Abstract
Background
Primary chest wall sarcoma is a rare disease with limited reports of surgical resection.
Methods
This retrospective review included 41 patients with primary chest wall sarcoma who underwent chest wall resection and reconstruction from 2001 to 2015. The clinical, histologic, and surgical variables were collected and analyzed by univariate and multivariate Cox regression analyses for overall survival (OS) and recurrence-free survival (RFS).
Results
The OS rates at 5 and 10 years were 73% and 61%, respectively. The RFS rate at 10 years was 57.1%. Multivariate Cox regression analysis revealed old age (hazard ratio [HR], 5.16; 95% confidence interval [CI], 1.71–15.48) as a significant risk factor for death. A surgical resection margin distance of less than 1.5 cm (HR, 15.759; 95% CI, 1.78–139.46) and histologic grade III (HR, 28.36; 95% CI, 2.76–290.87) were independent risk factors for recurrence.
Conclusion
Long-term OS and RFS after the surgical resection of primary chest wall sarcoma were clinically acceptable.
Keywords: Sarcoma, Chest wall, Undifferentiated pleomorphic sarcoma
Introduction
Primary chest wall sarcoma is a rare disease for which surgical resection is the treatment of choice. The 5-year overall survival (OS) rates reported by previous reviews are acceptable [1–9], ranging from 59% to 68%. Many risk factors are known to affect OS, including tumor histology, sex, resection margin status, and histologic grade [1–9]. However, only a few reports are available on cases of primary chest wall sarcoma followed up for >10 years.
In Korea, there are limited reports of surgical resection for primary chest wall sarcoma [10–16]. Because relatively few patients have primary chest wall sarcoma, survival analyses and risk factor analyses have been rarely conducted.
This study aimed to report the long-term outcomes of surgical resection for primary chest wall sarcoma and to analyze the prognostic factors for long-term OS and recurrence-free survival (RFS).
Methods
1) Participants
We reviewed the cases of 42 patients who underwent surgical resection of primary chest wall sarcomas from January 2001 to December 2015. All patients were followed up until September 2018.
All patients included in this study had histologically confirmed primary chest wall sarcomas other than metastatic chest wall sarcomas or benign chest wall tumors. Because only cases of sarcoma treated by curative resection were included for analysis in this study, we excluded 1 patient who underwent palliative resection for primary chest wall sarcoma with pleural seeding.
This study was approved by the Institutional Ethics Committee/Review Board of Samsung Medical Center, which waived the requirement for informed patient consent due to its retrospective nature (IRB approval no., SMC 2018-12-032-001).
2) Variables
We reviewed the following clinical, histological, and surgical variables that were analyzed and suggested as prognostic values in previous studies [1–9]: age, sex, symptoms at presentation, tumor maximal diameter, tumor histologic grade following the Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grade, specific anatomic structures resected, materials used for the chest wall reconstruction, neoadjuvant or adjuvant treatment, and resection margin status.
Two different methods can be used to describe the resection margin status, as reported in other reviews [1,2,5,6,8]: residual tumor (R) classification and measurement of the nearest distance of the resected specimen margin to the tumor. However, our data on the distance between the resection margin and the tumor were based on pathologic data, not surgical records, because some data about resection margin distance in the surgical records were missing or incorrect.
3) Surgical technique
Surgical resection of chest wall sarcoma included resection of the affected rib with at least a 4-cm margin, unaffected ribs above and below the tumor, adjacent muscles, and underlying pleura, and any other tissues adherent to the tumor. Pathologically negative margins were identified in all 4 directions from the tumor via frozen biopsy. When vital structures such as the spinal cord or vascular structures were present near the tumor, a 2-cm margin was permitted for surgical resection.
4) Statistical analysis
The descriptive statistics for categorical variables are reported as frequency and percentage, whereas continuous variables are reported as mean±standard deviation or median (range). Categorical variables were compared between the groups using the chi-square test or the Fisher exact test, while continuous variables were compared using the 2-sample t-test or the Wilcoxon rank-sum test. All statistical tests were 2-sided, with an alpha level of 0.05. OS and RFS curves were constructed using Kaplan-Meier estimates and compared using the log-rank test. The relationship between maximal diameter of the tumor and resection margin distance was studied by calculating the Pearson correlation coefficient (r). Statistical significance was inferred for p-values <0.05. The optimal resection margin distance cutoff was evaluated using the Youden index; receiver operating characteristic (ROC) curve analyses for OS and RFS were performed, and the area under the curve (AUC) for each analysis was calculated. Using this criterion, a test with no better accuracy than chance has an AUC of 0.5, whereas a test with perfect accuracy has an AUC of 1. Cox proportional hazard regression was used to identify predictors of OS and RFS. Variables with p-values <0.1 in the univariate analysis were considered for the multivariate analysis. The backward elimination method was used for model selection in the multivariate analysis. The results are reported as hazard ratio (HRs) and 95% confidence interval (CIs). The statistical analysis was performed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA).
