Abstract
Objective:
The optimal treatment of nonconvulsive seizures in critically ill patients is uncertain. We evaluated the comparative effectiveness of the antiseizure drugs lacosamide (LCM) and fosphenytoin (fPHT) in this population.
Methods:
The TRENdS (Treatment of Recurrent Electrographic Nonconvulsive Seizures) study was a noninferiority, prospective, multicenter, randomized treatment trial of patients diagnosed with nonconvulsive seizures (NCSs) by continuous electroen-cephalography (cEEG). Treatment was randomized to intravenous (IV) LCM 400mg or IV fPHT 20mg phenytoin equivalents/kg. The primary endpoint was absence of electrographic seizures for 24 hours as determined by 1 blinded EEG reviewer. The frequency with which NCS control was achieved in each arm was compared, and the 90% confidence interval (CI) was determined. Noninferiority of LCM to fPHT was to be concluded if the lower bound of the CI for relative risk was >0.8.
Results:
Seventy-four subjects were enrolled (37 LCM, 37 fPHT) between August 21, 2012 and December 20, 2013. The mean age was 63.6 years; 38 were women. Seizures were controlled in 19 of 30 (63.3%) subjects in the LCM arm and 16 of 32 (50%) subjects in the fPHT arm. LCM was noninferior to fPHT (p = 0.02), with a risk ratio of 1.27 (90% CI = 0.88–1.83). Treatment emergent adverse events (TEAEs) were similar in both arms, occurring in 9 of 35 (25.7%) LCM and 9 of 37 (24.3%) fPHT subjects (p = 1.0).
Interpretation:
LCM was noninferior to fPHT in controlling NCS, and TEAEs were comparable. LCM can be considered an alternative to fPHT in the treatment of NCSs detected on cEEG.
Seizures are common in critically ill patients admitted to intensive care units (ICUs). Up to 90% of these are nonconvulsive seizures (NCSs) that cannot be recognized without electroencephalography (EEG).1 Between 12 and 22% of patients undergoing continuous EEG monitoring (cEEG) have been found to have frequent NCSs or nonconvulsive status epilepticus (NCSE).2–5 NCSs are seen more frequently in patients admitted to neurological ICUs with coma, acute brain injury, and prior convulsive seizures, but are also found in patients admitted to medical ICUs.4,6–8
NCSs and NCSE are associated with worse short-and long-term outcomes in critically ill patients, including mortality, cognition, and development of epilepsy.9,10 Unlike convulsive seizures, there is no uniformly accepted treatment protocol for NCSs.11,12 With limited data, the expert consensus is that for intermittent focal NCSs, multiple trials of nonsedating antiseizure drugs (ASDs) are preferred before resorting to more aggressive treatment with anesthetic medications.13
Although ASDs are used to treat NCS and NCSE, prospective, randomized studies comparing the effectiveness of ASDs are lacking. Data from treatment trials of generalized convulsive status epilepticus (GCSE) are often extrapolated and used to guide treatment of NCS and NCSE. One of the most commonly used drugs for treating GCSE, phenytoin (PHT), or its prodrug, fosphenytoin (fPHT), is often used for NCS and NCSE. There are no studies evaluating the effectiveness of PHT or fPHT for NCSs. Lacosamide (LCM) is a newer ASD with an intravenous (IV) formulation. Case reports and a few open label studies suggest it is effective in controlling NCSs and NCSE in critically ill patients, with a potentially more favorable adverse event profile and fewer drug-drug interactions than PHT.14,15
The Treatment of Recurrent Electrographic Nonconvulsive Seizures (TRENdS) study was designed to evaluate whether IV LCM is noninferior to IV fPHT in controlling NCSs, using cessation of electrographic NCSs on cEEG as the primary endpoint. fPHT was chosen as it is considered the gold standard ASD in many countries for treating status epilepticus (SE) of all types; LCM was chosen as it is a well-tolerated, commonly used antiseizure medication available in an IV formulation and with preliminary evidence of efficacy for acute seizures and SE in animals and humans.14 Levetiracetam was not considered an option, because prestudy review showed that the majority of patients potentially qualifying for this study would already have been treated with this medication.
Patients and Methods
Study Design
TRENdS was a noninferiority, prospective, multicenter, randomized clinical trial with nonblinded caregivers and blinded EEG reviewers in which IV fPHT was compared to IV LCM in its ability to stop NCSs, with crossover to the other study drug if the first was ineffective. The study was designed by investigators from the Critical Care EEG Monitoring Research Consortium (CCEMRC). It was funded through an investigator-initiated study grant from UCB Pharmaceuticals. The investigators independently wrote the protocol, analyzed the data, and are publishing the results. UCB Pharmaceuticals was not involved in designing or conducting the trial or in data analysis. This study was performed under an Investigational New Drug application approved by the U.S. Food and Drug Administration (FDA), as the dosing for LCM used in TRENdS is not currently approved by the FDA.
Study sites were recruited from member centers of the CCEMRC and other centers performing high volumes of cEEG. Twelve centers participated in the trial. The Duke Clinical Research Institute was used as the clinical research organization. The study was approved by the Duke University Institutional Review Board (IRB) and the local IRB of each study site (ClinicalTrials.gov identifier: NCT01458522). Written informed consent was obtained from legally authorized representatives of subjects, because subjects lacked decision-making capacity due to their neurologic condition. To prevent delay of treatment, consenting prior to meeting full enrollment eligibility was permitted if investigators deemed them to be potential candidates for enrollment conditional on subsequent cEEG findings.
