Abstract
Background:
Cigarette smoke contains microbes and microbial toxins, such as endotoxin and , that may have adverse respiratory effects. To our knowledge, the potential for contamination of electronic cigarette (EC) products sold in the United States has not been investigated.
Objectives:
We aimed to determine whether popular cartridge and e-liquid EC products were contaminated with endotoxin or glucan and to examine differences according to the type and flavor of products.
Methods:
We selected 37 cartridges and 38 e-liquid products with the highest nicotine content from the ten top-selling U.S. brands. Flavors were classified into four groups: tobacco, menthol, fruit, and other. Endotoxin and glucan were measured using an endotoxin-specific kinetic turbidimetric assay and a Glucatell® Kinetic Assay (Associates of Cape Cod, Inc.), respectively.
Results:
Endotoxin concentrations were over the limit of detection (LOD) in 17 of 75 products tested (23%), and glucan concentrations were greater than LOD in 61 of 75 products (81%). After adjusting for brand and flavor, the mean glucan concentration was 3.2 times higher [95% confidence interval (CI): , 18.4] in cartridge vs. e-liquid samples. After adjusting for brand and type of product, glucan concentrations in tobacco- and menthol-flavored ECs were 10.4 (95% CI: 1.8, 44.9) and 3.5 (95% CI: 0.1, 17.3) times higher than concentrations found in fruit-flavored products.
Conclusions:
EC products may be contaminated with microbial toxins. Further studies with large representative samples of products are needed to confirm our findings, identify sources and routes of contamination, and evaluate health effects associated with the use of contaminated products. https://doi.org/10.1289/EHP3469
Introduction
The use of e-cigarettes (ECs), also called electronic nicotine delivery systems (ENDS), has increased substantially, with sales in the United States reaching an estimated in 2015, a 32% increase from in 2014 (Marynak et al. 2017). Based on the U.S. National Youth Tobacco Survey (NYTS), use among U.S. teens increased 1.5% (220,000 students) to 20.8% (3.05 million students) among high school students, and 0.6% (60,000 students) to 4.9% (570,000 students) among middle school students between 2011 and 2018 (Cullen et al. 2018), and EC products were the most commonly used by U.S. teens in 2017, exceeding reported use of cigarettes, chewing tobacco, cigars, and hookahs (Wang et al. 2018). Furthermore, in a nationally representative sample of U.S. teens (12–17 years of age) who never smoked a conventional cigarette before enrollment, those who reported ever using EC at baseline were 3.5 times [95% confidence interval (CI): 2.5, 4.9] more likely than never- EC users to have smoked at least one cigarette a year later (Watkins et al. 2018).
Although the use of ECs continues to climb, data on exposures and potential human health effects are lacking. Investigations have focused on chemical content, such as nicotine, tobacco-specific nitrosamines, carbonyl compounds, aldehydes, fine particulate matter, metals, volatile organic compounds (VOCs), flavorings, and other additives, which have been found in various EC matrices, including refill solutions, cartridges, aerosols, and environmental emissions, and in aqueous solution (Allen et al. 2016; Bekki et al. 2014; Cheng 2014; Farsalinos et al. 2014, 2015; Fernández et al. 2015; Geiss et al. 2015; Goniewicz et al. 2014a, 2014b; Ingebrethsen et al. 2012; Jensen et al. 2015; Klager et al. 2017; Lee et al. 2017; Melstrom et al. 2017; Orr 2014; Pankow et al. 2017; Park et al. 2019; Schober et al. 2014; Uchiyama et al. 2013; Williams et al. 2013). However, to our knowledge, no data are available on the contamination of EC products with microbes or microbial toxins.
Microbial agents such as endotoxin [or lipopolysaccharide (LPS)], part of the outer membrane of Gram-negative bacteria (Bos and Tommassen 2004), and , a fungal cell wall constituent, have been associated with adverse respiratory health outcomes (Adhikari et al. 2011; Douwes 2005; Maheswaran et al. 2014; Pauly and Paszkiewicz 2011).
Endotoxin is ubiquitous in the environment and is present at higher concentrations in tobacco smoke than in smoke-free indoor air (Larsson et al. 2004; Szponar et al. 2012). Endotoxin also has been identified in occupational settings, such as cotton-textile workplaces (Lai et al. 2012, 2015), agricultural environments (e.g., livestock, dairy) (Donham 2010; Basinas et al. 2015), and waste-incineration facilities (Park et al. 2011), as well as in residential environments (Carnes et al. 2017; Holst et al. 2015; Lee et al. 2018; Yoda et al. 2017). Endotoxin exposure causes emphysematous changes (Brass et al. 2008) and airway remodeling (Brass et al. 2003) in experimental animals. In humans, occupational endotoxin exposure has been associated with the development of airflow obstruction, respiratory symptoms, reduced lung function, and current atopic and nonatopic asthma (Carnes et al. 2017; Castellan et al. 1987; Lai et al. 2012, 2015). Household endotoxin exposure has been also associated with increased peripheral leucocyte count, a biomarker of inflammation (Fessler et al. 2017), and increased asthma prevalence in the National Health and Nutrition Examination Survey (NHANES) 2005–2006 (Thorne et al. 2015).
