Abstract
Background:
Although use of simulation-based team training for pediatric trauma resuscitation has increased, its impact on patient outcomes has not yet been shown. The purpose of this study was to determine the association between simulation use and patient outcomes.
Methods:
Trauma centers that participate in the American College of Surgeons (ACS) Pediatric Trauma Quality Improvement Program (TQIP) were surveyed to determine frequency of simulation use in 2014 and 2015. Center-specific clinical data for 2016 and 2017 were abstracted from the ACS TQIP registry (n=57,916 patients) and linked to survey responses. Center-specific risk-adjusted mortality was estimated using multivariable hierarchical logistic regression and compared across four levels of simulation-based training use: no training, low-volume training, high-volume training, and survey non-responders (unknown training use).
Results:
Survey response rate was 75% (94/125 centers) with 78% of the responding centers (73/94) reporting simulation use. The average risk-adjusted odds of mortality was lower in centers with a high volume of training compared to centers not using simulation (OR 0.58, 95% CI 0.37-0.92). The times required for resuscitation processes, evaluations, and critical procedures (endotracheal intubation, head computed tomography, craniotomy, and surgery for hemorrhage control) were not different between centers based on levels of simulation use.
Conclusions:
Risk-adjusted mortality is lower in TQIP-Pediatric centers using simulation-based training, but this improvement in mortality may not be mediated by a reduction in time to critical procedures. Further investigation into alternative mediators of improved mortality associated with simulation use is warranted, including assessment of resuscitation quality, improved communication, enhanced teamwork skills, and decreased errors.
Level of Evidence:
Level III therapeutic / care management
Keywords: pediatric, trauma, resuscitation, simulation-based training, outcomes
INTRODUCTION
Injury remains the leading cause of mortality in children 1-18 years old.1,2 More than 50% of deaths after injury are within the first 24 hours.3 This early mortality shows the importance of improving the quality of the resuscitation phase of care for critically injured children. Children may have better outcomes when treated at pediatric trauma centers,4-8 but a minority of severely injured children are initially resuscitated at pediatric centers.9,10 Due to the rarity of severe injury in children, providers in pediatric centers may lack experience caring for an injured child in extremis.11 Trauma resuscitation is a time-dependent process, and time to completion of critical evaluation and intervention may play a critical role in improving outcomes.12-17 Achieving a timely and high-quality multidisciplinary resuscitation requires experienced trauma providers working as a coordinated team.
Simulation-based training has been associated with improved team performance during trauma resuscitation.18-22 Simulation use has also been associated with improved outcomes for pediatric in-hospital cardiac arrest.23 The use of simulation-based training for pediatric trauma resuscitation has been reported by several single-center studies and is being increasingly utilized,18,22,24,25 but not in a standardized fashion.26 Demonstrated benefits of multidisciplinary simulation-based team training for trauma resuscitation also include faster time to completion of a) the primary survey, b) critical procedures (time to endotracheal intubation and time to computed tomography completion), and c) emergency surgery in single-center studies of adult trauma patients.27 The impact of simulation use on patient outcomes, however, has not been studied widely and has not been studied specifically for injured children.
The purpose of this study was to determine if the use of simulation-based training for trauma resuscitation is associated with improved performance measured by 1) time to critical evaluations and procedures, and 2) risk-adjusted mortality in pediatric trauma patients. We hypothesized that pediatric trauma centers that use simulation-based training would have lower risk-adjusted mortality and faster times to critical evaluations and procedures for injured children.
METHODS
Study Population
All trauma centers participating in the American College of Surgeons Pediatric Trauma Quality Improvement Program (ACS Pediatric TQIP) in 2016 (N=125) were selected for inclusion in this cross-sectional cohort study. Participation in the ACS Pediatric TQIP Program is limited to centers with either state designation or ACS verification as a pediatric trauma center. Trauma centers participating in the ACS Pediatric TQIP program contribute data to a national registry in accordance with the National Trauma Data Standard. The Pediatric TQIP registry includes patients eighteen years and under with at least one injury with an Abbreviated Injury Scale (AIS) severity score of two or higher. Patients are excluded from the registry if presenting without signs of life, are discharged home from the emergency department, have a pre-existing advanced directive, or have a major burn injury.28 Transfer-in patients were excluded from the primary mortality analyses and from the secondary time-to-procedure analyses, as simulation use would not be expected to impact outcomes in patients that underwent initial resuscitation at a referring facility. Transfer-in patients were, however, analyzed independently and used as an internal center-specific control for risk-adjusted modeling of mortality, as these patients should not be affected by any impact of simulation-based training. Human Subjects approval was obtained from the Children’s Hospital Los Angles Institutional Review Board.
Survey Development, Implementation, and Exposure Definition
A seventeen-item survey was developed by the study team and piloted for readability by a group of trauma program managers. Full details of the survey have been previously described.26 The survey was administered electronically via Qualtrics online software to trauma program managers at each participating center and subsequently sent to trauma medical directors at initially nonresponding centers. Additional follow-up by direct phone survey was attempted by ACS TQIP program staff, with verbal survey administration of the survey. Survey items included annual number of simulation-based training sessions for calendar years 2014 and 2015 (scaled 0-12 and 13+). Trauma center simulation use was categorized as no training (zero simulation-based training sessions in two years), low-volume training (1-10 simulation-based training sessions over two years), high-volume training (11 or more simulation-based training sessions over two years), or unknown training (survey non-responders). Categorization of ‘high’ and ‘low’ volume simulation use was arbitrarily defined based on the median number of reported pediatric simulation sessions in 2014 (5.5) for centers reporting simulation use.
