Abstract
Background.
Stem cell therapy is a promising treatment modality for injured cardiac tissue. A novel mechanism for this cardioprotection may include paracrine actions. Our lab has recently shown that gender differences exist in mesenchymal stem cell (MSC) paracrine function. Estrogen is implicated in the cardioprotection found in females. It remains unknown whether 17β-estradiol (E2) affects MSC paracrine function and whether E2 treated MSCs may better protect injured cardiac tissue. We hypothesize that E2 exposed MSCs infused into hearts prior to ischemia may demonstrate increased VEGF production and greater protection of myocardial function compared to untreated MSCs.
Methods.
Untreated and E2 treated MSCs were isolated, cultured, plated, and supernatants were harvested for VEGF assay (ELISA). Adult male Sprague-Dawley rat hearts (n=13) were isolated and perfused via Langendorff model, and subjected to 15min equilibration, 25min warm global ischemia, and 40min reperfusion. Hearts were randomly assigned to perfusate vehicle, untreated male MSC, or E2 treated male MSC. Transcoronary delivery of 1 million MSCs was performed immediately prior to ischemia in experimental hearts.
Results.
E2 treated MSCs provoked significantly more VEGF production than untreated MSCs (933.2±64.9 vs. 595.8±10.7pg/mL). Post-ischemic recovery of left ventricular developed pressure was significantly greater in hearts infused with E2 treated MSCs (66.9±3.3%) than untreated MSCs (48.7±3.7%) and vehicle (28.9±4.6%) at end reperfusion. There was also greater recovery of the end diastolic pressure with E2 treated MSCs than untreated MSCs and vehicle.
Conclusions.
Pre-ischemic infusion of MSCs protects myocardial function and viability. E2 treated MSCs may enhance this paracrine protection, which suggests that ex-vivo modification of MSCs may improve therapeutic outcome.
Keywords: sex hormones, cardioprotection, estrogen, paracrine, VEGF
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death for both men and women in the United States. However, treatment of this prevalent disease presents interesting challenges over other tissues because myocardium has limited regenerative abilities (1), and reperfusion therapy, the treatment of choice for infarction, is accompanied by the deleterious effects of ischemia / reperfusion (I/R) injury (2, 3). Considering the irreversible nature of cardiac tissue myopathies, remodeling of injured tissue may be a promising treatment modality (4).
Stem cells have generated ongoing interest from researchers and clinicians for their ability to specialize into mature cell types (5). Ferrari et al initially found that bone marrow derived stem cells (BMSCs) transplanted into injured muscle tissue participated in the regeneration process (6). However, it now appears that the cardioprotection afforded by stem cells may not result from differentiation (7, 8). Paracrine actions may also be responsible for the cardioprotective effects of stem cells (9, 10). Indeed, our lab has previously demonstrated that acute pretreatment of myocardium subjected to I/R with human BMSCs increased growth factor production, improved functional recovery, decreased proinflammatory cyotkine production and decreased activation of proapoptotic caspases without evidence of differentiation (11).
Gender differences have been observed in myocardial function following I/R injury. Clinical studies have shown that pre-menopausal women have a lower incidence of CVD and increased survival (12). Interestingly, our lab has previously shown that gender differences also exist in MSC growth factor and cytokine production (13). Indeed, female MSCs express greater vascular endothelial growth factor (VEGF) production and less tumor necrosis factor alpha (TNF) production, a proinflammatory cytokine that causes myocardial dysfunction and cardiomyocyte death (1), following insult than male MSCs (14). Hiasa and colleagues found that VEGF plays an important role in BMSC mediated cardioprotection from ischemia (15). Previous studies have found that estrogen is attributable to the cardioprotection found in females (16, 17). MSC sex differences may also reflect chronic/genomic effects of estrogen (14, 18). No study has addressed the role of estrogen on stem cell activation and protection. The main objectives of this study are to investigate the cardioprotective effects of male MSCs treated with 17β-estradiol (E2) on I/R injury and to compare them to the cardioprotective effects of untreated male MSCs on I/R injury. Therefore, we hypothesize that E2 treated MSCs infused into hearts prior to ischemia may demonstrate increased VEGF production and greater protection of myocardial function compared to untreated MSCs.
