Abstract
Oligodendrocyte precursor cells (OPCs) are one of the major cell types in cerebral white matter, which are generated from neural progenitor cells (NPCs) and give rise to mature oligodendrocytes. Although past studies have extensively examined how OPCs are generated from NPCs and how OPCs differentiate into mature oligodendrocytes, the underlying mechanisms remain unelucidated. In particular, the roles of DNA methylation and the related enzymes DNA methyltransferases (DNMTs) in oligodendrocyte lineage cells are still mostly unknown, although DNA methylation plays a critical role in cell fate decision in multiple cell types. Recently, OPCs were proposed as a promising source of cell-based therapy for patients with oligodendrocyte/myelin damage. Therefore, understanding the mechanisms underlying the involvement of DNMTs in OPCs would help to develop an approach for the efficient preparation of OPCs for cell-based therapy. As a part of the special issue for "Stem Cell Therapy" in Brain Research, this mini-review article first overviews the potential for clinical application of OPCs for cell-based therapy, and then summarizes the key findings of DNMT roles in OPCs, focusing on OPC generation and differentiation.
Keywords: oligodendrocyte precursor cell, cell-based therapy, DNA methylation, DNA methyltransferase
1. Introduction:
Oligodendrocyte precursor cells (OPCs) are a sub-type of glial cells that give rise to mature oligodendrocytes. OPCs are active in the developing central nervous system (CNS) and are primarily generated in germinal zones during development (Spassky et al., 2001). OPCs are generated from multipotential neural progenitor cells (NPCs), and so far, several waves for OPC generation have been identified in the developing mouse forebrain. The initial wave of OPC production begins in the medial ganglionic eminence at about embryonic day (E) 12.5 in mice. By E18 in mice, these ventrally-derived OPCs migrate and populate most of the embryonic telencephalon, including the cerebral cortex. At about E15.5, a second wave of OPC generation proceeds from the lateral and caudal ganglionic eminences and joins the OPCs from the first wave. Finally, around the time of birth, a third wave of OPC generation commences from the cortex. OPCs generated in the first wave disappear during postnatal life, and the majority of adult oligodendrocytes originates from the OPCs generated in the last two waves (Kessaris et al., 2006). Additionally, it was recently reported that in the postnatal period, OPCs are generated from the subventricular zone (SVZ) and are distributed into the neocortex, constituting a small component of the overall OPC population (Hill and Nishiyama, 2014). This indicates the specificity of age- and region-dependent differences in the OPC cell cycle, and therefore, OPCs may possess heterogeneity in brain development.
The major function of OPCs is to generate mature oligodendrocytes, which comprise the key source of myelin production. Because mature oligodendrocytes do not proliferate, OPCs play a critical role in increasing the number of oligodendrocytes during development. In addition, some of the population of OPCs remains in an undifferentiated state in the adult brain; these residual OPCs contribute to both physiological myelin sheath renewal and compensatory oligodendrogenesis after myelin damage in adult brain. Originally, based on immuno-reactivity to anti-A2B5 antibody, morphologically and physiologically distinct cells were purified as bipotential oligodendrocyte-type2 astrocyte progenitor cells (O-2A cells) (Raff et al., 1983), which were then called OPCs, since the cells produced oligodendrocytes. However, it should be noted that OPCs are not restricted to generating oligodendrocytes, as OPCs can produce astrocytes and neurons under some conditions (Kondo and Raff, 2000). Furthermore, O-2A cells express NG2 chondroitin sulfate proteoglycan (CSPG4) (Nishiyama et al., 1999), and therefore, the O-2A cells are now also referred as NG2 cells or polydendrocytes (Nishiyama et al., 2009).
Because OPCs work as multi-potent neuronal stem cells, they are a potential source of cell-based therapy for neurological diseases. In fact, there have already been several trials in pre-clinical studies to test the efficacy of OPCs as a source of cell-based therapy, though their efficacy has not been yet proven in clinic. One potential issue may be due to the lack of knowledge in the mechanisms of OPC generation and differentiation into oligodendrocytes. Several splendid works have identified numerous extrinsic/intrinsic factors for the underlying mechanisms, but how OPCs are generated from NPCs and how OPCs differentiate into mature oligodendrocytes remain to be elucidated. In particular, the mechanisms of DNA methyltransferases (DNMTs) are mostly unknown in those processes, although DNA methylation by DNMTs is heavily involved in cell proliferation and cell fate decision. Therefore, as a part of the special issue for "Stem Cell Therapy" in Brain Research, in this mini-review, we will first briefly discuss the therapeutic potential of OPCs as a source for cell transplantation, and then introduce key findings about DNMT roles in OPC generation and differentiation.
