peak stimulated DA release and h DA clearance in the presence of cocaine alone and in combination with L-741,626 (n = 5). Color plots in (a) and (e) depict representative changes in current in z axis (color) with time along the abscissa and applied cyclic potential along the ordinate. Current traces in (b) and (f) depict release and clearance of DA with time along the abscissa and DA concentration (normalized as a percentage of the mean of all samples collected at the baseline condition) along the ordinate. In (c) and (g), values are depicted as the mean ± SEM maximum DA concentration following stimulation (normalized as percentage of the mean peak DA release at baseline). In (d) and (h), values are depicted as the mean ± SEM calculated tau constant (normalized as percentage of the mean tau value at baseline) *p < 0.05, Dunnett’s test as compared to 1 µM cocaine
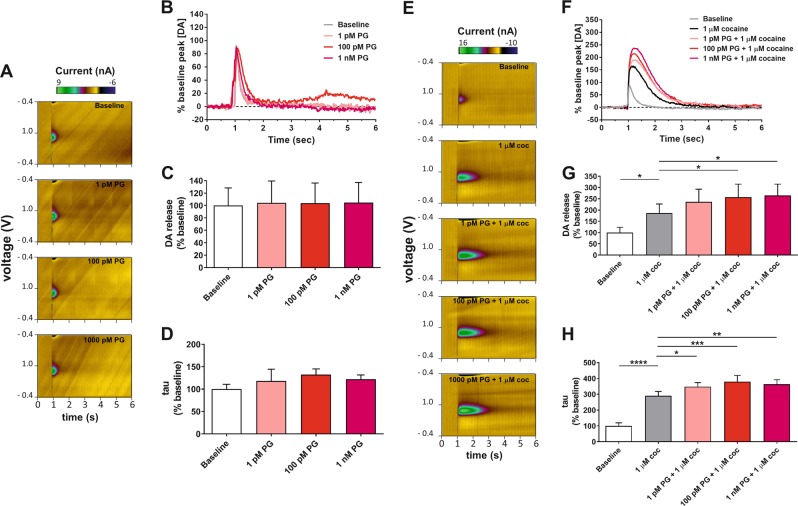
Figure 3a, b show representative color plots and current traces for DA detection with increasing concentrations of PG01037. Application of PG01037 alone did not alter peak stimulated DA release (one-way RM ANOVA: main effect of concentration [F(3,6) = 0.16, p = 0.921]; Fig. 3c) or DA clearance (one-way RM ANOVA: main effect of concentration [F(3,6) = 1.31, p = 0.354]; Fig. 3d). Figure 3e, f show representative color plots and current traces for DA detection following combined application of cocaine (1 µM) and increasing concentrations of PG01037. One-way RM ANOVA (main effect of treatment condition [F(4,24) = 12.94, p < 0.0001]) with post hoc Dunnett’s tests indicated that cocaine increased peak stimulated DA release, and this effect was further enhanced following application of 100 pM or 1 nM PG01037 (Fig. 3g). Cocaine also produced an expected increase in tau value, but in contrast to L-741,626, co-application of PG01037 potentiated this effect (one-way RM ANOVA with post hoc Dunnett’s tests: main effect of treatment condition [F(4,24) = 83.35, p < 0.0001]; Fig. 3h).
Fig. 3.
Effects of selective D3R antagonism alone or in combination with cocaine on stimulated DA release in the NAc as measured by ex vivo fast scan cyclic voltammetry. Representative a color plots and b current traces depict electrochemical detection of DA in the presence of varied concentrations of the selective D3R antagonist PG01037. Quantification of c peak stimulated DA release and d DA clearance as a function of applied concentrations of PG01037 (n = 3). Representative e color plots and f current traces depict electrochemical detection of DA in the presence of 1 µM cocaine alone and in combination with varied concentrations of PG01037. Quantification of g peak stimulated DA release and h DA clearance in the presence of cocaine alone and in combination with PG01037 (n = 7). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Dunnett’s test as compared to 1 µM cocaine. Additional details (e.g., units of measurement, axes, etc.) are as described in Fig. 2
Selective blockade of D2R or D3R produces differential effects on neuronal firing in NAc D1-MSNs, but not D2-MSNs
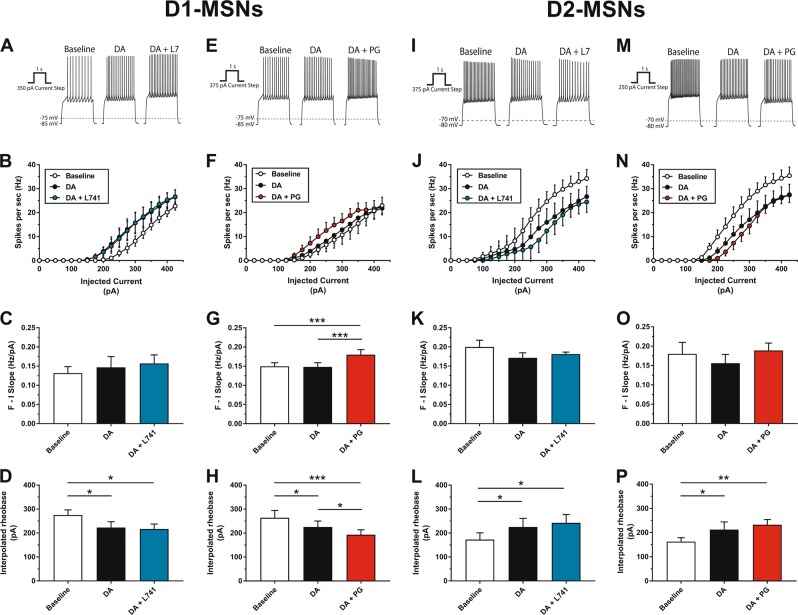
We next examined potential postsynaptic differences by performing ex vivo whole cell electrophysiology to examine changes in the activity of NAc D1-MSNs and D2-MSNs following combined application of DA and selective D2R or D3R antagonists. Shown in Fig. 4a–d are the effects of L-741,626 on DA-mediated changes in D1-MSN activity. Two-way RM ANOVA (main effect of current [F(12,60) = 67.05, p < 0.0001], main effect of condition [F(2,10) = 2.33, p = 0.148], interaction [F(24,120) = 1.93, p = 0.011]) followed by Holm–Sidak’s tests indicated that DA application alone significantly increased spike frequency as compared to baseline, but co-administration of L-741,626 did not modulate this effect of DA (Fig. 4b). Neither the application of DA alone nor the co-application of DA + L-741,626 affected F–I slope (one-way RM ANOVA: main effect of treatment condition [F(2,10) = 2.60, p = 0.124]; Fig. 4c), and the DA-mediated reduction of rheobase in D1-MSNs was