Participants
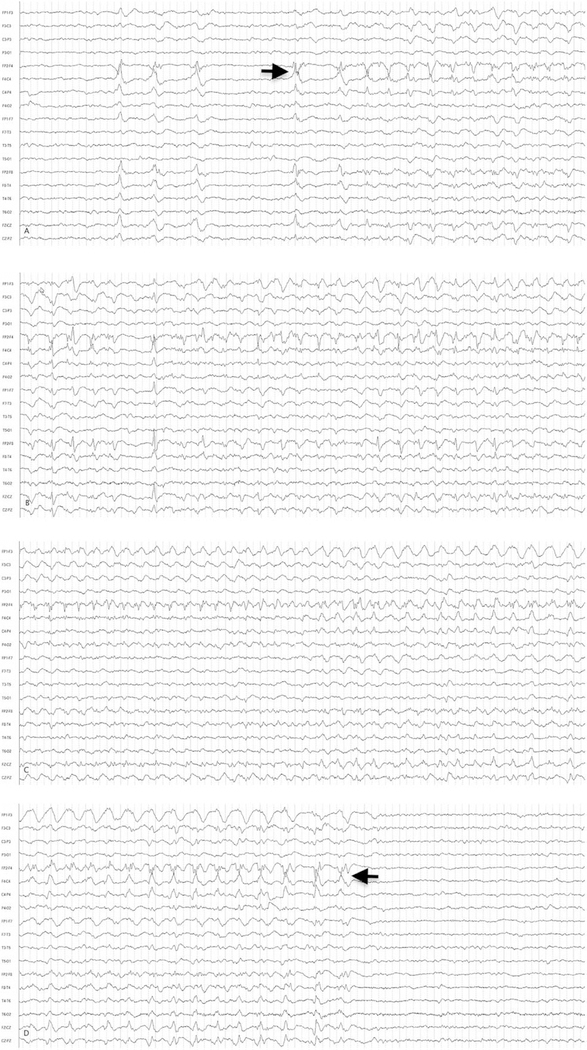
Inclusion criteria were: age 18 years or older, undergoing cEEG for detection of NCSs, at least 1 electrographic seizure with or without clinical correlate lasting at least 10 seconds and <30 minutes, seizures frequent enough to warrant intravenous ASD as part of the patient’s clinical care, and a seizure within 6 hours of randomization and initiation of the study drug. An example of an electrographic seizure from this trial is shown in Figure 1. Subjects administered other ASDs prior to randomization were eligible if the qualifying NCS occurred at least 2 hours after starting that ASD.
FIGURE 1:
Sample electrographic seizure. Electroencephalographic (EEG) example of a representative electrographic noncon-vulsive seizure from a 69-year-old male. An anterior-posterior bipolar montage is presented. (A) EEG shows sharp waves in the right hemisphere and seizure onset (arrow). (B) Seizure continuation. (C) Continuation of the seizure with maximum rhythmicity over the right anterior quadrant. (D) The termination (arrow) of the seizure. This seizure lasted about 50 seconds. Low-frequency filter = 1Hz; high-frequency filter = 70Hz; 60Hz filter is off.
Exclusion criteria were: already being treated with fPHT, PHT, or LCM; known hypersensitivity or contraindication to either drug; anoxic encephalopathy; undergoing therapeutic hypothermia; generalized convulsive seizures during cEEG; and electrographic SE with seizures lasting >30 minutes per hour of cEEG.
Randomization
Once subjects were enrolled in the trial, they were randomized in a 1:1 ratio to initial treatment with fPHT or LCM. The IXRS Interactive Voice and Web Response System was used to generate the randomization sequence. Randomization was stratified based on presence of known risk factors for NCS, including history of epilepsy (present or absent), Glasgow Coma Scale (<9 or ≥9), and age (<60 years or ≥60 years).
Procedures
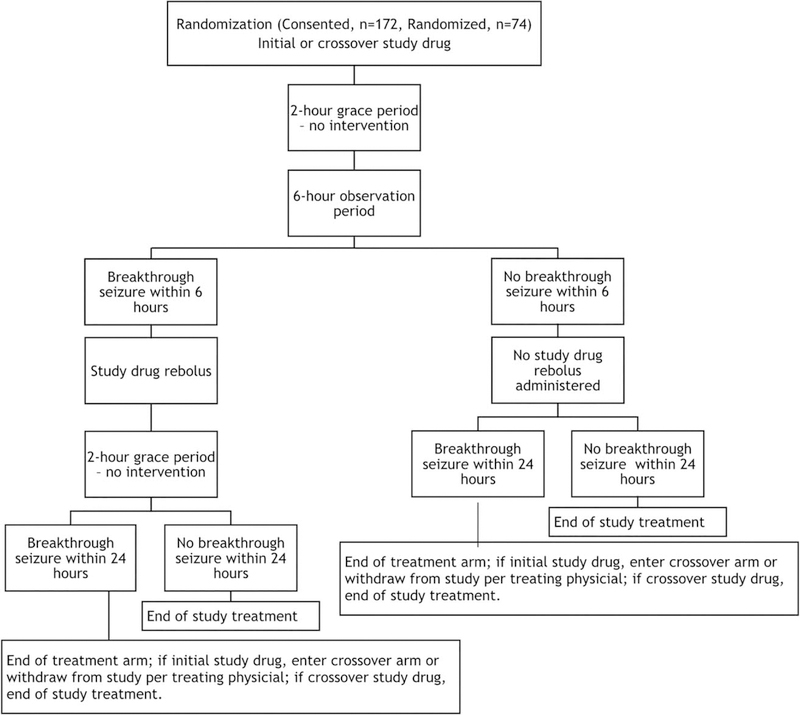
Subjects were randomized to initial treatment with fPHT or LCM. Subjects in the fPHT arm received an IV fPHT bolus of 20mg phenytoin equivalents (PE)/kg, whereas those in the LCM arm received an IV LCM bolus of 400mg, both over 30 minutes. A 2-hour grace period was allowed before efficacy assessments (control of seizures) were made. This allowed for completion of the study drug infusion and penetration into the central nervous system. After this 2-hour grace period, subjects were observed for a further 6-hour period for breakthrough seizures. If seizures were noted during this period, a rebolus of IV fPHT 5mg PE/kg was administered to subjects in the fPHT group, and a rebolus of IV LCM 200mg was administered to subjects in the LCM group. After this rebolus, another 2-hour grace period was allowed, and subjects were then observed for 24 hours for further seizures.