Outcome Measures and Cohort Definitions
The primary outcome measure for this study was risk-adjusted mortality compared across levels of training. Secondary outcomes included time to critical interventions (endotracheal intubation), evaluations (head CT), and procedures (emergent craniotomy and surgery for hemorrhage control). For secondary analyses, ‘traumatic brain injury (TBI)’ was defined as any patient with an AIS head severity score ≥ 1, excluding scalp laceration or skull fracture codes. ‘Isolated TBI’ was defined as any patient with TBI with no other AIS severity score ≥ 2 other than facial injuries. ‘Emergent craniotomy’ was defined as any craniotomy in a patient that had an ED disposition of ‘Operating Room’ and only received one head CT before ED discharge. Frequency of endotracheal intubation (as a marker of resuscitation quality) and time to endotracheal intubation were assessed for all trauma patients with initial known GCS total ≤ 8, as well as for patients with confirmed TBI and initial known GCS total ≤ 8. Time to first head CT was assessed for isolated TBI patients with an initial known GCS total ≤ 8, and for polytrauma patients with TBI and an initial known GCS total ≤ 8. Time to emergent craniotomy was assessed for isolated TBI patients and for polytrauma patients with TBI. Time to surgery for hemorrhage control was assessed for any patient that received packed red blood cells within four hours of ED admission, had a surgical (non-angiographic) procedure for control of bleeding (laparotomy, thoracotomy, sternotomy, neck exploration, extremity exploration, skin or soft tissue operation, or mangled extremity procedure), and had an ED disposition of ‘Operating Room’.
Patients were excluded from analyses of time to intubation if they had prehospital intubation (defined by time to procedure variable as 0), tracheostomy (defined by ICD-10 procedure codes) performed in the emergency room, or endotracheal intubation performed after discharge from the emergency room. Patients were excluded from analyses of time to head CT if the scan time was the same as the patient arrival time or if the scan occurred after ED discharge. The location and timing of endotracheal intubation or head CT origination was determined using time to procedure variables and ED length of stay variables.
Confounding Covariates
The TQIP program uses a validated multivariable model for risk-adjusted benchmarking of mortality between pediatric trauma centers.29 Covariates in this model include gender, race (white, black, Asian, other), age, comorbidities (respiratory diseases, substance abuse, major psychiatric illness, bleeding disorder, functional dependence, diabetes mellitus, hypertension, congenital anomalies, and prematurity), injury-specific survival risk ratios calculated and validated based on historic datasets, age-normalized initial ED systolic blood pressure, age-normalized initial ED heart rate, initial ED GCS motor score, maximum AIS severity scores by body region (head, face, neck, chest, abdomen, spine, upper extremity, lower extremity), mechanism of injury (fall, motor vehicle occupant, motorcyclist, struck by object, firearm, cut/pierce, pedestrian struck by bicycle, other), pre-hospital cardiac arrest, and interaction terms for AIS head by age, AIS head by infant, and systolic blood pressure by firearm injury. Trauma center state designation status, ACS verification level, and annual admission volume are specifically not included in the TQIP mortality model. We therefore performed sensitivity analyses including these covariates to assess for unmeasured confounding due to trauma center resources and due to trauma admission volume as a proxy for provider and team experience.
Adjusted analyses for all secondary outcomes included the same covariates in in the TQIP model in addition to several other specific factors. Additional covariates considered included ED respiratory rate, ED respiratory assistance, ED oxygen saturation, ED supplemental oxygen, and injury related to child abuse. Adjusted analyses for time to head CT, time to craniotomy, and time to surgery for hemorrhage control also included factors that may impact ED length of stay, including prehospital intubation, chest tube placement in the emergency room, transfusion in the emergency room, placement of a surgical airway in the emergency room, and CT of the abdomen and pelvis in the emergency room. Transfer-in patients were excluded from all secondary analyses.
Statistical Analyses
Baseline center characteristics assessed for differences across levels of simulation with omnibus tests of significance, including ANOVA for continuous variables and chi-squared test of independence for categorical variables. Missing physiological data for center-level mortality comparisons were managed using multiple imputation. The imputation model included age, gender, race, transfer status, the presence of a serious body region injury (AIS≥2 of the spine, abdomen, lower extremity, and upper extremity), injury mechanism (fall, firearm, motorcyclist, motor vehicle occupant, pedestrian, struck, and other mechanism), and age by vital interactions in the imputation model. Mortality was modeled using data from Fall 2017 TQIP report as outcomes in an hierarchical logistic regression model. Because of positive skew, resuscitation process times were normalized by log transformation. Missing data for multivariable time-to-event analyses were imputed by drawing from a random distribution with sample mean and standard deviation or from a binary distribution for proportions to minimize bias and preserve variability. To account for clustering of patients within centers, time-to-event outcomes were analyzed using hierarchical linear regression across levels of simulation-based training use. All significance tests were two-tailed, with α=0.05. All analyses were performed using SAS software v. 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
One hundred twenty-five ACS TQIP-Pediatric trauma centers were surveyed (Table 1a). Survey response rate was 75% (N = 94/125 centers). One center that responded to the survey did not submit registry data for 2016 and was therefore not included in the clinical outcomes analysis, leaving 124 centers for our analysis sample. Simulation use in 2014-15 was reported in 54 centers (43% of all centers and 58% among respondents). Among centers reporting simulation use in 2014-15, 19 (15% of all centers and 20% among respondents) centers reported low volume simulation use (median [IQR] of 6 [3-8] sessions over two years). Thirty-five (28% of all centers and 37% among respondents) centers reported high-volume simulation use (22 [14-26] sessions over two years). High-volume simulation centers were found to have significantly lower overall trauma volume and annual trauma admissions with serious injury (ISS≥16). No significant differences in pediatric (age ≤ 14) admissions were observed across levels of training. Centers reporting any level of training were more likely to be an ACS-verified or state-designated level 1 pediatric trauma center and were more likely to have a pediatric intensive care unit.