MATERIALS AND METHODS
Animals
Age-matched (250–300 g, 9- to 10-week) normal male Sprague–Dawley rats (Harlan, Indianapolis, IN) were fed a standard diet and acclimated in a quiet quarantine room for 1 week before the experiments. The animal protocol was reviewed and approved by the Indiana Animal Care and Use Committee of Indiana University. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, revised 1985).
Preparation of mouse bone marrow stromal cells
A single-step purification method using adhesion to cell culture plastic is used as previously described (19) with the following modifications: After killing 8-week old mice, mouse bone marrow stromal cells were collected from bilateral femurs and tibias by removing the epiphyses and flushing the shaft with complete media (Iscove modified Dulbecco medium [GIBCO Invitrogen, Carlsbad, Calif] and 10% fetal bovine serum [GIBCO Invitrogen]) using a syringe with a 23-G needle. Cells were disaggregated by vigorously pipetting several times. Cells were passed through a 30-μm nylon mesh to remove remaining clumps of tissue. Cells were washed by adding complete media, centrifuging for 5 min at 300 rpm at 24°C, and removing supernatant. The cell pellet was then resuspended and cultured in 75 cm2 culture flasks with complete media at 37°C. Mesenchymal stem cells were preferentially attached to the polystyrene surface; after 48 h, nonadherent cells in suspension were discarded. Fresh complete medium was added and replaced every 3 or 4 days thereafter. Mesenchymal stem cell cultures were maintained at 37°C in 5% CO2 in air. When the cultures reached 90% of confluence, the MSC culture was passaged; cells were recovered by the addition of a solution of 0.25% trypsi-EDTA (GIBCO Invitrogen) and replated in 75 cm2 culture flasks.
Measurement of MSC VEGF production
MSCs were plated in 12-well plates in a concentration of 1× 106 cells/well/mL, and divided into four groups: 1) untreated MSCs; 2) 10 nM E2 treated MSCs; 3) 100 nM E2 treated MSCs; and 4) 1000 nM E2 treated MSCs. The 17β-estradiol solution was prepared with water soluble β-Estradiol (Sigma-Aldrich, St. Louis, MO) and PBS. After 24 h, supernatants were harvested for VEGF assay (enzyme-linked immunosorbent assay [ELISA]).
To determine the dilution of estradiol for ex vivo isolated heart experiments, the latter dose response curve of estradiol measuring VEGF production was used. Treated MSCs were subjected to 1 μM 17β-estradiol for 24h.
Preparation of E2 treated MSCs for isolated heart
E2 treated MSCs were subjected to 1 μM estradiol for 24 h. After 24 h, the estradiol treated supernatant was removed, and the cells were washed with PBS to remove any residual E2 from the incubation medium. The cells were then recovered by the addition of a solution of 0.25% trypsi-EDTA and resuspended in perfusate vehicle to further eliminate any residual E2.
Experimental isolated heart groups
All isolated rat hearts were subjected to the same I/R protocol: 15 min equilibration period, 25 min of global index ischemia (37°C), and 40 min of total reperfusion. Male rats were randomly assigned to three experimental groups: 1) perfusate vehicle (n = 4); 2) untreated male mesenchymal stem cell (n = 5); and 3) 17β-estradiol treated male mesenchymal stem cell (n = 4). Experimental hearts were infused with 1 million mesenchymal stem cells in 1 mL over 1 minute immediately prior to ischemia. Vehicle and MSC solutions were infused through a port above the aortic root one minute prior to ischemia.