is a polymer of glucose that is present in the cell walls of most fungi, plants, certain bacteria, and algae (Douwes 2005). Airborne has been used as a surrogate to estimate the human exposure to fungi in indoor environments (Adhikari et al. 2011; Douwes et al. 2006; Iossifova et al. 2008; Schram‐Bijkerk et al. 2005). in house dust has been associated with a greater annual decline in forced expiratory volume in 1 s () among residents of a rowhouse (Thorn and Rylander 1998) and persistent atopic asthma and the onset of bronchial hyperresponsiveness (BHR) in adolescent children (Maheswaran et al. 2014). These bacterial and fungal components are believed to contribute, in part, to lung inflammation in smokers (Pauly and Paszkiewicz 2011).
Although contamination of e-liquid (also called e-juice) with microbes or microbial toxins is possible, to our knowledge no study of microbial contamination of EC products has been published. Therefore, as a first step toward assessing potential hazards to EC users, we assayed samples of EC cartridges and e-liquids sold by the top 10 retail brands in the United States for endotoxin and glucan contamination.
Methods
Selection of EC Cartridges and E-Liquids
The total number of products tested was determined by the availability of funds to cover the cost of the assays. Specific EC products included in our study sample (total ) were selected based on the following criteria. First, we identified the 10 top-selling EC product brands in the United States during 2013 (hereafter referred to as Brands A–J) based on Nielsen Scantrak Data, which reflect representative sales estimates that were derived from information from in-store scanners and field audits of retail outlets without scanners (Giovenco et al. 2015; Herzog et al. 2014). Next, we ranked all cartridge products (first generation, also known as cigalikes) and all e-liquid products (refillable e-liquid bottles) sold by the top 10 brands. This selection included all the flavors, except the mixed flavor, as indicated on product labels (Brand I, 8 samples). If labels listed more than one available nicotine content amount for each flavor by brand and type, we selected the product with the highest nicotine content as indicated on product labels. Finally, the total 75 samples included 37 cartridges and 38 e-liquids samples (Figure S1). All selected products were available online and purchased from the EC company websites (9 company websites, 69 samples), except the products from one brand (Brand G). We purchased the products from that brand (Brand G, 6 samples) from the convenience store located near the Harvard T.H. Chan School of Public Health based on convenience and costs. After purchase, products were stored in a dark room at room temperature about 20 to 22 degrees celsius in their original packages until they were shipped to the laboratory where all sample preparation and assays were performed (Associates of Cape Cod, Inc., East Falmouth, Massachusetts).
Quantification of Microbial Markers
Sample preparation.
All samples were extracted in a laminar flow hood using aseptic techniques.
Cartridges.
EC cartridges, which are cylinder-like chambers, contain fibrous pads that absorb the e-liquid and act as a wick to deliver the liquid to the atomizer. Depyrogenated forceps were used to remove the pad from each cartridge and squeeze the liquid from it. Liquid from three cartridges of the same product from the same package was pooled in a depyrogenated beaker and transferred to depyrogenated glass tubes for retention and testing. The samples were diluted with LRW in serial dilution and tested, in duplicate, to find minimum noninterfering dilutions.
E-Liquids.
E-liquid samples were not pooled, and a small portion of e-liquid was taken from an e-liquid bottle. In the same manner as the cartridge samples were taken, the e-liquid samples were diluted and tested to find minimum noninterfering dilutions.
Sample assays.
Endotoxin.
Endotoxin was measured using an endotoxin-specific kinetic turbidimetric in a Limulus amebocyte lysate (LAL) assay. Samples were tested in duplicate with Limulus reagent water (LRW) in serial dilutions ranging from 1:20 to 1:320,000 to find the minimum noninterfering dilution. The limits of detection (LODs) for the endotoxin assays ranged from 0.1 to 1.6 endotoxin units (EU)/mL. All absolute values for the correlation coefficients for the calibration curves using Control Standard Endotoxin (Associates of Cape Cod, Inc.) were . Negative controls (LRW) for each batch were lower than lowest standard ( or ), and recovery of product positive controls (; Control Standard Endotoxin) ranged 50% to 200% for endotoxin. These assay parameters, including correlation coefficients, negative controls, and positive product controls, met the U.S. Pharmacopeia (USP) requirement for the endotoxin assay (USP 2016).
.
was measured using a Glucatell® Kinetic Assay (Associates of Cape Cod, Inc.). Samples were tested in duplicate with LRW in serial dilutions from 1:20 to 1:320,000 to find the minimum noninterfering dilution. All absolute values for the correlation coefficients for the calibration curves using standard (Pachyman, Associates of Cape Cod, Inc.) were . LODs ranged from 0.0125 to . LRW controls for each batch were lower than lowest standard ( or ). USP requirements had not been set for the glucan assay at the time our study was performed.
