Abstract
Objective: The aim of this research was to evaluate uterine-wall integrity 12 months after transcervical fibroid ablation (TFA) of uterine fibroids with the Sonata® system (Gynesonics Inc., Redwood City, CA).
Materials and Methods: INTEGRITY is a secondary analysis of the FAST-EU clinical trial, a prospective, longitudinal, multicenter single-armed trial involving women with heavy menstrual bleeding secondary to fibroids who were treated at 7 academic and community hospitals in the United Kingdom, the Netherlands, and Mexico with transcervical, intrauterine, ultrasound-guided radiofrequency ablation (the Sonata system). TFA was performed on up to 5 fibroids per subject ranging from 1–5 cm in diameter as determined by magnetic resonance imaging (MRI). All measurements and comparisons, including uterine-wall thicknesses were derived from baseline and 12-month MRI scans by an independent core MRI center. Scans were analyzed to assess preservation of uterine-wall integrity and reviewed for uterine-wall anomalies after TFA with the Sonata system.
Results: Twenty-nine patients had baseline and 12-month MRI with contrast enhancement. Minimum uterine-wall thicknesses in all visible slices were >2.5 mm in diameter. No areas on MRI indicated any loss of uterine-wall integrity, compared with baseline imaging; comparison of baseline and postablation uterine-wall thicknesses revealed no significant changes.
Conclusion: Transcervical fibroid ablation with the Sonata system was associated with preservation of uterine-wall integrity in this patient cohort.
Keywords: uterine fibroids, radiofrequency ablation, transcervical fibroid ablation (TFA), uterine preserving
Introduction
Uterine rupture involves the complete interruption of all layers of the uterine wall.1 Unlike uterine dehiscence, in which serosal integrity is preserved and can be an incidental finding at delivery, uterine rupture can be an obstetric catastrophe.1,2 While uterine rupture is uncommon, it has been associated with procedures that involve uterine incision/resection or significant necrosis, including prior single and multiple cesarean sections (particularly via classical hysterotomies), operative hysteroscopies, myomectomies, and uterine-artery embolizations.3–7 In contrast, there have been no reports of uterine rupture or dehiscence in women who underwent image-guided volumetric radiofrequency (RF) ablation of uterine fibroids and who subsequently delivered neonates.8
Although it appears that clinical prediction of uterine rupture after prior cesarean section is unreliable, uterine imaging has been used to ascertain if uterine-wall scarring or reductions in thickness might suggest an increased risk of uterine rupture in subsequent or current pregnancies.2,4,9–11 In particular, a lower uterine segment (LES) thickness <2.0–2.3 mm has been proposed as predictive of an increased risk of uterine rupture after prior cesarean section.4,9 The presence of a uterine scar, however, might not be a useful marker for future uterine rupture. This is because, while uterine scarring is common after hysterotomy (it has been demonstrated in 99.1% of women with prior histories of cesarean section), uterine rupture is rare (35 of 10,000 women undergoing labor after prior cesarean sections).12 This suggests that prior hysterotomy or myometrial injury, while associated with an increased risk of uterine rupture in a future pregnancy, does not guarantee a rupture's occurrence. At the same time, the occurrence of uterine dehiscence/rupture becomes even more uncommon as uterine-wall thickness increases.13
The Sonata® system (Gynesonics Inc., Redwood City, CA) is a transcervical device that uses RF energy to ablate fibroids under integrated intrauterine sonographic guidance, and has been described in detail.14–17 As with other RF devices for treating uterine fibroids, pregnancy outcomes to date have been favorable.8,18 A multinational clinical trial (FAST-EU) of the Sonata system included a magnetic resonance imaging (MRI) comparison of fibroid volumes at baseline and at 12 months postablation. These imaging files also provide the ability to examine post-treatment scans for possible changes in uterine-wall integrity, particularly areas of abnormal thinning. This assessment of uterine-wall integrity (the INTEGRITY secondary analysis) is intended to address potential concerns about hyperthermic ablation of uterine fibroids in patients who desire future pregnancies.