If after the initial bolus the subject did not have a seizure during the initial 6-hour observation period (and 2-hour grace period), he/she was observed for another 18 hours (total 24+2 hours) for breakthrough seizures. If seizures occurred during this 24-hour period, the subject entered the crossover arm, where the second drug was administered using the same protocol.
The initial study drug was continued at maintenance doses regardless of the need to rebolus or cross over. The first maintenance dose was administered 12 hours after the initial bolus for all patients. If no further seizures occurred for 24 hours, the subject successfully exited the study. This information is presented in graphic form in Figure 2.
FIGURE 2:
Graphic representation of the Treatment of Recurrent Electrographic Nonconvulsive Seizures trial treatment paradigm.
EEG
All subjects were undergoing cEEG at the time of enrollment. The definitions for convulsive seizures, NCSs, NCSE, and other EEG patterns commonly seen in critically ill patients were established based on published criteria and agreed upon by the investigators prior to study commencement.16 These definitions are presented in the Supplementary Table. Investigators were instructed to enroll only patients with unequivocal electrographic seizures.
To minimize variability of EEG interpretation, a training manual was created that identified various EEG patterns and how they should be interpreted for the purpose of this trial. All electroencephalographers responsible for interpreting study EEGs, including principal investigators, subinvestigators, and central EEG readers, were required to review this manual and pass a competency test.
Interpretation of cEEG was performed in real time by the site electroencephalographer (principal or subinvestigator), who was blinded to the identity of the study drug(s). Decisions regarding rebolusing and crossover to second study drug were based on this interpretation. The treating physician, who was not blinded to the identity of the study drug, was permitted to stop the study drug if it was considered to be in the subject’s best interest. For the study’s primary outcome measurement, the cEEG was later transferred to a central database, from which 2 blinded central electroencephalographers independently marked all seizure onset and offset times.
Outcomes
The primary outcome measure was lack of electrographic seizure recurrence for 24 hours after the first study drug administration (or rebolus, if needed) and after the 2-hour grace period, based on cEEG as interpreted by the blinded central electroencephalographers. This outcome was met if at least 1 of the electroencephalographers observed no seizures during this period.
Secondary outcome measures included the percentage of subjects who required a rebolus in the first treatment arm, the second study drug, and a rebolus in the second treatment arm. The change in seizure burden (time spent in electrographic seizures per hour of EEG recording) before treatment versus at the end of the first treatment arm, and before treatment versus at the end of the second treatment arm, was also determined.
Safety assessments were performed and compared between the treatment arms. Adverse events of special interest included cardiac arrhythmias, hypotension, and multiorgan hypersensitivity reactions.
Statistical Analysis
Three analysis populations were defined: (1) the intention to treat (ITT) population consisted of all randomized subjects, (2) modified ITT (mITT) population consisted of randomized subjects who received study drug and had at least 67% (16 hours) of cEEG available during the 24-hour follow-up period, and (3) the safety population consisted of all subjects who were randomized and received study drug. A sample size of 200 with a 10% dropout rate was determined based on a plausible enrollment target, and power was evaluated under different scenarios for response rates in both arms. Because efficacy data of fPHT and other ASDs in NCSs is not available, sample size determination was made based on a consensus of the investigators. Ultimately, 74 subjects were enrolled due to withdrawal of funding.
Primary analysis was performed in the mITT population and analyzed according to the randomized initial treatment arm. The objective of the primary efficacy analysis was to determine whether the response rate (percentage of subjects with no recurrence of seizures) in the LCM arm was noninferior to the fPHT arm. The noninferiority margin was set to 0.8. Because there were no available historic data regarding efficacy of fPHT in this patient population, the noninferiority margin was based on consensus of the investigators. We prespecified that noninferiority of LCM would be concluded if the lower bound of the 90% confidence interval (CI) for relative risk of no recurrence in LCM versus the fPHT arm was determined to be >0.8, corresponding to a 1-sided significance level of 0.05. If noninferiority was found, a test for superiority of LCM versus fPHT would be performed using a 1-sided significance level of 0.05. A generalized linear model using a binomial distribution with a log link was used to derive the relative risk and 90% CI. The primary analysis included only treatment in the model.
Secondary efficacy analyses were performed in the ITT population using 2-sided tests at a 5% significance level. The percentage of subjects in each arm requiring rebolus and crossover treatment were compared using Fisher exact test with a mid-p correction. Percentage change in seizure burden from baseline to the end of the initial treatment arm was analyzed using Poisson regression. Supporting analyses used the Wil-coxon signed-rank test to compare absolute and percentage change in seizure burden from baseline to the end of the initial treatment arm.