Replicate samples.
We used simple random samplings (SAS® PROC SURVEYSELECT; version 9.4, SAS Institute Inc.) to select 4 cartridge samples out of 37 cartridge products and 4 e-liquid samples out of 38 e-liquid products, respectively (more than 10% of total sample size) for replicate assays (Table S1). Samplings were without replacement, and the selection probability for each cartridge and e-liquid equals 0.108 and 0.105, respectively. For cartridge samples, we pooled liquid from three cartridges for the replicate sample from the same package for each product selected (i.e., testing a primary sample and a replicate sample from the same package). For e-liquid samples, we assayed liquid from the same bottle (i.e., testing a primary sample and a replicate sample from the same bottle). For glucan, the coefficient of variance (CV) for each of the 8 pairs of replicate samples ranged from 1.3% to 22.4% (Table S1). Endotoxin concentrations were less than LOD in all samples tested.
Statistical Analysis
Data were analyzed using the SAS Statistical Package (version 9.4; SAS Institute Inc.). Values below the LOD for endotoxin or glucan were imputed as the LOD/2 (Allen et al. 2016). Descriptive statistics include the mean, median, and range of microbial markers, according to product type (cartridge or e-liquid), flavor (4 groups), and brand. Product flavors were categorized as tobacco, menthol, fruit, or other (Table 1), based on product names and descriptions provided on distributor websites, consistent with Nielsen classifications (Giovenco et al. 2015). The fruit category included any flavor that referenced a fruit or fruit-like product (e.g., peach, strawberry); the category titled other included miscellaneous flavors, such as vanilla, chocolate, and coffee. We also derived Spearman correlations between endotoxin and glucan concentrations in each sample. We used linear regressions to estimate differences in endotoxin and glucan concentrations, according to product type (cartridge vs. e-liquid) and flavor (tobacco, menthol, or other vs. fruit), before and after adjusting for brand (modeled using indicator terms) and either flavor or type, as appropriate. Model estimates were converted to percent differences as , where is the estimated regression coefficient.
Table 1.
EC Flavor categories according Nielsen classification.
| Flavor type | Flavors in this group |
|---|---|
| Tobacco | Classic tobacco, platinum label tobacco, bold tobacco, gold tobacco, regular, traditional tobacco, tobacco-bold, original, tobacco, traditional, classic, American blend (tobacco), pro-platinum label tobacco |
| Menthol | Magnificent menthol, platinum label menthol, menthol, menthol-bold, cool ice blend (menthol), pro-platinum label menthol |
| Fruit | Cherry crush, peach schnapps, pomegranate, berry, acai berry, strawberry, peach, Washington red, ocean mist (melon), grape, mango, apple, berry, pineapple, watermelon, menthol citrus, citrus crush, Havana, tropical fruit, , peach tea |
| Others | Java jolt, vivid vanilla, piña colada, mint, cream, chai, vanilla, fusion, winter mint, java (coffee), vanilla bean |
Results
Among the 75 EC products tested, endotoxin concentrations were greater than LOD in 17 products (23%) and glucan concentrations were greater than LOD in 61 products (81%) (Table 2). One product (a fruit-flavored e-liquid, brand F, Table S2) was greater than LOD for endotoxin but less than LOD for glucan; otherwise, all products that were greater than LOD for endotoxin were also greater than LOD for glucan. Endotoxin concentrations were greater than LOD in 12 of 37 cartridge products, and in 4 of 16, 1 of 15, 7 of 29, and 5 of 15 tobacco-, menthol-, fruit-, and other-flavored products, respectively. Glucan concentrations were greater than LOD in all cartridge products and in 16 of 16 tobacco-flavored products, 13 of 15 menthol-flavored products, 19 of 29 fruit-flavored products, and 13 of 15 other-flavored, respectively. When evaluated by brand, endotoxin concentrations were greater than LOD in at least one product from 7 of the 10 brands, whereas glucan concentrations were greater than LOD in every product tested for 8 brands, and in 3 of 7 and 8 of 18 products from Brands F and I, respectively. Both microbial contaminants were less than LOD in 13 products (17%), including 3 of 7 products from Brand F, and 10 of 18 products from Brand I. Information on the brand, type, flavor, and endotoxin and glucan concentrations of each of the 75 samples tested is provided in Table S2.
Table 2.