Isolated heart preparation (Langendorff)
Rats were anesthetized (sodium pentobarbital, 60 mg/kg intraperitoneal) and heparinized (500 U intraperitoneal), and hearts were rapidly excised via median sternotomy and placed in 4°C Krebs–Henseleit solution. The aorta was cannulated and the heart was perfused (70 mmHg) with oxygenated (95% O2/5% CO2) Krebs–Henseleit solution (37 °C). A left atrial resection was performed prior to insertion of a water-filled latex balloon through the left atrium into the left ventricle. The preload volume (balloon volume) was held constant during the entire experiment to allow continuous recording of the LVDP and EDP. The balloon was adjusted to a mean EDP of 5 mmHg (range 4–8 mmHg) during the initial equilibration. Pacing wires were fixed to the right atrium and hearts were paced at approximately 6 Hz, 3 V, 2 ms (350 bpm) throughout perfusion. A three-way stopcock above the aortic root was used to create global ischemia, during which the heart was placed in a 37°C degassed organ bath. Coronary flow was measured by collecting pulmonary artery effluent. Data were continuously recorded using a PowerLab 8 preamplifier/digitizer (AD Instruments Inc., Milford, MA) and an Apple G4 PowerPC computer (Apple Computer Inc., Cupertino, CA). The +dP/dt and −dP/dt were calculated using PowerLab software. After reperfusion, the heart was removed from the apparatus, immediately sectioned, and snap frozen in liquid nitrogen.
Presentation of data and statistical analysis
All reported values are mean ± S.E.M. Data were compared using two-way analysis of variance (ANOVA) with post-hoc Bonferroni test or Student’s t-test. A two-tailed probability value of less than 0.05 was considered statistically significant.
RESULTS
MSC Activation
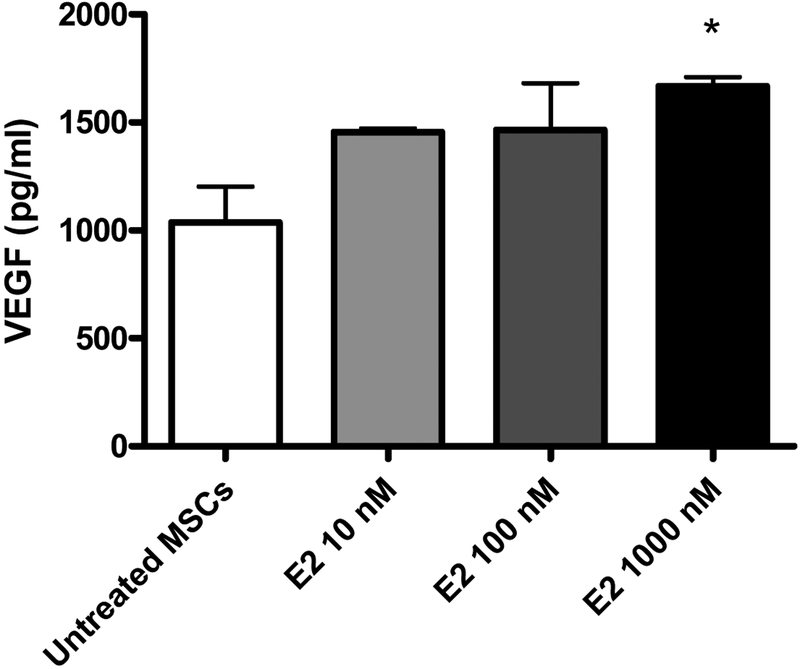
E2 exposure at 1000 nM, but not 10 nM or 100 nM, resulted in significantly increased production of VEGF. 1000 nM E2 treated MSCs provoked significantly more (p<.05) VEGF production (pg/mL) than untreated MSCs (1667 ± 44.4 vs. 1037 ± 165), as shown in Figure 1.
FIG. 1.
Expression of VEGF in untreated male MSCs and MSCs exposed to increasing doses of 17β Estradiol (E2). *p<0.05 vs. untreated MSC; results are expressed as pg/mL, mean ± SEM.