Materials and Methods
The FAST-EU clinical trial was performed to establish the effectiveness and confirm the safety of transcervical fibroid ablation (TFA) of symptomatic uterine fibroids with the Sonata system (formerly known as VizAblate®).15 Seven hospitals in The Netherlands, the United Kingdom, and Mexico participated in FAST-EU from January 2011 through March 2014. Patients were enrolled and treated with the Sonata system if they had symptomatic fibroids ranging from 1 to 5 cm in maximum diameter, heavy menstrual bleeding, and no intent for future fertility, along with other inclusion and exclusion requirements. All patients were required to have at least 1 indenting fibroid (type 1, type 2, or types 2–5). The primary endpoint was the change in perfused fibroid volume assessed by contrast-enhanced magnetic resonance imaging (MRI) at 3 months. Secondary endpoints, evaluated at 6 and 12 months, included safety, reductions in menstrual bleeding, reductions in symptom severity, improvements in health-related quality of life and other measures.
The FAST-EU protocol was approved by the ethics committees of the respective institutions as well as by the Federal Commission for Protection against Health Risks (COFEPRIS) in Mexico. All enrolled patients provided written informed consent for treatment prior to enrollment. Clinical results of the FAST-EU trial at 3, 6, and 12 months have been reported and included significant reductions in bleeding, fibroid volumes, and improvements in quality of life measures.14,15
As part of a protocol amendment during the FAST-EU clinical trial, additional contrast-enhanced MRI was performed at 12-months on a subgroup of patients who provided informed consent to do so. This 12-month MRI was used for this evaluation of uterine-wall integrity along with previously-published reductions in fibroid volume at 12 months post-treatment.
All digital MRI series in the FAST-EU clinical trial were reviewed by an independent, third-party imaging facility (MedQIA, Los Angeles, CA). This evaluation included quality control and computer-assisted evaluation of the minimum and maximum anterior and posterior, in-plane, full uterine wall-thicknesses performed on T1-weighted sagittal post-contrast scans. Fibroid diameters were subtracted from these measurements when present, to avoid confounding the actual uterine-wall thicknesses. In order to exclude a potential loss of myometrial integrity, a minimum uterine-wall thickness threshold >2.5 mm was required, as this was more stringent than the 2.0 mm and 2.3 mm thresholds in the literature.4,9 If any 12-month MRI scans showed a measured uterine-wall thickness ≤2.5 mm, that would prompt an additional focused review by a board-certified radiologist at the independent imaging facility, and any verified radiologic signs of thinning or compromise of the uterine wall, compared to baseline, would be noted.
Statistical analysis was performed with Microsoft Excel 16.14 (Microsoft, Redmond, WA). Changes in variables were assessed using a paired t-test. Values were considered significant at the level of α = 0.05.
Results
Of 50 patients who were enrolled and treated in the FAST-EU clinical trial, 29 provided consent to undergo an additional elective 12-month MRI study as part of a protocol amendment that was enacted after patients had already been enrolled and treated in the clinical trial. The baseline and 12-month MRI studies on these 29 patients were deemed adequate for an evaluation of uterine-wall integrity by the independent third-party imaging facility.
Review of the 29 12-month MRI scans showed no evidence of loss of uterine-wall integrity after TFA. There were no new myometrial scars (nonperfused linear areas) associated with fibroid ablation; 6 patients had visible anterior LES scars from prior cesarean hysterotomies. As shown in Table 1, the minimum full uterine-wall thicknesses (anterior and posterior) were all >3.0 mm, and, thus, did not require additional imaging reviews. On average, maximum and minimum anterior and posterior, 12-month wall thicknesses did not show significant changes from baseline.
Table 1.