Characteristics and endotoxin and glucan concentrations of 75 products included in the study sample according to product type, flavor, and brand.
| Endotoxin (EU/mL)a | Glucan (ng/mL)a | Bothb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | Median | Min | Max | (%) | Median | Min | Max | (%) | |||
| Overall | 75 (100) | 17 (23) | 0.10 | 0.05 | 1.64 | 61 (81) | 1.06 | 0.01 | 1,450.00 | 13 (17) | ||
| By type | ||||||||||||
| Cartridge | 37 (49) | 12 (32) | 0.20 | 0.05 | 1.64 | 37 (100) | 7.15 | 0.37 | 1,450.00 | 0 (0) | ||
| E-liquid | 38 (51) | 5 (13) | 0.05 | 0.05 | 0.89 | 24 (63) | 0.10 | 0.01 | 202.80 | 13 (34) | ||
| By flavor | ||||||||||||
| Tobacco | 16 (21) | 4 (25) | 0.08 | 0.05 | 0.74 | 16 (100) | 9.30 | 0.03 | 202.80 | 0 (0) | ||
| Menthol | 15 (20) | 1 (7) | 0.05 | 0.05 | 0.80 | 13 (87) | 5.73 | 0.01 | 38.20 | 2 (13) | ||
| Fruit | 29 (39) | 7 (24) | 0.10 | 0.05 | 0.89 | 19 (66) | 0.09 | 0.01 | 113.40 | 9 (31) | ||
| Others | 15 (20) | 5 (33) | 0.05 | 0.05 | 1.64 | 13 (87) | 5.40 | 0.02 | 1,450.00 | 2 (13) | ||
| By brand | ||||||||||||
| A | 7 (9) | 4 (57) | 0.40 | 0.10 | 1.64 | 7 (100) | 5.40 | 3.30 | 1,450.00 | 0 (0) | ||
| B | 4 (5) | 0 (0) | 0.08 | 0.05 | 0.10 | 4 (100) | 0.63 | 0.12 | 1.24 | 0 (0) | ||
| C | 19 (25) | 4 (21) | 0.10 | 0.05 | 0.89 | 19 (100) | 0.45 | 0.08 | 36.20 | 0 (0) | ||
| D | 2 (3) | 0 (0) | 0.05 | 0.05 | 0.05 | 2 (100) | 1.30 | 1.24 | 1.36 | 0 (0) | ||
| E | 2 (3) | 1 (50) | 0.77 | 0.74 | 0.80 | 2 (100) | 18.15 | 16.40 | 19.90 | 0 (0) | ||
| F | 7 (9) | 2 (29) | 0.20 | 0.05 | 0.80 | 3 (43) | 0.02 | 0.01 | 202.80 | 3 (43) | ||
| G | 6 (8) | 1 (17) | 0.05 | 0.05 | 0.33 | 6 (100) | 7.03 | 1.60 | 9.73 | 0 (0) | ||
| H | 6 (8) | 4 (67) | 0.73 | 0.20 | 0.83 | 6 (100) | 12.35 | 2.20 | 13.70 | 0 (0) | ||
| I | 18 (24) | 1 (6) | 0.05 | 0.05 | 0.80 | 8 (44) | 0.03 | 0.01 | 113.40 | 10 (56) | ||
| J | 4 (5) | 0 (0) | 0.05 | 0.05 | 0.20 | 4 (100) | 21.75 | 16.70 | 25.30 | 0 (0) | ||
Note: See Table S2 for characteristics and concentrations in each product tested.
Concentrations less than LOD were replaced by when calculating distributions. LODs ranged from for endotoxin and from for glucan.
Number (%) of samples with measured concentrations less than LOD for both endotoxin and glucan.
After substituting values less than LOD with LOD/2, geometric mean concentrations () of endotoxin and glucan in all tested EC products were (, range ) and (, range ), respectively (Table 2). Endotoxin and glucan concentrations in individual samples were positively correlated (Spearman correlation coefficient , ).
On average, glucan concentrations were 4,123% higher (95% CI: 1,408, 11,727%, ) in EC cartridges relative to e-liquid samples (Table 3 and Figure 1), though the difference decreased to 318% (95% CI: , 1,842%, ) when adjusted for brand and flavor (Table 3). When adjusted for brand and product type, glucan concentrations were 1,042% (95% CI: 184, 4,489%, ) and 350% (95% CI: 11, 1,733%, ) higher in tobacco- and menthol-flavored products than concentrations found in fruit-flavored products, but similar for other-flavored products (112% higher, 95% CI: , 710%; ). Endotoxin concentrations did not show clear differences according to product type or flavor.
Table 3.
Percent differences [95% confidence intervals (CIs)] for endotoxin and glucan levels associated with type and flavor.
| Endotoxin | Glucan | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted models | Adjusted model | Unadjusted models | Adjusted model | |||||
| % Difference | p-Value | % Difference | p-Value | % Difference | p-Value | % Difference | p-Value | |
| Typea | ||||||||
| Cartridge | 66 (, 178) | 0.06 | 23 (, 159) | 0.6 | 4123 (1408, 11727) | 318 (, 1842) | 0.07 | |
| E-liquid | Ref | Ref | Ref | Ref | ||||
| Flavorb | ||||||||
| Tobacco | (, 50) | 0.4 | (, 19) | 0.1 | 2421 (391, 12845) | 1042 (184, 4489) | 0.001 | |
| Menthol | (, 52) | 0.4 | (, 20) | 0.2 | 822 (73, 4800) | 0.01 | 350 (11, 1733) | 0.04 |
| Others | (, 94) | 0.9 | (, 56) | 0.5 | 928 (93, 5363) | 0.01 | 112 (, 710) | 0.3 |
| Fruit | Ref | Ref | Ref | Ref | ||||
Note: Sample levels below LOD are substituted by a half the LOD. LODs ranged from 0.1 to for endotoxin and from 0.0125 to for glucan. Ref, reference.
