Abstract
Introduction
Cervical cancer imposes a substantial health burden worldwide including in Australia and is caused by persistent infection with one of 13 sexually transmitted high-risk human papillomavirus (HPV) types. The objective of this study was to assess the cost-effectiveness of adding a nonavalent new Gardasil-9® (9vHPV) vaccine to the national immunisation schedule in Australia across three different delivery strategies.
Materials and methods
The Papillomavirus Rapid Interface for Modelling and Economics (PRIME) model was used to examine the cost-effectiveness of 9vHPV vaccine introduction to prevent HPV infection. Academic literature and anecdotal evidence were included on the demographic variables, cervical cancer incidence and mortality, treatment costs, and vaccine delivery costs. The incremental cost-effectiveness ratios (ICERs) were measured per disability-adjusted life years (DALYs) averted, using the heuristic cost-effectiveness threshold defined by the World Health Organisation (WHO). Analyses and data from international agencies were used in scenario analysis from the health system and societal perspectives.
Results
The 9vHPV vaccination was estimated to prevent 113 new cases of cervical cancer (discounted) during a 20-year period. From the health system and societal perspectives, the 9vHPV vaccination was very cost-effective in comparison with the status quo, with an ICER of A$47,008 and A$44,678 per DALY averted, respectively, using the heuristic cost-effectiveness threshold level. Considering delivery strategies, the ICERs per DALY averted were A$47,605, A$46,682, and A$46,738 for school, health facilities, and outreach-based vaccination programs from the health system perspective, wherein, from the societal perspective, the ICERs per DALY averted were A$46,378, A$43,729, A$43,930, respectively. All estimates of ICERs fell below the threshold level (A$73,267).
Conclusions
This cost-effectiveness evaluation suggests that the routine two-dose 9vHPV vaccination strategy of preadolescent girls against HPV is very cost-effective in Australia from both the health system and societal perspectives. If equally priced, the 9vHPV option is the most economically viable vaccine. Overall, this analysis seeks to contribute to an evidence-based recommendation about the new 9vHPV vaccination in the national immunisation program in Australia.
Introduction
Cervical cancer is both a leading cancer and the leading cause of cancer deaths in women globally [1]. An estimated 570,000 new cases of cervical cancer were diagnosed in 2018, composing 6.6% of all cancers in women [1]. In Australia, over the last couple of years, the age-specific cervical cancer incidence has slightly reduced to 7.1 cases per 100,000 females in 2018 from 7.4 cases per 100,000 females in 2014 [2]. However, the incidence is quite high among young adult females at 15.0 cases per 100,000 females and was the most frequently diagnosed cancer among women in 2018 [2]. Persistent infections with human papillomavirus (HPV) are a key cause of cervical cancer and an established carcinogen of cervical cancer [3]. HPV is predominantly transmitted to reproductive-aged women through sexual contact [4]. Most HPV infections are transient and can be cleared up within a short duration, usually a few months after their acquisition. However, HPV infections can continue and evolve in cancer in some cases. There are more than 100 types of HPV infections that have been identified and divided into low- and high-risk types develop into cervical cancer [5]. Thirteen high-risk HPV types are known to be predominantly responsible for malignant and premalignant lesions of the anogenital area [6] and are the leading causes of most aggressive cervical cancers [7]. Further, HPV is also responsible for the majority of anogenital cervical cancers such as anal cancers (88%), vulvar cancers (43%), invasive vaginal carcinomas (70%), and all penile cancers (50%) globally [5]. The incidence of neck and head cancers caused by HPV infection is low but not negligible [8]. Cervical cancer is preventable through implementation of a primary prevention strategy such as vaccination worldwide including Australia [9,10]. Therefore, a reduction in cervical cancer incidence and associated cancer mortality along with the improvement of survival rates have the potential to reduce the burden of cervical cancer.
The high burden of cervical cancer in terms of incidence and associated mortality rates across the world could be reduced by incorporating a comprehensive primary prevention mechanism. Prevention mechanisms includes early vaccination, diagnosis, effective screening, adequate referral and advanced course of treatment procedures. In this context, HPV vaccinations (i.e., bivalent and quadrivalent) has been introduced in many countries in the past decade [10]. Currently, available HPV vaccines can promote herd immunity against cancer-causing types of HPV that helps to reduce the high-risk of cervical cancer burden. These vaccines have played a significant role in preventing HPV infection types 16 and 18 [10], which cause more than 70% of cervical cancers in Australia [7].
Australia was the first country to implement a publicly-funded National HPV Immunisation Program (NHIP), starting with preadolescent girls in 2007, using the quadrivalent Gardasil® vaccine (4vHPV; Merck & Co., Kenilworth, NJ, USA) [11]. The goals of the immunisation program were to reduce the acquisition and spread of HPV infections and to achieve optimum coverage through the school-based delivery system [12]. This program for adolescent employed a three-dose schedule of the 4vHPV vaccine [13]. The 4vHPV vaccine provides protection against HPV infection types 6, 11, 16, and 18 [14]. In the context of Australia, the 4vHPV vaccine was replaced by the two-dose nonavalent Gardasil®-9 vaccine (9vHPV; Merck Sharp & Dohme) in 2018 [15]. According to the underlying distribution of HPV infection types of cervical cancers, the 9vHPV vaccine builds population-level strong immunity against HPV-6, 11, 16, 18, 31, 33, 45, 52, and 58 infections [6] that cumulatively contribute to approximately 89% of all cervical cancers globally [16] and 93% in Australia [17]. Considering the primary prevention of HPV infection, the 9vHPV vaccine is anticipated to reduce by 10% more the lifetime risk of diagnosis of cervical cancer in immunised cohorts than the 4vHPV vaccine and by 52% more compared to non-vaccinated cohorts [18].
With the availability of vaccines against the different HPV infection types, there are good opportunities for primary prevention to add to continuing efforts on secondary prevention strategies. However, the decision for any country to add a new vaccine to national immunization programs requires careful assessment of the relative value of the vaccine compared with alternative uses of the required resources (i.e., cost-effectiveness) and its affordability (i.e., budgetary impact). Cost-effectiveness analysis (CEA) is a pragmatic approach which aims to examine the outcomes and costs of interventions or programs designed to improve health. CEA evolves measuring the net or incremental costs and effects of an intervention or program in terms of costs and health outcomes as compared with some comparator. There is considerable evidence of assessing the cost-effectiveness of the 9vHPV vaccine in different country settings. In Canada, the 9vHPV was found to be highly cost-effective compared with the 4vHPV vaccine taking into consideration the shorter duration of protection (9vHPV = 20 years vs. 4vHPV = lifelong), along with a lower vaccine efficacy (85% vs. 95%) [19]. In other studies conducted in the United States (US), the 9vHPV vaccine was also found to be very cost-effective compared to the 4vHPV vaccine [20]. However, findings from cost-effective evaluations will differ based on study settings, funding, perspectives and coverage of vaccination For example, in the US, Chesson et al. (2016) found that the 9vHPV vaccine was not cost-effective, with an incremental cost-effectiveness ratio (ICER) of $146,200 per quality-adjusted life year (QALY) gained that exceeded the cost-effectiveness threshold ($100,000) [21]. Some cost-effective evaluations were performed using the same vaccine (i.e., 9-valent) in the US to capture the different dimensions of its economic viability [22–27]. These studies incorporated different study participants, designs, perspectives, vaccine delivery routes and model specifications. Simms et al. (2016) evaluated the 9vHPV vaccine in a primary HPV screening scenario in both Australia and Canada [18]. They found that 9vHPV had a significant impact on reducing cervical cancer incidence from the health system perspective. Further, they claimed that the incremental cost per dose in girls should not exceed a median of A$35.99. However, this study emphasised the impact of vaccines to prevent cervical cancer rather than their economic viability. Sufficient evidence did not arise for health policymakers to use the findings to develop cost-effective intervention strategies. In Germany, universal immunisation with 9vHPV was suggested as it had an ICER of €22,987/QALY gained, which was below the threshold [28]. In Spain, a recent study evaluated a vaccine program in adolescent girls, wherein the 9vHPV vaccine was found to be more highly cost-effective, with an ICER of €7,718 per QALY compared to the 4vHPV vaccine [29]. In the African setting, in Kenya and Uganda, a study recommended that the 9vHPV vaccine was very cost-effective in both countries, wherein the additional cost of the 9vHPV vaccine did not exceed I$8.3 per immunised girl [30].