similarly unaffected following co-administration with L-741,626 (one-way RM ANOVA: main effect of treatment condition [F(2,10) = 5.69, p = 0.022]; Fig. 4d). By contrast, application of PG01037 modulated several DA-mediated changes in D1-MSN activity (Fig. 4e–h). Two-way RM ANOVA (main effect of current [F(12,96) = 35.45, p < 0.0001], main effect of condition [F(2,16) = 7.67, p = 0.005], interaction [F(24,192) = 0.958, p = 0.523]) followed by Holm–Sidak’s tests revealed that combined administration of DA + PG01037 increased spike frequency as compared to both baseline and DA alone conditions (Fig. 4f), an observation that was accompanied by an increase in the slope of the F–I curve (one-way RM ANOVA: main effect of treatment condition [F(2,16) = 13.4, p = 0.0004]; Fig. 4g). Furthermore, application of DA significantly reduced rheobase as compared to baseline, and PG01037 potentiated this effect (one-way RM ANOVA: main effect of treatment condition [F(2,16) = 12.74, p = 0.002]; Fig. 4h). While these data suggest that D3R antagonism enhances the stimulatory effects of DA on D1-MSNs, it is alternatively possible that PG01037 alters D1-MSN activity independent of DA application, and the potentiated response with their co-application represents an additive, rather than interactive, effect. To address this, we measured D1-MSN activity following application with 1 nM PG01037 alone, and found that it did not affect D1-MSN firing rate (two-way RM ANOVA: main effect of current [F(12,48) = 14.16, p < 0.0001], main effect of condition [F(1,4) = 0.22, p = 0.662], interaction [F(12,48) = 2.00, p = 0.05]; Supplementary Figure S5A), F–I slope (paired t-test: t(4) = 0.87, p = 0.433; Supplementary Figure S5B), or rheobase (paired t-test: t(4) = 0.46, p = 0.668; Supplementary Figure S5C).
Fig. 4.
Effects of selective D2R or D3R antagonism on neuronal activity in D1-MSNs and D2-MSNs as measured by ex vivo slice electrophysiology. a Representative traces depict the change in firing rate of an individual D1-MSN after application of 60 µM DA followed by 60 µM DA + 1 nM L-741,626. b Firing rate as a function of injected current in D1-MSNs (n = 6) following application of 60 µM DA alone or in combination with 1 nM L-741,626. c F–I slope, quantified through the primary linear range of the F–I curve for each individual D1-MSN (n = 6), following perfusion of 60 µM DA alone or in combination with 1 nM L-741,626. d Interpolated rheobase in D1-MSNs (n = 6) following application of 60 µM DA alone or in combination with 1 nM L-741,626. e Representative traces depict the change in firing rate of an individual D1-MSN after application of 60 µM DA followed by 60 µM DA + 1 nM PG01037. f Firing rate as a function of injected current in D1-MSNs (n = 9) following application of 60 µM DA alone or in combination with 1 nM PG01037. g F–I slope, quantified through the primary linear range of the F–I curve for each individual D1-MSN (n = 9), following perfusion of 60 µM DA alone or in combination with 1 nM PG01037. h Interpolated rheobase in D1-MSNs (n = 9) following application of 60 µM DA alone or in combination with 1 nM PG01037. i Representative traces depict the change in firing rate of an individual D2-MSN after application of 60 µM DA followed by 60 µM DA + 1 nM L-741,626. j Firing rate as a function of injected current in D2-MSNs (n = 5) following application of 60 µM DA alone or in combination with 1 nM L-741,626. k F–I slope, quantified through the primary linear range of the F–I curve for each individual D2-MSN (n = 5), following perfusion of 60 µM DA alone or in combination with 1 nM L-741,626. l Interpolated rheobase in D2-MSNs (n = 5) following application of 60 µM DA alone or in combination with 1 nM L-741,626. m Representative traces depict the change in firing rate of an individual D2-MSN after application of 60 µM DA followed by 60 µM DA + 1 nM PG01037. n Firing rate as a function of injected current in D2-MSNs (n = 5) following application of 60 µM DA alone or in combination with 1 nM PG01037. o F–I slope, quantified through the primary linear range of the F–I curve for each individual D2-MSN (n = 5), following perfusion of 60 µM DA alone or in combination with 1 nM PG01037. p Interpolated rheobase in D2-MSNs (n = 5) following application of 60 µM DA alone or in combination with 1 nM PG01037. Data points are depicted as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, significant difference by Holm–Sidak’s test