Safety endpoints were analyzed in the safety population. The number and percentage of subjects with treatment emergent adverse events (TEAEs) occurring within 24 hours after administration of the initial and crossover drug (if given) were reported according to the initial and crossover treatments, using MedDRA Preferred Term and System Organ Class.
All analyses were performed using SAS v9.2 (SAS Institute, Cary, NC).
Results
Enrollment and Subjects
The TRENdS trial enrolled from June 5, 2012 to April 1, 2014. The study was terminated because of with-drawal of funding. Because subjects often gave consented before all inclusion criteria were met, 172 subjects gave consent and 74 were enrolled in the trial. The ITT population included 37 subjects in each arm. The mITT population included 62 subjects, 30 in the LCM arm and 32 in the fPHT arm, whereas the safety population included 72 subjects, 35 in the LCM arm and 37 in the fPHT arm. The demographics and neurologic comorbidities of the subjects are presented in Table 1. The LCM and fPHT arms were comparable in terms of age, gender, race, medical and neurologic history, history of epilepsy, and use of ASDs. Details of the ASDs used are in Table 2. The time since last seizure before randomization in the mITT population was also similar ]between the two arms, with a median of 0.35 (interquartile range [IQR = 0.09–1.23) hours in the LCM arm and 0.48 (IQR = 0.18–1.15) hours in the fPHT arm (p = 0.65).
TABLE 1.
Demographics and History, Intention-to-Treat Population
| Characteristic | LCM, n = 37 | fPHT, n = 37 | Total, n = 74 | |
|---|---|---|---|---|
| Age, yr | ||||
| Mean, SD | 63.8 (12.1) | 63.4 (20.4) | 63.6 (16.6) | |
| ≥60 years | 25 (67.6%) | 23 (62.2%) | 48 (64.9%) | |
| Gender, F | 17 (45.9%) | 21 (56.8%) | 38 (51.4%) | |
| Race | ||||
| White | 26 (70.3%) | 32 (86.5%) | 58 (78.4%) | |
| Black or African American | 9 (24.3%) | 3 (8.1%) | 12 (16.2%) | |
| Asian | 2 (5.4%) | 2 (5.4%) | 4 (5.4%) | |
| Neurologic history, concurrent conditionsa | 26 (70.3%) | 20 (54.1%) | 46 (62.2%) | |
| Subdural hemorrhage | 6 (16.2%) | 5 (13.5%) | 11 (14.9%) | |
| Brain tumor, intra-axial | 3 (8.1%) | 3 (8.1%) | 6 (8.1%) | |
| Hemorrhagic stroke, intraparenchymal | 3 (8.1%) | 2 (5.4%) | 5 (6.8%) | |
| Subarachnoid hemorrhage | 2 (5.4%) | 3 (8.1%) | 5 (6.8%) | |
| Toxic/metabolic encephalopathy | 2 (5.4%) | 1 (2.7%) | 3 (4.1%) | |
| Other | 3 (8.1%) | 6 (16.2%) | 9 (12.2%) | |
| History of status epilepticus | 3 (8.6%) | 0 | 3 (4.2%) | |
| History of provoked seizures | 2 (5.4%) | 1 (2.7%) | 3 (4.1%) | |
| History of epilepsy | 11 (29.7%) | 12 (32.4%) | 23 (31.1%) | |
| Focal onset | 6 (54.5%) | 8 (66.7%) | 14 (60.9%) | |
| Generalized onset | 1 (9.1%) | 2 (16.7%) | 3 (13.0%) | |
| Unknown | 4 (36.4%) | 4 (33.3%) | 8 (34.8%) | |
| Witnessed seizure activity prior to subject’s current illness | 7 (63.6%) | 6 (50.0%) | 13 (56.5%) |
Subjects may have had one or more of these conditions
F = female; fPHT = fosphenytoin; LCM = lacosamide; SD = standard deviation
TABLE 2.
ASDs Used prior to Enrollment
| LCM, n = 37 | fPHT, n = 37 | Total, n = 74 | |
|---|---|---|---|
| Patients who received at least 1 prior ASD, n (%) | 26 (70.3%) | 28 (75.7%) | 54 (73.0%) |
| Levetiracetam | |||
| n | 26 | 27 | 53 |
| Median mg/day (IQR) | 1,500 (1,000–2,000) | 2,000 (2,000–3,000) | 2,000 (1,500–2,000) |
| Lorazepam | |||
| n | 4 | 8 | 12 |
| Median mg/day (IQR) | 1.5 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Phenobarbital | |||
| n | 2 | 0 | 2 |
| Median mg/day (IQR) | 97.2 (64.8–129.6) | 97.2 (64.8–129.6) | |
| Valproic acid | |||
| n | 2 | 1 | 3 |
| Median mg/day (IQR) | 875 (750–1,000) | 2,000 (2,000–2,000) | 1,000 (750–2,000) |
| Carbamazepine | |||
| n | 1 | 0 | 1 |
| Median mg/day (IQR) | 400 (400–400) | 400 (400–400) | |
| Lamotrigine | |||
| n | 1 | 0 | 1 |
| Median mg/day (IQR) | 600 (600–600) | 600 (600–600) | |
| Midazolam | |||
| n | 0 | 1 | 1 |
| Median mg/day (IQR) | 6.0 (6.0–6.0) | 6.0 (6.0–6.0) | |
| Oxcarbazepine | |||
| n | 1 | 1 | 1 |
| Median mg/day (IQR) | 600 (600–600) | 600 (600–600) | 600 (600–600) |
| Pregabalin | |||
| n | 0 | 1 | 1 |
| Median mg/day (IQR) | 300 (300–300) | 300 (300–300) |
ASD = antiseizure drug; fPHT = fosphenytoin; IQR = interquartile range; LCM = lacosamide.