In Australia, the 9vHPV vaccine was introduced in 2018. There is limited current comprehensive evidence about the cost-effectiveness of the 9vHPV vaccine in Australia across delivery strategies (e.g., school-based, health facility-based and outreach-based) from the health system and societal perspectives. The present study evaluates the cost-effectiveness of the 9vHPV vaccine from both health system and societal perspectives across three delivery routes. However, the previous cost-effective evaluation considered only one perspective nor health system or societal, or both perspectives along with single vaccine delivery route. Further, the findings of the present study will provide evidence about the cost-effectiveness of the 9vHPV vaccine to policymakers. These cost-effectiveness findings will also be significant for determining the optimal pricing of delivery strategies in the vaccination program in order to maximise the societal benefits of the introduction of the new 9vHPV vaccine to Australia.
The objectives of this study are (1) to examine the cost-effectiveness of the 9vHPV vaccine by considering three different vaccine delivery strategies in the setting of Australia from the health system and societal perspectives and (2) compare the ICER per case, disability-adjusted life years (DALYs), and life-years saved across delivery strategies such as school-based, health facility-based, and outreach-based programs.
Materials and methods
Study perspective
This study was designed from both the health system and societal perspectives. The societal perspective refers to all types of costs that can be identified, quantified, estimated, and valued no matter who incurred them and it is considered to be the summation of both provider and household costs. This is the recommended standard for undertaking cost-effectiveness analysis [31].
Model overview
The study used the Papillomavirus Rapid Interface for Modelling and Economics (PRIME) model. PRIME is a user-friendly model designed and developed by the World Health Organisation (WHO) in collaboration with the Johns Hopkins Bloomberg School of Public Health in Baltimore, the School of Hygiene and Tropical Medicine in London, and the Universite Laval in Quebec [10]. PRIME is a Microsoft Excel based (Microsoft Corp., Armonk, NY, USA) static model that measures the health and economic effects of the vaccination of adolescent girls against HPV infection. It is not designed to examine other dimensions, such as immunised males or older women or the impact of cervical cancer screening services [10]. Several spreadsheets are contained in this model to input different parameter-level data on demographics, an age-dependent incidence of cervical cancer, associated mortality, vaccine efficacy, vaccine coverage, and associated costs (e.g., vaccination costs, treatment costs). This model does not consider indirect effects like herd immunity.
Methodological assumptions
Methodological assumptions follow the WHO guidelines for cost-effectiveness analysis [32]. The use of cost-effectiveness analysis is recommended when considering health system and societal perspectives. In the context of the health system perspective, the average cost parameters associated with treating a woman with cervical cancer (per episode, over the lifetime), and the cost of the HPV vaccination program were both considered. From the societal viewpoint, both direct medical (e.g., drugs, diagnostics) and non-medical (e.g., transportation) costs as well as indirect costs (e.g., productivity loses or income loss due to cervical cancer) were considered in the analysis. All future costs and health benefits were adjusted by a discount rate of 5% annually [5,29,32], which was validated in the sensitivity analysis. The primary outcome measure is the ICERs per DALYs averted. DALY estimation was undertaken by summing up the fatal burden (years of life lost; YLL) due to premature cervical cancer related mortality and the non-fatal burden (years lost due to disability; YLD) for patients surviving the condition.
| (1) |
| (2) |
| (3) |
where, N = number of deaths; L (YLL) = standard life expectancy at the age of death in that year; I = number of people with cervical cancer cases; DW = disability weight; r = discount rate; and L (YLD) = duration of disability in years.
Cost-effectiveness analysis
The performance of competing strategies was explained using the ICER which were calculated by dividing the difference in cost with and without HPV vaccination by the difference in health outcomes (e.g., the number of DALYs averted, the number of deaths and cases averted) with and without vaccination in Australia. The ICER is used to examine whether the 9vHPV vaccine is economically viable in Australia. In the context of Australia, no explicit cost-effectiveness threshold has been approved [33,34], although research has confirmed that there is a correlation between the incremental cost per health outcomes (e.g., QALY gained or DALY averted) and the probability of rejection of a health intervention or a new medicine [35]. The pharmaceutical industry claim that an acceptable threshold was in the range of AUD 45,000 to AUD 60,000 per additional QALY gained [36]. Some studies also stated that “Pharmaceutical Benefits Advisory Committee (PBAC) decisions in the past have shown that the ICER per QALY gained was of the order of $50,000” [18,37]. The present study intended to evaluate the cost-effectiveness of the 9vHPV vaccine in terms of the ICER per DALYs averted. Further, DALYs and QALYs differ in concept and application. The concept of DALYs was used to measure the disease burden using life lost due to premature death and the time spent in worse healthy states. Empirical evidence in the Australian context is limited to the use of the willingness-to-pay (WTP) threshold values for the ICER per DALYs averted. In reporting the cost-effectiveness scenario, the present study used the heuristic cost-effectiveness threshold as defined by the WHO Commission on Macroeconomics and Health (CMH) [38]. The gross domestic product (GDP)-related cost-effectiveness thresholds were based on assumptions about leisure time, non-health consumption, longevity and health-related quality of life. An intervention is cost-effective if the ICER per DALY averted is less than three times of a country’s annual per capita GDP. According to this guideline, the CMH recommended three broad decision rules, as follows: (1) a program or intervention is defined as very cost-effective if the ICER per DALY averted is less than one time the GDP per capita; (2) a program or intervention is cost-effective if the ICER per DALY averted is one or more times the GDP per capita but less than or equal to three times the GDP per capita; and (3) a program or intervention is not cost-effective if the ICER per DALY averted is more than three times the GDP per capita [31].