Myocardial function
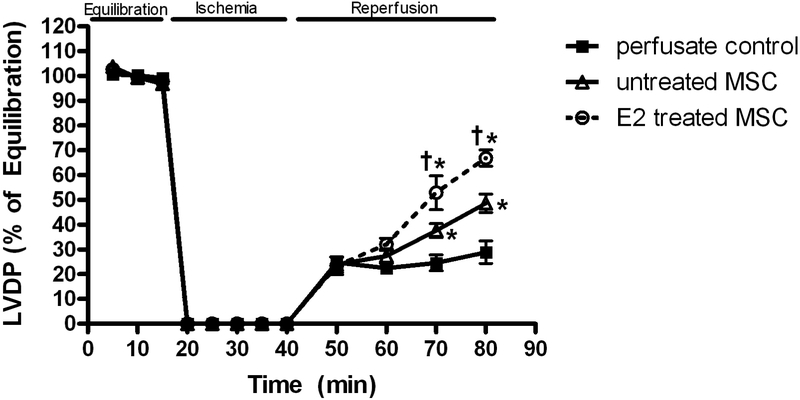
Ischemia / reperfusion resulted in markedly decreased left ventricular developed pressure (LVDP) in all groups (Fig 2). Postischemic recovery of LVDP (expressed as percentage of preischemic function) was significantly greater (p < .001) in the E2 treated group (66.9 ± 3.3%) than the untreated MSC group (48.7 ± 3.7%) and vehicle (28.9 ± 4.6%) at end reperfusion (Fig 2).
FIG. 2.
Changes in left ventricular developed pressure (LVDP % of equilibration) during I/R. E2 treated MSC group compared to untreated MSC group and control. Results are mean ± SEM, *p<0.001 vs control, †p<0.01 vs untreated MSCs.
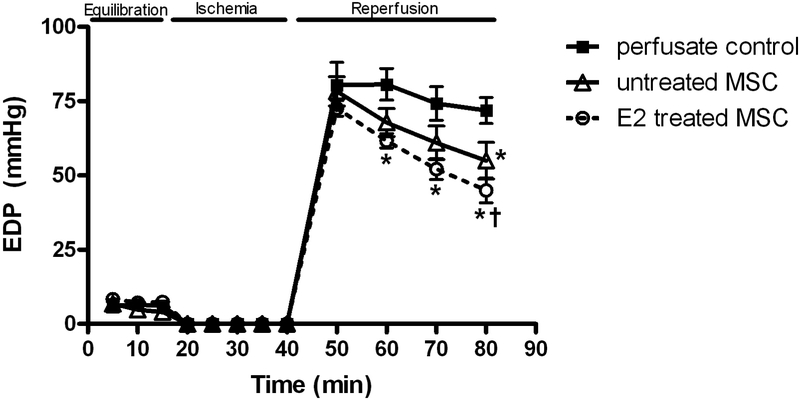
Left ventricular end diastolic pressure (mmHg) was elevated in response to I/R in all groups (Figure 3). However, isolated hearts in the E2 treated group (45.0 ± 4.2mmHg) had significantly decreased EDP after forty minutes of reperfusion compared to those who received untreated MSCs (55.0 ± 6.1mmHg, p<.05) or vehicle (71.9 ± 4.4mmHg, p < .001) as shown in Fig 3.
FIG. 3.
Changes in left ventricular end diastolic pressure (EDP) during I/R. E2 treated MSC group compared to untreated MSC group and control. Results are mean ± SEM, *p<0.01 vs control, †p<0.05 vs untreated MSCs.