Primary Outcome
Seizures ceased after initial treatment (no seizure recurrence for 24 hours, with or without rebolus) in 19 of 30 (63.3%) subjects in the LCM arm and in 16 of 32 (50%) subjects in the fPHT arm. The risk ratio was 1.27 (90% CI = 0.88–1.83); significance for noninferiority of LCM to fPHT was reached (p = 0.02), whereas significance for superiority of LCM over fPHT was not (p = 0.29). Thus, LCM was noninferior to fPHT.
Secondary Outcomes
In the LCM arm, 16 of 35 (45.7%) subjects received a rebolus, versus 13 of 37 (35.1%) subjects in the fPHT arm (p = 0.41). The crossover study drug was administered in 15 of 35 (42.9%) subjects in the LCM arm and 10 of 37 (27.0%) subjects in the fPHT arm (p = 0.18). Among subjects who received the crossover treatment, in the LCM arm, fPHT was rebolused in 4 of 15 (26.7%) subjects, whereas in the fPHT arm, 3 of 10 (30%) of subjects received a rebolus of LCM.
The initial seizure burdens for the LCM and fPHT groups were statistically indistinguishable (median = 2.00, IQR = 0.35–7.91 vs median = 1.97, IQR = 0.40–4.48min/h, respectively). After treatment with the initial drug, the seizure burden decreased by 98% (IQR = 68–100%, p = 0.001) in the LCM arm and by 76% (48–100%, p < 0.001) in the fPHT arm. However, the difference between the absolute percentage reductions (22%) in LCM arm versus the fPHT arm did not reach significance (p = 0.129). After crossover treatment, the median seizure burden was reduced compared to baseline by 99% (IQR = 79–100%) in the LCM group (ie, after receiving fPHT) and by 100% (IQR = 80–100%) in the fPHT group (ie, after receiving LCM). A summary of the outcome measures is presented in Table 3.
TABLE 3.
Summary of Primary and Secondary Endpoints
| Outcome | LCM, n = 37 | fPHT, n = 37 | p |
|---|---|---|---|
| Primary outcome | |||
| No seizure recurrence, with or without rebolus | 19/30 (63.3%)a | 16/32 (50%) | 0.02b,c |
| Secondary outcome | |||
| Initial treatment arm: subjects receiving rebolus | 16/35 (45.7%)d | 13/37 (35.1%) | 0.41 |
| Crossover treatment arm: drug administered | 15/35 (42.9%)d | 10/37 (27.0%) | 0.18 |
| Crossover treatment arm: subjects receiving rebolus | 4/15 (26.7%) | 3/10 (30%) | |
| Seizure burden reduction compared to baseline after initial study drug | 98% (68–100%) | 76% (48–100%) | <0.01b,e |
| Seizure burden reduction compared to baseline after crossover study drug | 99% (79–100%) | 100% (80–100%) |
Modified intention-to-treat population.
Statistically significant.
Probability value for noninferiority; superiority of LCM over fPHT was not significant (p = 0.29).
LCM not administered in 2 subjects.
Comparison to pretreatment baseline, not to each other. Comparison with each other was not significant (p = 0.129).
fPHT = fosphenytoin; LCM = lacosamide.
Safety Measures
At least 1 TEAE occurred up to 24 hours after the last dose of the first study drug in 9 of 35 subjects (25.7%) in the LCM group and in 9 of 37 (24.3%) in the fPHT group (p = 1.0). Serious adverse events (SAEs) occurred in 5 of 35 subjects (14.3%) in the LCM group and 4 of 37 (10.8%) in the fPHT group (p = 0.73). TEAEs of special interest (cardiac arrhythmias, hypotension, respiratory failure, and multiorgan hypersensitivity reactions) also occurred with comparable frequency in the two treatment groups: 4 of 35 subjects (11.4%) in the LCM group and 5 of 37 subjects (13.5%) in the fPHT group (p = 1.0). A summary of the safety measures is presented in Table 4. The frequency of SAEs grouped according to system organ class in the LCM and fPHT groups is presented in Table 5. No significant differences were found in the TEAE analyses.
TABLE 4.
Summary of AEs up to 24 Hours after Last Dose of Study Drug, Safety Population
| LCM, n = 35 | fPHT, n = 37 | Total, = 72 | p | |
|---|---|---|---|---|
| Patients with at least 1 TEAE after initial study drug | 9 (25.7%) | 9 (24.3%) | 18 (25.0%) | 1.0 |
| Patients with at least 1 TEAE after crossover study drug: LCM group, n = 15; fPHT group, n = 10 | 5 (33.3%) | 1 (10.0%) | 6 (24.0%) | |
| Patients with at least 1 SAE after initial study drug | 5 (14.3%) | 4 (10.8%) | 9 (12.5%) | 0.73 |
| Patients with at least 1 SAE after crossover study drug: LCM group, n = 15; fPHT group, n = 10 | 3 (20.0%) | 1 (10.0%) | 4 (16.0%) | |
| Patients with TEAE of special interest after initial study druga | 4 (11.4%) | 5 (13.5%) | 9(12.5%) | 1.0 |
| Patients with TEAE of special interest after crossover study drug: LCM group, n = 15; fPHT group, n = 10a | 5 (33.3%) | 2 (20.0%) | 7 (28.0%) | |
| Patients who discontinued treatment with initial study drug due to AE | 2 (5.7%) | 3 (8.1%) | 5 (6.9%) | 1.0 |
| Patients who discontinued treatment with crossover study drug due to AE: LCM group, n = 15; fPHT group, n = 10 | 3 (20.0%) | 0 | 3 (12.0%) |
TEAEs of special interest are: cardiac arrhythmias, hypotension, respiratory failure, and multiorgan hypersensitivity reactions.