The effects of L-741,626 on D2-MSN spike frequency are shown in Fig. 4i–l. Two-way RM ANOVA (main effect of current [F(12,48) = 60.99, p < 0.0001], main effect of condition [F(2,8) = 12.43, p = 0.004], interaction [F(24,96) = 2.65, p = 0.0004]) followed by Holm–Sidak’s tests showed that application of either DA alone or DA + L-741,626 significantly reduced spike frequency as compared to baseline, but the effects of combined administration of DA + L-741,626 did not significantly differ from the DA alone condition (Fig. 4j). None of the treatments significantly affected the slope of the F–I curve as compared to baseline (one-way RM ANOVA: main effect of treatment condition [F(2,8) = 2.63, p = 0.132]; Fig. 4k). In agreement with results on firing rate, rheobase values were significantly increased in the DA alone and DA + L-741,626 conditions as compared to baseline, but did not significantly differ from each other (one-way RM ANOVA: main effect of treatment condition [F(2,8) = 7.22, p = 0.016]; Fig. 4l). We next tested the impact of PG01037 application on D2-MSN spike frequency (Fig. 4m–p). Two-way RM ANOVA (main effect of current [F(12,48) = 53.11, p < 0.0001], main effect of condition [F(2,8) = 13.85, p = 0.003], interaction [F(24,96) = 4.01, p < 0.0001]) followed by Holm–Sidak’s tests found that the application of either DA alone or DA + PG01037 reduced spike frequency when compared to baseline, but the DA alone and DA + PG01037 conditions did not significantly differ from each other (Fig. 4n). F–I slope was unaffected by any treatment compared to baseline (one-way RM ANOVA: main effect of treatment condition [F(2,8) = 0.82, p = 0.478]; Fig. 4o). Finally, application of DA alone or DA + PG01037 significantly increased rheobase values compared to baseline, but did not differ from each other (one-way RM ANOVA: main effect of treatment condition [F(2,8) = 13.79, p = 0.003]; Fig. 4p).
The results obtained from recordings in D2-MSNs indicated that DA reduced the excitability of these neurons, but selective blockade of either D2Rs or D3Rs alone was incapable of reversing this effect. Because D2-MSNs co-express D2Rs and D3Rs, we speculated that singular pharmacological blockade of either receptor alone fails to alleviate DA-mediated inhibition because DA binding at the spared receptor subtype is sufficient to exert efficacious inhibitory action on the cell. To test this hypothesis, we first assessed DA-mediated inhibition of spike frequency in D2-MSNs following administration of the nonselective D2R/D3R antagonist sulpiride. Two-way RM ANOVA (main effect of current [F(12,48) = 83.87, p < 0.0001], main effect of condition [F(2,8) = 3.31, p = 0.090], interaction [F(24,96) = 2.93, p < 0.0001]) with post hoc Holm–Sidak’s tests indicated that DA alone again produced an expected reduction in spike frequency, but the addition of sulpiride completely abolished this effect (Supplementary Figure S6A-B). We next tested whether co-administration of both L-741,626 and PG01037 would recapitulate the effects of sulpiride. Two-way RM ANOVA (main effect of current [F(12,84) = 60.48, p < 0.0001], main effect of condition [F(2,14) = 14.14, p = 0.0004], interaction [F(24,168) = 5.21, p < 0.0001]) with post hoc Holm–Sidak’s tests revealed that DA alone produced the expected reduction in spike frequency, and simultaneous co-administration of 1 nM L-741,626 + 1 nM PG01037 significantly attenuated this effect (Supplementary Figure S6C-D). These findings suggest that DA-mediated inhibition of D2-MSN activity involves DA binding at both D2Rs and D3Rs, and that simultaneous blockade of both receptor subtypes may be necessary to temper the impact of DA on the D2-MSN’s firing properties.