2. OPC transplantation in rodent demyelinated diseases:
In 1999, Brustle et al reported that mouse ESC-derived A2B5+ glial precursors (e.g. OPC-like cells) interacted with host neurons and efficiently myelinated axons in the brain and spinal cord after transplantation into myelin-deficient rats (Brustle et al., 1999), which was the first proposal of the potential of OPCs as a source of cell-based therapy. Thereafter, several studies showed that primary OPCs were effective after transplantation to rodent spinal cord injury (SCI) models (Bambakidis and Miller, 2004; Cao et al., 2005; Cao et al., 2010; Sun et al., 2014; Wu et al., 2012). These studies used primary OPC cultures prepared from neonatal rodent brains. While primary rodent OPC cultures may not be optimal for clinical application, these findings laid the groundwork for OPC therapy for demyelinating diseases. Transplanted OPCs may have at least three benefits for damaged/injured white matter: (i) promoting remyelination, (ii) filling the core lesion or cavity, and (iii) enhancing endogenous self-repairing mechanisms by the systematic regulation of the immune response. The first benefit seems to be the primary mechanism for the approach of cell-based therapy with OPCs, but some studies suggest that remyelination may not always be necessary for the survival of demyelinated axons (Loers et al., 2004; Smith et al., 2013). Thus, the other two benefits may also require further investigation to maximize the therapeutic potential of OPCs for clinical application. Although the precise mechanism as to how transplanted OPCs support damaged white matter is still understudied, several pre-clinical studies using rodent demyelinated diseases have demonstrated the efficacy of transplanted OPCs. In this section, therefore, we provide an overview of the potential for clinical application of OPCs for cell-based therapy by introducing the efficacy of OPC therapy in pre-clinical studies.
SCI is a white matter trauma and has been used to test the efficacy of OPCs as a source for cell-based therapy. Oligodendrocyte/myelin damage appears within hours and lasts for weeks after SCI, due to ischemic stress, free radical production, and/or phagocytosis by immunoreactive cells. Keirstead et al showed that transplanted OPCs, which were derived from human ESCs (hESCs), enhanced remyelination and promoted improvement locomotor ability at early time points after thoracic SCI (Keirstead et al., 2005). Sharp et al also used hESC-derived OPCs and demonstrated the efficacy of transplanted OPCs in a rat model of SCI. The study showed that the transplanted OPCs survived, differentiated into oligodendrocytes, and preserved motor neurons at the injury site, which was correlated with the improvement of motor function of the forelimb (Sharp et al., 2010). Thereafter, All et al and Priest et al also confirmed the efficacy of hESC-derived OPCs in SCI model rodents (All et al., 2012; Priest et al., 2015). These studies suggest that injectable OPCs can be prepared from human ESCs and that the transplanted ESC-derived OPCs may functionally contribute to remyelinating motor neurons in the SCI site and other additional protective effects.
Transplanted OPCs may also be effective for radiation-induced demyelination. X-irradiation therapy has been applied to many primary and metastatic cancers in intracranial lesions, though it may cause cognitive impairment. Demyelination is one side effect of X-irradiation therapy, because the main target of radiation is the large pool of mitotically active cells, including OPCs (Chari et al., 2006; Panagiotakos et al., 2007). Piao et al reported that radiation decreased the number of OPCs along with the expression of MBP, but when ESC-derived OPCs were grafted onto the brain tissue, they migrated throughout the white matter and re-myelinated the irradiated brain, restoring some cognitive function (Piao et al., 2015). The study also demonstrated that the transplantation of OPCs into the cerebellum improved motor function (Piao et al., 2015); however, it should be noted that it is still under debate whether the improvement of cognitive and locomotive function by OPC transplantation is dependent on remyelination. Mouse-derived neural progenitor cells transplanted into irradiated mice increased axon survival by restoring remyelinating capacity, and there were no signs of axonal degeneration in the chronic stage of genetically mutated MBP mice (Smith et al., 2013). Therefore, the transplanted OPCs may contribute to repairing the demyelinated region through mechanisms other than myelin sheath production. In addition, we may also need to pay attention to the balance between endogenous OPCs and exogenous (e.g. transplanted) OPCs when we consider the efficacy of transplanted OPCs under radiation-induced demyelinating conditions. Even after radiation exposure, endogenous OPCs may still be present in the injured white matter region, although they may have decreased ability to differentiate and remyelinate the demyelinated axons. These remaining aberrant OPCs may disturb re-myelination by transplanted OPCs. The establishment of exogenous OPCs would require the presence of inflammation to ensure that the endogenous aberrant OPCs are completely eliminated (Blakemore and Irvine, 2008; Chari et al., 2006).