Adjusted for flavor and brand (A to J).
Adjusted for type and brand (A to J).
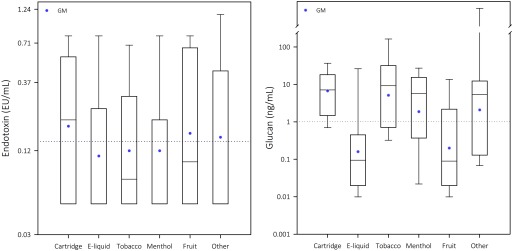
Figure 1.
Boxplots showing the median (horizontal line in box), interquartile range (the central rectangle), and the fifth and the 95th percentiles (whiskers above and below the box) of endotoxin and glucan levels by EC type and flavor. Geometric means (GM) by type and flavor are shown as dots. Overall GMs ( for endotoxin and for glucan) are shown as horizontal dotted lines. Median endotoxin concentrations in e-liquid samples, and menthol- and other-flavor samples are not visible as separate lines because they are equal to the concentration at the fifth percentile ().
Discussion
To our knowledge, this study is the first to identify endotoxin and glucan in EC cartridges and e-liquids sold in the United States. Endotoxin was detected in 17 of 75 (23%) of EC samples, and glucan was detected in 61 of 75 (81%) of EC samples. After adjusting for brand and flavor, the estimated mean glucan concentration in EC cartridges was 3.2 times higher (95% CI: , 18) than in concentrations found in e-liquids. When adjusted for brand and product type, glucan concentrations in tobacco- and menthol-flavored ECs were 10 times (95% CI: 1.8, 45) and 3.5 times (95% CI: 0.11, 17) higher than concentrations in fruit-flavored ECs, respectively. The products included in our analysis were sampled from high-nicotine products sold by popular brands and were not intended to be a representative sample of all EC products sold in the United States (overall, or by brand, type of product, or flavor). Contamination might have occurred at any point during the production of ingredients or finished EC products, and further research is needed to determine whether microbial contaminants that are present in e-liquids prior to aerosolization result in exposures or health risks to users. However, differences in glucan concentrations among the products included in our study sample may provide clues about sources of contamination and suggest that risks might vary by EC type and flavor.
Previous studies have not evaluated endotoxins in EC products, but Hasday et al. reported the first evidence of bioactive LPS (bacterial endotoxin) in cigarette smoke particles and noted higher bioactive LPS in particles collected from mainstream smoke than in particles collected from sidestream smoke samples (Hasday et al. 1999). Larsson et al. reported that the concentration of bacterial endotoxin in airborne particles sampled from a room in which 15 cigarettes were smoked over 7 h was 120 times higher than in indoor air samples collected from the same room in the absence of smoking (Larsson et al. 2004). Although no scientific evidence supports a hypothesis that current observed levels of endotoxin and glucan in ECs raise health concerns, adverse responses of respiratory and immunological systems to exposure to endotoxin and glucan in epidemiologic studies (responses such as reduced lung function; increase in nonatopic asthma, bronchial hyper-responsiveness and peripheral leucocyte count; inflammation; and airflow obstruction) suggest the potential effects on the inhalation exposure route during EC smoking (Carnes et al. 2017; Castellan et al. 1987; Lai et al. 2012, 2015; Maheswaran et al. 2014; Thorn and Rylander 1998; Thorne et al. 2015).
Cartridge ECs contained wicks made of cotton or other fibers (Chun et al. 2017). Endotoxin and glucan are biological contaminants of cotton fibers (Lane and Sewell 2006); thus, contamination of cartridge wicks may be a source of endotoxin and glucan contamination and might contribute to higher concentrations of glucans in cartridge ECs than in e-liquids. Tobacco-, menthol-, and other-flavored ECs had higher glucan concentrations than concentrations found in fruit-flavored ECs, but differences were attenuated after adjusting for EC type and brand. In contrast, endotoxin concentrations were higher in fruit-flavored products than concentrations found in other products, though differences were not significant. Raw materials used to manufacture flavors might be a source of microbial contamination, but contamination during the manufacture of flavors, other EC components, or finished EC products is also possible.
In 2016, the U.S. FDA issued a final rule to begin regulating ECs as tobacco products under the Family Smoking Prevention and Tobacco Control Act (2009), which defines tobacco products as “any product made or derived from tobacco that is intended for human consumption, including any component, part, or accessory of a tobacco product.” We analyzed EC products containing the highest strength of nicotine in each brand and flavor, but we do not know the origin of the nicotine used in the tested EC products, which may be natural nicotine obtained from tobacco leaves or tobacco-free synthetic nicotine. Nicotine derived from tobacco leaves might be a source of endotoxin or glucan contamination, but synthetic nicotine also might be contaminated during manufacturing.