AE = adverse event; fPHT = fosphenytoin; LCM = lacosamide; SAE = serious AE; TEAE = treatment emergent AE.
TABLE 5.
Serious Adverse Events up to 24 Hours after Last Dose of Study Drug, Safety Population
| System Organ Class/ | Initial Treatment Arm |
Crossover Treatment Arm |
|||
|---|---|---|---|---|---|
| Preferred Term | LCM, n = 35 | fPHT, n = 37 | p | LCM, n = 15 | fPHT, n = 10 |
| Patients with at least 1 SAE | 5 (14.3%) | 4 (10.8%) | 0.73 | 3 (20.0%) | 1 (10.0%) |
| Cardiac disorders | 1 (2.9%) | 1 (2.7%) | 1.0 | 0 | 0 |
| Bradycardia | 1 (2.9%) | 0 | 0.49 | 0 | 0 |
| Atrial tachycardia | 0 | 1 (2.7%) | 1.0 | 0 | 0 |
| Injury, poisoning, procedural complications | 2 (5.7%) | 1 (2.7%) | 0.61 | 1 (6.7%) | 0 |
| Subdural hematoma | 2 (5.7%) | 0 | 0.23 | 0 | 0 |
| Toxicity to various agents | 0 | 1 (2.7%) | 1.0 | 1 (6.7%) | 0 |
| Nervous system disorders | 0 | 1 (2.7%) | 1.0 | 1 (6.7%) | 1 (10.0%) |
| Cerebral hemorrhage | 0 | 1 (2.7%) | 1.0 | 0 | 1 (10.0%) |
| Intracranial pressure increased | 0 | 0 | 1.0 | 1 (6.7%) | 0 |
| Vascular disorders | 2 (5.7%) | 1 (2.7%) | 0.61 | 1 (6.7%) | 0 |
| Hypotension | 2 (5.7%) | 1 (2.7%) | 0.61 | 1 (6.7%) | 0 |
| Respiratory, thoracic, and mediastinal disorders | 1 (2.9%) | 0 | 0.49 | 0 | 0 |
| Acute respiratory failure | 1 (2.9%) | 0 | 0.49 | 0 | 0 |
| Respiratory failure | 0 | 0 | 1.0 | 0 | 0 |
fPHT = fosphenytoin; LCM = lacosamide; SAE =serious adverse event.
Discussion
This prospective, randomized, crossover, clinical trial found that IV LCM was noninferior to IV fPHT in the treatment of NCS in critically ill subjects. There were no statistically significant differences in adverse event profiles, including hypotension and bradycardia.
TRENdS is the first prospective, randomized trial to compare the effectiveness of ASDs in the treatment of NCSs. It is also the first interventional clinical trial to use cEEG findings as the primary endpoint. Whereas the initial target enrollment based on sample size assessments was 200 subjects, 74 were ultimately enrolled due to withdrawal of funding. Despite this, the study was successful in reaching its primary outcome measure of non-inferiority with statistical significance.
ASDs, particularly PHT and fPHT, have been used for patients with NCSs because practitioners are familiar with these agents, and they have demonstrated utility in GCSE.17 Valproic acid and levetiracetam, both available as IV ASDs, have been compared to each other and to PHT in SE with variable results.18–25 Many of these trials were randomized, but most were not blinded. None has focused on patients with NCSs, and none has included cEEG as a marker for sustained, successful treatment of seizures.
Uncontrolled observational studies and 1 randomized trial suggest the effectiveness of LCM in controlling SE is between 33 and 88%.26–32 A review of all studies reporting effectiveness of LCM in treating refractory SE found that it was successful in 56% of patients14. These studies reported effectiveness of LCM in SE, not NCSs. The present study only evaluated patients with NCSs, a different population than what has been reported previously. The effectiveness of LCM for controlling NCSs in the TRENdS study was 63.3%. Additionally, this study showed that fPHT was successful in controlling NCSs in 50% of patients. These data can be used as benchmarks for future ASD treatment trials for acute seizures.
The concept of seizure burden, the minutes of electrographic seizure activity per hour of EEG recording, was explored in this trial. Ictal discharges lasting ≥10 seconds and <30 minutes were counted as seizures and contributed to the seizure burden (see Supplementary Table).16 A high seizure burden (>12 minutes per hour) has been shown to be associated with neurologic decline in children.9 In this study, the seizure burden was about 2min/h before treatment. Although this may seem low, it was calculated based on 6 hours of EEG data obtained prior to randomization. Only 7 subjects had a high seizure burden of >12 minutes at baseline, and 2 continued to have such a high seizure burden after treatment with both drugs. As expected, there was significant decrease in the seizure burden after treatment with either LCM or fPHT; the difference between the two arms was not statistically significant. The subjects with a high seizure burden responded in a manner comparable to those with a lower seizure burden, although due to the small number of subjects in this category, a definite conclusion cannot be drawn. Often NCSs in critically ill patients do not end abruptly after administration of an ASD, but instead gradually decrease in duration and frequency. Consequently, measuring the seizure burden may be a possible way to gauge improvement.
Whereas the initial bolus (“loading”) dose of fPHT of 20mg PE/kg is well established, the optimal LCM dose is unknown. In this study, LCM 400mg was used, as there were safety data for this dose administered as a bolus in non-critically ill epilepsy patients.33 A rebolus of LCM 200mg and fPHT 5mg PE/kg was used if breakthrough seizures were observed. Whether a higher initial bolus dose of either drug would be more effective remains unanswered.