Table 1a.
Center Characteristics by Level of Simulation Use.
| Total Number of Pediatric Simulation Sessions, 2014-2015 | Omnibus p-value† |
||||
|---|---|---|---|---|---|
| Unknown | None | 1-10 | 11+ | ||
| Number of Facilities | 30 | 40 | 19 | 35 | |
| Quantification of Clinical Trauma Volume | |||||
| Annual Trauma Admissions | 1,682.5 [873-2,690] | 1,829.5 [1,233-2,460] | 1,462 [747-2,847] | 1,367 [776-1,987] | 0.01 |
| Annual Admissions with ISS ≥16, Excluding Transfers | 297.5 [162-543] | 346 [204.5-622.5] | 289 [105-855] | 182 [116-500] | 0.02 |
| Annual Admissions, Age ≤ 14yo | 398.5 [169-607] | 229.5 [155.5-588.5] | 347 [261-555] | 355 [185-922] | 0.32 |
| Annual Admissions, Age ≤ 14yo with ISS ≥16 | 57 [24-83] | 37 [21.5-60] | 54 [33-82] | 46 [18-115] | 0.82 |
| Annual Admissions, Age ≤ 14yo with ISS≥16, excluding transfers | 21 [10-34] | 17 [11.5-31] | 24 [11-33] | 16 [10-47] | 0.65 |
| Hospital Teaching Status | |||||
| University Hospital | 19 (63.3) | 25 (62.5) | 13 (68.4) | 26 (74.3) | 0.75 |
| Community Teaching Hospital | 10 (33.3) | 12 (30.0) | 5 (26.3) | 9 (25.7) | |
| Nonteaching Hospital | 1 (3.3) | 3 (7.5) | 1 (5.3) | 0 (0.0) | |
| Hospital Type | |||||
| For Profit | 3 (10.0) | 3 (7.5) | 1 (5.3) | 0 (0.0) | 0.28 |
| Not for Profit | 27 (90.0) | 37 (92.5) | 18 (94.7) | 35 (100.0) | |
| ACS Pediatric Verification | |||||
| Level 1 Pediatric Verification | 8 (28.6) | 7 (18.0) | 9 (47.4) | 17 (50.0) | 0.01 |
| Level 2 Pediatric Verification | 6 (21.4) | 16 (41.0) | 7 (36.8) | 5 (14.7) | |
| Not Verified for Pediatric Trauma | 14 (50.0) | 16 (41.0) | 3 (15.8) | 12 (35.3) | |
| State Pediatric Designation | |||||
| Level 1 State Designation | 12 (40.0) | 15 (37.5) | 8 (42.1) | 23 (65.7) | 0.03 |
| Level 2 State Designation | 5 (16.7) | 16 (40.0) | 4 (21.1) | 4 (11.4) | |
| No State Designation | 13 (43.3) | 9 (22.5) | 7 (36.8) | 8 (22.9) | |
| Patient Care Characteristics | |||||
| Associated with a Pediatric Hospital | 24 (80.0) | 32 (80.0) | 16 (84.2) | 29 (82.9) | 0.99 |
| Have a Pediatrics Ward | 30 (100.0) | 39 (97.5) | 19 (100.0) | 35 (100.0) | 1.00 |
| Have a Pediatric Intensive Care Unit | 28 (93.3) | 40 (100.0) | 19 (100.0) | 35 (100.0) | 0.08 |
| Transfer severely injured children to other centers | 5 (16.7) | 3 (7.5) | 1 (5.3) | 1 (2.9) | 0.25 |
| Provide all Acute Care Services to Injured Children | 29 (96.7) | 37 (92.5) | 18 (94.7) | 35 (100.0) | 0.40 |
Data expressed as median [IQR] or N(%).
One-way analysis of variance (ANOVA) for continuous variables and Fisher’s exact test for categorical variables.
The mortality analysis included 57,916 patients treated at 124 centers. There were statistically significant differences in most patient-level characteristics across levels of simulation use, but the differences were small (effect size ≤0.20) for all variables except mechanism of injury (Table 1b). Centers with a high-volume of simulation-based training use had significantly less motor vehicle crash occupants (14% versus 21-23%, p<0.01, d=−0.25) and significantly more patients treated after a fall (53% versus 41%, p<0.01, d=0.24) when compared to centers with a low volume of or no simulation-based training use.
Table 1b:
Demographic, Physiologic, and Injury Characteristics for Children Treated at Pediatric TQIP Centers by Level of Simulation Use.