Vaccine and efficacy
The Australian Technical Advisory Group on Immunisation (ATAGI) in 2018 advised moving from using the quadrivalent 4vHPV to using the nonavalent Gardasil-9 (9vHPV) vaccine [39]. The 9vHPV vaccine has been registered for use in Australia [40]. The vaccine is funded through the national immunisation program (NIP) and delivered primarily by state and territory school-based immunisation programs in Australia [39]. This vaccine is manufactured using a procedure similar to that of the 4vHPV vaccine, which contains 0.5mg of aluminium hydroxyphosphate sulphate and a yeast expression system [40]. The 4vHPV vaccine contains five more virus-like particles than the original vaccine, identical to those in the protective capsule around the nine included strains (HPV-6, 11, 16, 18, 31, 33, 45, 52, and 58) with the aim to further reduce the HPV disease burden. The high prophylactic efficacy of the 9vHPV vaccine (93%) against HPV infection is evident both in Australia (77% for HPV types 16, 18 and 16% for HPV types 6, 31, 33, 45, 52, 58) [17] and globally (89%) [16]. However, no herd immunity was considered. It was recommended for the target cohort of adolescents aged 12 years to receive a two-dose 9vHPV vaccination for several reasons [39]. First, administering a vaccination at this age is more likely to ensure it is being given before their first sexual encounter (and HPV exposure). Also, the immune response tends to be stronger and more long-lasting when the vaccine is given to pre-adolescents. However, 9vHPV is not recommended for use during pregnancy. Similarly, vaccination is delayed if the person is unwell or has a high temperature, medical advice is recommended if the person is allergic to yeast or has had a severe reaction to a previous vaccine, and anyone who receives the vaccine is recommended to sit for 15 minutes thereafter to reduce the risk of fainting.
Vaccine delivery strategies
The HPV vaccine delivery strategy is an important aspect that needs to be considered carefully by each country. According to the country-specific context, the costs of vaccine delivery may vary. The WHO has recommended several types of common vaccine delivery strategies for different country settings. One example is vaccine delivery at healthcare facilities and via outreach routes (e.g. school-based program) and campaigns. It may be required to use a combined vaccine delivery strategy to ensure access among the entire target population. The 9vHPV vaccine has been delivered in Australia through school-based NIP in all states and territories to the target population cohort of school-going adolescents since January 2018. Two doses of 9vHPV are recommended to be administered at a minimum interval of six to 12 months between doses [39]. In some cases, general practitioner (GP) and other primary health care providers are generally engaged to catch up doses missed in the routinely school-based NIP. All providers are proactively involved in delivering and ensuring the completion of all doses of the 9vHPV vaccine to those individuals with special requirements, vaccine hesitancy, or immunocompromise. However, individuals who have already been fully immunised with HPV vaccines are not eligible for free 9vHPV vaccination. The present study incorporated another two hypothetical vaccine delivery strategies, as health facility-based and outreach-based, both from the health system and societal perspectives.
Vaccine delivery costs
The present study considered vaccine delivery related costs across three delivery strategies (e.g., school-based, health facility-based and outreach-based). Costs were derived from an existing costing study [41]. This study captured both financial and economic costs according to the WHO guidelines [42], included eight cost parameters, and focused on the investment and recurrent cost impacts of HPV vaccination on existing vaccination services. Furthermore, the investment costs were defined as microplanning (e.g., per diems and travel allowances, venue rental, transport and personnel time spent), training (e.g., training materials, stationery), social mobilisation (e.g., facilitator time in meetings, production of television/radio spots, posters, leaflets, value of teacher and volunteer time), and cold chain supplement. In addition, recurrent costs were covered including vaccines, service delivery, monitoring and evaluation, and waste disposal.
Cervical cancer treatment costs
Direct medical costs
Cervical cancer treatment costs were derived from a previous cost-of-illness study considering four treatment procedures: localised cancer treatment, regional cancer treatment, distant cancer treatment and terminal care [43]. The treatment costs were estimated based on different parameters such as surgical (e.g., conisation, hysterectomy, radical hysterectomy) and non-surgical (e.g., radiation therapy, adjuvant radiation therapy, chemo-radiation) [43]. Different types of activities included in cancer diagnosis were the direct medical costs such as colposcopy, chest X-ray, computerised tomography scan, positron-emission tomography scan, magnetic resonance imaging, bone scan and cystoscopy. Other costs included those related to inpatient care, emergency care, medicine costs, rehab, complex continuing care, long-term care, home care services, physician consultations, and non-physician provider costs.
Indirect costs
Indirect costs of cervical cancer patients and vaccine receivers were restricted to the loss of labour productivity due to ill health. Absenteeism-related data were obtained for a cervical cancer episode from elsewhere [32]. Other indirect costs were estimated using the human capital approach (S1 Table). The production losses were measured in both monetary and quantitative terms (e.g., days of productivity loss) [44]. The value of unpaid time devoted to own care and family defined caregivers [45]. The value of daily productivity was measured based on an age-specific average wage [46]. The average daily wage of cervical cancer patients were used for adult patients, and one-half of that wage was applied to teenager patients. Intangible costs related to pain, discomfort and grief were excluded [46]. All costs were converted into 2018 Australian dollars using the Consumer Price Index of Health Care [47].
Dynamic modelling of HPV transmission and the impact of vaccination
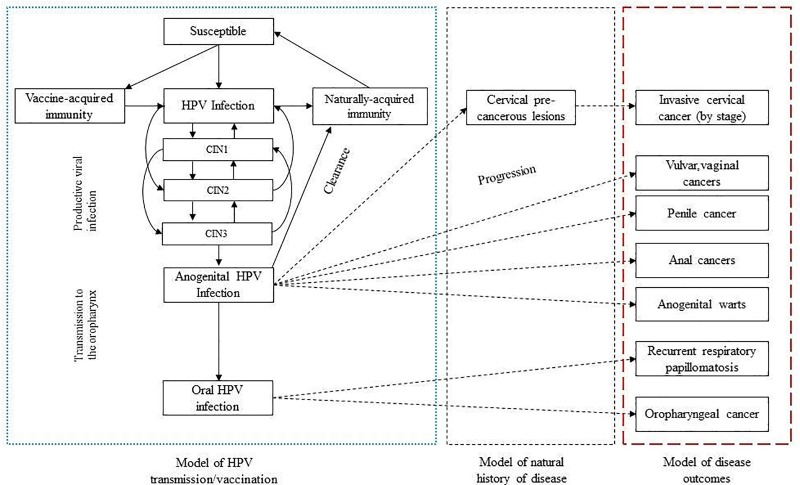
A dynamic cancer disease model was introduced to cover HPV transmission, HPV vaccination and cervical pre-cancer (Fig 1). The model incorporates demographics, economics, HPV attributable fractions in cervical cancer and vaccine uptake assumptions, as detailed in Table 1. When modelling the impact of HPV vaccination, the model captured the effects of herd protection (i.e., naturally acquired immunity) on the unvaccinated cohort. It was assumed that 9vHPV vaccine type-specific (HPV types) efficacy in girls was 100% and that the duration of protection was 20 years [19,27].
Fig 1. Simplified diagram of the model of HPV transmission, human impact of vaccination and disease outcomes in Australia.