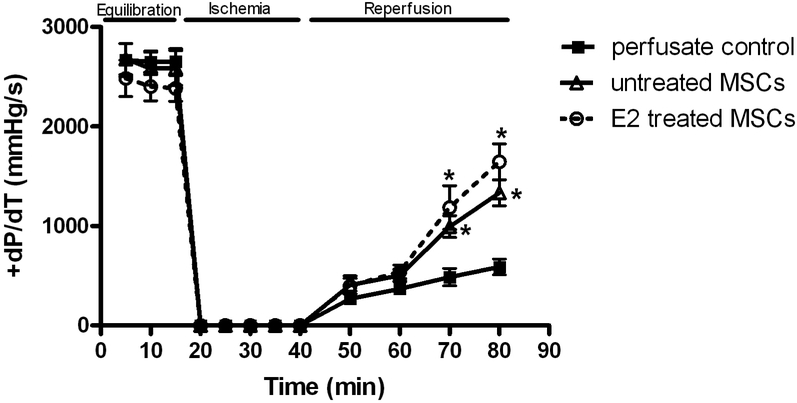
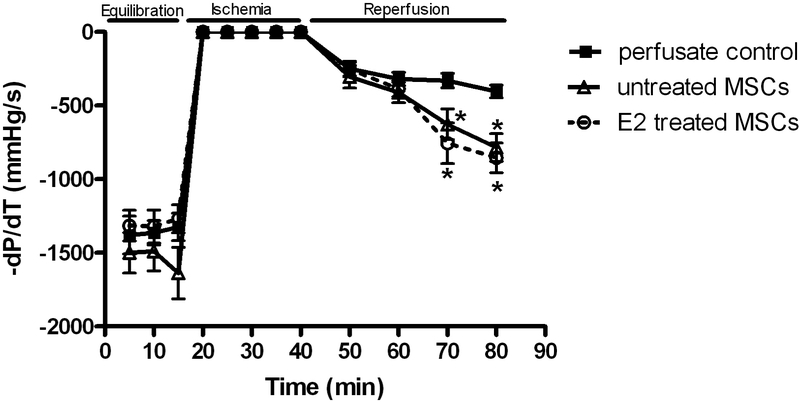
Postischemic recovery of the maximal positive and negative values of the first derivative of pressure (+dP/dt and −dP/dt) declined in response to I/R. Interestingly, recovery of +dP/dt and −dP/dt was very similar with E2 treated MSC (1645.4±180.9mmHg/s and −854.9 ± 100.2mmHg/s, respectively, Fig. 4 and 5) and untreated MSC (1334.9 ± 132.3mmHg/s and −784.4 ± 94.0mmHg/s, respectively). Both E2 treated MSC and untreated MSC groups showed better recovery than vehicle (589.7 ± 79.8mmHg/s and −403.6 ± 43.5mmHg/s, respectively, Fig. 4 and 5) at end reperfusion.
FIG. 4.
Changes in the maximal positive values of the first derivative of pressure (+dP/dt). E2 treated MSC group and untreated MSC group compared to control. Results are mean ± SEM, *p<0.01 vs control.
FIG. 5.
Changes in the maximal negative values of the first derivative of pressure (-dP/dt). E2 treated MSC group and untreated MSC group compared to control. Results are mean ± SEM, *p<0.01 vs control.