We acknowledge several limitations. We tested only for contamination of samples from cartridges and e-liquids, which may differ from other types of EC products, such as second-generation (pens), third-generation (tanks/MODs), and fourth-generation (pods) devices. We did not test multiple samples of the same product to assess variation among different batches or packages of the same product. In addition, we identified endotoxin and glucan in samples from cartridges and bottles of e-liquids, but we did not evaluate contamination of aerosols inhaled by users. Finally, we analyzed small numbers of products that were selected from popular brands. Future studies should include larger numbers of products, should test products that have been systematically sampled to be representative of all EC products sold in the United States, should perform repeat tests of individual products from different production batches to assess within-product variability, should conduct targeted testing to evaluate specific sources of contamination and variation among different types of EC products, and should measure endotoxin and glucan concentrations in aerosol samples.
In conclusion, our findings indicate that some popular EC brands and flavors may be contaminated with microbial toxins. Additional research is needed to confirm our findings and assess potential exposures and health effects in EC users.
Supplementary Material
Acknowledgments
This research was made possible by grant no. T42 OH008416, from the National Institute for Occupational Safety and Health (NIOSH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIOSH.
References
- Adhikari A, Gupta J, Wilkins JR 3rd, Olds RL, Indugula R, Cho KJ. 2011. Airborne microorganisms, endotoxin, and (1→3)-beta-d-glucan exposure in greenhouses and assessment of respiratory symptoms among workers. Ann Occup Hyg 55:272–285, PMID: 21177263, 10.1093/annhyg/meq082. [DOI] [PubMed] [Google Scholar]
- Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH. 2016. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect 124(6):733–739, PMID: 26642857, 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinas I, Sigsgaard T, Kromhout H, Heederik D, Wouters IM, Schlünssen V. 2015. A comprehensive review of levels and determinants of personal exposure to dust and endotoxin in livestock farming. J Expo Sci Environ Epidemiol 25(2):123–137, PMID: 24280684, 10.1038/jes.2013.83. [DOI] [PubMed] [Google Scholar]
- Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. 2014. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health 11(11):11192–11200, PMID: 25353061, 10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MP, Tommassen J. 2004. Biogenesis of the Gram-negative bacterial outer membrane. Curr Opin Microbiol 7(6):610–616, PMID: 15556033, 10.1016/j.mib.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Brass DM, Hollingsworth JW, Cinque M, Li Z, Potts E, Toloza E, et al. . 2008. Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis. Am J Respir Cell Mol Biol 39(5):584–590, PMID: 18539952, 10.1165/rcmb.2007-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass DM, Savov JD, Gavett SH, Haykal-Coates N, Schwartz DA. 2003. Subchronic endotoxin inhalation causes persistent airway disease. Am J Physiol Lung Cell Mol Physiol 285(3):L755–L761, PMID: 12794002, 10.1152/ajplung.00001.2003. [DOI] [PubMed] [Google Scholar]
- Carnes MU, Hoppin JA, Metwali N, Wyss AB, Hankinson JL, O'Connell EL, et al. . 2017. House dust endotoxin levels are associated with adult asthma in a U.S. farming population. Ann Am Thorac Soc 14(3):324–331, PMID: 27977294, 10.1513/AnnalsATS.201611-861OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan RM, Olenchock SA, Kinsley KB, Hankinson JL. 1987. Inhaled endotoxin and decreased spirometric values. An exposure-response relation for cotton dust. N Engl J Med 317(10):605–610, PMID: 3614274, 10.1056/NEJM198709033171005. [DOI] [PubMed] [Google Scholar]
- Cheng T. 2014. Chemical evaluation of electronic cigarettes. Tob Control 23 (Suppl 2):ii11–ii17, 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. 2017. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol 313(2):L193–L206, PMID: 28522559, 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. 2018. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students - United States, 2011-2018. MMWR Morb Mortal Wkly Rep 67(45):1276–1277, PMID: 30439875, 10.15585/mmwr.mm6745a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donham KJ. 2010. Community and occupational health concerns in pork production: a review. J Anim Sci 88(13 Suppl):E102–E111, PMID: 20154166, 10.2527/jas.2009-2554. [DOI] [PubMed] [Google Scholar]
- Douwes J. 2005. (1→3)-Beta-D-glucans and respiratory health: a review of the scientific evidence. Indoor Air 15(3):160–169, PMID: 15865616, 10.1111/j.1600-0668.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- Douwes J, Van Strien R, Doekes G, Smit J, Kerkhof M, Gerritsen J, et al. . 2006. Does early indoor microbial exposure reduce the risk of asthma? The Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J Allergy Clin Immunol 117(5):1067–1073, PMID: 16675334, 10.1016/j.jaci.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Family Smoking Prevention and Tobacco Control Act. 2009. Pub. L No. 111–31 (111th Congress, 22 June 2009) https://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm246129.htm [accessed 30 October 2016].