Results
The baseline characteristics of the patients are listed in Table 1. Their mean age was 47.5 years, and 23 patients (56%) were male. Eighteen patients (44%) presented with a palpable mass. Twelve patients (29%) had a previous history of breast or thyroid cancer. The mean maximal diameter of the tumor was 6.19 cm, with a standard deviation of 3.5 cm. Eight patients (20%) had the cellular type of chondrosarcoma, and 7 (17%) had the cellular type of undifferentiated pleomorphic sarcoma. Fifteen patients (37%) had FNCLCC histologic grade II tumors, while 9 (22%) had FNCLCC histologic grade III tumors. The median number of ribs resected per patient was 3; there were 3 cases of partial resection of the sternum and 4 of partial resection of the vertebrae. Gore-Tex was used for reconstructing the chest wall in 11 patients (27%), and 20 patients (49%) were treated using neoadjuvant or adjuvant treatment with surgery.
Table 1.
Clinical, histological, and surgical characteristics of all patients (N=41)
| Characteristic | Value |
|---|---|
| Age (yr) | 47.51±19 |
| Male sex | 23 (56) |
| Previous cancer history | 12 (29) |
| Symptom at presentation | |
| Mass | 18 (44) |
| Pain | 11 (27) |
| None | 11 (27) |
| Tumor maximal diameter (cm) | 6.19±3.5 |
| Tumor histology | |
| Chondrosarcoma | 8 (20) |
| Undifferentiated pleomorphic sarcoma | 7 (17) |
| Liposarcoma | 6 (15) |
| Fibrosarcoma | 5 (12) |
| Ewing’s sarcoma | 5 (12) |
| Osteosarcoma | 3 (7) |
| Leiomyosarcoma | 2 (5) |
| Others | 5 (12) |
| Histologic grade (FNCLCC) | |
| Grade I | 8 (20) |
| Grade II | 15 (37) |
| Grade III | 9 (22) |
| Resection | |
| No. of resected ribs | 3 (1–3) |
| Sternum | 3 (7) |
| Vertebra | 4 (10) |
| Reconstruction | |
| Gore-Tex | 11 (27) |
| Muscle flap | 5 (12) |
| Mesh | 2 (5) |
| Neoadjuvant or adjuvant therapy | 20 (49) |
Values are presented as mean±standard deviation, number (%), or median (range).
FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer.
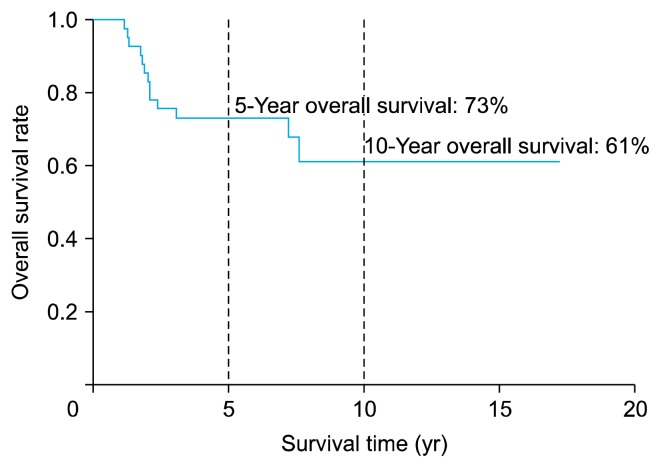
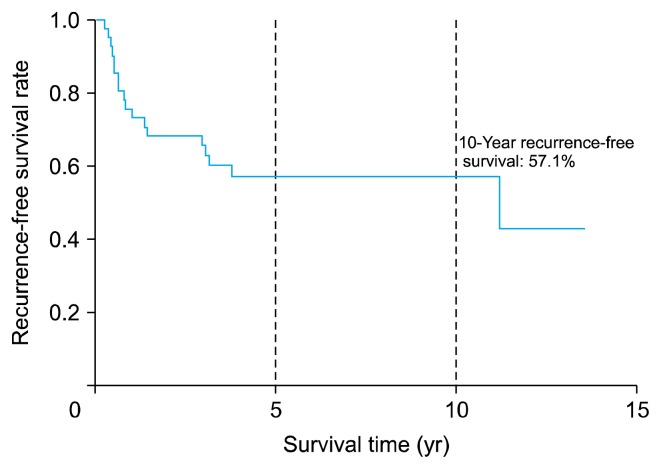
Fig. 1 shows the OS curve: 30 patients (73%) survived for >5 years after surgical resection of the primary chest wall sarcoma, while 25 patients (61%) survived for >10 years. Fig. 2 shows the RFS curve. The 10-year RFS was 57.1%.