Besides SCI-induced or radiation-induced demyelination, cell-based OPC therapy would also be effective for other demyelinating diseases. Xu et al used a rat model of traumatic brain injury to prove the efficacy of transplanted OPCs. The study transplanted ESC-derived OPCs into the deep sensorimotor cortex and confirmed that the injected OPCs migrated massively along the white matter tract and differentiated into ensheathing oligodendrocytes (Xu et al., 2015). Also in rodent models of metabolic disorders of myelin, OPC transplantation was proven effective for damaged white matter (Chen et al., 2015; Kuai et al., 2015). In these studies, transplanted OPCs promoted BDNF signaling, which could enhance the endogenous self-repairing system by supporting the proliferation of NSC. Because engrafted NSCs would also be effective for some white matter-related diseases, future studies are warranted to pursue the possibility of the combined transplantation of NSCs and OPCs to maximize the efficacy of OPC-based cell therapy.
3. Source for OPCs in cell-based therapy:
Table 1 summarizes cell sources of injectable OPCs for cell-based therapy (Table 1). The primary source for preparation of OPCs in cell-based therapy is ESCs. As introduced in the previous section, ESC-derived OPCs were confirmed to be effective for multiple demyelinating diseases in pre-clinical studies. Another source for OPC preparation is induced pluripotent stem cells (iPSCs), which have an advantage in autologous engraftment of disease-relevant cells by directed differentiation using defined factors. Czepiel et al successfully differentiated iPSCs into functional oligodendrocytes through the embryonic bodies floating culture. They first obtained NSCs from iPSCs using FGF2 and EGF and then differentiated the iPSC-derived NSCs into oligodendrocyte lineage cells using PDGF-AA, T3 and NT3. These cells formed myelin around the axons of co-cultured DRG neurons in vitro and in the demyelinated corpus callosum of cuprizone-fed mice without teratoma formation in vivo (Czepiel et al., 2011). Wang et al also prepared OPCs from iPSCs by first forming EBs from iPSCs and then using FGF2 to induce the differentiation into the neuroepithelial stage. After this, they used RA, B27, and purmophamine to obtain pre-OPCs. Finally, Wang et al differentiated the iPSC-derived pre-OPCs into OPCs using T3, NT3, IGF, PDGF-AA and purmophamine. After further purifying the iPSC-derived OPCs by FACS sorting with A2B5, CD140a/PDGFRα, and CD9 antibodies, Wang et al transplanted these cells into homozygous shiverer rag2-null mice, and they showed that the mice with OPC transplantation lived almost two times longer than control mice, along with densely myelinated corpus callosums with no evidence of tumorigenesis (Wang et al., 2013). In the study, Wang et al also prepared tissue-derived CD140a-sorted OPCs based on the methods by Sim et al (Sim et al., 2011), and they confirmed that the myelination efficiency of implanted iPSCs-derived OPCs were as high as that of the tissue-derived OPCs (Wang et al., 2013). More recently, All et al also confirmed the efficacy of iPSC-derived OPCs in a rat SCI model (All et al., 2015), and Kawabata et al showed that iPS-derived OPC-enriched neural stem/progenitor cells successfully differentiated into mature oligodendrocytes after transplantation in a mouse SCI model (Kawabata et al., 2016).
Table 1:
Cell Sources of injectable OPCs to rodent disease models
| Source | Animal model | Reference |
|---|---|---|
| Human Embryonic Stem Cell | Spinal cord injury (rat) | Keirstead et al. 2005; |
| Spinal cord injury (rat) | Sharp et al. 2010; | |
| Spinal cord injury (rat) | All et al. 2012; | |
| Spinal cord injury (rat) | Priest et al. 2015; | |
| Radiation-induced demyelination (rat) | Piao et al. 2015; | |
| Traumatic brain injury (rat) | Xu et al. 2015; | |
| Mouse Embryonic Stem Cell | Spinal cord injury (rat) | Brustle et al. 1999; |
| Periventricular leukomalacia (rat) | Chen et al. 2015; | |
| Globoid cell leukodystrophy (mouse) | Kuai et al. 2015; | |
| Human iPSC | Congenital hypomyelination (mouse) | Wang et al. 2013; |
| Spinal cord injury (rat) | All et al. 2015; | |
| Spinal cord injury (mouse) | Kawabata et al. 2016; | |
| Mouse iPSC | Cuprizone-treated MS model (mouse) | Czepiel et al. 2011; |
| Rat BMSC | LPC-induced demyelination (rat) | Nazm Bojnordi et al. 2014; |
| Mouse Fibroblast | Spinal cord injury (mouse) | Kim et al. 2015; |
| Extracted human OPC | lysolecithin-induced demyelination (rat) | Windrem et al. 2002; |
| Demyelination (shiverer mouse) | Windrem et al. 2004; | |
| Primary OPCs | ||
| from neonatal rat spinal cords | Spinal cord injury (rat) | Bambakidis and Miller 2004; |
| from embryonic rat spinal cords | Spinal cord injury (rat) | Cao et al. 2005; |
| from adult rat spinal cords | Spinal cord injury (rat) | Cao et al. 2010; |
Both ESCs and iPSCs are promising sources for OPC preparation in cell-based therapy. However, one challenging aspect of using ESC- or iPSC-derived OPCs in clinical application is the lengthy culture period for differentiation from ESCs/iPSCs into OPCs (Tabar and Studer, 2014), which prevents patients from receiving immediate cell-based therapy. Previous reports showed that almost 3 months are required to obtain functional OPCs from ESCs and almost 4 months for the process of iPSCs-to-OPCs. Furthermore, an additional 3 months is needed for transplanted OPCs to mature into oligodendrocytes before they can produce dense and compact myelin. One potential solution for this issue is the usage of dual SMAD and Wnt/β-catenin inhibitors, because these inhibitors can rapidly induce neuronal lineage cells from stem cells, which is therefore expected to shorten the differentiation period into oligodendrocyte lineage cells (Chambers et al., 2012). Another