Table 1. Input parameter assumptions and sensitivity analysis.
| Input parameters | Health system perspective | Societal perspective |
Sensitivity analysis and potential sources |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | School-based | Health facilities-based | Outreach-based | Overall | School-based | Health facilities-based | Outreach-based | ||
| Population | |||||||||
| Population cohort at birth (female) (‘000) | 191.34 | 191.34 | 191.34 | 191.34 | 191.34 | 191.34 | 191.34 | 191.34 | [50] |
| Population cohort at vaccination age (female) (‘000) | 118.68 | 118.68 | 118.68 | 118.68 | 118.68 | 118.68 | 118.68 | 118.68 |
[40,51] |
| Target age group (yrs) | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | [39] |
| Vaccination and vaccine delivery costs | |||||||||
| Vaccination coverage (full doses) | 86% | 86% | 86% | 86% | 86% | 86% | 86% | 86% | 72.00% -90.1% [51–55] |
| Vaccine effectiveness vs HPV types1 | 95% | 95% | 95% | 95% | 95% | 95% | 95% | 95% | 85% -100% [25,52,53,56] |
| Price of vaccine per fully immunised girl (FIG) (A$) | 280 | 280 | 280 | 280 | 280 | 280 | 280 | 280 | 270–320 [32,41,57] |
| Direct costs of vaccine delivery per FIG (A$) | 31.77 | 35.27 | 29.86 | 30.19 | 31.77 | 35.27 | 29.86 | 30.19 | [5,32,41] |
| Indirect costs of vaccine delivery per FIG (A$) | - | - | - | - | 17.59 | 24.05 | 13.93 | 14.79 | |
| Total cost of vaccine delivery cost per FIG (A$) | 31.77 | 35.27 | 29.86 | 30.19 | 49.36 | 59.32 | 43.80 | 44.98 | |
| Total costs of vaccination per FIG (A$) | 311.77 | 315.27 | 309.86 | 310.19 | 329.36 | 339.32 | 323.80 | 324.98 | 300–500 [5,32,41] |
| Treatment cost per episode | |||||||||
| Direct costs A$ (‘000) | 32.95 | 32.95 | 32.95 | 32.95 | 32.95 | 32.95 | 32.95 | 32.95 | [32,43] |
| Indirect costs (including caregiver costs) A$ (‘000) | - | - | - | - | 28.32 | 28.32 | 28.32 | 28.32 | [32,43] |
| Total treatment costs A$ (‘000) | 32.95 | 32.95 | 32.95 | 32.95 | 61.27 | 61.27 | 61.27 | 61.27 | 36.05–71.05 [32,43] |
| Methodological assumptions | |||||||||
| Disability weight for cancer diagnosis | 0.09 | 0.09 | 0.09 | 0.09 | 0.07 | 0.09 | 0.09 | 0.09 | 0.061–0.095 [10,32,58,59] |
| Disability weight for non-terminal (per year) | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.065–0.091 [10,32] |
| Disability weight for terminal cancer | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.70–0.90 (assumption) |
| Vaccine protection (years) | 20 yrs | 20 yrs | 20 yrs | 20 yrs | 20 yrs | 20 yrs | 20 yrs | 20 yrs | 20 years [19] |
| Discount rate | 5.0% | 5.0% | 5.0% | 5.0% | 5.0% | 5.0% | 5.0% | 5.0% | 3.0% -5.0% [23,24,30,32,58,60] |
| Proportion of cervical cancer cases that are due to 1HPV-types |
90.3% | 90.3% | 90.3% | 90.3% | 90.3% | 90.3% | 90.3% | 90.3% | 70% -95.0% [9,15,17,39] |
| Economic growth | |||||||||
| GDP per capita, A$ | 73,267 | 73,267 | 73,267 | 73,267 | 73,267 | 73,267 | 73,267 | 73,267 | 73,267 [61] |
1HPV-6, 11, 16,18,31,33,45,52,58
Sensitivity analysis
A deterministic sensitivity analysis was performed to examine the robustness of the results. The output estimates varied for each value of the input parameters. These prices were derived from the academic and anecdotal literature and aimed to determine the impact of uncertainty in input assumptions on the ICERs.
Results
Model input parameters
Table 1 shows several input parameters, including the population cohort at birth, coverage of full dose vaccine, vaccine effectiveness versus HPV-9 types, the price of vaccine, and vaccine delivery costs per fully immunised girl. Cervical cancer treatment related costs per episode included direct costs (e.g., medical and non-medical costs) and indirect costs (e.g., loss of labour productivity for patients and caregivers during treatment). DALYs incurred for nonfatal and fatal cervical cancer episodes, and epidemiological data related to cervical cancer incidence were used. The sizes of the female birth cohort and the cohort at immunisation age were 191,340 and 118,679, respectively. Vaccination coverage was 86%, whereas vaccine effectiveness against HPV infections was 95%. The price of the vaccine and direct and indirect vaccine delivery costs were A$280, A$31.77, and A$17.59, respectively. Cervical cancer treatment costs were A$61,272, wherein 53.78% (A$32,952) were direct costs and 46.22% (A$28,322) indirect costs. These varied depending on the types of treatment and stages of cancer. Cervical cancer incidence and mortality-related data were extracted from national sources and the GLOBOCAN-2018 study [48]. Methodological assumptions such as disability weights (for cancer diagnosis, non-terminal and terminal) are shown in Table 1. Vaccine protection was considered to be 20 years as suggested by an expert panel and earlier research [49].
Cost-effectiveness estimates
The model estimates in Table 2 show the cost-effectiveness of the 9vHPV vaccination in Australia under various assumptions about the cost of cervical cancer treatment and the cost of vaccination across delivery strategies. The 9vHPV vaccination in Australia would cost the public approximately A$28.11 million for this target population cohort, although several types of treatment procedures would be transferred from the health system perspective and the value of A$26.72 million from a societal perspective across the various vaccine delivery strategies (e.g., school-based, health facility-based, outreach-based) would result. State and territory school-based immunisation programs primarily implement the 9vHPV vaccination through the NIP in Australia. Another two possible delivery strategies (e.g., health facility-based and outreach-based) were also included for comparison. Overall, the ICER per DALY averted was A$47,008 from a health system perspective and A$44,678 from a societal perspective, respectively. Considering delivery strategies, the ICERs per DALY averted were A$47,605, $46,682 and $46,738 for school-based, health facility-based and outreach-based programs, respectively, from the health system perspective. Whereas, from the societal perspective, the values were A$46,378, A$43,729, and A$43,930 respectively. Both perspectives for ICERs per DALY averted fell below the 2018 fiscal year GDP per capita in Australia (A$73,267), which is used as a threshold for examining the cost-effectiveness of an intervention. Similarly, consistent results were presented for the ICERs per life-year saved for both perspectives across delivery strategies (Table 2). This evaluation signifies the cost-effectiveness of the 9vHPV vaccination from both perspectives in Australia.
Table 2. Outcomes of the vaccination program*.