The enhancement of cocaine’s locomotor stimulant effects by D3R antagonism requires intact D1R signaling
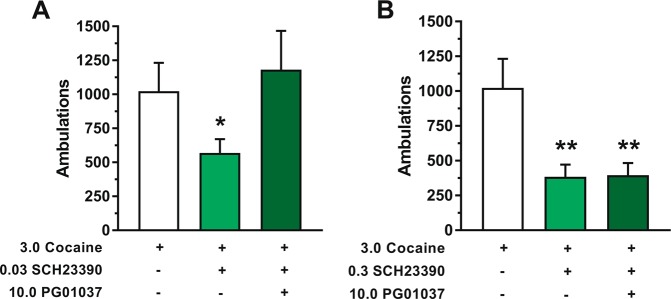
Based on the results of our FSCV and electrophysiological experiments, we hypothesized that D3R antagonism potentiates cocaine-induced locomotion in mice via a combination of two distinct neuropharmacological mechanisms in the NAc. First, D3R antagonism enhances cocaine-induced increases in DA release, likely via blockade of presynaptic D3Rs on DA terminals. Second, D3R antagonism results in potentiated excitation of D1-MSNs by DA, likely via blockade of postsynaptic D3Rs that normally provide inhibitory tone on these cells. These combined effects of D3R blockade create a scenario in which cocaine increases DA levels beyond its normal capacity, and this DA in turn hyperexcites D1-MSNs, thereby producing greater levels of locomotion as compared to cocaine alone. An important facet of this system is that although D1R signaling and D3R signaling oppose each other in D1-MSNs, the presence of some basal level of D1R-mediated excitation within these cells is ultimately required for the behavioral effects of cocaine to emerge. We therefore reasoned that a low level of D1R blockade should reduce cocaine-induced locomotion, and that this effect could be reversed with combined treatment of a D3R antagonist. However, if the level of D1R blockade was substantially strengthened, the effects of a D3R antagonist should be rendered ineffective because its capacity to enhance cocaine’s behavioral effects is dependent upon some threshold amount of intact D1R signaling. To test these hypotheses, we performed a final set of locomotor activity studies in mice. One-way RM ANOVA (main effect of treatment condition, [F(2,14) = 6.49, p = 0.010]) with post hoc Dunnett’s tests revealed that pretreatment with a low dose (0.03 mg/kg) of the D1-like receptor antagonist SCH23390 caused a modest ~45% attenuation of the locomotor-activating effects of 3.0 mg/kg cocaine, and this effect was reversed by co-administration of 10.0 mg/kg PG01037 (Fig. 5a; time course, Supplementary Figure S7A). Increasing the dose of SCH23390 10-fold caused a greater (~63%) reduction in cocaine-induced locomotion, but co-administration of 10.0 mg/kg PG01037 had no impact on the effects of this higher dose of SCH23390 (one-way RM ANOVA with post hoc Dunnett’s tests: main effect of treatment condition, [F(2,14) = 11.78, p = 0.001]; Fig. 5b; time course, Supplementary Figure S7B). Collectively, these findings support the notion that D3R antagonism potentiates the stimulant effects of cocaine via a D1-like receptor-dependent mechanism.
Fig. 5.
Effects of combined pretreatment with the D3R antagonist PG01037 and the D1-like receptor antagonist SCH23390 on acute cocaine-induced locomotor activity in mice. a Total number of ambulations in the 60 min period following administration of 3.0 mg/kg cocaine. Thirty min prior to cocaine administration, animals had been injected with a combination of PG01037 (vehicle or 10.0 mg/kg) and SCH23390 (vehicle or 0.03 mg/kg SCH23390). b Conditions were identical to panel (a), with the exception that the dose of SCH23390 was increased 10-fold (vehicle or 0.3 mg/kg). Each data point represents mean ± SEM ambulations. All mice received all treatments (n = 8). Drug treatments are depicted in a table below each figure; a plus symbol indicates the active dose of the drug was administered, while a minus symbol indicates that its vehicle was administered. *p < 0.05, **p < 0.01, significant difference by Dunnett’s test compared to vehicle/vehicle/3.0 cocaine condition (empty bar)
Discussion
In the present study, we demonstrate that selective D3R antagonism enhances several facets of NAc DA neurotransmission and potentiates the behavioral-stimulant effects of cocaine, while selective D2R antagonism produces an opposite response, functionally attenuating cocaine’s locomotor-activating effects. Furthermore, we for the first time have identified hyperexcitation of D1-MSNs as a putative mechanism by which D3R blockade uniquely facilitates NAc DA neurotransmission and associated behavioral output.
While nonselective antagonism of D2-like receptors has previously been reported to attenuate the locomotor-activating effects of cocaine [55, 56], the specific contributions of the D2-like receptor subtypes in mediating these effects have remained unclear. Results from our behavioral studies show that systemic pretreatment with the selective D2R antagonist L-741,626 attenuates the acute locomotor-stimulant effects of cocaine and abolishes the development of locomotor sensitization, while administration of the selective D3R antagonist PG01037 enhances cocaine’s acute locomotor-stimulant effects and renders a low, ineffective dose of cocaine capable of inducing behavioral sensitization. Intra-NAc infusion of PG01037 recapitulated the effects of systemic administration, thus confirming the NAc as a key neuroanatomical substrate for modulation of cocaine-induced locomotion by D3R antagonism. The effects of D3R blockade are therefore in direct opposition to those reported previously for nonselective D2-like receptor antagonism [55, 56] and for selective D2R antagonism in the present study, suggesting that the effect of nonselective D2-like receptor blockade on cocaine-induced locomotion is mediated predominantly by antagonism of the D2R in the NAc. This is supported by evidence that genetic deletion of the D2R subtype within striatal MSNs in mice diminishes the locomotor response to cocaine [58]. Moreover, our present results with PG01037 are in agreement with the previous finding that administration of another highly-selective D3R antagonist, NGB 2904, potentiates amphetamine-induced locomotor activity in mice [41], as well as the reported hypersensitivity of various lines of D3R knockout mice to the behavioral-stimulant effects of cocaine [42, 59] and amphetamine [40, 42]. Our observed enhancement of acute and repeated cocaine-induced locomotion following PG01037 administration can be attributed to changes in NAc DA neurotransmission exclusively for several reasons. First, psychostimulant-induced increases in rodent locomotion are strongly linked to the modulation of DA levels specifically within the NAc [60, 61]. Second, the D3R is enriched in the mouse NAc as compared to other regions [19, 20]. Finally, we show in the present study that intra-NAc infusion of PG01037 is sufficient to produce the enhanced behavioral response to cocaine.