Uterine-Wall Thicknesses (in mm) at Baseline and 12 Months After TFA (N = 29 patients)
| Parameter | Baseline | 12 months | Change |
|---|---|---|---|
| MAXA | |||
| Mean | 13.2 ± 5.6 | 13.0 ± 3.3 | –0.2 ± 4.5 |
| Min, Max | 6.5, 32.8 | 6.8–19.8 | –16.7, 6.0 |
| Median | 11.9 | 13.3 | +1.2 |
| p | .848 | ||
| MAXP | |||
| Mean | 13.9 ± 3.7 | 14.5 ± 3.2 | +0.7 ± 3.0 |
| Min, Max | 5.5, 21.6 | 6.9–20.9 | –5.0, 6.6 |
| Median | 14.1 | 14.4 | +1.4 |
| p | 0.238 | ||
| MINA | |||
| Mean | 7.0 ± 2.6 | 7.0 ± 2.2 | 0.0 ± 2.4 |
| Min, Max | 3.5, 13.5 | 3.1–11.1 | –6.8, 3.6 |
| Median | 6.5 | 7 | 0 |
| p | 0.970 | ||
| MINP | |||
| Mean | 8.3 ± 2.0 | 8.5 ± 2.3 | +0.2 ± 2.0 |
| Min, Max | 4.6, 12.7 | 3.8–12.5 | –5.3, 4.0 |
| Median | 8.1 | 8.2 | +0.3 |
| p | 0.692 | ||
TFA, transcervical fibroid ablation; MAXA, maximum anterior uterine-wall thickness; Min, minimum; Max, maximum; MAXP, maximum posterior uterine-wall thickness; MINA, minimum anterior uterine-wall thickness; MINP, minimum posterior uterine-wall thickness.
Discussion
Analysis of baseline and postablation MR images after TFA treatment with the Sonata system showed no ablation-related myometrial scarring nor significant reduction in full uterine-wall thickness. The minimum anterior and posterior wall thicknesses at 12 months were all ≥3.1 mm and ≥3.8 mm, respectively, with little or no change in minimum wall thicknesses (– 0.2 mm ±0.7 mm).
A minimum baseline >2.5 mm was used to determine if there was a priori evidence of uterine-wall compromise after TFA. This was based on studies involving sonographic imaging. Fukuda and colleagues evaluated 84 pregnant women in the third trimester (prior to labor onset) with prior cesarean section scars visible on transabdominal sonography.11 These researchers noted that “good healing” of a prior hysterotomy was associated with a uterine wall thickness >3 mm, while “poor healing” was associated with a uterine wall thickness <2 mm and that LES continuity was lost. Of 14 patients who underwent repeat cesarean section, prompted by the finding of a uterine-wall thickness <2 mm on sonography, all had detectable thinning of the lower segment at the time of delivery, with a loss of uterine continuity noted. Five patients had fetal hair visible through the defects. In contrast, of 46 patients with a lower-segment thickness >3 mm who underwent repeat cesarean section for obstetric indications, 42 had no evidence of any wall thinning and 4 had some degree of thinning but fetal hair was not visible.
Subsequently, Quereshi and colleagues performed a prospective, randomized controlled study of 43 pregnant women with histories of cesarean sections and 80 gravidae without prior uterine surgery.4 A lower-segment thickness >2 mm on transvaginal sonography was considered to represent adequate healing for the prior cesarean–section cohort and <2 mm represented poor healing and patients were considered for elective cesarean sections (women at exactly 2 mm had their managements individualized). Based on the obstetric and histopathologic outcomes, a lower-segment thickness ≤2 mm was 86.7% sensitive and 100% specific, with a 100% positive predictive value for uterine-scar thinning. No patients with full thickness >2 mm experienced uterine dehiscence or rupture in the peripartum period. Similar recommendations for a uterine-wall baseline were proposed by Osser and colleagues (≥ 2.2 mm),19 Bujold and colleagues (≥ 2.3 mm),9 and Sen and colleagues (≥ 2.5 mm).20 Of note, the Bujold study9 included 3 cases of uterine rupture, none of which occurred in women with a full lower-segment thickness ≥2.5 mm. While some researchers have suggested a higher threshold of 3.1 mm, this suggestion came with reduced specificity regarding obstetrical decision-making, potentially resulting in unnecessary cesarean sections.2,13,21
Uterine rupture is uncommon after both abdominal (≤ 0.2%) and laparoscopic myomectomy (≤ 0.3%), and is at the level of anecdote for uterine artery embolization.3,6,22 While rare, uterine rupture can result in maternal and/or fetal mortality. Postcesarean uterine-wall thinning is associated with an increased incidence of uterine dehiscence and rupture, thus, it is apparent that uterine injury appears to be necessary for uterine rupture to occur (in the absence of obstetric factors such as inappropriate use of oxytocics), even if this is not sufficient, as the presence of a visible uterine-wall defect does not invariably lead to uterine rupture in a subsequent or current pregnancy.