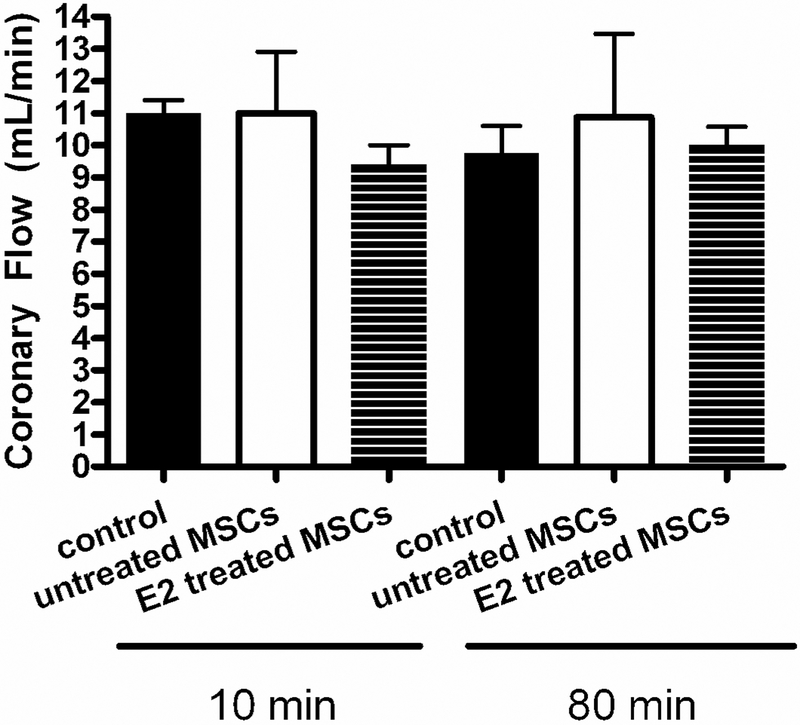
No significant differences in coronary flow were observed between the control (11.0 ± 0.4 to 9.8 ± 0.9ml/min) and the untreated MSC (11.0 ± 1.9 to 11.8 ± 4.2ml/min) and E2 treated MSC groups (9.5 ± 0.5 to 10.0 ± 0.6ml/min, Fig. 6).
FIG. 6.
Coronary flow at 10 minutes (during equilibration and before ischemia) and 80 minutes (at the end of reperfusion). E2 treated MSC group and untreated MSC group compared to control.
DISCUSSION
The results of this study indicate that acute exposure of MSCs to 24h of 17β-estradiol increases MSC VEGF production. Furthermore, isolated hearts infused with these E2 treated MSCs via transcoronary delivery prior to ischemia demonstrate: 1) greater post-ischemic end reperfusion LVDP; and 2) decreased end reperfusion EDP compared to untreated MSCs and vehicle. This study is the first demonstration that suggests that estrogen may have a positive influence on mesenchymal stem cell VEGF production as well as mesenchymal stem cell induced myocardial protection during I/R injury.
These findings give further evidence that untreated MSCs are cardioprotective (20–22). We found that untreated MSCs significantly increased left ventricular developed pressure, contractility (+dP/dt) and rate of relaxation (-dP/dt) at the end of reperfusion. Additionally, left ventricular end diastolic pressure was significantly decreased at end reperfusion compared to control, indicating perhaps a reduction in the unstressed volume of the ventricle, possibly due to myocardial contracture or edema. Previous studies by our lab (9, 11) have also demonstrated that untreated MSCs are myocardial protective. Wang et al (11) demonstrated that pretreatment with human MSCs improved functional recovery, decreased myocardial proinflammatory cytokine production, and decreased myocardial pro-apoptotic caspases following I/R.
Paracrine effects may help explain why stem cell delivery into injured tissue confers cardioprotection in an acute setting (< 24 hrs), without evidence of differentiation. Macrophages and cardiomyocytes release proinflammatory cytokines following injury (1). In response to the inflammatory environment, stem cells may release growth factors such as VEGF (9), hepatocyte growth factor (HGF) (23), and insulin-like growth factor 1 (IGF-1) (23) that interact with neighboring cardiomyocytes. These growth factors may promote angiogenesis (24, 25), decrease proinflammatory cytokine production (26, 27), and reduce apoptosis (28, 29).