| Unknown | No Sim Use | 0-10 hours | 11+ hours | |||
|---|---|---|---|---|---|---|
| Number of Facilities | 30 | 39 | 19 | 35 | ||
| Patients (All) | 14,576 | 17,118 | 8,645 | 19,114 | ||
| Transfer In | 7,307 (50.1) | 7,434 (43.4) | 4,556 (52.7) | 8,976 (47.0) | ||
| Patients (No Transfers) | 7,269 | 9,684 | 4,089 | 10,138 | p-value | effect size |
| Race - White | 4263 (59.6) | 6031 (65.7) | 2296 (57.1) | 6292 (64.1) | <0.01 | ≤0.18 |
| Gender - Male | 4619 (63.5) | 6267 (64.7) | 2620 (64.1) | 6387 (63) | 0.08 | |
| Age | 8 (4-14) | 9 (4-15) | 10 (4-15) | 8 (4-13) | <0.01 | ≤0.17 |
| ED GCS Motor Score | 6 (6-6) | 6 (6-6) | 6 (6-6) | 6 (6-6) | <0.01 | ≤0.09 |
| ED Systolic Blood Pressure | 120 (110-132) | 120 (110-132) | 122 (111-134) | 119 (108-130) | <0.01 | ≤0.20 |
| ED Heart Rate | 103 (88-121) | 103 (88-121) | 104 (88-123) | 104 (88-121) | 0.36 | ≤0.04 |
| Prehospital Cardiac Arrest | 62 (0.9) | 83 (0.9) | 29 (0.7) | 51 (0.5) | <0.01 | ≤0.09 |
| Comorbidity | ||||||
| Functional Dependence | 18 (0.2) | 45 (0.5) | 14 (0.3) | 35 (0.3) | 0.11 | |
| Substance Abuse | 194 (2.7) | 302 (3.2) | 169 (4.1) | 172 (1.7) | <0.01 | ≤0.15 |
| Congenital Anomalies | 73 (1) | 160 (1.7) | 62 (1.5) | 182 (1.8) | <0.01 | ≤0.06 |
| Prematurity | 111 (1.5) | 162 (1.7) | 60 (1.5) | 173 (1.7) | 0.62 | |
| Mechanism | ||||||
| Firearm | 206 (2.8) | 376 (3.9) | 164 (4) | 228 (2.2) | <0.01 | ≤0.10 |
| Motorcyclist | 42 (0.6) | 121 (1.2) | 40 (1) | 60 (0.6) | <0.01 | ≤0.07 |
| Motor Vehicle Crash - Occupant | 1247 (17.2) | 2069 (21.4) | 962 (23.5) | 1407 (13.9) | <0.01 | ≤0.25† |
| Cut/pierce | 109 (1.5) | 149 (1.5) | 51 (1.2) | 151 (1.5) | 0.62 | |
| Fall | 3495 (48.1) | 4013 (41.4) | 1661 (40.6) | 5341 (52.7) | <0.01 | ≤0.24‡ |
| Pedestrian | 704 (9.7) | 1105 (11.4) | 429 (10.5) | 1043 (10.3) | <0.01 | ≤0.06 |
| Other | 657 (9) | 765 (7.9) | 374 (9.1) | 797 (7.9) | <0.01 | ≤0.05 |
| Worst AIS Severity Score | ||||||
| Head | 0 (0-2) | 0 (0-2) | 0 (0-2) | 0 (0-2) | <0.01 | ≤0.19 |
| Face | 0 (0-0) | 0 (0-1) | 0 (0-0) | 0 (0-0) | <0.01 | ≤0.10 |
| Neck | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | <0.01 | ≤0.04 |
| Chest | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | <0.01 | ≤0.18 |
| Abdomen | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | <0.01 | ≤0.09 |
| Spine | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | <0.01 | ≤0.04 |
| Upper Extremity | 0 (0-2) | 0 (0-2) | 0 (0-2) | 0 (0-2) | <0.01 | ≤0.15 |
| Lower Extremity | 0 (0-2) | 0 (0-2) | 0 (0-2) | 0 (0-2) | 0.04 | ≤0.03 |
| Severe TBI1 | 266 (3.7) | 422 (4.4) | 198 (4.8) | 287 (2.8) | <0.01 | ≤0.10 |
| Infant with Severe TBI1 | 13 (4.9) | 10 (2.4) | 6 (3.0) | 10 (3.5) | 0.35 | |
| Firearm Injury with Hypotension | 3 (1.1) | 10 (2.4) | 5 (2.5) | 8 (2.8) | 0.53 |
Data expressed as frequency (%) or median (interquartile range).
AIS Head ≥3 and GCS≤8. Reported effect sizes represent the largest post-hoc pairwise difference between groups. Variables with effect size differences >0.20 are detailed as follows: Mechanism-MVC Occupant: unknown simulation versus no simulation, p<0.01, d=0.11; no simulation versus 0-10 hours, p<0.01, d=0.05; 0-10 hours versus 11+ hours of simulation, p<0.01, d=0.25. Mechanism-Fall: unknown simulation versus no simulation, p<0.01, d=0.13; no simulation versus 0-10 hours, p=0.37; 0-10 hours versus 11+ hours of simulation, p<0.01, d=0.24.
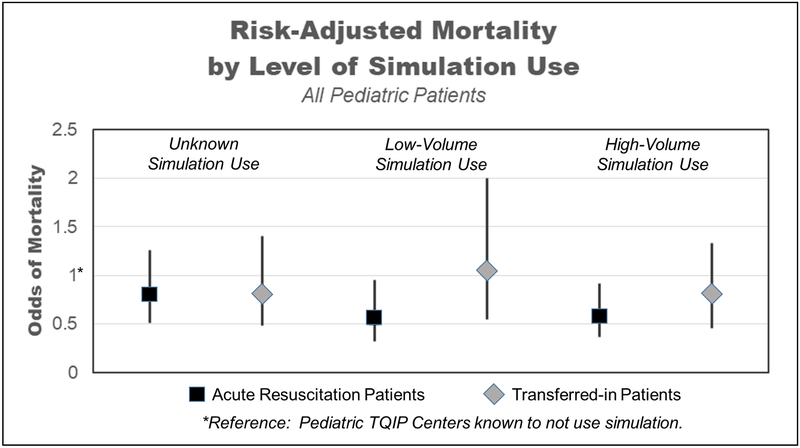
Average center-specific unadjusted mortality rate was lowest in centers with a high volume of simulation-based training use (Table 2). Centers using either low- or high-volume training had significantly lower mortality when compared to centers that do not use simulation. Additional adjustment for ACS trauma center verification level and for annual trauma admission volume did not impact these results. Using transfer patients within centers as internal controls, we did not see a significant impact of simulation use on risk-adjusted mortality for these patients that underwent initial resuscitation at a referring center (Figure 1). Adjusted odds ratios for covariates included in the multivariable mortality model are shown in Supplemental Table 1.