Fig. 1.
Overall survival time of 41 patients who underwent surgical resection of primary chest wall sarcoma.
Fig. 2.
Recurrence-free survival time of 41 patients who underwent surgical resection of primary chest wall sarcomas.
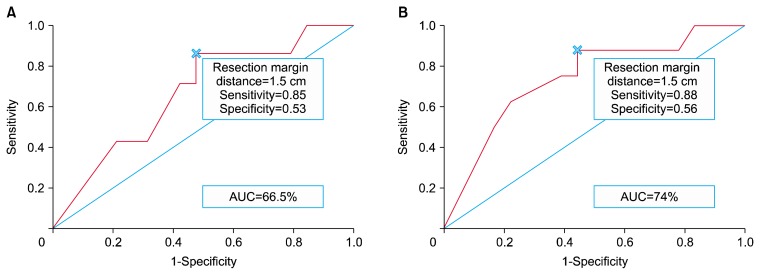
The AUC of the ROC curve for OS was 66.5% (Fig. 3A) and the AUC of the ROC curve for RFS was 74% (Fig. 3B). The cutoff value in both ROC curves was a resection margin distance of 1.5 cm, which corresponded to the maximum Youden index (in the ROC curve for OS, the cutoff value of 1.5 cm had a sensitivity of 0.85 and a specificity of 0.53; in the ROC curve for RFS, the cutoff value of 1.5 cm had a sensitivity of 0.88 and a specificity of 0.56.
Fig. 3.
ROC curve and cutoff analysis with the Youden index for determination of the optimal resection margin distance. (A) ROC curve and cutoff analysis for overall survival. (B) ROC curve and cutoff analysis for recurrence-free survival. ROC, receiver operating characteristic; AUC, area under the curve.
The relationship between the maximal diameter of the tumor and the resection margin distance was studied using Pearson correlation coefficients owing to a tendency for the resection margin distance to be shorter in patients with large tumors in clinical practice. The Pearson correlation coefficient was −0.371 (p=0.107).
Table 2 compares the baseline characteristics of the patients without recurrence (group 1, n=23) and those showing recurrence (group 2, n=18). The patients in group 2 were older and had higher rates of histologic grade III tumors and undifferentiated pleomorphic sarcomas. More patients in group 2 than those in group 1 had a resection margin distance <1.5 cm and R1 resection. However, the proportion of patients receiving adjuvant or neoadjuvant treatment did not significantly differ between the groups.
Table 2.
Clinical, histological, and surgical characteristics by recurrence
| Variable | No recurrence (n=23) | Recurred (n=18) | p-value |
|---|---|---|---|
| Age (yr) | 37.20±3.75 | 57.33±3.52 | <0.001 |
| Male sex | 15 (65) | 8 (44) | 0.219 |
| Tumor histology | 0.067 | ||
| Soft tissue sarcoma | 12 (52) | 6 (33) | |
| Bone-originating sarcoma | 10 (44) | 6 (33) | |
| Undifferentiated pleomorphic sarcoma | 1 (4) | 6 (33) | |
| Histologic grade (FNCLCC) | 0.002 | ||
| Grade I | 7 (30) | 1 (5) | |
| Grade II | 10 (43) | 5 (27) | |
| Grade III | 1 (4) | 8 (44) | |
| Resection margin distance (cm) | 0.011 | ||
| ≥1.5 | 10 (44) | 1 (6) | |
| <1.5 | 13 (56) | 17 (94) | |
| Resection margin classification | 0.136 | ||
| R0 resection | 18 (86) | 10 (63) | |
| R1 resection | 3 (14) | 6 (37) | |
| Previous cancer history | 4 (17) | 8 (44) | 0.087 |
| Neoadjuvant or adjuvant therapy | 11 (48) | 9 (50) | 1.000 |
Values are presented as mean±standard deviation or number (%).
FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer.
In the univariate analysis for OS (Table 3), male sex, old age, tumor histology with undifferentiated pleomorphic sarcoma, histologic grade III, R1 resection, and resection margin distance had p-values <0.1 and were included in the multivariate analysis. In the multivariate analysis (Table 3), old age was the only independent risk factor for death.
Table 3.
Univariate and multivariate analysis of overall survival
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Male sex | 2.68 (0.87–8.23) | 0.085 | ||
|
| ||||
| Age >65 yr | 5.49 (1.84–16.42) | 0.002 | 5.16 (1.72–15.48) | 0.003 |
|
| ||||
| Previous cancer history | 1.83 (0.59–5.62) | 0.289 | ||
|
| ||||
| Tumor maximal diameter | 1.13 (0.97–1.32) | 0.113 | ||
|
| ||||
| Tumor histology | ||||
|
| ||||
| Soft tissue sarcoma | 1 | |||
|
| ||||
| Bone-originating sarcoma | 1.56 (0.41–5.81) | 0.508 | ||
|
| ||||
| Undifferentiated pleomorphic sarcoma | 3.33 (0.82–13.37) | 0.091 | ||
|
| ||||
| Histologic grade (FNCLCC) | ||||
|
| ||||
| Grade I | 1 | |||
|
| ||||
| Grade II | 5.025 (0.61–44.96) | 0.130 | ||
|
| ||||
| Grade III | 16.85 (1.85–153.19) | 0.012 | ||
|
| ||||
| Resection margin classification | ||||
|
| ||||
| R0 resection | 1 | |||
|
| ||||
| R1 resection | 2.99 (0.99–9.03) | 0.051 | ||
|
| ||||
| Resection margin distance | 0.55 (0.27–1.17) | 0.073 | ||
|
| ||||
| Resection margin distance classification | ||||
|
| ||||
| Resection margin distance ≥1.5 cm | 1 | |||
|
| ||||
| Resection margin distance <1.5 cm | 4.43 (0.57–34.33) | 0.118 | ||
|
| ||||
| Neoadjuvant or adjuvant therapy | 1.00 (0.33–3.02) | 0.988 | ||
HR, hazard ratio; CI, confidence interval; FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer.
In the univariate analysis for RFS (Table 4), old age, previous history of cancer, maximal diameter of the tumor, tumor histology with undifferentiated pleomorphic sarcoma, histologic grade III, resection margin distance, and a resection margin distance <1.5 cm had p-values <0.1. In the multivariate analysis (Table 4), histologic grade III and a resection margin distance <1.5 cm were risk factors for recurrence.
Table 4.
Univariate and multivariate analysis of recurrence-free survival
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Male sex | 0.48 (0.18–1.23) | 0.121 | ||
|
| ||||
| Age >65 yr | 4.44 (1.61–12.21) | 0.002 | ||
|
| ||||
| Previous cancer history | 2.55 (1.05–6.48) | 0.041 | ||
|
| ||||
| Tumor maximal diameter | 1.15 (1.00–1.32) | 0.040 | ||
|
| ||||
| Tumor histology | ||||
|
| ||||
| Soft tissue sarcoma | 1 | |||
|
| ||||
| Bone-originating sarcoma | 1.08 (0.41–2.83) | 0.868 | ||
|
| ||||
| Undifferentiated pleomorphic sarcoma | 3.75 (1.25–11.22) | 0.018 | ||
|
| ||||
| Histologic grade (FNCLCC) | ||||
|
| ||||
| Grade I | 1 | 1 | ||
|
| ||||
| Grade II | 3.13 (0.36–27.03) | 0.298 | 2.48 (0.28–21.75) | 0.410 |
|
| ||||
| Grade III | 18.02 (2.16–149.92) | 0.007 | 28.36 (2.76–290.87) | 0.005 |
|
| ||||
| Resection margin classification | ||||
|
| ||||
| R0 resection | 1 | |||
|
| ||||
| R1 resection | 1.99 (0.71–5.61) | 0.181 | ||
|
| ||||
| Resection margin distance | 0.47 (0.22–0.98) | 0.022 | ||
|
| ||||
| Resection margin distance classification | ||||
|
| ||||
| Resection margin distance ≥1.5 cm | 1 | 1 | ||
|
| ||||
| Resection margin distance <1.5 cm | 8.85 (1.17–66.77) | 0.011 | 15.759 (1.78–139.46) | 0.013 |
|
| ||||
| Neoadjuvant or adjuvant therapy | 1.42 (0.56–3.61) | 0.452 | ||
HR, hazard ratio; CI, confidence interval; FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer.