There have been no reported cases of uterine dehiscence or rupture associated with the use of volumetric RF ablation to treat symptomatic uterine fibroids.8 After TFA with the Sonata system no uterine defects were noted at the time of term an elective repeat cesarean section in a patient enrolled in the FAST-EU trial who conceived 3 months post-treatment.18 Berman and colleagues reported a successful pregnancy after laparoscopic RF ablation of a uterine fibroid.23 There was no placental adherence at delivery and an MRI study performed 3 months postpartum revealed a uterine-wall thickness of 9.6 mm. A series of 6 patients who delivered after laparoscopic RF ablation, similarly, had no cases of uterine dehiscence or rupture, nor was any uterine thinning noted at the time of the cesarean sections.24
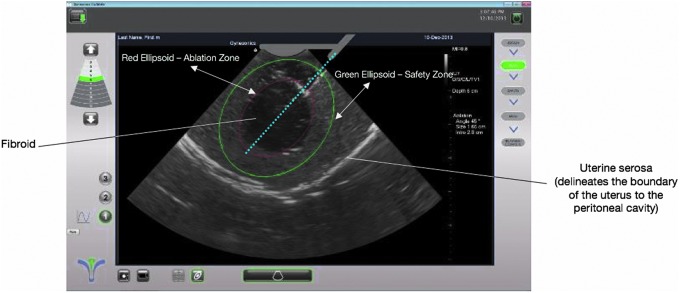
In a larger number of published cases of pregnancies, fibroid treatment with focused ultrasound (another form of targeted thermal ablation) has also not been associated with uterine rupture or dehiscence.25–29 Unlike laparoscopic myomectomy, which involves the considerable use of bipolar or monopolar electrosurgery and one or more hysterotomies, image-guided hyperthermic ablation methods with RF energy or focused ultrasound typically involve a more controlled application of energy directly to one or more fibroids, with collateral injury to the myometrium minimized or eliminated due to image-guided precision targeting (Fig. 1). There are also no incisions or use of sutures within the uterus, which could contribute to the lack of adverse effects on the uterine wall to date after TFA or focused ultrasound.24
FIG 1.
Image-guided precision targeting with the Sonata® system's SMART Guide™ (Gynesonics Inc., Redwood City, CA). Permission granted by David Toub, MD, MBA, FACOG, Medical Director of Gynesonics, for use of this image.
In 2015, the U.S. Food and Drug Administration specified that hyperthermic ablation of uterine fibroid tissue with MRI-guided focused ultrasound (MRgFUS) could be considered for women with symptomatic uterine fibroids who desired to retain their fertility and conserve their uteri.30 This was based on the accumulated outcomes of 118 pregnancies treated with MRgFUS.31 Because TFA with the Sonata system similarly provides image-guided, volumetric ablation of uterine fibroids and has not been associated with uterine-wall compromise, there is no a priori evidence to suggest that focal RF ablation would result in different outcomes from that of MRgFUS regarding future pregnancies.
A key strength of this study of uterine wall integrity after TFA was the independent review and analysis of all MRI data by a single core-imaging facility, which utilized computer-assisted analysis for baseline and 12-month uterine-wall measurements (excluding fibroid dimensions when fibroids were present), such that these measurements were not affected by radiologist biases. Another advantage was the use of a more-sensitive full uterine-wall measurement (≤ 2.5 mm) as a definition of myometrial compromise rather than the <2.0–2.3-mm threshold proposed by other researchers that have greater specificity.
Some limitations of this study included the lack of pregnancy outcomes (the 1 patient who became pregnant during the FAST-EU clinical trial did not undergo an additional MRI study at 12 months postablation before delivering at term) and the inclusion of only 29 patients from the 50-patient cohort of the FAST-EU trial, due to the elective nature of the protocol change permitting the additional 12-month MRI study. Finally, as there is nothing in the gynecologic literature that has evaluated the risk of uterine rupture for myometrial thicknesses at other sites within the uterus such as the corpus, the only evidence-based metric that could be utilized regarding increased risk for uterine rupture was the sonography-derived lower-segment thickness of ≤2.3 mm.
It is not known what the minimum uterine-wall thickness must be in other areas of the uterus to define “integrity.” However, in this cohort of patients treated with TFA, there was no significant change in myometrial thickness at 12 months, compared to baseline, suggesting that ablation did not materially increase any existing risk of uterine rupture.