| Scenario | Scenario– 1 | Scenario—2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Perspective | Health system perspective | Societal perspective | ||||||
| Vaccine delivery strategies | Overall | School-based | Health facilities-based | Outreach-based | Overall | School-based | Health facilities-based |
Outreach-based |
| Output parameters | ||||||||
| Cohort size at birth (female), (‘000) | 191.34 | 191.34 | 191.34 | 191.34 | 191.34 | 191.34 | 191.34 | 191.34 |
| Cohort size at vaccination age (female) (‘000) | 118.68 | 118.68 | 118.68 | 118.68 | 118.68 | 118.68 | 118.68 | 118.68 |
| Total costs of vaccination, A$ (‘000) | 31,820.48 | 32,177.70 | 31,625.53 | 31,659.21 | 33,615.78 | 34,632.34 | 33,048.30 | 33,168.74 |
| Total treatment costs averted, A$ (‘000) | 3,709.75 | 3,709.75 | 3,709.75 | 3,709.75 | 6,898.41 | 6,898.41 | 6,898.41 | 6,898.41 |
| Net costs of the vaccination, A$ (‘000) | 28,110.73 | 28,467.95 | 27,915.78 | 27,949.47 | 26,717.37 | 27,733.93 | 26,149.90 | 26,270.33 |
| Number of averted- | ||||||||
| - Cervical cancers case averted | 113 | 113 | 113 | 113 | 113 | 113 | 113 | 113 |
| - Deaths averted | 23 | 23 | 23 | 23 | 23 | 23 | 23 | 23 |
| - Life years saved | 543 | 543 | 543 | 543 | 543 | 543 | 543 | 543 |
| Nonfatal DALYs averted | 55 | 55 | 55 | 55 | 55 | 55 | 55 | 55 |
| Incremental cost-effectiveness ratio (ICER) per- | ||||||||
| - Cervical cancers case averted, A$ | 248,767 | 251,929 | 247,042 | 247,340 | 236,437 | 245,433 | 231,415 | 232,481 |
| - Life saved, A$ | 1,222,205 | 1,237,737 | 1,213,730 | 1,215,194 | 1,161,625 | 1,205,823 | 1,136,952 | 1,142,188 |
| - Life year saved1, A$ | 51,769 | 52,427 | 51,410 | 51,472 | 49,203 | 51,075 | 48,158 | 48,380 |
| - DALYs averted1, A$ | 47,008 | 47,605 | 46,682 | 46,738 | 44,678 | 46,378 | 43,729 | 43,930 |
| Cost-effectiveness threshold | ||||||||
| GDP per capita, A$ | 73,267 | 73,267 | 73,267 | 73,267 | 73,267 | 73,267 | 73,267 | 73,267 |
| Decision rules | ||||||||
| - Very cost-effective1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| - Cost-effective2 | ||||||||
| - No cost-effective3 | ||||||||
1Very cost-effective if ICER per DALYs averted < 1 time GDP per capita
2cost-effective if ICER per DALYs averted ≥ 1 times GDP per capita and ≤ 3 times GDP per capita
3no cost-effective if ICER per DALYs averted > 3 times GDP per capita.
*Costs and DALYs were discounted at 5% per year.
Sensitivity analysis
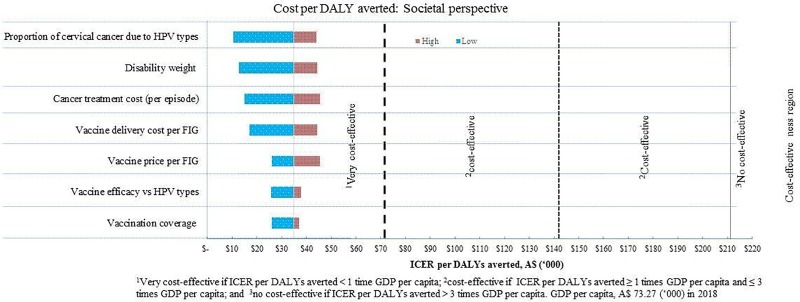
Model uncertainty was investigated by changing the values of input parameters in the cost-effectiveness model from the health system (Fig 2) and societal perspectives (Fig 3). The output of the deterministic sensitivity analysis showed that the price of vaccine, vaccine delivery costs, the incidence of cervical cancer, vaccination coverage, vaccine efficacy, and cervical cancer treatment costs were the dominating parameters that influence the ICERs per DALY averted. These findings are conservative, as only a simple static model of 9vHPV vaccination was considered. According to the WHO-CHOICE threshold, the 9vHPV vaccination is a very cost-effective and favourable option for introduction in Australia. This analysis indicates that the model outputs are robust to variation in the values of all parameters; however, there is a necessity to confirm that the pricing of the vaccine is appropriate in the context of Australia.
Fig 2. Changes in input model parameters on ICER per DALY averted from a health system perspective.
Fig 3. Changes in input model parameters on ICER per DALY averted from a societal perspective.
Discussion
The present study is a comprehensive cost-effectiveness evaluation of the introduction of a NIP with the new 9vHPV vaccine to adolescent girls in Australia. The impact of 9vHPV vaccination on health and economic outcomes was measured using various model scenarios allowing for the testing of three different vaccine delivery strategies.
The findings show that the new 9-valent vaccination of 12-year-old adolescent girls is highly cost-effective, with ICERs per DALY of A$47,008 and A$44,678, from the health system and societal perspectives, respectively. Although the 9vHPV vaccination has been implemented as part of the school-based delivery strategy, the present study has emphasised two other hypothetical delivery strategies, namely health facility-based and outreach-based programs. If the 9vHPV vaccination program is extended to these delivery outlets, the ICER remains highly cost-effective at A$46,682/DALY averted for health facility-based and A$46,738/DALY averted for outreach-based vaccination programs compared with a school-based vaccination program (ICER = A$47,605/DALY averted). Considering the societal perspective, the 9vHPV vaccination also reports a very cost-effective outcome, with an ICER of A$46,378/DALY averted, A$43,729/DALY averted, and A$43,930/DALY averted for the school-based, health facility-based and outreach-based vaccination programs, respectively. It is noteworthy that the ICERs are comparatively lower from the societal perspective in terms of vaccine delivery strategies compared with the health system perspective.
Immunisation would still be very cost-effective from both the health system and societal perspectives if the program is extended to encompass other delivery strategies. However, no herd immunity was considered in the context of these strategies. This evaluation provides a piece of initial evidence for the value of money of investments in the 9vHPV vaccination and protection against transient and persistent infections of HPV. Under the input model assumptions, the present evaluation of the two-dose 9vHPV vaccination would be very cost-effective across delivery strategies. From the societal perspective, the ICER per DALYs averted was comparatively lower than the health system perspective in terms of delivery strategies. The cost-effectiveness evaluation is significant even allowing for different vaccine delivery strategies and vaccination model assumptions.
This study findings are consistent with the conclusions from the evaluation of cost-effectiveness of the 9vHPV vaccination in other country settings including Austria [62], Canada [19], Germany [28], Italy [5], Kenya and Uganda [30], South Africa [52], and the US [21,63]. These studies estimated that an immunisation programs with the 9vHPV vaccine was likely to belong within an acceptance heuristic threshold level of cost-effectiveness or even reach cost-saving status in different country settings. In Canada, the 9vHPV vaccine was offered to school-aged girls and evidenced to be cost-effective at a price increment lower than CAN$24 [19]. Further, 9vHPV was found to be cost-effective in the US, if the incremental cost per dose of the 9vHPV was less than US$13 for a gender-neutral strategy (school-aged girls only) from a health system perspective [64]. From a societal perspective, the 9vHPV vaccine would also be considered very cost-effective at the national and state levels in the US if the vaccine price of 9vHPV was US$148 per dose (in 2016) [65], whereas two-dose schedules of the 9vHPV vaccine were likely more cost-efficient compared with three-dose schedules considering the population-level effectiveness [18]. Another recent study showed that introducing a universal 9vHPV vaccination in Germany would yield noteworthy incremental public health benefits and be highly cost-effective [28].
The present evaluation was performed among school-aged preadolescent girls (i.e., 12 years of age). Previous studies confirmed that vaccination of girls only was commonly more effective versus vaccination of both genders in different settings [28,32]. The two-dose 9vHPV vaccination approach is recommended for the target cohort of adolescent girls aged 12 to 14 years for several reasons [39]. Giving the vaccination at this age is likely to ensure immunization before their first sexual encounter and HPV exposure. As a result, the immune response tends to be stronger and more long-lasting when the vaccine is present in preadolescent girls. A vaccination schedule against HPV would allow for a more efficient primary strategy by protecting females exposed to male partners and unvaccinated females to prevent HPV transmission [9,28,63]. Eventually it would provide additional benefits to potentially accomplish virus eradication [28].