potential solution to avoid the lengthy culture period is to use other cells, such as bone marrow stromal cells (BMSCs) and fibroblast cells. Nazm Bojnordi et al transplanted BMSC-derived OPCs into the lysophosphatidylcholine (LPC)-injected demyelinated rodent brain, which successfully mitigated demyelination and augmented remyelination (Nazm Bojnordi et al., 2014). In addition, OPCs can also be generated from fibroblasts by direct reprogramming through ectopic expression of defined transcriptional factors (Kim et al., 2015; Yang et al., 2013). The ectopic expression of transcriptional factor Oct4 in fibroblasts generates self-renewing and bipotent OPCs (iOPCs), which can be a powerful and promising source for OPC cell-based therapy. Interestingly, this iOPC is available about one month after reprograming and can proliferate through over 30 self-renewals. Therefore, this method would bypass the undifferentiated stem cell state and limit the tumorigenic potential after transplantation (Fong et al., 2010). More importantly, transplanted iOPCs were confirmed to enhance the recovery of locomotion in a rodent spinal cord injury model (Kim et al., 2015). Usage of extracted OPCs from fetal or adult brain may be another strategy for preparing OPCs in cell-based therapy, as the extracted OPCs were reported to be effective when xenografted to congenitally or chemically demyelinated mice (Windrem et al., 2002; Windrem et al., 2004). However, this approach would be still challenging for clinical application because the post-mitotic state of extracted OPCs may impede their large-scale expansion. Ultimately, a deeper understanding of the mechanisms that regulate OPC generation and proliferation would help to develop an effective method for preparing injectable OPCs for cell-based therapy.
4. Roles of DNA methyltransferases in OPC generation and differentiation:
As stated in previous sections, several pre-clinical studies have demonstrated that cell-based therapy with OPCs may have the beneficial cellular effects on the injured region, through (i) promoting remyelination, (ii) replacing heterogeneous OL/astrocytes in the cavity, and (iii) supporting endogenous NSC function. However, the clinical efficacy of cell-based therapy with OPCs has not been proven yet, and further investigation into the mechanisms of OPC generation and differentiation is required to elucidate the clinical benefits of OPC therapy for patients with demyelinating disorders. Thus far, several intrinsic/extrinsic signaling molecules have been identified as regulators of OPC generation from NSCs and OPC differentiation into oligodendrocytes (Domingues et al., 2017; Takebayashi and Ikenaka, 2015; Zhang et al., 2016b). However, though the epigenetic system is known to participate in cell fate decisions and DNA chromatin homeostasis has been implied to participate in the regulation of oligodendrocyte lineage cells (Huang et al., 2015), mechanisms as to how these epigenetic regulators contribute to OPC differentiation are still mostly unknown. Nonetheless, recent studies have revealed the critical roles of epigenetic regulators on OPC generation and differentiation. Therefore, in this section, we introduce the key findings of epigenetic regulation in oligodendrocyte lineage cells, focusing on the DNA methyltransferases (DNMTs) that mediate DNA methylation.
Epigenetic regulation is a process that modifies DNA chromatin by altering gene expressions and is closely related to cell differentiation in most organs (Yao et al., 2016). Roughly speaking, epigenetic regulation can be categorized into histone modification and DNA methylation. Different combinations of methylated DNA with acetylated histones are associated with “open” or “closed” epigenetic conditions for gene expression. The degree of histone acetylation is generally determined by the balance between histone acetyltransferases (HATs) and histone deacetylases (HDACs), which acetylate and deacetylate lysine residues of histones, respectively. Although the roles of HATs in oligodendrocyte lineage cells are mostly unknown, HDACs have been shown to be deeply involved in OPC generation and differentiation. Two studies from different research groups demonstrated that HDAC inhibiters suppressed OPC differentiation both during the developmental stage and under diseased conditions in the adult CNS (Liu et al., 2012; Lyssiotis et al., 2007; Shen et al., 2008). In addition, Ye et al demonstrated that HDAC1/2 double-knockdown in OPCs suppressed OPC differentiation during the perinatal period in vivo (Ye et al., 2009). Our group also confirmed that HDAC1/2 double-knockdown suppressed OPC differentiation in cell culture conditions (Egawa et al., 2019). Furthermore, we also showed that HDAC1 knockdown suppressed OPC differentiation but HDAC2 knockdown promoted OPC differentiation (Egawa et al., 2019), suggesting that HDAC1 and HDAC2 have different roles in OPC function. HDAC1 and HDAC2 both belong to the Class I HDAC family, and HDAC3, another member of the Class I HADC family, also plays critical roles in regulating the fate switch to oligodendrocytes. Zhang et al reported that HDAC3 ablation led to an increase of astrocytes alongside the loss of oligodendrocytes (Zhang et al., 2016a). In their study, a genome-wide occupancy analysis showed that HDAC3 interacted with p300 HAT to activate oligodendroglial lineage-specific genes (Zhang et al., 2016a), indicating that HATs are also involved in OPC generation. Besides the Class I HDAC family, other HDAC families also play roles in OPC differentiation. Jagielska et al showed that although the expression of HDAC11, a member of Class IV HDAC family, was low in OPCs under resting state, the expression level of HDAC11 is increased during strain-induced oligodendrocyte maturation (Jagielska et al., 2017).