Some studies have failed to detect enhanced stimulant-induced locomotor activity following D3R antagonism in rodents, but these results could be attributed to many factors including; the use of genetically-altered mice (i.e., D3R−/− mice) that may have compensatory changes to basal neurobiological systems affecting locomotor responsivity due to developmental and/or chronic loss of the receptor, use of pharmacological compounds with low selectivity for D3R vs. D2R, different routes of administration, use of various doses of psychostimulants to induce locomotor activity, use of various psychostimulants with diverse mechanisms of action, species differences, etc. For example, pretreatment with the highly-selective D3R antagonist S33084 reportedly failed to alter amphetamine- or cocaine-induced locomotion [46], but only single/high doses of these psychostimulants were tested which may have precluded detection of potentiation—indeed, visual inspection of those data suggests that S33084 pretreatment trended towards enhancing the amphetamine-induced locomotor response, but may have failed to reach statistical significance due to a ceiling effect. In other studies, administration of the selective D3R antagonist SB-277011A was reported to inhibit cocaine-induced locomotor activity [62] or have no effect on amphetamine-induced locomotion [35], but the inhibitory effect was observed only at a very high dose of SB-277011A which may have lacked selectivity for D3R vs. D2R, and the lack of effect on amphetamine-induced locomotion occurred following oral administration of SB-277011A. By contrast, we (present study) and others [38, 41] have found that selective D3R antagonism potentiates locomotor responses to cocaine or amphetamine across species. Our within-subjects, multi-dose approach in particular represents a significant advancement over previous investigations and reveals for the first time a clear leftward/upward shift of the cocaine dose–response function, interpreted unequivocally as a pharmacological potentiation. While a full reconciliation of our present findings with the rich body of previous work exploring effects of D3R antagonism on stimulant-induced locomotor activity is beyond the scope of the present discussion, it is clear that experimental parameters can profoundly affect the data and may explain why such varying results have been reported. We would also caution that the impact of D3R antagonism on psychostimulant-induced locomotor activity should not be compared to their impact on other behavioral effects of psychostimulants. Indeed, there is general agreement across studies that D3R antagonists do not alter self-administration of psychostimulants under schedules of reinforcement that provide easy access to the drug (e.g., FR1), but that D3R antagonists are effective in reducing break points under a progressive ratio (PR) schedule and also block, rather than enhance, drug-seeking elicited by various stimuli [14]. The reasons for these different effects on behaviors elicited by psychostimulants remain unclear, but likely involve distinct signaling pathways associated with motivation that will require further research to clarify.
D2Rs and D3Rs within the NAc are expressed both presynaptically on DAergic terminals and postsynaptically on MSNs [6, 63]. Because blockade of presynaptic DA autoreceptors typically results in enhanced presynaptic DA release [64, 65], it seemed plausible that D3R antagonism may potentiate cocaine-induced locomotion, at least in part, by exacerbating cocaine-induced increases in extracellular DA. In support of this hypothesis, we found that the application of PG01037 enhanced cocaine-induced increases in DA release and also significantly facilitated cocaine’s inhibitory action on DA clearance. The effects we observed with PG01037 in our FSCV experiments here are similar to those reported for another highly-selective D3R antagonist, SB-277011-A, suggesting that D3R antagonism is the primary pharmacological mechanism of action for our observed effects [31, 34]. It should be noted that these experiments were conducted in striatal slice, which inevitably eliminates basal DA tone due to severed axonal connections. Our FSCV studies are therefore unable to determine whether PG01037 may modulate basal DA tone in an intact animal. However, previous in vivo microdialysis experiments found that pretreatment with the selective D3R antagonist NGB 2904 potentiated cocaine-induced NAc DA increases, but failed to alter basal NAc DA levels [33]. This finding, coupled with the lack of observable locomotor effects following PG01037 administration alone in awake behaving mice in our present work, suggests that D3R antagonism does not appreciably modulate basal DA tone within the NAc. Taken together, these studies and our present behavioral and FSCV results indicate that presynaptic D3R antagonism potentiates stimulant-induced increases in NAc DA levels in the absence of effects on basal DA concentrations. The exact mechanisms by which D3R antagonists exert these effects on DA release and clearance and why they only do so under conditions of increased DA levels remain to be determined, but possible explanations include disinhibition of the presynaptic terminal via autoreceptor blockade [6, 21, 22] and/or modulation of the structure and/or function of the dopamine transporter itself [31, 66]. Regardless, these actions are unlikely to wholly explain the faciliatory effects of D3R antagonism on cocaine-induced locomotion because the application of L-741,626 to NAc slices produced effects similar to, and not opposite from, those produced by PG01037 in our FSCV studies. The opposing influences of selective D2R and D3R antagonism on behavioral output related to NAc DA signaling do not therefore appear to be mediated by disparate effects on presynaptic DA release.