- Farsalinos KE, Kistler KA, Gillman G, Voudris V. 2015. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res 17(2):168–174, PMID: 25180080, 10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. 2014. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep 4:4133, PMID: 24569565, 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández E, Ballbé M, Sureda X, Fu M, Saltó E, Martinez-Sánchez JM. 2015. Particulate matter from electronic cigarettes and conventional cigarettes: a systematic review and observational study. Curr Environ Health Rep 2(4):423–429, PMID: 26452675, 10.1007/s40572-015-0072-x. [DOI] [PubMed] [Google Scholar]
- Fessler MB, Carnes MU, Salo PM, Wilkerson J, Cohn RD, King D, et al. . 2017. House dust endotoxin and peripheral leukocyte counts: results from two large epidemiologic studies. Environ Health Perspect 125(5):057010, PMID: 28599265, 10.1289/EHP661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss O, Bianchi I, Barahona F, Barrero-Moreno J. 2015. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int J Hyg Environ Health 218(1):169–180, PMID: 25455424, 10.1016/j.ijheh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Giovenco DP, Hammond D, Corey CG, Ambrose BK, Delnevo CD. 2015. E-cigarette market trends in traditional U.S. retail channels, 2012-2013. Nicotine Tob Res 17(10):1279–1283, PMID: 25542918, 10.1093/ntr/ntu282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Hajek P, McRobbie H. 2014a. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction 109(3):500–507, PMID: 24345184, 10.1111/add.12410. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. . 2014b. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23(2):133–139, PMID: 23467656, 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W. 1999. Bacterial endotoxin is an active component of cigarette smoke. Chest 115(3):829–835, PMID: 10084499, 10.1378/chest.115.3.829. [DOI] [PubMed] [Google Scholar]
- Herzog B, Gerberi J, Scott A. 2014. Equity Research: Tobacco-Nielsen C-Store Data-E-Cig $ Sales Decline Moderates. Charlotte, NC: Wells Fargo Securities; http://www.c-storecanada.com/attachments/article/153/Nielsen%20C-Stores%20-%20Tobacco.pdf [accessed 30 August 2015]. [Google Scholar]
- Holst G, Høst A, Doekes G, Meyer HW, Madsen AM, Sigsgaard T. 2015. Determinants of house dust, endotoxin, and beta-(1,3)-d-glucan in homes of Danish children. Indoor Air 25(3):245–259, PMID: 25039673, 10.1111/ina.12143. [DOI] [PubMed] [Google Scholar]
- Ingebrethsen BJ, Cole SK, Alderman SL. 2012. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol 24(14):976–984, PMID: 23216158, 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- Iossifova Y, Reponen T, Sucharew H, Succop P, Vesper S. 2008. Use of (1-3)-β-D-glucan concentrations in dust as a surrogate method for estimating specific fungal exposures. Indoor Air 18(3):225–232, 10.1111/j.1600-0668.2008.00526.x. [DOI] [PubMed] [Google Scholar]
- Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. 2015. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med 372(4):392–394, PMID: 25607446, 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- Klager S, Vallarino J, MacNaughton P, Christiani DC, Lu Q, Allen JG. 2017. Flavoring chemicals and aldehydes in e-cigarette emissions. Environ Sci Technol 51(18):10806–10813, PMID: 28817267, 10.1021/acs.est.7b02205. [DOI] [PubMed] [Google Scholar]
- Lai PS, Fresco JM, Pinilla MA, Macias AA, Brown RD, Englert JA, et al. . 2012. Chronic endotoxin exposure produces airflow obstruction and lung dendritic cell expansion. Am J Respir Cell Mol Biol 47(2):209–217, PMID: 22517795, 10.1165/rcmb.2011-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai PS, Hang JQ, Valeri L, Zhang FY, Zheng BY, Mehta AJ, et al. . 2015. Endotoxin and gender modify lung function recovery after occupational organic dust exposure: a 30-year study. Occup Environ Med 72(8):546–552, PMID: 25666844, 10.1136/oemed-2014-102579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SR, Sewell RD. 2006. Correlative measurement of four biological contaminants on cotton lint, and their implications for occupational health. Int J Occup Environ Health 12(2):120–125, PMID: 16722191, 10.1179/oeh.2006.12.2.120. [DOI] [PubMed] [Google Scholar]