Compared to mechanisms of histone modification by HDACs, the roles of DNA methylation and its related enzymes DNMTs in OPC function are still understudied. DNA methylation is mediated by DNA methyltransferase enzymes (DNMTs), which are composed of DNMT1, DNMT3A, and DNMT3B. In general, DNMT1 is necessary for the maintenance of DNA methylation, and DNMT3A/3B are necessary for de novo DNA methylation. DNMT1 knockout mice are embryonic lethal (Li et al., 1992) and DMNT3A/3B knockout mice develop to term but die within a few weeks after birth (Okano et al., 1999), suggesting that DNMTs play a fundamental role in cellular homeostasis. In addition, using DNMT-mutant embryonic stem cells, Liao et al showed that DNMT1 is essential for cell viability in undifferentiated stem cells (Liao et al., 2015). Furthermore, Cui et al reported that DNMT3A would promote cell proliferation by accelerating the G1/S transition in gastric carcinogenesis (Cui et al., 2015). DNMT dysfunction is also implied to be related to CNS disorders. Mutations of DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss (Klein et al., 2011) or autosomal dominant cerebellar ataxia, deafness, and narcolepsy (Winkelmann et al., 2012). Also in animal models, deletion of DNMT1 leads to embryonic lethality, and conditional knockout of either DNMT1 or DNMT3A in post-mitotic neuron of forebrain results in abnormal hippocampal CA1 long-term plasticity and deficits of learning and memory (Feng et al., 2010). In addition, disruption or mutation of methyl-CpG binding domain protein 2 (MECP2), which binds to methylated DNA sites in the methylated gene promoters, causes the X-linked Rett syndrome, a neurological disorder associated with axon growth deficiency and autistic symptoms (Gabel et al., 2015). DNA methylation may also affect neuronal recovery after a CNS injury. DNA methylation is increased after brain ischemia in a DNMT1-activity-dependent manner, and mice with a heterozygous mutation for DNMT1 are resistant to mild ischemic damage (Endres et al., 2000).
All the studies introduced above strongly suggest the critical roles of DNMTs in CNS function, but so far, roles of DNMTs in OPC function are mostly unknown. Nonetheless, Moyon et al recently demonstrated that DNMT1 is important for OPC differentiation during prenatal stage (Moyon et al., 2016). However, the roles of DNMTs in OPC proliferation/differentiation change through aging. Using transgenic mouse lines of OPC-specific DNMTs knockdown, the same group demonstrated that DNMT3A, but not DNMT1, plays an important role in remyelination after LPC-induced myelin damage in adult mice (Moyon et al., 2017). More recently, Zhou et al prepared OPC samples from rat spinal cords of different ages, and they examined the age-related changes of the genomic DNA methylation level and the activity of DNMTs in OPCs. Both mRNA and protein levels of DNMT3A/3B increased, but mRNA and protein levels of DNMT1 were significantly downregulated by aging (Zhou et al., 2019). Because the efficacy for remyelination after white matter damage decreases with age (Miyamoto et al., 2013), the DNA hypomethylation in aged OPCs by the downregulation of DNMT1 is one of the reasons for a lower capacity for remyelination in aged animals. In addition, our group also examined the roles of DNMTs in OPC proliferation and differentiation using a cell culture system. We prepared OPC cultures from the neonatal rat cortex, and under normal conditions, these OPC cultures expressed DNMT1 and DNMT3A, but not DNMT3B (Egawa et al., 2019). When DNMT1 expression was downregulated by the siRNA approach before differentiation, OPC differentiation was significantly suppressed; however, when DNMT1 expression was downregulated during differentiation, OPC differentiation was not affected (Egawa et al., 2019), suggesting the stage-specific