To the best of our knowledge, the present study represents the first assessment of selective D2R or D3R antagonism on DA-induced changes in D1-MSN and D2-MSN activity within the NAc. With respect to D2-MSNs, although neither L-741,626 nor PG01037 application alone blocked DA-mediated inhibition of D2-MSN activity, we found that their combined administration produced a partial blockade of DA’s effects that resembled the effects of the nonselective D2/D3 receptor antagonist sulpiride, suggesting that simultaneous D2R and D3R antagonism may be required to fully prevent the inhibitory actions of DA on these cells. The finding that ineffective doses, when combined, produced a measurable change suggests that these compounds exert qualitatively similar rather than dissimilar effects on D2-MSN activity and that their combined effects are at least additive, if not synergistic, in nature in these cells. In contrast to their effects on D2-MSNs, we observed qualitative differences between selective D2R and D3R antagonism on DA-mediated excitation of D1-MSNs. The finding that L-741,626 failed to significantly alter the excitatory impact of DA on D1-MSNs was not surprising given the rarity of D2R co-expression in these cells [26]. However, a significant proportion of D1-MSNs in the NAc co-express the D3R [24–26], and accordingly we found that blockade of D3Rs via application of PG01037 modulated the activity of D1-MSNs, rendering them hyperexcitable to DA application. D1-like receptors are positively coupled to adenylyl cyclase via Gαs protein activation and typically produce intracellular effects that promote neuronal excitation, while the D3R is negatively coupled to adenylyl cyclase via Gαi/o protein activation and causes neuronal inhibition [6]. When co-expressed in the same cells, these receptors may exert opposing influences on neuronal activity [67]. Therefore, the hypersensitivity to DA-mediated excitation exhibited by D1-MSNs in the presence of PG01037 likely reflects the removal of tonic inhibitory tone that would otherwise be provided by DA’s activation of the D3R. As D1-like receptor activation has been positively linked with stimulant-induced locomotor output [68, 69], our findings provide a plausible mechanistic explanation as to how D3R antagonism results in exacerbated stimulant-induced locomotion, i.e., through increased activation of NAc D1-MSNs via reductions in D3R-mediated inhibition. Consistent with this model, we found that D3R antagonism functionally opposed the reductions in cocaine-induced locomotion exerted by a low dose of the D1-like receptor antagonist SCH23390, likely because some residual level of D1-like receptor signaling remained that was sufficient to engender increased locomotor output when tonic D3R-mediated inhibition was antagonized. However, when the dose of SCH23390 was increased 10-fold, the D3R antagonist was rendered ineffective, probably because the level of D1-like receptor signaling was no longer sufficient to increase locomotor output. Our electrophysiological and behavioral studies are uniformly consistent with the hypothesis that D3R antagonism renders NAc D1-MSNs hyper-responsive to DA-induced excitation, and also provide a possible mechanism to explain how D3R antagonism functionally enhances the behavioral, cellular, and neurochemical effects of cocaine observed herein and previously by others [28, 31, 34, 59, 70, 71]. Further investigation will be required to confirm that disinhibition of NAc D1-MSNs mediates the potentiation of cocaine-induced behavioral effects in vivo.
In summary, we report that pretreatment with the selective D3R antagonist PG01037 enhances, while the selective D2R antagonist L-741,626 attenuates, the behavioral-stimulant effects of cocaine in mice. We also provide evidence that suggests the effects of PG01037 may be mediated by a two-pronged neuropharmacological modulation of NAc DA neurotransmission. First, D3R antagonism potentiates cocaine-induced increases in presynaptic DA release, and second, causes hypersensitivity of NAc D1-MSNs to the excitatory effects of DA while lacking appreciable effects on D2-MSNs. Our results help to resolve a complicated and mixed literature regarding the impact of D3R antagonism on NAc-related signaling and behavioral output that will be of importance to those exploring D3R antagonists as potential pharmacotherapeutics for various DA-related disorders.
Funding and disclosure
This work was supported by the following funding sources: National Institutes of Health Grants K99DA039991 and R00DA039991 to D.F.M.; T32ES012870 to R.C.B.; F31DA044726 to S.L.F.; F32NS098615 to K.A.P.S.; F31DA037652 and F32AG058396 to K.A.S.; ZIADA000424 to A.H.N.; R01ES023839 to G.W.M.; R21MH113341 and R01MH079276 to C.A.P.; R01DA038453 to D.W. and C.A.P.; R21DA040788 to D.W., and The Brown Foundation Inc. Fellowship to A.K.P. Portions of this work have previously been reported in poster presentations at the Society for Neuroscience (2016) and the Gordon Research Conference: Catecholamines (2015, 2017).
Supplementary information
Competing interests
The authors have no conflicts of interest to disclose.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Daniel F. Manvich, Alyssa K. Petko, Rachel C. Branco
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0371-2).