Most previous studies pay little attention to comparing the cost-effectiveness from the health system and societal point of views across vaccine delivery strategies. Thus, the evidence produced is not sufficient for health policymakers to decide upon effective or conclusive strategies. This study findings however provides effective and efficient empirical evidence of its economic viability. Health policymakers can use this evidence for the allocation of health resources and extend their vaccination program to other country settings to ensure optimal health gains.
This study has some strengths that should be highlighted. This vaccination is justified overall by epidemiological and health and economic outcomes. Under the input model assumptions, this study demonstrates that the 9vHPV vaccination is economically viable from both the health system and societal perspectives. A broader societal perspective calculates additional benefits of the new vaccine that are mainly associated with reduced productivity losses. HPV-related cervical lesions lead to a loss or reduction of women’s household income due to high productivity loss (presenteeism) and absenteeism [66]. Ultimately, HPV related diseases lead to a decrease in a victim’s socioeconomic position, which is costly for working women, their employers, and the economy. The study findings show distinctly that three vaccine delivery strategies (e.g., school-based, health facilities and outreach-based) are cost-effective. This is significant for health policymakers, strategic leaders, health scientists, cancer experts and public health professionals to help promote further implementation and extension of vaccination via a universal immunisation strategy.
This study also has some caveats. Little evidence is available on the health and economic burden of cervical cancer in Australia. Some of the model parameters related to indirect costs for cervical cancer treatment and costs of vaccination across vaccine delivery strategies (e.g., school-based, health facility-based, and outreach-based) are not available for Australia. Indirect costs of patients (e.g., opportunity costs) in terms of absenteeism due to cervical cancer and caregiver time were taken from the academic literature and anecdotal evidence in Australia and international sources. In this context, the cost-of-illness study would be appropriate for measuring the productivity losses of patients and their caregivers. However, due to a limited timeframe it was not able to conduct a cost-of-illness study among cervical cancer patients. It was presumed that the 9vHPV vaccine would be delivered to both boys and girls, but that it would only be cost-effective among girls, as the direct health impacts for 9vHPV is expected to be small for boys. This study used the GDP per capita thresholds level as defined by CMH. The GDP threshold might be a suitable screening method but should not be the only consideration for vaccination investment as there are other issues such as feasibility, affordability, alternative interventions and other local considerations which are not accounted for in the threshold level decision rule. Finally, the study findings were generated for the national context in Australia and might vary by state or regional settings, depending on cervical cancer outcomes (e.g., incidence, mortality), treatment procedures, cancer stages, costs of vaccination, and coverage of immunisation.
Conclusions
This study is an extensive cost-effectiveness analysis of 9vHPV vaccination in Australia from both the health system and societal perspectives. The introduction of the 9vHPV immunisation is assessed to be very cost-effective from both perspectives. It incorporated three delivery strategies (school-based, health facility-based, and outreach-based). However, this high-value vaccination would need substantial upfront investments. Considering a two-dose schedule, the 9vHPV vaccination demonstrated ‘good value for money’, if the vaccination could accomplish a high vaccination coverage and provide protection. The findings of this evaluation contribute to decision-making about the incorporation of the 9vHPV vaccine into a universal cervical cancer vaccination program in Australia. With continued assessment of the potential vaccine properties as well as vaccine delivery and scale-up strategies, the two-dose 9vHPV vaccine would provide significant health and economic benefits for preadolescents and society. Finally, the success of 9vHPV vaccination will be contingent on several predominating factors including value for money, feasibility, acceptability, and affordability.
Supporting information
(XLSX)
Acknowledgments
The study is part of the first author’s PhD research works. The PhD program was funded by the University of Southern Queensland, Australia. We would also like to thank the Australian Institute of Health and Welfare, Central Cancer Registry, Australian Bureau of Statistics, and Australian Burden of Disease Study. We would like to gratefully acknowledge the reviewers and editors of our manuscript. Special thanks also to my PhD fellow colleague (Syed Afroz Keramat) for his cordial support during data collection and model selection.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organization (WHO). National cancer contro programmes: Cervical cancer statistics [Internet]. 2019. [cited 29 Aug 2019]. Available: https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/ [Google Scholar]
- 2.Australian Institute of Health and Welfare. Cervical cancer in Australia: Cervical cancer statistics [Internet]. 2018. [cited 14 Feb 2019]. Available: https://cervical-cancer.canceraustralia.gov.au/statistics [Google Scholar]
- 3.Forman D, Lortet-Tieulent J, de Martel C, Ferlay J, Franceschi S, Plummer M, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30: F12–F23. 10.1016/j.vaccine.2012.07.055 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control (CDC). Genital HPV infection—fact sheet. Centers for disease control and prevention [Internet]. 2015. [cited 14 Feb 2019]. Available: http://www.cdc.gov/std/hpv/stdfact-hpv.htm [Google Scholar]
- 5.Mennini FS, Bonanni P, Bianic F, Waure C, Baio G, Plazzotta G, et al. Cost-effectiveness analysis of the nine-valent HPV vaccine in Italy. Cost Effectiveness and Resource Allocation. 2017;15: 1–14. 10.1186/s12962-017-0063-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan P, Howell-Jones R, Li N, Bruni L, De Sanjosé S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. International Journal of Cancer. 2012;131: 2349–2359. 10.1002/ijc.27485 [DOI] [PubMed] [Google Scholar]