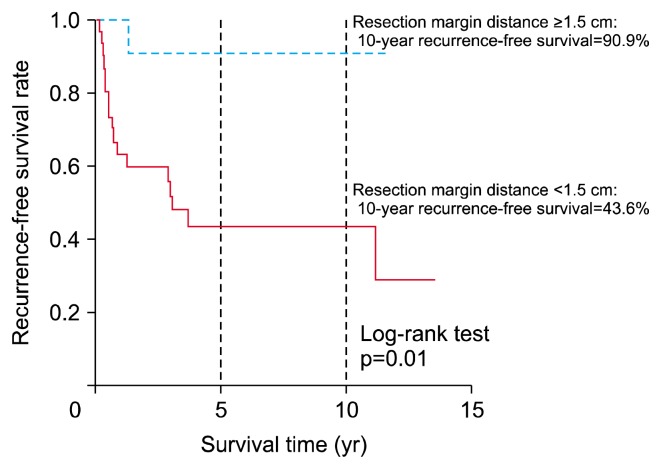
Fig. 4 shows the RFS curve of patients according to the resection margin distance. The 10-year RFS of patients with a resection margin distance ≥1.5 cm was 90.9%, while that of patients with a resection margin distance <1.5 cm was 43.6%. In the log-rank test, the RFS rates differed significantly among the 3 groups (p=0.01).
Fig. 4.
Recurrence-free survival time according to resection margin distance.
Table 5 shows the recurrence patterns of both groups. Only 1 patient with a resection margin distance ≥1.5 cm experienced recurrence (local recurrence at the excision site). In contrast, 17 patients (58.6%) with a resection margin distance <1.5 cm experienced recurrence, of whom 11 (37.9%) showed local recurrence at the resection site, while 6 (20.6%) experienced distant metastasis in the lungs, bones, and lymph nodes. The median time of recurrence was 3.67 years after surgery in patients with a resection margin distance <1.5 cm.
Table 5.
Recurrence pattern of all patients with known resection margin distance (N=40)
| Variable | Resection margin distance | p-value | |
|---|---|---|---|
| ≥1.5 cm (n=11) | <1.5 cm (n=29) | ||
| No recurrence | 10 (90.9) | 13 (44.8) | 0.011 |
| Total recurrence | 1 (9.1) | 17 (58.6) | |
| Local recurrence | 1 (9.1) | 11 (37.9) | |
| Distant metastasis | 0 | 6 (20.6) | |
| Lung | 0 | 3 (10.3) | |
| Bone | 0 | 2 (6.8) | |
| Lymph node | 0 | 1 (3.4) | |
| Time to recurrence (yr) | 1.33 | 3.67 (0.16–11.18) | |
Values are presented as number (%) or median (range).
Discussion
The aim of this study was to determine long-term OS and RFS and to identify risk factors for death and recurrence. The 5- and 10-year OS rates were 73% and 61%, respectively, and the 10-year RFS rate was 57.1%; these rates are better than those in previous studies [1–6,8,9].
Prior to the risk factor analysis, we performed an analysis of the ROC curve and the cutoff for the optimal resection margin distance. The AUC of the ROC curve for OS was 0.66, and the AUC of the ROC curve for RFS was 0.74. The cutoff value with the maximum Youden index was 1.5 cm. In many reviews, a wide surgical resection margin has been considered a prognostic factor for long-term survival [6,8]. Authors have described specific methods of achieving a wide resection margin for primary chest wall sarcoma [1–8]. Although the optimal resection margin for good long-term survival was 4 cm according to the previous results of a study by King et al. [6], we identified a 1.5-cm resection margin distance to be the cutoff point for an optimal resection margin in this study. There might be 2 reasons for this difference. First, our data about the distance between the resection margin and the tumor were based on pathologic data, not surgical records, because some data about resection margin distance were missing or incorrect in the surgical records. The resection margin distance in the pathologic review can be more accurate than that in surgical records. The second issue relates to sample size. The number of all patients with primary chest wall sarcoma and the number of patients with a resection margin distance >4 cm (n=3) might have been too small for determining the optimal resection margin distance. However, some previous reviews [1,2,6] also used a 2-cm resection margin as an indicator of better survival.