roles of DNMT1 in OPC differentiation. On the other hand, DNMT3A knockdown decreased the number of OPCs (Egawa et al., 2019), confirming that the general roles of DNMT3A in stem cells (i.e. DNMT3A contributes to cell proliferation) apply for OPCs as well. The importance of DNMTs/DNA-methylation in oligodendrocyte homeostasis is also supported by the findings that the ten-eleven translocation (TET) enzymes regulate oligodendrocyte differentiation. The TET enzymes are responsible for DNA demethylation, and so far, two studies have demonstrated the involvement of TETs in oligodendrocyte function. Zhao et al. showed that three TET family members possess unique protein expression patterns in oligodendrocyte lineage cells (Zhao et al., 2014). The study showed that TET1 expression was high in OPCs, but was downregulated in oligodendrocytes. In contrast, expression levels of TET2 and TET3 were stable in both OPCs and oligodendrocytes. Furthermore, their cell culture experiments with the siRNA approach suggested that TET2 and TET3 would have stronger effects on oligodendrocyte differentiation compared to TET1. More recently, Kwon et al. reported that TET1 overexpression mice showed a slight increase in oligodendrocyte differentiation in the hippocampus (Kwon et al., 2018). Although the precise roles of TETs in OPCs/oligodendrocytes are still mostly unknown, all these findings show that DNMTs (and DNA methylation) are heavily involved in OPC homeostasis (Figure 1). Nevertheless, DNMT roles in OPCs would change depending on the surrounding conditions, such as aging and external stress, and therefore, for the purpose of developing an approach to prepare injectable OPCs in clinic, further investigation into the roles of DNMTs in oligodendrocyte lineage cells is necessary.
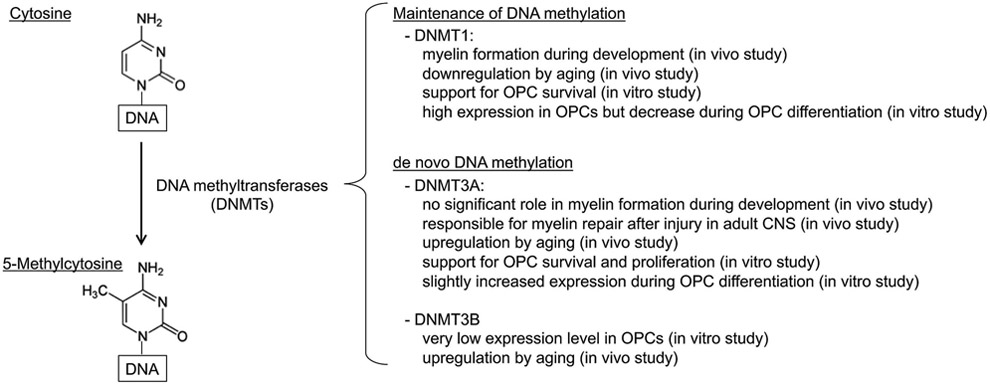
Figure 1. Schematics for DNMT roles in OPC function:
DNMTs are responsible for DNA methylation by converting cytosine into 5-methylcytosine. In general, DNMT1 is necessary for the maintenance of DNA methylation, and DNMT3A/3B are necessary for de novo DNA methylation. In OPCs, DNMT1 and DNMT3A are extensively expressed. Although DNMT3B expression level in OPCs seems very low under cell culture conditions, the level increases through aging in vivo. DNMT1 may regulate myelin formation during development, but in the adult CNS, DNMT3A is responsible for oligodendrocyte regeneration after injury.
5. Conclusions and Future perspective:
There is no doubt that cell-based therapy is a promising therapeutic approach for CNS diseases, and OPCs can be a source of injectable cells for patients with myelin damage. Several pre-clinical studies have provided a proof-of-concept that cell-based therapy with OPCs would be effective for demyelinating diseases (Figure 2). However, there are several hurdles for the clinical application of OPC therapy. As discussed in this mini-review, one of the challenges in OPC therapy is to develop a method to shorten the duration for preparing injectable OPCs. For this purpose, it would be necessary to understand the precise mechanisms of OPC generation from stem cells. Recent findings demonstrate the critical roles of DNMTs in OPC generation, proliferation, and differentiation. Therefore, manipulating DNMT-related mechanisms can be expected to shorten the duration for OPC proliferation. In addition, because DNMTs regulate OPC survival and differentiation, understanding the role of DNMTs in OPCs would help to maximize the efficacy of transplanted OPCs. The research for the roles of DNMTs in OPCs is still limited, and future studies are warranted to find out how DNMT manipulation can accelerate the OPC generation from stem cells and what DNMT levels (and DNA methylation status) in transplanted OPCs would increase the efficacy of stabilizing the cells in the recipient organs.