Table 2:
Center-Specific Mortality, Risk-Adjusted Center-Specific Mortality, and Sensitivity Analysis of Center-Specific Risk-Adjusted Mortality Including Additional Adjustment for ACS Pediatric Trauma Center Verification and for Annual Trauma Volume for Pediatric TQIP Centers by Level of Simulation Use.
| Total Number of Pediatric Simulation Sessions, 2014-2015 | ||||
|---|---|---|---|---|
| Unknown | None | 1-10 | 11+ | |
| N=30 | N=40 | N=19 | N=35 | |
| Unadjusted center-specific mortality rate | 1.37% | 1.88% | 1.59% | 1.03% |
| Risk-adjusted mortality (TQIP model) | 0.80 [0.51-1.26] | Ref | 0.55 [0.32-0.96] | 0.58 [0.37-0.92] |
| Risk-adjusted mortality with additional adjustment for ACS verification level and annual trauma volume | 0.80 [0.51-1.27] | Ref | 0.56 [0.33-0.94] | 0.65 [0.41-1.03] |
Data presented as percentages or odds ratio with 95% confidence interval. TQIP: Trauma Quality Improvement Program.
Figure 1:
Risk-Adjusted Mortality in Transferred and Non-Transferred Trauma Patients Treated at Centers Using No Simulation, Low-Volume (0-10 hrs) Simulation, High-Volume (11+ hrs) Simulation, and Unknown Simulation Use (Survey Non-Respondents).
We observed no significant difference in time to intubation, head CT, emergent craniotomy, or surgery for hemorrhage control between centers of differing levels of simulation-based training use (Table 3). Trauma patients with an initial ED GCS total ≤ 8 had a slightly higher frequency of intubation at centers using high-volume training compared to centers not using simulation-based training (94% versus 91%, p=0.02, Table 4).
Table 3:
Resuscitation Process Times for Centers of Varying Levels of Simulation Use and Adjusted Odds Ratios Comparing Centers Using High-Volume Simulation to Centers Using No Simulation.
| Unknown Simulation | No Simulation | Low Volume Simulation | High Volume Simulation | Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean [95% CI] |
N | Mean [95% CI] |
N | Mean [95% CI] |
N | Mean [95% CI] |
Omnibus p-value | Adjusted OR [95% CI] |
|
| Time to Endotracheal Intubation | ||||||||||
| All Patients with GCS 8 or less | 111 | 8 [7-10] | 175 | 9 [8-11] | 83 | 9 [7-11] | 117 | 10 [8-12] | 0.44 | 1.00 [0.80-1.26] |
| Isolated TBI with GCS 8 or less | 41 | 9 [6-11] | 48 | 8 [6-11] | 21 | 8 [5-11] | 33 | 12 [8-16] | 0.31 | 0.80 [0.55-1.16] |
| Time to Head CT | ||||||||||
| All Patients with GCS 8 or less | 183 | 21 [18-26] | 296 | 27 [23-33] | 136 | 27 [21-34] | 220 | 25 [21-29] | 0.30 | 1.17 [0.91-1.50] |
| Isolated TBI with GCS 8 or less | 53 | 21 [16-27] | 68 | 23 [18-29] | 38 | 22 [16-29] | 61 | 24 [19-31] | 0.84 | 1.0 [0.74-1.4] |
| Time to Emergent Craniotomy | ||||||||||
| All patients | 43 | 107 [91-128] | 83 | 128 [113-146] | 35 | 113 [93-136] | 53 | 128 [109-150] | 0.32 | 1.07 [0.84-1.35] |
| Isolated TBI | 24 | 107 [85-135] | 33 | 122 [101-148] | 21 | 117 [92-148] | 28 | 124 [101-152] | 0.80 | 0.92 [0.72-1.17] |
| Time to Surgery for | ||||||||||
| Hemorrhage Control | 55 | 70 [54-90] | 101 | 53 [43-66] | 54 | 51 [39-68] | 70 | 61 [48-76] | 0.31 | 0.97 [0.64-1.21] |
Table 4:
Frequency of Endotracheal Intubation for Pediatric Trauma Patients that Present with a Glasgow Coma Scale of 8 or Less by Trauma Center Level of Simulation Use.
| Total Number of Pediatric Simulation Sessions, 2014-2015 | ||||||
|---|---|---|---|---|---|---|
| No Survey | No Simulation | 1-10 | 11+ | TOTAL | Omnibus p- value† |
|
| N | 784 | 932 | 486 | 882 | 3,084 | |
| Not intubated | 72 (9.1) | 84 (9.0) | 55 (11.3) | 53 (6.0) | 264 | 0.02 |
| Intubated | 712 (90.8) | 848 (91.0) | 431 (88.7) | 829 (94.0) | 2,820 | |
Data expressed as N(%).
Chi-square test of independence.
DISCUSSION
This retrospective study of simulation-based training use in ACS Pediatric TQIP centers found an association between increased use of simulation-based training and lower risk-adjusted mortality for acutely injured children. We did not find a difference in time to critical interventions, evaluations, or procedures, but found an increased rate of intubation for patients with a GCS ≤ 8 in centers with a high volume of training. These findings suggest that simulation-based training may improve resuscitation quality and outcomes in pediatric trauma patients, but improved outcomes may not be mediated by faster time to critical interventions, evaluations, and procedures.