Recently, some researchers have focused on the modification of stem cells in order to maximize therapeutic benefit. Transduction of MSCs to overexpress VEGF has been studied. Matsumoto et al infused MSCs transduced with VEGF into hearts following coronary ligation and found decreased infarct size and improved functional recovery (30). Similarly, Wang et al determined that over-expression of VEGF by bone marrow mesenchymal stem cells improved recovery after experimental coronary LAD ligation (31). Enhancing the survival of transplanted stem cells may also improve stem cell efficacy. Li et al infused MSCs that overexpressed an anti-apoptotic Bcl-2 gene into hearts and reported decreased apoptosis and increased functional recovery following myocardial infarction compared to normal MSCs (32). Similar to Bcl-2, Uemura and colleagues found that MSC proliferation and survival may depend on Akt, a prosurvival gene (8). Accordingly, transduction of Akt1 into MSCs followed by an infusion of these Akt1 overexpressing stem cells conferred increased functional recovery and decreased inflammation and apoptosis (33). Further study of these Akt1 overexpressing MSCs revealed that the cardioprotective effects observed may be from paracrine actions (34, 35). The modification of stem cells to increase potency and cell survival may lead to improved outcomes.
Estrogen has been widely implicated in the increased myocardial protection found in females via direct interactions with the heart. Interestingly, the findings of this study suggest that estrogen may also have other indirect cardioprotective effects via enhancement of MSC paracrine pathways. When E2 binds with estrogen receptor alpha, it may cause MSCs to release growth factors. Indeed, in this study, E2 treated MSCs showed an increased production of VEGF in cell culture as compared to untreated MSCs (Fig. 1). Further, estrogen may inhibit part of the inflammatory cascade, which produces proinflammatory cytokines and increases apoptosis. Xu et al showed that exogenous estrogen mediates protection from I/R injury by decreasing TNF production (36). Estrogen may be a novel adjunct to improve MSC paracrine mediated protection. This study demonstrates that isolated hearts infused with E2 treated MSCs infused prior to ischemia exhibit greater recovery of myocardial function (LVDP and EDP) than untreated MSCs. Therefore, ex vivo modification of MSCs prior to therapeutic implementation may confer increased benefit.
This study suggests that estrogen enhances a stem cell’s ability to improve myocardial functional recovery following I/R injury. Future studies are necessary to understand the gender differences associated with estrogen treated stem cells as well as to elucidate the mechanism by which E2 mediates this enhancement.
Acknowledgments
This work was supported in part by NIH R01GM070628, NIH R01HL085595, NIH K99/R00 HL0876077, NIH F32HL085982, AHA Grant-in-aid, and AHA Post-doctoral Fellowship 0725663Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Meldrum DR Tumor necrosis factor in the heart. Am J Physiol 274: R577–595, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Crisostomo PR, Wang M, Wairiuko GM, Morrell ED, Terrell AM, Seshadri P, Nam UH, and Meldrum DR High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock 26: 575–580, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura K, Jeremy RW, Schaper J, and Becker LC Progression of myocardial necrosis during reperfusion of ischemic myocardium. Circulation 97: 795–804, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Nagy RD, Tsai BM, Wang M, Markel TA, Brown JW, and Meldrum DR Stem cell transplantation as a therapeutic approach to organ failure. J Surg Res 129: 152–160, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, and Anversa P Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, and Mavilio F Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279: 1528–1530, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, and Field LJ Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428: 664–668, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Uemura R, Xu M, Ahmad N, and Ashraf M Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res 98: 1414–1421, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Crisostomo PR, Wang M, Markel TA, Lahm T, Abarbanell AM, Herrmann JL, and Meldrum DR Stem cell mechanisms and paracrine effects: potential in cardiac surgery. Shock 28: 375–383, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, and Meldrum DR Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: Role of the 55 kDa TNF receptor (TNFR1). J Mol Cell Cardiol 42: 142–149, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Tsai BM, Crisostomo PR, and Meldrum DR Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock 25: 454–459, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hayward CS, Kelly RP, and Collins P The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res 46: 