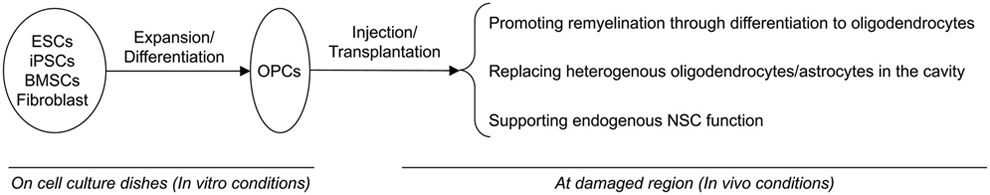
Figure 2. Schematics for therapeutic potential of OPCs in stem cell therapy.
OPCs can work as multi-potent neuronal stem cells, and they are a potential source of cell-based therapy for white matter-related diseases with demyelination. There are several sources of injectable OPCs: ESCs, iPSCs, BMSCs, and fibroblasts. After injection into damaged region, transplanted OPCs would (i) promote remyelination through differentiating into mature oligodendrocytes, (ii) replace heterogeneous OL/astrocytes in the cavity, and (iii) support endogenous NSC function. One potential hurdle for OPC-therapy is the length of time to prepare injectable OPCs in the in-vitro stage. Shortening the duration for expansion/differentiation is required, and for this purpose, we need to understand the precise intracellular mechanisms of OPC generation/proliferation.
Highlights:
Oligodendrocyte precursor cells (OPCs) are one of the major cell types in cerebral white matter
OPCs can be a potential source of cell-based therapy for neurological diseases
DNA methyltransferases (DNMTs) play important roles in OPC generation and differentiation
Acknowledgements:
Supported in part by National Institutes of Health.
Footnotes
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- All AH, et al. , 2012. Human embryonic stem cell-derived oligodendrocyte progenitors aid in functional recovery of sensory pathways following contusive spinal cord injury. PLoS One. 7, e47645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- All AH, et al. , 2015. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS One. 10, e0116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambakidis NC, Miller RH, 2004. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 4, 16–26. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Irvine KA, 2008. Endogenous or exogenous oligodendrocytes for remyelination. J Neurol Sci. 265, 43–6. [DOI] [PubMed] [Google Scholar]
- Brustle O, et al. , 1999. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 285, 754–6. [DOI] [PubMed] [Google Scholar]
- Cao Q, et al. , 2005. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 25, 6947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, et al. , 2010. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 30, 2989–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, et al. , 2012. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 30, 715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari DM, et al. , 2006. Oligodendrocyte progenitor cell (OPC) transplantation is unlikely to offer a means of preventing X-irradiation induced damage in the CNS. Exp Neurol. 198, 145–53. [DOI] [PubMed] [Google Scholar]
- Chen LX, et al. , 2015. Neuroprotective effects of oligodendrocyte progenitor cell transplantation in premature rat brain following hypoxic-ischemic injury. PLoS One. 10, e0115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, et al. , 2015. DNA methyltransferase 3A promotes cell proliferation by silencing CDK inhibitor p18INK4C in gastric carcinogenesis. Sci Rep. 5, 13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czepiel M, et al. , 2011. Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia. 59, 882–92. [DOI] [PubMed] [Google Scholar]
- Domingues HS, et al. , 2017. Mechanical plasticity during oligodendrocyte differentiation and myelination. Glia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa N, et al. , 2019. Differential roles of epigenetic regulators in the survival and differentiation of oligodendrocyte precursor cells. Glia. 67, 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, et al. , 2000. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci. 20, 3175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, et al. , 2010. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 13, 423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong CY, Gauthaman K, Bongso A, 2010. Teratomas from pluripotent stem cells: A clinical hurdle. J Cell Biochem. 111, 769–81. [DOI] [PubMed] [Google Scholar]
- Gabel HW, et al. , 2015. Disruption of DNA-methylation-dependent long gene repression in Rett syndrome. Nature. 522, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Nishiyama A, 2014. NG2 cells (polydendrocytes): listeners to the neural network with diverse properties. Glia. 62, 1195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, et al. , 2015. Oligodendroglial Development: New Roles for Chromatin Accessibility. Neuroscientist. 21, 579–88. [DOI] [PubMed] [Google Scholar]
- Jagielska A, et al. , 2017. Mechanical Strain Promotes Oligodendrocyte Differentiation by Global Changes of Gene Expression. Front Cell Neurosci. 11, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, et al. , 2016. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Reports. 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, et al. , 2005. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 25, 4694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, et al. , 2006. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 9, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, et al. , 2015. Oct4-induced oligodendrocyte progenitor cells enhance functional recovery in spinal cord injury model. EMBO J. 34, 2971–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CJ, et al. , 2011. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat Genet. 43, 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M, 2000. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 289, 1754–7. [DOI] [PubMed] [Google Scholar]
- Kuai XL, et al. , 2015. Transplantation of mouse embryonic stem cell-derived oligodendrocytes in the murine model of globoid cell leukodystrophy. Stem Cell Res Ther. 6, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon W, et al. , 2018. Tet1 overexpression leads to anxiety-like behavior and enhanced fear memories via the activation of calcium-dependent cascade through Egr1 expression in mice. FASEB J. 32, 390–403. [DOI] [PubMed] [Google Scholar]