Simulation-based training has been shown to improve trauma team performance, mainly measured by number of tasks completed and time to task completion in acute resuscitation.18-22 Few studies have found an association between training with simulation and improved trauma resuscitation performance in a real-world setting.19,21,27 Simulation-based training has been associated with faster times to critical procedures and evaluations, including time to endotracheal intubation, time to head CT, and time to the operating room in experimental studies using this methodology as an educational intervention.21 Systematic implementation of simulation-based training for all providers in a trauma center led to faster time to critical operations after implementation.27 In addition to faster resuscitation processes, simulation-based training has also been shown to decrease missed critical steps during trauma resuscitation.19 While none of these single-center studies demonstrated a mortality benefit from training with simulation, they all postulate benefit from improved resuscitation times and faster time to critical procedures.
Based on our literature review, we hypothesized that simulation-based training would be associated with a more efficient resuscitation with shorter times to critical evaluations and procedures, but we found no difference in time to intubation, head CT, emergent craniotomy or emergent surgery for hemorrhage control. Conversely, we did demonstrate an association with simulation use and decreased risk-adjusted mortality, which brings into question the mechanism by which training with simulation may improve mortality. Our findings suggest that earlier performance of these procedures is not necessarily the only factor associated with lower mortality and are consistent with other evidence where quality of care for severely injured patients might be of greater value than small differences in time to intervention.30 Faster resuscitation time has been associated with improved survival,31 while early intubation13 and faster time to laparotomy12,14,15 have been shown to improve outcomes. Among patients with severe TBI requiring craniotomy, however, the association between time to surgery and outcome is not certain.12,16,17,32-34 The outcome of some reversible clinical scenarios, such as hemorrhagic shock, may be more dependent on rapid intervention.14,15,35 We did not find an association between the use of simulation-based training and time to surgery for hemorrhage control, but the median time to laparotomy was much longer than observed by previous adult data (10-36 min).14,15 This longer time to surgery for hemorrhage control in our cohort suggests that many of the patients we defined as ‘emergent’ may not have truly had immediate life-threatening hemorrhage, and we would not expect simulation-based training to have a profound impact on less urgent operative times. This longer time to laparotomy may also be a pediatric center-specific effect, with a greater emphasis on non-operative management. Furthermore, using our definition of ‘emergent craniotomy’, we demonstrated times to OR of approximately two hours – again questioning the true emergent nature of these operations, as one would expect a “crash” craniotomy to be in the operating room in 30-60 minutes from arrival to the trauma bay. In the absence of more granular physical exam findings and GCS trends over time, we are not able to better define a cohort of ‘emergent’ craniotomy patients using the TQIP dataset which may have led to our lack of demonstrating an impact on time to critical operations.
The survival advantage found in our study may be attributed to center-specific factors that are not measured in this study. While we have attributed the impact on mortality to simulation-based training, the use of simulation may alternatively serve as a proxy for other factors such as organizational culture that embraces teamwork, communication, and quality improvement. These latter factors may directly improve outcomes in the centers that have adopted simulation use. The use of simulation may also be a marker for more institutional resources, as the use of simulation requires significant financial investment. Children treated at pediatric trauma centers may have improved mortality compared to those treated at adult trauma centers,4,6,7,36 but we found no impact of center verification level (as a marker for resources) on the association of simulation use with improved mortality. Centers that use a higher-volume of simulation may also be more likely to adopt evidence-based practices. We attempted to control for these unmeasured factors using transfer-in patients from the same institutions as internal controls, assuming that hospital-wide factors would have an impact on both acutely resuscitated patients and transferred-in patients equally. We only saw an association with a mortality benefit in acutely resuscitated patients and not in transfer-in patients that were resuscitated at referring institutions – suggesting that whatever factor we are measuring does lead to some improvement in the initial resuscitation.
There are several limitations to this study. Our analysis was limited to centers that participate in ACS Pediatric TQIP – centers that, by definition, have significant resources. Our findings, therefore, may not be generalizable to all trauma centers. We would expect, however, the impact of simulation would be larger in lower-resourced centers that have more opportunity for improvement in initial resuscitation practices. Response bias may have contributed to the results as the frequency of simulation use was based on a survey in which we had a 25% non-response rate. Outcomes in non-response centers were similar to those in centers that do not use simulation suggesting the effect size may indeed be larger than we have shown. We accounted for this limitation by including non-responders as a separate category in our multivariable analysis. Outcomes in non-response centers were similar to those in centers that do not use simulation, suggesting the effect size may indeed be larger than we observed.
Our findings are inherently subject to issues common to a retrospective design, most prominent of which is that the level of simulation-based training was not randomly assigned. High-volume simulation centers had lower annual trauma admission volumes, suggesting the use of simulation-based training may be an attempt to supplement provider and team experience in the presence of lower clinical volumes. We noted minor differences in patient demographics and injury characteristics and significant differences in mechanism of injury between trauma centers using differing levels of simulation-based training. These variables were all adjusted for in the multivariable model, which should limit the impact of these differences. The cross-sectional design limits our ability to assess the impact of simulation on individual programs over time. Low-volume and lower-performing centers may have implemented simulation-based training to address inefficiencies in resuscitation. The lack of difference in time to procedures, therefore, may reflect a true improvement from baseline at these centers. Unmeasured confounding likely remained despite controlling for known independent predictors of mortality. For instance, we could not measure change in condition over time during the initial resuscitation. An initial GCS of 8 may rapidly improve during resuscitation and thus obviate the need for intubation. Experienced providers may elect to avoid intubation for a brief period to assess for response, which may provide an alternative explanation of the differences in intubation frequency and in the less than 100% intubation frequency for GCS of 8 or less. It also should be noted that ‘high volume’ simulation was arbitrarily defined for this study as over ten simulated scenarios in two years, and that median frequency of simulation use in ‘high-volume’ centers was 22 sessions over two years – or slightly less than once per month. While the ideal frequency with which teams should undergo simulation-based training is unknown, the expected effect size from this infrequent use may be modest.