We studied the relationship between the maximal diameter of the tumor and the resection margin distance, because in clinical practice, there might be a tendency for a shorter resection margin distance, especially in patients with large tumors, because of proximity to vital structures. Using the Pearson correlation test (r=−0.371, p=0.107), we failed to prove a correlation between the maximal diameter of the tumor and the resection margin distance. However, in our data, all patients with a maximal diameter of the tumor more than 7.5 cm had a resection margin distance less than 1 cm. Although we were unable to prove the correlation due to the small number of patients, there might be an inverse relationship between the maximal diameter of the tumor and the resection margin distance, especially in larger tumors.
In risk factor analysis, old age was the only independent risk factor for overall death. High histologic grade and resection margin distance less than 1.5 cm were 2 independent risk factors for recurrence, which is consistent with the results of previous reviews [2,4,5,9].
An adequate resection margin distance is a well-known prognostic factor for recurrence after chest wall sarcoma resection [6]. Many centers have reported variable descriptions of wide resection of chest wall sarcoma, accompanied by variation in OS. King et al. [6], who published the first report on the importance of a wide surgical resection margin for primary chest wall sarcoma, defined wide resection as a margin of 4 cm with several partial ribs above and below for rib tumors and entire bone resection for sternal or manubrial cancers [6]. However, they concluded that a 2-cm margin would be safe for low-grade sarcomas, such as chondrosarcoma. Walsh et al. [2] and Athanassiadi et al. [8] reported similar descriptions of wide resection; however, Athanassiadi et al. [8] reported different methods for sternal tumors depending on the location of the sarcoma. Perry et al. [4] and Jonsson et al. [7] described at least 1 uninvolved rib and intercostal space above and below the surgical resection of a primary chest wall sarcoma, and Wouters et al. [1] described a 1- to 2-cm margin of normal tissue as their principle of surgical resection for primary chest wall sarcoma.
In the present study, we analyzed long-term RFS according to the surgical resection margin distance based on pathology reports, not surgical records. Many surgical records in our study with a planned resection margin distance of 4 cm revealed an inadequate resection margin distance (<1.5 cm) in the gross pathological reports after surgery. This difference may imply that the resection margin distance measured by the surgeon during the operation might be incorrect or inadequate because the resection margin distance may have been considered in only 1 of the 4 directions from the tumor. Because a sufficient resection margin distance is important for favorable surgical outcomes, we suggest that the resection margin distance be measured in all directions to ensure an adequate margin.
Surgeons may achieve insufficient resection margins during chest wall sarcoma resection owing to insufficient adherence to surgical principles, low-grade chest wall sarcomas, or a tumor location near vital structures. If it is impossible to ensure a sufficient resection margin due to a tumor location near vital structures, a 1.5-cm resection margin distance could be justified considering our findings. Fig. 3 shows a 90.9% 10-year RFS of patients with a resection margin distance ≥1.5 cm if the resection margin distance is evaluated correctly. However, if possible, every effort should be made to ensure a resection margin distance of 4 cm to ensure a good long-term disease-free survival rate based on previous reviews [2,5,6,8].
This study has some limitations. First, this was a retrospective case-control study. For ethical reasons, however, it is impossible to conduct a randomized controlled study of differences in the surgical resection margin. Second, values were missing for some variables. Nine patients (21.1%) had no data on the histologic grade. This may have limited the interpretation of risk factor analysis because histologic grade was an independent risk factor for RFS. However, most of the data, including survival and resection margin, were completely collected for most patients.
In conclusion, the long-term OS and RFS after the surgical resection of primary chest wall sarcoma were clinically acceptable. Old age was the only independent risk factor for death, while a resection margin distance <1.5 cm and histologic grade III were prognostic factors for recurrence. Surgeons should aim to achieve an adequate resection margin distance to ensure favorable long-term RFS.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund no. KTCS04-127).