- Larsson L, Szponar B, Pehrson C. 2004. Tobacco smoking increases dramatically air concentrations of endotoxin. Indoor Air 14(6):421–424, PMID: 15500635, 10.1111/j.1600-0668.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- Lee MK, Carnes MU, Butz N, Azcarate-Peril MA, Richards M, Umbach DM, et al. . 2018. Exposure related to house dust microbiota in a U.S. farming population. Environ Health Perspect 126(6):067001, PMID: 29863827, 10.1289/EHP3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. 2017. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 16(1):42, PMID: 28449666, 10.1186/s12940-017-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran D, Zeng Y, Chan-Yeung M, Scott J, Osornio-Vargas A, Becker AB, et al. . 2014. Exposure to Beta-(1,3)-D-glucan in house dust at age 7-10 is associated with airway hyperresponsiveness and atopic asthma by age 11-14. PLoS One 9(6):e98878, PMID: 24905346, 10.1371/journal.pone.0098878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marynak KL, Gammon DG, Rogers T, Coats EM, Singh T, King BA. 2017. Sales of nicotine-containing electronic cigarette products: United States, 2015. Am J Public Health 107(5):702–705, PMID: 28323467, 10.2105/AJPH.2017.303660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melstrom P, Koszowski B, Thanner MH, Hoh E, King B, Bunnell R, et al. . 2017. Measuring PM2.5, ultrafine particles, air nicotine and wipe samples following the use of electronic cigarettes. Nicotine Tob Res 19(9):1055–1061, PMID: 28340080, 10.1093/ntr/ntx058. [DOI] [PubMed] [Google Scholar]
- Orr MS. 2014. Electronic cigarettes in the USA: a summary of available toxicology data and suggestions for the future. Tob Control 23(Suppl 2):ii18–ii22, PMID: 24732158, 10.1136/tobaccocontrol-2013-051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, et al. . 2017. Benzene formation in electronic cigarettes. PLoS One 12(3):e0173055, PMID: 28273096, 10.1371/journal.pone.0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DU, Ryu SH, Kim SB, Yoon CS. 2011. An assessment of dust, endotoxin, and microorganism exposure during waste collection and sorting. J Air Waste Manag Assoc 61(4):461–468, PMID: 21516941. [DOI] [PubMed] [Google Scholar]
- Park HR, O'Sullivan M, Vallarino J, Shumyatcher M, Himes BE, Park JA, et al. . 2019. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci Rep 9:1400, PMID: 30710127 10.1038/s41598-018-37913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JL, Paszkiewicz G. 2011. Cigarette smoke, bacteria, mold, microbial toxins, and chronic lung inflammation. J Oncol 2011:819129, PMID: 21772847, 10.1155/2011/819129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, et al. . 2014. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health 217(6):628–637, PMID: 24373737, 10.1016/j.ijheh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Schram‐Bijkerk D, Doekes G, Douwes J, Boeve M, Riedler J, et al. . 2005. Bacterial and fungal agents in house dust and wheeze in children: the PARSIFAL study. Clin Exp Allergy 35(10):1272–1278, PMID: 16238785, 10.1111/j.1365-2222.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- Szponar B, Pehrson C, Larsson L. 2012. Bacterial and fungal markers in tobacco smoke. Sci Total Environ 438:447–451, PMID: 23026151, 10.1016/j.scitotenv.2012.08.067. [DOI] [PubMed] [Google Scholar]
- Thorn J, Rylander R. 1998. Airways inflammation and glucan in a rowhouse area. Am J Respir Crit Care Med 157(6 Pt 1):1798–1803, PMID: 9620908, 10.1164/ajrccm.157.6.9706081. [DOI] [PubMed] [Google Scholar]
- Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, et al. . 2015. Endotoxin exposure: predictors and prevalence of associated asthma outcomes in the United States. Am J Respir Crit Care Med 192(11):1287–1297, PMID: 26258643, 10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S, Ohta K, Inaba Y, Kunugita N. 2013. Determination of carbonyl compounds generated from the e-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci 29(12):1219–1222, PMID: 24334991. [DOI] [PubMed] [Google Scholar]
- USP (United States Pharmacopeia). 2016. Bacterial Endotoxins. 39, NF 34 Chapter <85> Bacterial Endotoxins Test.
- Wang TW, Gentzke A, Sharapova S, Cullen KA, Ambrose BK, Jamal A. 2018. Tobacco product use among middle and high school students - United States, 2011-2017. MMWR Morb Mortal Wkly Rep 67(22):629–633, PMID: 29879097, 10.15585/mmwr.mm6722a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SL, Glantz SA, Chaffee BW. 2018. Association of noncigarette tobacco product use with future cigarette smoking among youth in the population assessment of tobacco and health (PATH) study, 2013-2015. JAMA Pediatr 172(2):181–187, PMID: 29297010, 10.1001/jamapediatrics.2017.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells Fargo Securities. 2014. Equity Research: Tobacco—Nielsen C-Store Data—ecig$Sales Decline Moderates. Wells Fargo Securities.
- Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. 2013. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One 8(3):e57987, PMID: 23526962, 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda Y, Tamura K, Shima M. 2017. Airborne endotoxin concentrations in indoor and outdoor particulate matter and their predictors in an urban city. Indoor Air 27(5):955–964, PMID: 28161889, 10.1111/ina.12370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.