- 7.Li N, Franceschi S, Howell-Jones R, Snijders PJF, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. International Journal of Cancer. 2011;128: 927–935. 10.1002/ijc.25396 [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AR, Nyitray AG, Kreimer AR, Pierce Campbell CM, Goodman MT, Sudenga SL, et al. EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. International Journal of Cancer. 2015;136: 2752–2760. 10.1002/ijc.29082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel C, Brotherton JML, Pillsbury A, Jayasinghe S, Donovan B, Macartney K, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: What additional disease burden will a nonavalent vaccine prevent? Eurosurveillance. 2018;23: 30–40. 10.2807/1560-7917.ES.2018.23.41.1700737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jit M, Brisson M, Portnoy A, Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: A PRIME modelling study. Lancet Global Health. 2014;2: e406–e414. 10.1016/S2214-109X(14)70237-2 [DOI] [PubMed] [Google Scholar]
- 11.Georgousakis M, Jayasinghe S, Brotherton J, Gilroy N, Chiu C, Macartney K. Population-wide vaccination against human papillomavirus in adolescent boys: Australia as a case study. Lancet Infectious Diseases. 2012;12: 627–634. 10.1016/S1473-3099(12)70031-2 [DOI] [PubMed] [Google Scholar]
- 12.Ward K, Quinn H, Bachelor M, Bryant V, Campbell-Lloyd S, Newbound A, et al. Adolescent school-based vaccination in Australia. Commun Dis Intell Q Rep. 2013;37: E156–67. [PubMed] [Google Scholar]
- 13.Department of Health and Ageing. Immunise Australia program: human papillomavirus (HPV) [Internet]. [cited 13 Feb 2019]. Available: http://www.health.gov.au/internet/immunise/publishing.nsf/%0AContent/immunise-hpv
- 14.Smith MA, Canfell K. Projected impact of HPV vaccination and primary HPV screening on cervical adenocarcinoma: Example from Australia. Papillomavirus Research. 2017;3: 134–141. 10.1016/j.pvr.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Office of the Prime Minister of Australia. A new vaccine to strengthen the health of young Australians [Internet]. 2017. [cited 13 Feb 2019]. Available: https://parlinfo.aph.gov.au/parlInfo/search/display/display.w3p;query=Id:%22media/pressrel/5562151%22 [Google Scholar]
- 16.Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infectious Agents and Cancer. 2012;7: 1–13. 10.1186/1750-9378-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brotherton JML, Tabrizi SN, Phillips S, Pyman J, Cornall AM, Lambie N, et al. Looking beyond human papillomavirus (HPV) genotype 16 and 18: Defining HPV genotype distribution in cervical cancers in Australia prior to vaccination. International Journal of Cancer. 2017;141: 1576–1584. 10.1002/ijc.30871 [DOI] [PubMed] [Google Scholar]
- 18.Simms KT, Laprise JF, Smith MA, Lew J Bin, Caruana M, Brisson M, et al. Cost-effectiveness of the next generation nonavalent human papillomavirus vaccine in the context of primary human papillomavirus screening in Australia: A comparative modelling analysis. Lancet Public Health. 2016;1: e66–e75. 10.1016/S2468-2667(16)30019-6 [DOI] [PubMed] [Google Scholar]
- 19.Drolet M, Laprise JF, Boily MC, Franco EL, Brisson M. Potential cost-effectiveness of the nonavalent human papillomavirus (HPV) vaccine. International Journal of Cancer. 2014;134: 2264–2268. 10.1002/ijc.28541 [DOI] [PubMed] [Google Scholar]
- 20.Chesson HW, Meites E, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of nonavalent HPV vaccination among males aged 22 through 26years in the United States. Vaccine. 2018;36: 4362–4368. 10.1016/j.vaccine.2018.04.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesson HW, Laprise JF, Brisson M, Markowitz LE. Impact and cost-effectiveness of 3 doses of 9-valent human papillomavirus (HPV) vaccine among US females previously vaccinated with 4-valent hpv vaccine. Journal of Infectious Diseases. 2016;213: 1694–1700. 10.1093/infdis/jiw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brisson M, Laprise JF, Chesson HW, Drolet M, Malagón T, Boily MC, et al. Health and economic impact of switching from a 4-Valent to a 9-valent HPV vaccination program in the United States. Journal of the National Cancer Institute. 2016;108: 1–9. 10.1093/jnci/djv282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laprise JF, Markowitz LE, Chesson HW, Drolet M, Brisson M. Comparison of 2-dose and 3-dose 9-valent human papillomavirus vaccine schedules in the United States: A cost-effectiveness analysis. Journal of Infectious Diseases. 2016;214: 685–688. 10.1093/infdis/jiw227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesson HW, Markowitz LE, Hariri S, Ekwueme DU, Saraiya M. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: Estimates from a simplified transmission model. Human Vaccines and Immunotherapeutics. 2016;12: 1363–1372. 10.1080/21645515.2016.1140288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chesson HW, Laprise JF, Brisson M, Markowitz LE. Impact and cost-effectiveness of 3 doses of 9-valent human papillomavirus (HPV) vaccine among US females previously vaccinated with 4-valent hpv vaccine. Journal of Infectious Diseases. 2016;213: 1694–1700. 10.1093/infdis/jiw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markowitz LE, Drolet M, Laprise J-F, Brisson M, Chesson HW. Comparison of 2-dose and 3-dose 9-valent humanpapillomavirus vaccine schedules in the United States: A cost-effectiveness analysis. Journal of Infectious Diseases. 2016;214: 685–688. 10.1093/infdis/jiw227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesson HW, Laprise JF, Brisson M, Markowitz LE. Impact and Cost-effectiveness of 3 Doses of 9-Valent Human Papillomavirus (HPV) vaccine among US females previously vaccinated with 4-valent hpv vaccine. Journal of Infectious Diseases. 2016;213: 1694–1700. 10.1093/infdis/jiw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Largeron N, Petry KU, Jacob J, Bianic F, Anger D, Uhart M. An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. Expert Review of Pharmacoeconomics and Outcomes Research. 2017;17: 85–98. 10.1080/14737167.2016.1208087 [DOI] [PubMed] [Google Scholar]
- 29.De La Fuente J, Hernandez Aguado JJ, Martín MS, Boix PR, Gómez SC, López N. Estimating the epidemiological impact and cost-effectiveness profile of a nonavalent hpv vaccine in Spain. Human Vaccines & Immunotherapeutics. 2019;15: 1949–1961. 10.1080/21645515.2018.1560770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiatpongsan S, Kim JJ. Costs and cost-effectiveness of 9-valent human papillomavirus (HPV) vaccination in two east african countries. PLoS ONE. 2014;9: 1–6. 10.1371/journal.pone.0106836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edejer TT-T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans D., et al. Making choices in health: WHO guide to cost-effective analysis. 20 Avenue Appia, 1211 Geneva 27, Switzerland; 2003. Report No.: 9241546018.