Footnotes
This study was presented at the 2018 Fall Conference of the Korean Society of Thoracic and Cardiovascular Surgery (English Competition Forum 10th).
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Wouters MW, van Geel AN, Nieuwenhuis L, et al. Outcome after surgical resections of recurrent chest wall sarcomas. J Clin Oncol. 2008;26:5113–8. doi: 10.1200/JCO.2008.17.4631. [DOI] [PubMed] [Google Scholar]
- 2.Walsh GL, Davis BM, Swisher SG, et al. A single-institutional, multidisciplinary approach to primary sarcomas involving the chest wall requiring full-thickness resections. J Thorac Cardiovasc Surg. 2001;121:48–60. doi: 10.1067/mtc.2001.111381. [DOI] [PubMed] [Google Scholar]
- 3.Sabanathan S, Shah R, Mearns AJ. Surgical treatment of primary malignant chest wall tumours. Eur J Cardiothorac Surg. 1997;11:1011–6. doi: 10.1016/S1010-7940(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 4.Perry RR, Venzon D, Roth JA, Pass HI. Survival after surgical resection for high-grade chest wall sarcomas. Ann Thorac Surg. 1990;49:363–8. doi: 10.1016/0003-4975(90)90239-3. [DOI] [PubMed] [Google Scholar]
- 5.Bagheri R, Haghi SZ, Kalantari MR, et al. Primary malignant chest wall tumors: analysis of 40 patients. J Cardiothorac Surg. 2014;9:106. doi: 10.1186/1749-8090-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King RM, Pairolero PC, Trastek VF, Piehler JM, Payne WS, Bernatz PE. Primary chest wall tumors: factors affecting survival. Ann Thorac Surg. 1986;41:597–601. doi: 10.1016/S0003-4975(10)63067-6. [DOI] [PubMed] [Google Scholar]
- 7.Jonsson P, Gyllstedt E, Hambraeus G, Lillogil R, Rydholm A. Chest wall sarcoma: outcome in 22 patients after resection requiring thoracic cage reconstruction. Sarcoma. 1998;2:143–7. doi: 10.1080/13577149877894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Athanassiadi K, Kalavrouziotis G, Rondogianni D, Loutsidis A, Hatzimichalis A, Bellenis I. Primary chest wall tumors: early and long-term results of surgical treatment. Eur J Cardiothorac Surg. 2001;19:589–93. doi: 10.1016/S1010-7940(01)00655-8. [DOI] [PubMed] [Google Scholar]
- 9.Gross JL, Younes RN, Haddad FJ, Deheinzelin D, Pinto CA, Costa ML. Soft-tissue sarcomas of the chest wall: prognostic factors. Chest. 2005;127:902–8. doi: 10.1378/chest.127.3.902. [DOI] [PubMed] [Google Scholar]
- 10.Sohn ST, Chon SH, Shinn SH, et al. Review of primary chest wall tumors. Korean J Thorac Cardiovasc Surg. 1998;31:988–94. [Google Scholar]
- 11.Mah JS, Choi BW, Yu HS. Primary tumors of the chest wall. Korean J Thorac Cardiovasc Surg. 1974;7:61–5. [Google Scholar]
- 12.Kim SM, Park SD, Jeong JH, Cho SR, Lee SH. Clinical experience of chest wall tumors: a review of twenty one cases. Korean J Thorac Cardiovasc Surg. 1987;20:723–9. [Google Scholar]
- 13.Lee MG, Oh TY, Chang WH. Clinical evaluation of chest wall tumors: review of 33 cases. Korean J Thorac Cardiovasc Surg. 1995;28:778–83. [Google Scholar]
- 14.Park KH, Kim KB, Sung SW, Kim JH. Surgical management of chest wall tumors. Korean J Thorac Cardiovasc Surg. 1991;24:547–54. [Google Scholar]
- 15.Paik HC, Kang JH, Choi SS, Chung KY. Clinical review of primary chest wall tumors. Korean J Thorac Cardiovasc Surg. 2003;36:175–81. [Google Scholar]
- 16.Kim CG, Kuh JH, Kim KS. Clinical study of primary chest wall tumors. Korean J Thorac Cardiovasc Surg. 1998;31:155–61. [Google Scholar]