Conclusions
This independent, third-party analysis of baseline and 12-month pelvic MRI (INTEGRITY) showed preservation of uterine-wall integrity with no myometrial abnormalities observed in a patient cohort after TFA with the Sonata system. There was no evidence of myometrial thinning or scarring and no significant changes in minimum and maximum wall thicknesses anteriorly or posteriorly.
Acknowledgments
David Toub, MD, MBA, the medical director of Gynesonics, contributed to the writing and development of this article, while Taraneh G. Farazi, PhD, vice-president of clinical affairs at Gynesonics, contributed to the review of this article.
Author Disclosure Statement
Drs. Bongers and Garza-Leal are consultants for Gynesonics and Dr. Garza-Leal has stock options. The remaining authors have no competing financial interests.
References
- 1. Fox NS, Gerber RS, Mourad M, Saltzman DH, Klauser CK, Gupta S, Rebarber A. Pregnancy outcomes in patients with prior uterine rupture or dehiscence. Obstet Gynecol 2014;123:785. [DOI] [PubMed] [Google Scholar]
- 2. Jastrow N, Chaillet N, Roberge S, Morency AM, Lacasse Y, Bujold E. Sonographic lower uterine segment thickness and risk of uterine scar defect: A systematic review. J Obstet Gynaecol Can 2010;32:321. [DOI] [PubMed] [Google Scholar]
- 3. Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol 2010;17:551. [DOI] [PubMed] [Google Scholar]
- 4. Qureshi B, Inafuku K, Oshima K, Masamoto H, Kanazawa K. Ultrasonographic evaluation of lower uterine segment to predict the integrity and quality of cesarean scar during pregnancy: A prospective study. Tohoku J Exp Med 1997;183:55. [DOI] [PubMed] [Google Scholar]
- 5. Sentilhes L, Sergent F, Roman H, Verspyck E, Marpeau L. Late complications of operative hysteroscopy: Predicting patients at risk of uterine rupture during subsequent pregnancy. Eur J Obstet Gynecol Reprod Biol 2005;120:134. [DOI] [PubMed] [Google Scholar]
- 6. Yeaton-Massey A, Loring M, Chetty S, Druzin M. Uterine rupture after uterine artery embolization for symptomatic leiomyomas. Obstet Gynecol 2014;123(2[pt2(suppl2)]):418. [DOI] [PubMed] [Google Scholar]
- 7. [American College of Obstetricians and Gynecologists] Practice Bulletin No. 184 Summary: Vaginal Birth After Cesarean Delivery. Obstet Gynecol 2017;130:1167. [DOI] [PubMed] [Google Scholar]
- 8. Keltz J, Levie M, Chudnoff S. Pregnancy outcomes after direct uterine myoma thermal ablation: Review of the literature. J Minim Invasive Gynecol 2017;24:538. [DOI] [PubMed] [Google Scholar]
- 9. Bujold E, Jastrow N, Simoneau J, Brunet S, Gauthier RJ. Prediction of complete uterine rupture by sonographic evaluation of the lower uterine segment. Am J Obstet Gynecol 2009;201:320 e1. [DOI] [PubMed] [Google Scholar]
- 10. Kok N, Wiersma IC, Opmeer BC, de Graaf IM, Mol BW, Pajkrt E. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: A meta-analysis. Obstet Gynecol Surv 2014;69:123. [DOI] [PubMed] [Google Scholar]
- 11. Fukuda M, Fukuda K, Mochizuki M. Examination of previous caesarean section scars by ultrasound. Arch Gynecol Obstet 1988;243:221. [DOI] [PubMed] [Google Scholar]
- 12. Ofili-Yebovi D, Ben-Nagi J, Sawyer E, Yazbek J, Lee C, Gonzalez J, Jurkovic D. Deficient lower-segment Cesarean section scars: Prevalence and risk factors. Ultrasound Obstet Gynecol 2008;31:72. [DOI] [PubMed] [Google Scholar]
- 13. Umelo F, Eigbefoh J, Eifediyi R, Okome G, Isabu P. Can sonographic evaluation of lower uterine segment predict women at risk of uterine rupture/dehiscence? Int J Gynecol Obstet Res 2015;3:13 [Google Scholar]