References
- 1.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–32. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jellinger KA. The pathomechanisms underlying Parkinson’s disease. Expert Rev Neurother. 2014;14:199–15. doi: 10.1586/14737175.2014.877842. [DOI] [PubMed] [Google Scholar]
- 3.Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol. 2017;20:1036–46. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–25. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 7.Newman-Tancredi A, Cussac D, Audinot V, Nicolas JP, De Ceuninck F, Boutin JA, et al. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor. J Pharmacol Exp Ther. 2002;303:805–14. doi: 10.1124/jpet.102.039875. [DOI] [PubMed] [Google Scholar]
- 8.Nordstrom AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, et al. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry. 1993;33:227–35. doi: 10.1016/0006-3223(93)90288-O. [DOI] [PubMed] [Google Scholar]
- 9.Muench J, Hamer AM. Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81:617–22. [PubMed] [Google Scholar]
- 10.Banks ML, Hutsell BA, Schwienteck KL, Negus SS. Use of preclinical drug vs. food choice procedures to evaluate candidate medications for cocaine addiction. Curr Treat Options Psychiatry. 2015;2:136–50. doi: 10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishi T, Matsuda Y, Iwata N, Correll CU. Antipsychotics for cocaine or psychostimulant dependence: systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2013;74:e1169–80. doi: 10.4088/JCP.13r08525. [DOI] [PubMed] [Google Scholar]
- 12.Maramai S, Gemma S, Brogi S, Campiani G, Butini S, Stark H, et al. Dopamine D3 receptor antagonists as potential therapeutics for the treatment of neurological diseases. Front Neurosci. 2016;10:451. doi: 10.3389/fnins.2016.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokoloff P, Le Foll B. The dopamine D3 receptor, a quarter century later. Eur J Neurosci. 2017;45:2–19. doi: 10.1111/ejn.13390. [DOI] [PubMed] [Google Scholar]
- 14.Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shorter D, Kosten TR. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 2011;9:119. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov Today. 2005;10:917–25. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- 17.Joyce JN, Millan MJ. Dopamine D3 receptor agonists for protection and repair in Parkinson’s disease. Curr Opin Pharmacol. 2007;7:100–5. doi: 10.1016/j.coph.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Leggio GM, Salomone S, Bucolo C, Platania C, Micale V, Caraci F, et al. Dopamine D(3) receptor as a new pharmacological target for the treatment of depression. Eur J Pharmacol. 2013;719:25–33. doi: 10.1016/j.ejphar.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–19. doi: 10.1016/0006-8993(91)91456-B. [DOI] [PubMed] [Google Scholar]
- 20.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–51. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 21.Chen PC, Lao CL, Chen JC. The D(3) dopamine receptor inhibits dopamine release in PC-12/hD3 cells by autoreceptor signaling via PP-2B, CK1, and Cdk-5. J Neurochem. 2009;110:1180–90. doi: 10.1111/j.1471-4159.2009.06209.x. [DOI] [PubMed] [Google Scholar]
- 22.Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–84. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu XY, Mao LM, Zhang GC, Papasian CJ, Fibuch EE, Lan HX, et al. Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron. 2009;61:425–38. doi: 10.1016/j.neuron.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Moine C, Bloch B. Expression of the D3 dopamine receptor in peptidergic neurons of the nucleus accumbens: comparison with the D1 and D2 dopamine receptors. Neuroscience. 1996;73:131–43. doi: 10.1016/0306-4522(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, et al. Functional implications of multiple dopamine receptor subtypes: the D1/D3 receptor coexistence. Brain Res Brain Res Rev. 1998;26:236–42. doi: 10.1016/S0165-0173(97)00046-5. [DOI] [PubMed] [Google Scholar]
- 26.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–91. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, et al. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–9. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Congestri F, Formenti F, Sonntag V, Hdou G, Crespi F. Selective D3 receptor antagonist SB-277011-A potentiates the effect of cocaine on extracellular dopamine in the nucleus accumbens: a dual core–shell voltammetry study in anesthetized rats. Sensors. 2008;8:6936–51. doi: 10.3390/s8116936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph JD, Wang YM, Miles PR, Budygin EA, Picetti R, Gainetdinov RR, et al. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors. Neuroscience. 2002;112:39–49. doi: 10.1016/S0306-4522(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 30.Koeltzow TE, Xu M, Cooper DC, Hu XT, Tonegawa S, Wolf ME, et al. Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice. J Neurosci. 1998;18:2231–8. doi: 10.1523/JNEUROSCI.18-06-02231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGinnis MM, Siciliano CA, Jones SR. Dopamine D3 autoreceptor inhibition enhances cocaine potency at the dopamine transporter. J Neurochem. 2016;138:821–9. doi: 10.1111/jnc.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci USA. 2012;109:17675–80. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–59. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zapata A, Shippenberg TS. D(3) receptor ligands modulate extracellular dopamine clearance in the nucleus accumbens. J Neurochem. 2002;81:1035–42. doi: 10.1046/j.1471-4159.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- 35.Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–65. [PubMed] [Google Scholar]
- 36.Roberts C, Cummins R, Gnoffo Z, Kew JN. Dopamine D3 receptor modulation of dopamine efflux in the rat nucleus accumbens. Eur J Pharmacol. 2006;534:108–14. doi: 10.1016/j.ejphar.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, et al. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA. 1996;93:1945–9. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahi A, Boyer F, Bussard G, Dreyer JL. Silencing dopamine D3-receptors in the nucleus accumbens shell in vivo induces changes in cocaine-induced hyperlocomotion. Eur J Neurosci. 2005;21:3415–26. doi: 10.1111/j.1460-9568.2005.04157.x. [DOI] [PubMed] [Google Scholar]