- 32.Van Minh H, My NTT, Jit M. Cervical cancer treatment costs and cost-effectiveness analysis of human papillomavirus vaccination in Vietnam: A PRIME modeling study. BMC Health Services Research. 2017;17: 1–7. 10.1186/s12913-016-1943-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris AH, Hill SR, Chin G, Jing Jing Li, Walkom E. The role of value for money in public insurance coverage decisions for drugs in australia: A retrospective analysis 1994–2004. Medical Decision Making. 2008;28: 713–722. 10.1177/0272989X08315247 [DOI] [PubMed] [Google Scholar]
- 34.George B, Harris A, Mitchell A. Cost-effectivenessanalysisandthe consistency of decision making: Evidence from pharmaceutical reimbursement in Australia (1991to1996). Pharmacoeconomics. 2001;19: 1103–1109. 10.2165/00019053-200119110-00004 [DOI] [PubMed] [Google Scholar]
- 35.Henry DA, Hill SR, Harris A. Drug prices and value for money: The Australian Pharmaceutical Benefits Scheme. JAMA. 2005;294: 2630–2632. 10.1001/jama.294.20.2630 [DOI] [PubMed] [Google Scholar]
- 36.Roche Products Pty Ltd. Access to oncology medicines in Australia, Roche response to Medicines Australia Oncology Industry Taskforce report. [Internet]. 2013. Available: medicinesaustralia.com.au/files/2013/07/%0A131021_OIT_Roche_response_FINAL_.pdf
- 37.Lowe A, Dyson S. New therapies for advanced cancers: Can our society afford them? Is it Ethical to deny patients access to them? Hilton Sydney, Australia; 2013. [Google Scholar]
- 38.WHO Commission on Macroeconomics and Health. Macroeconomics and health: Investing in health for economic development. Report of the Commission on Macroeconomics and Health [Internet]. 20 Avenue Appia, 1211 Geneva 27, Switzerland; 2001. Available: http://whqlibdoc.who.int/publications/2001/924154550x.pdf
- 39.Australian Technical Advisory Group on Immunisation (ATAGI). Clinical advice and fact sheet: Introduction of GARDASIL-9 (9vHPV) in a 2 dose schedule under the school-based national immunisation program (NIP). In: Department of Health, Australian Government; 2018. [Google Scholar]
- 40.Brotherton JM. Human papillomavirus vaccination update: Nonavalent vaccine and the two-dose schedule. Australian journal of general practice. 2018;47: 417–421. 10.31128/AJGP-01-18-4462 [DOI] [PubMed] [Google Scholar]
- 41.Botwright S, Holroyd T, Nanda S, Bloem P, Griffiths UK, Sidibe A, et al. Experiences of operational costs of HPV vaccine delivery strategies in Gavi-supported demonstration projects. PLoS ONE. 2017;12: 1–13. 10.1371/journal.pone.0182663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization (WHO). WHO Cervical Cancer Prevention and Control Costing Tool (C4P): Immunization, Vaccines and Biologicals [Internet]. 2018. Available: https://www.who.int/immunization/diseases/hpv/cervical_cancer_costing_tool/en/ [Google Scholar]
- 43.Lew JB, Howard K, Gertig D, Smith M, Clements M, Nickson C, et al. Expenditure and resource utilisation for cervical screening in Australia. BMC Health Services Research. 2012;12: 1 10.1186/1472-6963-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubas-Jakóbczyk K, Kocot E, Seweryn M, Koperny M. Production lost due to cervical cancer in Poland in 2012. Medycyna Pracy. 2016;67: 289–299. 10.13075/mp.5893.00378 [DOI] [PubMed] [Google Scholar]
- 45.Rice D. Estimating the cost of illness. American Journal of Public Health and the Nations Health. 2008;57: 424–440. 10.2105/ajph.57.3.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarker AR, Islam Z, Khan IA, Saha A, Chowdhury F, Khan AI, et al. Cost of illness for cholera in a high risk urban area in Bangladesh: an analysis from household perspective. BMC Infectious Diseases. 2013;13: 2–7. 10.1186/1471-2334-13-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The Australian Bureau of Statistics. Consumer price index, Australia, December 2018. [Internet]. 2019. [cited 11 Mar 2019]. Available: http://www.abs.gov.au/ausstats/abs@.nsf/mf/6401.0 [Google Scholar]
- 48.Bray F, Ferlay J, Soerjomataram I. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018;68: 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 49.Drolet M, Laprise JF, Boily MC, Franco EL, Brisson M. Potential cost-effectiveness of the nonavalent human papillomavirus (HPV) vaccine. International Journal of Cancer. 2014;134: 2264–2268. 10.1002/ijc.28541 [DOI] [PubMed] [Google Scholar]
- 50.The Australian Bureau of Statistics (ABS). Births registered, summary statistics for Australian: Australian Demographic Statistics (cat. no. 3101.0). 2018. [Google Scholar]
- 51.Donovan B, Marshall H, Macartney K, Patel C, Pillsbury A, Brotherton JM, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Eurosurveillance. 2018;23 10.2807/1560-7917.es.2018.23.41.1700737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan N, Sharma M, Winer R, Galloway D, Rees H, Barnabas R V. Model-estimated effectiveness of single dose 9-valent HPV vaccination for HIV-positive and HIV-negative females in South Africa. Vaccine. 2018;36: 4830–4836. 10.1016/j.vaccine.2018.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z, Zhang J, Xia N, Zhao Q. Expanded strain coverage for a highly successful public health tool: Prophylactic 9-valent human papillomavirus vaccine. Human Vaccines and Immunotherapeutics. 2017;13: 2280–2291. 10.1080/21645515.2017.1346755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brotherton JML, Murray SL, Hall MA, Andrewartha LK, Banks CA, Meijer D, et al. Human papillomavirus vaccine coverage among female Australian adolescents: Success of the school-based approach. Medical Journal of Australia. 2013;199: 614–617. 10.5694/mja13.10272 [DOI] [PubMed] [Google Scholar]
- 55.Brotherton JML, Winch KL, Bicknell L, Chappell G, Saville M. HPV vaccine coverage is increasing in Australia. Medical Journal of Australia. 2017;206: 262 10.5694/mja16.00958 [DOI] [PubMed] [Google Scholar]
- 56.Brisson M, Laprise JF, Chesson HW, Drolet M, Malagón T, Boily MC, et al. Health and economic impact of switching from a 4-Valent to a 9-valent HPV vaccination program in the United States. Journal of the National Cancer Institute. 2016;108: 1–9. 10.1093/jnci/djv282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Explore Community Development Council. Immunisation Services: Immunisation price list 2018–19. In: City of Playford [Internet]. 2019. [cited 11 Mar 2019]. Available: https://www.playford.sa.gov.au/live/around-me/immunisation-services [Google Scholar]
- 58.Simms KT, Laprise JF, Smith MA, Lew J Bin, Caruana M, Brisson M, et al. Cost-effectiveness of the next generation nonavalent human papillomavirus vaccine in the context of primary human papillomavirus screening in Australia: a comparative modelling analysis. Lancet Public Health. 2016;1: e66–e75. 10.1016/S2468-2667(16)30019-6 [DOI] [PubMed] [Google Scholar]
- 59.Chesson HW, Meites E, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of nonavalent HPV vaccination among males aged 22 through 26years in the United States. Vaccine. 2018;36: 4362–4368. 10.1016/j.vaccine.2018.04.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jit M, Brisson M, Portnoy A, Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: A PRIME modelling study. Lancet Global Health. 2014;2: e406–e414. 10.1016/S2214-109X(14)70237-2 [DOI] [PubMed] [Google Scholar]
- 61.The Australian Bureau of Statistics (ABS). Australian System of National Accounts, 2017–18: Population estimates are as published in the Australian Demographic Statistics (cat. no. 3101.0) and ABS projections. [Internet]. 2019. Available: https://search.abs.gov.au/s/search.html?collection=abs&form=simple&profile=_default&query=GDP+per+capita+in+2018 [Google Scholar]
- 62.Boiron L, Joura E, Largeron N, Prager B, Uhart M. Estimating the cost-effectiveness profile of a universal vaccination programme with a nine-valent HPV vaccine in Austria. BMC Infectious Diseases. 2016;16: 1–15. 10.1186/s12879-015-1330-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chesson HW, Markowitz LE, Hariri S, Ekwueme DU, Saraiya M. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: Estimates from a simplified transmission model. Human Vaccines and Immunotherapeutics. 2016;12: 1363–1372. 10.1080/21645515.2016.1140288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malagón T, Brisson M, Laprise J-F, Chesson HW, Markowitz LE, Drolet M, et al. Health and economic impact of switching from a 4-Valent to a 9-valent HPV vaccination program in the United States. Journal of the National Cancer Institute. 2015;108: 1–9. 10.1093/jnci/djv282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Durham DP, Ndeffo-Mbah ML, Skrip LA, Jones FK, Bauch CT, Galvani AP. National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proceedings of the National Academy of Sciences. 2016;113: 5107–5112. 10.1073/pnas.1515528113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lerner D, Parsons SK, Justicia-Linde F, Chelmow D, Chang H, Rogers WH, et al. The impact of precancerous cervical lesions on functioning at work and work productivity. Journal of Occupational and Environmental Medicine. 2010;52: 926–933. 10.1097/JOM.0b013e3181f12fb0 [DOI] [PubMed] [Google Scholar]