TEAEs and SAEs occurred at comparable rates in both arms after initial study drug treatment. The rates of adverse events of special interest (cardiac arrhythmias, hypotension, respiratory failure, and multiorgan hypersensitivity reactions) were also similar. No unusual or unexpected adverse events were noted. Other retrospective and open label studies have noted similar adverse events occurring at a comparable frequency.14, 26 However, some retrospective studies have noted fewer adverse events with LCM.27–29 This discrepancy may be due to the nature of retrospective and open label studies. Nevertheless, assessment of adverse events in critically ill subjects is challenging due to multiple comorbidities and interventions, and the inability to assess subjective symptoms due to impaired mental status.
There were some challenges in performing this study that slowed enrollment.34 Coordination between the electroencephalographers and the treating providers (usually neurocritical care physicians) was important; when this was not present, enrollment slowed. Availability of the study coordinator after usual business hours was necessary, as that is when many of the subjects were diagnosed with NCSs. Sites with around-the-clock coordinator availability enrolled more efficiently. Education of providers in the emergency department, neurology wards, and critical care units was essential for identification of appropriate subjects. Prior to this education, potentially eligible patients were treated with ASDs without considering study enrollment.
One of the limitations of this study is that the treating physician was not blinded to treatment. Because this was the first prospective study of its type, it was considered important to allow the treating physician to be aware of the treatment in a critically ill subject, particularly as the ASDs had potentially differing side effect profiles. The electroencephalographers interpreting the cEEG at the site and centrally, however, were blinded to treatment.
Subjects with many different etiologies for NCSs were included. Patients with NCSs due to various etiologies may respond differently to ASDs or preferentially to a particular drug. This study was designed to mirror clinical practice, so multiple etiologies were intentionally allowed. The only exception was anoxic encephalopathy, due to its markedly different seizure types and poor prognosis. More-over, there is no convincing evidence that any ASD is more effective in controlling seizures from a specific etiology.
There was no placebo arm in this study; thus, it remains possible that neither drug was beneficial and that seizures improved spontaneously or for other reasons. Standard clinical practice, however, is to treat seizures with ASDs, and a placebo-controlled trial in this population would not be possible. Finally, this study does not address the issue of whether NCSs are injurious to the brain or related to prognosis, or whether successful treatment improves outcome.
Clinical manifestations of NCSs and how they evolved with treatment with study drugs were not evaluated in this study. Additionally, more severe forms of seizures, such as NCSE, were not enrolled, but rather were specifically excluded. Whether LCM or fPHT would be equally effective in patients with NCSE was not addressed in this study.
This trial was a prospective randomized study comparing 2 ASDs in critically ill patients having frequent electrographic seizures, using cEEG to assess adequacy of treatment, with central, blinded electroencephalographers identifying seizures. It establishes that performing rigorous treatment studies in critically ill patients having frequent seizures is possible, and that cEEG can be used as a primary endpoint for assessing treatment effectiveness. The data obtained here establish a blueprint by which future studies can be conducted in similar patient populations.
In summary, this study demonstrates that LCM is noninferior to fPHT in controlling nonconvulsive seizures in critically ill subjects. Adverse events occurred with comparable frequency for both drugs. Thus, LCM is a reasonable treatment alternative in critically ill subjects having frequent seizures, regardless of the etiology.
Supplementary Material
Acknowledgment
This study was funded by UCB through an Investigator Initiated Study grant.
We thank the following study personnel: Rebekka Arias, Project Manager, and staff of the Duke Clinical Research Institute; and the site study coordinators: Samantha Donovan (Massachusetts General Hospital), Nichelle Llewellyn (Brigham and Women’s Hospital), Brian Mace (Duke University Hospital), Jennifer Bonito (Yale University School of Medicine), Ashley Gnatt (Medical University of South Carolina), Jan Cameron-Watts (University of Texas Southwestern Medical Center Dallas), Sadie Seto (Huntington Hospital), Leslie Shell (Mission Hospital), Melanee Newman (Emory University School of Medicine), and Elizabeth Bachman (Beth Israel Deaconess Medical Center). We also thank the members of the Data Safety Monitoring Board: Peter Kaplan, Chair (Baltimore, MD), Christopher Granger (Durham, NC), Panayiotis Varelas (Detroit, MI), Eugen Trinka (Salzburg, Austria), and Carl Pieper (Durham, NC).
Footnotes
Potential Conflicts of Interest
Support from UCB, the company that manufactures LCM, was provided to B.J.K. and Y.L. for salary support, A.M.H. and S.R.S. for consulting, and A.M.H. and S.T.H. for investigator-initiated study grants. The remaining authors report no potential conflicts.