- Li E, Bestor TH, Jaenisch R, 1992. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 69, 915–26. [DOI] [PubMed] [Google Scholar]
- Liao J, et al. , 2015. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat Genet. 47, 469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, et al. , 2012. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience. 220, 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loers G, et al. , 2004. Comparison of myelin, axon, lipid, and immunopathology in the central nervous system of differentially myelin-compromised mutant mice: a morphological and biochemical study. Mol Cell Neurosci. 27, 175–89. [DOI] [PubMed] [Google Scholar]
- Lyssiotis CA, et al. , 2007. Inhibition of histone deacetylase activity induces developmental plasticity in oligodendrocyte precursor cells. Proc Natl Acad Sci U S A. 104, 14982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, et al. , 2013. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke. 44, 2573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyon S, et al. , 2016. Functional Characterization of DNA Methylation in the Oligodendrocyte Lineage. Cell Rep. 15, 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyon S, et al. , 2017. Efficient Remyelination Requires DNA Methylation. eNeuro. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazm Bojnordi M, Ghasemi HH, Akbari E, 2014. Remyelination after Lysophosphatidyl Choline-Induced Demyelination Is Stimulated by Bone Marrow Stromal Cell-Derived Oligoprogenitor Cell Transplantation. Cells Tissues Organs. 200, 300–6. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD, 1999. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 58, 1113–24. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, et al. , 2009. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 10, 9–22. [DOI] [PubMed] [Google Scholar]
- Okano M, et al. , 1999. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 99, 247–57. [DOI] [PubMed] [Google Scholar]
- Panagiotakos G, et al. , 2007. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One. 2, e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao J, et al. , 2015. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 16, 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest CA, et al. , 2015. Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen Med. 10, 939–58. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M, 1983. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 303, 390–6. [DOI] [PubMed] [Google Scholar]
- Sharp J, et al. , 2010. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 28, 152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, et al. , 2008. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 11, 1024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, et al. , 2011. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat Biotechnol. 29, 934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Cooksey E, Duncan ID, 2013. Myelin loss does not lead to axonal degeneration in a long-lived model of chronic demyelination. J Neurosci. 33, 2718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, et al. , 2001. Sonic hedgehog-dependent emergence of oligodendrocytes in the telencephalon: evidence for a source of oligodendrocytes in the olfactory bulb that is independent of PDGFRα signaling. Development. 128, 4993–5004. [DOI] [PubMed] [Google Scholar]
- Sun L, et al. , 2014. Inhibition of TROY promotes OPC differentiation and increases therapeutic efficacy of OPC graft for spinal cord injury. Stem Cells Dev. 23, 2104–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar V, Studer L, 2014. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet. 15, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Ikenaka K, 2015. Oligodendrocyte generation during mouse development. Glia. 63, 1350–6. [DOI] [PubMed] [Google Scholar]
- Wang S, et al. , 2013. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 12, 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, et al. , 2002. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 69, 966–75. [DOI] [PubMed] [Google Scholar]
- Windrem MS, et al. , 2004. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 10, 93–7. [DOI] [PubMed] [Google Scholar]
- Winkelmann J, et al. , 2012. Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Hum Mol Genet. 21, 2205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, et al. , 2012. Transplantation of oligodendrocyte precursor cells improves myelination and promotes functional recovery after spinal cord injury. Injury. 43, 794–801. [DOI] [PubMed] [Google Scholar]
- Xu L, et al. , 2015. Transplantation of human oligodendrocyte progenitor cells in an animal model of diffuse traumatic axonal injury: survival and differentiation. Stem Cell Res Ther. 6, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, et al. , 2013. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 31, 434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, et al. , 2016. Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci. 17, 537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, et al. , 2009. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the betacatenin-TCF interaction. Nat Neurosci. 12, 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, et al. , 2016a. Hdac3 Interaction with p300 Histone Acetyltransferase Regulates the Oligodendrocyte and Astrocyte Lineage Fate Switch. Dev Cell. 36, 316–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Chopp M, 2016b. Function of neural stem cells in ischemic brain repair processes. J Cereb Blood Flow Metab. 36, 2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. , 2014. Dynamics of ten-eleven translocation hydroxylase family proteins and 5-hydroxymethylcytosine in oligodendrocyte differentiation. Glia. 62, 914–26. [DOI] [PubMed] [Google Scholar]
- Zhou J, et al. , 2019. Age-related Changes in the Global DNA Methylation Profile of Oligodendrocyte Progenitor Cells Derived from Rat Spinal Cords. Curr Med Sci. 39, 67–74. [DOI] [PubMed] [Google Scholar]