- 39.Corbin AE, Pugsley TA, Akunne HC, Whetzel SZ, Zoski KT, Georgic LM, et al. Pharmacological characterization of PD 152255, a novel dimeric benzimidazole dopamine D3 antagonist. Pharmacol Biochem Behav. 1998;59:487–93. doi: 10.1016/S0091-3057(97)00442-5. [DOI] [PubMed] [Google Scholar]
- 40.McNamara RK, Logue A, Stanford K, Xu M, Zhang J, Richtand NM. Dose–response analysis of locomotor activity and stereotypy in dopamine D3 receptor mutant mice following acute amphetamine. Synapse. 2006;60:399–405. doi: 10.1002/syn.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pritchard LM, Newman AH, McNamara RK, Logue AD, Taylor B, Welge JA, et al. The dopamine D3 receptor antagonist NGB 2904 increases spontaneous and amphetamine-stimulated locomotion. Pharmacol Biochem Behav. 2007;86:718–26. doi: 10.1016/j.pbb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, et al. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–48. doi: 10.1016/S0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- 43.Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ. Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology. 1999;38:555–65. doi: 10.1016/S0028-3908(98)00213-5. [DOI] [PubMed] [Google Scholar]
- 44.Xu M, Koeltzow TE, Cooper DC, Tonegawa S, White FJ. Dopamine D3 receptor mutant and wild-type mice exhibit identical responses to putative D3 receptor-selective agonists and antagonists. Synapse. 1999;31:210–5. doi: 10.1002/(SICI)1098-2396(19990301)31:3<210::AID-SYN6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 45.Karasinska JM, George SR, Cheng R, O’Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci. 2005;22:1741–50. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- 46.Millan MJ, Dekeyne A, Rivet JM, Dubuffet T, Lavielle G, Brocco M. S33084, a novel, potent, selective, and competitive antagonist at dopamine D(3)-receptors: II. Functional and behavioral profile compared with GR218,231 and L741,626. J Pharmacol Exp Ther. 2000;293:1063–73. [PubMed] [Google Scholar]
- 47.Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, et al. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–48. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- 48.Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, et al. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem. 2007;50:4135–46. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- 49.Grundt P, Husband SL, Luedtke RR, Taylor M, Newman AH. Analogues of the dopamine D2 receptor antagonist L741,626: binding, function, and SAR. Bioorg Med Chem Lett. 2007;17:745–9. doi: 10.1016/j.bmcl.2006.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, et al. 3-((4-(4-Chlorophenyl)piperazin-1-yl)-methyl)-1H-pyrrolo-2,3-b-pyridine: an antagonist with high affinity and selectivity for the human dopamine D4 receptor. J Med Chem. 1996;39:1941–2. doi: 10.1021/jm9600712. [DOI] [PubMed] [Google Scholar]
- 51.Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter RD. Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci USA. 2002;99:13873–7. doi: 10.1073/pnas.212519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohr KM, Bernstein AI, Stout KA, Dunn AR, Lazo CR, Alter SP, et al. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci USA. 2014;111:9977–82. doi: 10.1073/pnas.1402134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–64. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–39. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Neisewander JL, O’Dell LE, Redmond JC. Localization of dopamine receptor subtypes occupied by intra-accumbens antagonists that reverse cocaine-induced locomotion. Brain Res. 1995;671:201–12. doi: 10.1016/0006-8993(94)01317-B. [DOI] [PubMed] [Google Scholar]
- 56.Baker DA, Khroyan TV, O’Dell LE, Fuchs RA, Neisewander JL. Differential effects of intra-accumbens sulpiride on cocaine-induced locomotion and conditioned place preference. J Pharmacol Exp Ther. 1996;279:392–401. [PubMed] [Google Scholar]
- 57.Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–31. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kharkwal G, Radl D, Lewis R, Borrelli E. Dopamine D2 receptors in striatal output neurons enable the psychomotor effects of cocaine. Proc Natl Acad Sci USA. 2016;113:11609–14. doi: 10.1073/pnas.1608362113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carta AR, Gerfen CR, Steiner H. Cocaine effects on gene regulation in the striatum and behavior: increased sensitivity in D3 dopamine receptor-deficient mice. Neuroreport. 2000;11:2395–9. doi: 10.1097/00001756-200008030-00012. [DOI] [PubMed] [Google Scholar]
- 60.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–10. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–22. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 62.Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, et al. YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict Biol. 2012;17:259–73. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 64.Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 2001;21:9134–41. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem. 1992;59:449–55. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- 66.Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, et al. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem. 2007;282:35842–54. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Xu M. Opposite regulation of cocaine-induced intracellular signaling and gene expression by dopamine D1 and D3 receptors. Ann N Y Acad Sci. 2006;1074:1–12. doi: 10.1196/annals.1369.001. [DOI] [PubMed] [Google Scholar]
- 68.Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Behav. 2002;72:857–63. doi: 10.1016/S0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- 69.Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, et al. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–55. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Huang L, Lu K, Liu Y, Tu G, Zhu M, et al. Cocaine-induced synaptic structural modification is differentially regulated by dopamine D1 and D3 receptors-mediated signaling pathways. Addict Biol. 2017;22:1842–55. doi: 10.1111/adb.12462. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, et al. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–54. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.