CONCLUSIONS
Risk-adjusted mortality is lower among children treated at pediatric TQIP centers that use simulation. Whether this effect is directly attributable to simulation use or to a center-level factor that simulation-based training use serves as a proxy for remains unknown. Simulation use was not associated with faster time to critical evaluations and procedures, but intubation frequency for patients with low GCS was higher in centers using high-volume simulation, suggesting that quality (or fewer missed steps) may be a greater mediator of mortality than the speed of the resuscitation or its critical components. Prospective evaluation of simulation-based training within trauma centers is needed to show a casual improvement in mortality over time, as is further delineation of the mechanisms by which simulation contributes to a clinical benefit for trauma patients.
Supplementary Material
Acknowledgments
This work was supported by grant #KFVS6290 from the National Institute for Child Health and Development (NICHD) and grant #KL2TR001854 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This work was presented as a poster at the 77th Annual Meeting of the American Association for the Surgery of Trauma, San Diego, CA, September 26, 2018.
None of the authors have any conflicts of interest to disclose. This work is not under consideration for publication in any other journal.
REFERENCES
- 1.Borse NN, Gilchrist J, Dellinger AM, Rudd RA, Ballesteros MF, Sleet DA. Unintentional childhood injuries in the United States: key findings from the CDC childhood injury report. J Saf Res. 2009;40:71–74. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Injury Prevention and Control, CDC using WISQARS. Ten Leading Causes of Death by Age Group, United States - 2015 Available from: https://www.cdc.gov/injury/wisqars/LeadingCauses.html. Accessed May 17, 2017.
- 3.McLaughlin C, Zagory JA, Fenlon M, Park C, Lane CJ, Meeker D, Burd RS, Ford HR, Upperman JS, Jensen AR. Timing of Mortality in Pediatric Trauma Patients: A National Trauma Databank Analysis. J Pediatr Surg. 2018;53(2):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amini R, Lavoie A, Moore L, Sirois MJ, Emond M. Pediatric trauma mortality by type of designated hospital in a mature inclusive trauma system. J Emerg Trauma Shock. 2011;4:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potoka DA, Schall LC, Ford HR. Improved functional outcome for severely injured children treated at pediatric trauma centers. J Trauma. 2001;51:824–832; discussion 832-834. [DOI] [PubMed] [Google Scholar]
- 6.Potoka DA, Schall LC, Gardner MJ, Stafford PW, Peitzman AB, Ford HR. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000;49:237–245. [DOI] [PubMed] [Google Scholar]
- 7.Sathya C, Alali AS, Wales PW, Scales DC, Karanicolas PJ, Burd RS, Nance ML, Xiong W, Nathens AB. Mortality Among Injured Children Treated at Different Trauma Center Types. JAMA Surg. 2015;150:874–881. [DOI] [PubMed] [Google Scholar]
- 8.Walther AE, Falcone RA, Pritts TA, Hanseman DJ, Robinson BR. Pediatric and adult trauma centers differ in evaluation, treatment, and outcomes for severely injured adolescents. J Pediatr Surg. 2016;51:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen EH, Cho CS, Shofer FS, Mills AM, Baren JM. Resident exposure to critical patients in a pediatric emergency department. Pediatr Emerg Care. 2007;23:774–778. [DOI] [PubMed] [Google Scholar]
- 10.Avraham JB, Bhandari M, Frangos SG, Levine DA, Tunik MG, DiMaggio CJ. Epidemiology of paediatric trauma presenting to US emergency departments: 2006-2012. Inj Prev. Epub ahead of print October 22, 2017. DOI: 10.1136/injuryprev-2017-042435. [DOI] [PubMed] [Google Scholar]
- 11.Mittiga MR, Geis GL, Kerrey BT, Rinderknecht AS. The spectrum and frequency of critical procedures performed in a pediatric emergency department: implications of a provider-level view. Ann Emerg Med. 2013;61:263–270. [DOI] [PubMed] [Google Scholar]
- 12.Davidson GH, Maier RV, Arbabi S, Goldin AB, Rivara FP. Impact of operative intervention delay on pediatric trauma outcomes. J Trauma Acute Care Surg. 2012;73:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miraflor E, Chuang K, Miranda MA, Dryden W, Yeung L, Strumwasser A, Victorino GP. Timing is everything: delayed intubation is associated with increased mortality in initially stable trauma patients. J Surg Res. 2011;170:286–290. [DOI] [PubMed] [Google Scholar]
- 14.Meizoso JP, Ray JJ, Karcutskie CA, Allen CJ, Zakrison TL, Pust GD, Koru-Sengul T, Ginzburg E, Pizano LR, Schulman CI, et al. Effect of time to operation on mortality for hypotensive patients with gunshot wounds to the torso: The golden 10 minutes. J Trauma Acute Care Surg. 2016;81:685–691. [DOI] [PubMed] [Google Scholar]
- 15.Barbosa RR, Rowell SE, Fox EE, Holcomb JB, Bulger EM, Phelan HA, Alarcon LH, Myers JG, Brasel KJ, Muskat P, et al. Increasing time to operation is associated with decreased survival in patients with a positive FAST examination requiring emergent laparotomy. J Trauma Acute Care Surg. 2013;75:S48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinh MM, Bein K, Roncal S, Byrne CM, Petchell J, Brennan J. Redefining the golden hour for severe head injury in an urban setting: the effect of prehospital arrival times on patient outcomes. Injury. 2013;44:606–610. [DOI] [PubMed] [Google Scholar]