- 14. Bongers M, Brölmann H, Gupta J, Garza-Leal JG, Toub D. Transcervical, intrauterine ultrasound-guided radiofrequency ablation of uterine fibroids with the VizAblate® System: Three- and six-month endpoint results from the FAST-EU study. Gynecol Surg 2015;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brölmann H, Bongers M, Garza-Leal JG, Gupta J, Veersema S, Quartero R, Toub D. The FAST-EU trial: 12-month clinical outcomes of women after intrauterine sonography-guided transcervical radiofrequency ablation of uterine fibroids. Gynecol Surg 2016;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garza-Leal JG, Toub D, León IH, et al. Transcervical, intrauterine ultrasound-guided radiofrequency ablation of uterine fibroids with the VizAblate System: safety, tolerability, and ablation results in a closed abdomen setting. Gynecol Surg. 2011;8:327 [Google Scholar]
- 17. Toub DB. A new paradigm for uterine fibroid treatment: Transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the Sonata System. Curr Obstet Gynecol Rep 2017;6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garza-Leal JG, León IH, Toub D. Pregnancy after transcervical radiofrequency ablation guided by intrauterine sonography: Case report. Gynecol Surg 2014;11:145 [Google Scholar]
- 19. Osser OV, Jokubkiene L, Valentin L. High prevalence of defects in Cesarean section scars at transvaginal ultrasound examination. Ultrasound Obstet Gynecol 2009;34:90. [DOI] [PubMed] [Google Scholar]
- 20. Sen S, Malik S, Salhan S. Ultrasonographic evaluation of lower uterine segment thickness in patients of previous cesarean section. Int J Gynecol Obstet 2004;87:215. [DOI] [PubMed] [Google Scholar]
- 21. Kok N, Wiersma IC, Opmeer BC, de Graaf IM, Mol BW, Pajkrt E. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol 2013;42(2):132. [DOI] [PubMed] [Google Scholar]
- 22. Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol 2007;14:453. [DOI] [PubMed] [Google Scholar]
- 23. Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid: A case report. J Reprod Med 2012;57(3–4):159. [PubMed] [Google Scholar]
- 24. Berman JM, Bolnick JM, Pemueller RR, Garza Leal JG. Reproductive outcomes in women following radiofrequency volumetric thermal ablation of symptomatic fibroids. A retrospective case series analysis. J Reprod Med 2015;60(5–6):194. [PubMed] [Google Scholar]
- 25. Bohlmann MK, Hoellen F, Hunold P, David M. High-intensity focused ultrasound ablation of uterine fibroids—potential impact on fertility and pregnancy outcome. Geburtshilfe Frauenheilkd 2014;74:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gavrilova-Jordan LP, Rose CH, Traynor KD, Brost BC, Gostout BS. Successful term pregnancy following MR-guided focused ultrasound treatment of uterine leiomyoma. J Perinatol 2007;27:59. [DOI] [PubMed] [Google Scholar]
- 27. Rabinovici J, David M, Fukunishi H, Morita Y, Gostout BS, Stewart EA; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril 2010;93:199. [DOI] [PubMed] [Google Scholar]
- 28. Rabinovici J, Inbar Y, Eylon SC, Schiff E, Hananel A, Freundlich D. Pregnancy and live birth after focused ultrasound surgery for symptomatic focal adenomyosis: A case report. Hum Reprod 2006;21:1255. [DOI] [PubMed] [Google Scholar]
- 29. Zou M, Chen L, Wu C, Hu C, Xiong Y. Pregnancy outcomes in patients with uterine fibroids treated with ultrasound-guided high-intensity focused ultrasound. BJOG 2017;124(suppl3):30. [DOI] [PubMed] [Google Scholar]
- 30. INSIGHTEC Women's Health. MR-Guided Focused Ultrasound (MRgFUS): A Non-Invasive Solution for Treating Uterine Fibroids & Adenomyosis. Tirat Carmel, Israel; 2015. Online document at: www.insightec.com/media/31068/womens-health-brochure_ce_digital.pdf Accessed July31, 2018 [Google Scholar]
- 31. U.S. Food and Drug Administration. ExAblate Ablation System, High Intensity Focused Ultrasound (HiFU), MR-Guided. 2015. Online document at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P040003S015 Accessed July31, 2018