28–49, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, and Meldrum DR In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery 142: 215–221, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Crisostomo PR, Wang M, Herring CM, Morrell ED, Seshadri P, Meldrum KK, and Meldrum DR Sex dimorphisms in activated mesenchymal stem cell function. Shock 26: 571–574, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Hiasa K, Egashira K, Kitamoto S, Ishibashi M, Inoue S, Ni W, Zhao Q, Nagata S, Katoh M, Sata M, and Takeshita A Bone marrow mononuclear cell therapy limits myocardial infarct size through vascular endothelial growth factor. Basic Res Cardiol 99: 165–172, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn ME, and Karas RH The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801–1811, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Yu HP, Shimizu T, Choudhry MA, Hsieh YC, Suzuki T, Bland KI, and Chaudry IH Mechanism of cardioprotection following trauma-hemorrhagic shock by a selective estrogen receptor-beta agonist: up-regulation of cardiac heat shock factor-1 and heat shock proteins. J Mol Cell Cardiol 40: 185–194, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Schneider CP, Schwacha MG, and Chaudry IH Impact of sex and age on bone marrow immune responses in a murine model of trauma-hemorrhage. J Appl Physiol 102: 113–121, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, and Prockop DJ Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103: 1662–1668, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Haider H, Royta U, and Ashraf M Role of pharmacologically mobilized endogenous bone marrow stem cells for cardiac repair. J Heart Lung Transplant 24: 1996–1997, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, Wang Y, Wani MA, Xu M, Ayub A, and Ashraf M Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol 35: 1113–1119, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Thompson RB, van den Bos EJ, Davis BH, Morimoto Y, Craig D, Sutton BS, Glower DD, and Taylor DA Intracardiac transplantation of a mixed population of bone marrow cells improves both regional systolic contractility and diastolic relaxation. J Heart Lung Transplant 24: 205–214, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Crisostomo PR, Herring C, Meldrum KK, and Meldrum DR Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 291: R880–884, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Klinkner DB, Densmore JC, Kaul S, Noll L, Lim HJ, Weihrauch D, Pritchard KA Jr., Oldham KT, and Sander TL Endothelium-derived microparticles inhibit human cardiac valve endothelial cell function. Shock 25: 575–580, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Pickkers P, Sprong T, Eijk L, Hoeven H, Smits P, and Deuren M Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock 24: 508–512, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Yang S, Hu S, Hsieh YC, Choudhry MA, Rue LW 3rd, Bland KI, and Chaudry IH Mechanism of IL-6-mediated cardiac dysfunction following trauma-hemorrhage. J Mol Cell Cardiol 40: 570–579, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Zheng R, Hu S, Ma Y, Choudhry MA, Messina JL, Rue LW 3rd, Bland KI, and Chaudry IH Mechanism of cardiac depression after trauma-hemorrhage: increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am J Physiol Heart Circ Physiol 287: H2183–2191, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Ayala A, Herdon CD, Lehman DL, Ayala CA, and Chaudry IH Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood 87: 4261–4275, 1996. [PubMed] [Google Scholar]
- 29.Ayala A, Xin Xu Y, Ayala CA, Sonefeld DE, Karr SM, Evans TA, and Chaudry IH Increased mucosal B-lymphocyte apoptosis during polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated process. Blood 91: 1362–1372, 1998. [PubMed] [Google Scholar]
- 30.Matsumoto R, Omura T, Yoshiyama M, Hayashi T, Inamoto S, Koh KR, Ohta K, Izumi Y, Nakamura Y, Akioka K, Kitaura Y, Takeuchi K, and Yoshikawa J Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol 25: 1168–1173, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, and Ashraf M Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol 40: 736–745, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, and Steinhoff G Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 25: 2118–2127, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, and Dzau VJ Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9: 1195–1201, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, and Dzau VJ Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11: 367–368, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, and Dzau VJ Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J 20: 661–669, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Arenas IA, Armstrong SJ, Plahta WC, Xu H, and Davidge ST Estrogen improves cardiac recovery after ischemia/reperfusion by decreasing tumor necrosis factor-alpha. Cardiovasc Res 69: 836–844, 2006. [DOI] [PubMed] [Google Scholar]