- 17.Tien HCN, Jung V, Pinto R, Mainprize T, Scales DC, Rizoli SB. Reducing time-to-treatment decreases mortality of trauma patients with acute subdural hematoma. Ann Surg. 2011;253:1178–1183. [DOI] [PubMed] [Google Scholar]
- 18.Auerbach M, Roney L, Aysseh A, Gawel M, Koziel J, Barre K, Caty MG, Santucci K. In situ pediatric trauma simulation: assessing the impact and feasibility of an interdisciplinary pediatric in situ trauma care quality improvement simulation program. Pediatr Emerg Care. 2014;30:884–891. [DOI] [PubMed] [Google Scholar]
- 19.Steinemann S, Berg B, Skinner A, DiTulio A, Anzelon K, Terada K, Oliver C, Ho HC, Speck C. In situ, multidisciplinary, simulation-based teamwork training improves early trauma care. J Surg Educ. 2011;68:472–477. [DOI] [PubMed] [Google Scholar]
- 20.Holcomb JB, Dumire RD, Crommett JW, Stamateris CE, Fagert MA, Cleveland JA, Dorlac GR, Dorlac WC, Bonar JP, Hira K, et al. Evaluation of trauma team performance using an advanced human patient simulator for resuscitation training. J Trauma. 2002;52:1078–1085. [DOI] [PubMed] [Google Scholar]
- 21.Capella J, Smith S, Philp A, Putnam T, Gilbert C, Fry W, Harvey E, Wright A, Henderson K, Baker D, et al. Teamwork training improves the clinical care of trauma patients. J Surg Educ. 2010;67:439–443. [DOI] [PubMed] [Google Scholar]
- 22.Falcone RA Jr, Daugherty M, Schweer L, Patterson M, Brown RL, Garcia VF. Multidisciplinary pediatric trauma team training using high-fidelity trauma simulation. J Pediatr Surg. 2008;43:1065–1071. [DOI] [PubMed] [Google Scholar]
- 23.Andreatta P, Saxton E, Thompson M, Annich G. Simulation-based mock codes significantly correlate with improved pediatric patient cardiopulmonary arrest survival rates. Pediatr Crit Care Med. 2011;12:33–38. [DOI] [PubMed] [Google Scholar]
- 24.Popp J, Yochum L, Spinella PC, Donahue S, Finck C. Simulation training for surgical residents in pediatric trauma scenarios. Conn Med. 2012;76:159–162. [PubMed] [Google Scholar]
- 25.Couto TB, Kerrey BT, Taylor RG, FitzGerald M, Geis GL. Teamwork skills in actual, in situ, and in-center pediatric emergencies: performance levels across settings and perceptions of comparative educational impact. Simul Healthc. 2015;10:76–84. [DOI] [PubMed] [Google Scholar]
- 26.Jensen AR, McLaughlin C, Wong CF, McAuliff K, Nathens AB, Barin E, Meeker D, Ford HR, Burd RS, Upperman JS. Simulation-based training for trauma resuscitation among ACS TQIP-Pediatric centers: Understanding prevalence of use, associated center characteristics, training factors, and implementation barriers. Am J Surg. 2019;217:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy M, Curtis K, Lam MK, Palmer CS, Hsu J, McCloughen A. Simulation-based multidisciplinary team training decreases time to critical operations for trauma patients. Injury. 2018;49:953–958. [DOI] [PubMed] [Google Scholar]
- 28.American College of Surgeons. Pediatric TQIP Benchmark Report Cycle References: Spring 2016 Cycle. Chicago, IL. [Google Scholar]
- 29.Newgard CD, Fildes JJ, Wu L, Hemmila MR, Burd RS, Neal M, Mann NC, Shafi S, Clark DE, Goble S, Nathens AB. Methodology and analytic rationale for the American College of Surgeons Trauma Quality Improvement Program. J Am Coll Surg. 2013;216:147–157. [DOI] [PubMed] [Google Scholar]
- 30.Haas B, Jurkovich GJ, Wang J, Rivara FP, Mackenzie EJ, Nathens AB. Survival advantage in trauma centers: expeditious intervention or experience? J Am Coll Surg. 2009;208:28–36. [DOI] [PubMed] [Google Scholar]
- 31.Townsend RN, Clark R, Ramenofsky ML, Diamond DL. ATLS-based videotape trauma resuscitation review: education and outcome. J Trauma. 1993;34:133–138. [DOI] [PubMed] [Google Scholar]
- 32.Walcott BP, Khanna A, Kwon C-S, Phillips HW, Nahed BV, Coumans JV. Time interval to surgery and outcomes following the surgical treatment of acute traumatic subdural hematoma. J Clin Neurosci. 2014;21:2107–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma: morbidity and mortality related to timing of operative intervention. J Trauma. 1990;30:733–736. [PubMed] [Google Scholar]
- 34.Matsushima K, Inaba K, Siboni S, Skiada D, Strumwasser AM, Magee GA, Sung GY, Benjaminm ER, Lam L, Demetriades D. Emergent operation for isolated severe traumatic brain injury: Does time matter? J Trauma Acute Care Surg. 2015;79:838–842. [DOI] [PubMed] [Google Scholar]
- 35.Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC. Earlier Endpoints are Required for Hemorrhagic Shock Trials Among Severely Injured Patients. Shock. 2017;47:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webman RB, Carter EA, Mittal S, Wang J, Sathya C, Nathens AB, Nance ML, Madigan D, Burd RS. Association Between Trauma Center Type and Mortality Among Injured Adolescent Patients. JAMA Pediatr. 2016;170:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.