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Claassen J, Mayer SA, Kowalski RG, et al. Detection of electro-graphic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–1748. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez V, Rodriguez Ruiz AA, LaRoche S, et al. The use and yield of continuous EEG in critically ill patients: a comparative study of three centers. Clin Neurophysiol 2017;128:570–578. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez Ruiz A, Vlachy J, Lee JW, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol 2017;74:181–188. [DOI] [PubMed] [Google Scholar]
- 4.Sinha SR, Hirsch LJ. Continuous EEG monitoring in the ICU In: Ebersole JS, Husain AM, Nordli DR, eds. Current practice of clinical electroencephalography, 4th ed Philadelphia, PA: Wolters Kluwer, 2014:543–598. [Google Scholar]
- 5.Swisher CB, Shah D, Sinha SR, Husain AM. Baseline EEG pattern on continuous ICU EEG monitoring and incidence of seizures. J Clin Neurophysiol 2015;32:147–151. [DOI] [PubMed] [Google Scholar]
- 6.Gilmore EJ, Gaspard N, Choi HA, et al. Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med 2015;41:686–694. [DOI] [PubMed] [Google Scholar]
- 7.Kamel H, Betjemann JP, Navi BB, et al. Diagnostic yield of electroencephalography in the medical and surgical intensive care unit. Neurocrit Care 2013;19:336–341. [DOI] [PubMed] [Google Scholar]
- 8.Oddo M, Carrera E, Claassen J, et al. Continuous electroencephalography in the medical intensive care unit. Crit Care Med 2009; 37:2051–2056. [DOI] [PubMed] [Google Scholar]
- 9.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology 2014;82:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan PW. Assessing the outcomes in patients with nonconvulsive status epilepticus: nonconvulsive status epilepticus is underdiagnosed, potentially overtreated, and confounded by comorbidity. J Clin Neurophysiol 1999;16:341–352; discussion 353. [DOI] [PubMed] [Google Scholar]
- 12.Wasim M, Husain AM. Nonconvulsive seizure control in the intensive care unit. Curr Treat Options Neurol 2015;17:340. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch LJ. Finding the lesser of two evils: treating refractory status epilepticus. Epilepsy Curr 2015;15:313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofler J, Trinka E. Lacosamide as a new treatment option in status epilepticus. Epilepsia 2013;54:393–404. [DOI] [PubMed] [Google Scholar]
- 15.Kellinghaus C, Berning S, Besselmann M. Intravenous lacosamide as successful treatment for nonconvulsive status epilepticus after failure of first-line therapy. Epilepsy Behav 2009;14:429–31. [DOI] [PubMed] [Google Scholar]
- 16.Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol 2005;22:79–91. [DOI] [PubMed] [Google Scholar]
- 17.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 1998;339:792–798. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal P, Kumar N, Chandra R, et al. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure 2007;16:527–532. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez V, Januel JM, Burnand B, Rossetti AO. Second-line status epilepticus treatment: comparison of phenytoin, valproate, and levetiracetam. Epilepsia 2011;52:1292–1296. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarthi S, Goyal MK, Modi M, et al. Levetiracetam versus phenytoin in management of status epilepticus. J Clin Neurosci 2015;22:959–963. [DOI] [PubMed] [Google Scholar]
- 21.Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J Neurol 2012;259:645–648. [DOI] [PubMed] [Google Scholar]
- 22.Misra UK, Kalita J, Patel R. Sodium valproate vs phenytoin in status epilepticus: a pilot study. Neurology 2006;67:340–342. [DOI] [PubMed] [Google Scholar]
- 23.Mundlamuri RC, Sinha S, Subbakrishna DK, et al. Management of generalised convulsive status epilepticus (SE): a prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam—pilot study. Epilepsy Res 2015;114:52–58. [DOI] [PubMed] [Google Scholar]
- 24.Navarro V, Dagron C, Elie C, et al. Prehospital treatment with levetiracetam plus clonazepam or placebo plus clonazepam in status epilepticus (SAMUKeppra): a randomised, double-blind, phase 3 trial. Lancet Neurol 2016;15:47–55. [DOI] [PubMed] [Google Scholar]
- 25.Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: a meta-analysis of published studies. Seizure 2014;23:167–174. [DOI] [PubMed] [Google Scholar]
- 26.Cherry S, Judd L, Muniz JC, et al. Safety and efficacy of lacosa-mide in the intensive care unit. Neurocrit Care 2012;16:294–298. [DOI] [PubMed] [Google Scholar]
- 27.Hofler J, Unterberger I, Dobesberger J, et al. Intravenous lacosa-mide in status epilepticus and seizure clusters. Epilepsia 2011;52: e148–e152. [DOI] [PubMed] [Google Scholar]
- 28.Kellinghaus C, Berning S, Stogbauer F. Intravenous lacosamide or phenytoin for treatment of refractory status epilepticus. Acta Neurol Scand 2014;129:294–299. [DOI] [PubMed] [Google Scholar]
- 29.Miro J, Toledo M, Santamarina E, et al. Efficacy of intravenous lacosamide as an add-on treatment in refractory status epilepticus: a multicentric prospective study. Seizure 2013;22:77–79. [DOI] [PubMed] [Google Scholar]
- 30.Misra UK, Dubey D, Kalita J. A randomized controlled trial of lacosamide versus sodium valproate in status epilepticus. Epilepsia 2017;58:919–923. [DOI] [PubMed] [Google Scholar]
- 31.Santamarina E, Toledo M, Sueiras M, et al. Usefulness of intravenous lacosamide in status epilepticus. J Neurol 2013;260:3122–3128. [DOI] [PubMed] [Google Scholar]
- 32.Sutter R, Marsch S, Ruegg S. Safety and efficacy of intravenous lacosamide for adjunctive treatment of refractory status epilepticus: a comparative cohort study. CNS Drugs 2013;27:321–329. [DOI] [PubMed] [Google Scholar]
- 33.Fountain NB, Krauss G, Isojarvi J, et al. Safety and tolerability of adjunctive lacosamide intravenous loading dose in lacosamidenaive patients with partial-onset seizures. Epilepsia 2013;54:58–65. [DOI] [PubMed] [Google Scholar]
- 34.Husain AM. Lacosamide in status epilepticus: update on the TRENdS study. Epilepsy Behav 2015;49:337–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.