Abstract
Background:
The effects of weather on diarrhea could influence the health impacts of climate change. Children have the highest diarrhea incidence, especially in India, where many lack safe water and sanitation.
Objectives:
In a prospective cohort of 1,284 children under 5 y of age from 900 households across 25 villages in rural Tamil Nadu, India, we examined whether high temperature and heavy rainfall was associated with increased all-cause diarrhea and water contamination.
Methods:
Seven-day prevalence of diarrhea was assessed monthly for up to 12 visits from January 2008 to April 2009, and hydrogen sulfide () presence in drinking water, a fecal contamination indicator, was tested in a subset of households. We estimated associations between temperature and rainfall exposures and diarrhea and using binomial regressions, adjusting for potential confounders, random effects for village, and autoregressive-1 error terms for study week.
Results:
There were 259 cases of diarrhea. The prevalence of diarrhea during the 7 d before visits was 2.95 times higher (95% CI: 1.99, 4.39) when mean temperature in the week before the 7-d recall was in the hottest versus the coolest quartile of weekly mean temperature during 1 December 2007 to 15 April 2009. Diarrhea prevalence was 1.50 times higher when the 3 weeks before the diarrhea recall period included (vs. 0 d) with rainfall of (95% CI: 1.12, 2.02), and 2.60 times higher (95% CI: 1.55, 4.36) for heavy rain weeks following a 60-d dry period. The prevalence in household water was not associated with heavy rain prior to sample collection.
Conclusions:
The results suggest that, in rural Tamil Nadu, heavy rainfall may wash pathogens that accumulate during dry weather into child contact. Higher temperatures were positively associated with diarrhea 1–3 weeks later. Our findings suggest that diarrhea morbidity could worsen under climate change without interventions to reduce enteric pathogen transmission through multiple pathways. https://doi.org/10.1289/EHP3711
Introduction
Diarrhea is the fourth leading cause of death of children worldwide (Liu et al. 2012), and recurrent and prolonged episodes early in life have been linked to stunted growth and cognitive development (Checkley et al. 1998; Guerrant et al. 2013). Diarrhea is seasonal in many settings and all-cause diarrhea is associated with temperature and rainfall in both high- and low-income countries (Carlton et al. 2016; Checkley et al. 2000; Levy et al. 2016). Bacterial and protozoal diarrhea is also associated with increased temperature (Carlton et al. 2016). For bacterial pathogens that cause diarrhea, higher temperatures may promote bacterial population growth in stored food and drinking water, increasing the risk of food- and waterborne illnesses (D’Souza et al. 2004; McCabe-Sellers and Beattie 2004). Lower temperatures were associated with a higher incidence rate of rotavirus in two meta-analyses (Jagai et al. 2012; Levy et al. 2009); however, a meta-analysis of any viral diarrhea found no association with temperature (Carlton et al. 2016). Meta-analyses of rainfall have also revealed a positive association with all-cause diarrheal disease and an inverse association with rotavirus-caused diarrhea (Levy et al. 2009, 2016). Because climate change is expected to increase global temperature and the frequency and intensity of heavy rainfall events, understanding how weather influences diarrhea is increasingly important [Hales et al. 2014; Interagency Working Group on Climate Change and Health (U.S.) 2010].
In India, diarrhea causes an estimated 3.65% of disability adjusted life-years (DALYs) lost (7.1% of DALYs lost in children ) (Murray et al. 2012). Despite this large burden, the contribution of climate-related stressors to child diarrhea in India is largely unknown. Evidence to date suggests there are strong seasonal patterns consistent with potential climate-related drivers: A study in Chennai, India, found increased diarrhea hospitalizations after heavy rainfall (Bush et al. 2014), and a study in Tamil Nadu found diarrhea seasonality and found that rainfall, but not temperature, increased diarrheal risk in rural populations (Kulinkina et al. 2016). Moreover, studies of the relationship between temperature, rainfall, and diarrhea risk across several low-income countries have mainly focused on populations that rely on surface water (e.g., springs, rivers, lakes) for drinking water (Adkins et al. 1987; Carlton et al. 2014, 2016; Luque Fernández et al. 2009; Jagai et al. 2012; Lama et al. 2004; Levy et al. 2016; Singh et al. 2001). In rural India, hundreds of millions of people obtain drinking water through systems that draw groundwater using tube wells, store it in village-level overhead storage tanks, and distribute it to standpipes through small, gravity-fed networks. In 2015, an estimated 85% of rural Indians had access to at least one of the following basic drinking-water sources: piped water, boreholes or tube wells, protected dug wells or springs, or packaged water (WHO and UNICEF 2017). It is, therefore, possible that rural Indian children are more protected from rainfall-driven changes in enteric pathogen transmission compared with populations that rely on surface drinking-water sources. Yet, several outbreak investigations in higher-income countries have traced diarrhea outbreaks to heavy rainfall-driven groundwater contamination (Auld et al. 2004; Gelting et al. 2005; Fong et al. 2007), and a study in Nigeria found increased borehole fecal pathogen contamination after rainfall, possibly due to poorly constructed or maintained boreholes (Kumpel et al. 2017).
Our objective was to use coupled in-household child health surveillance and water quality data to estimate the relationship between temperature, rainfall, and child all-cause diarrhea risk in southern India and to investigate a possible causal pathway through water contamination. We combined climatological data with monthly longitudinal diarrhea surveillance in a community-based prospective cohort of children enrolled in a study of diarrhea following interventions to improve water supplies, sanitation, and hygiene in 25 rural villages in the Tiruchirappalli District of the State of Tamil Nadu in southern India (Arnold et al. 2010). We also tested for the presence of hydrogen sulfide (), a by-product of -producing bacteria and an indicator of fecal contamination, in stored household drinking water and samples collected from the primary water source for each village (Khush et al. 2013; McMahan et al. 2011). We sought to answer three research questions. a) Are high temperature and heavy rainfall associated with increased diarrhea prevalence in this study population? b) Are heavy rainfall and increased temperature associated with an increased prevalence of household water contamination? c) Are associations between heavy rainfall and diarrhea or water contamination modified by longer-term (60-d) rain trends?
Our rationale was that studies conducted in multiple locations have documented diarrheal disease outbreaks after heavy rain events (Smith et al. 1989; Trærup et al. 2011; Willocks et al. 1998; Yamamoto et al. 2000) and associations between increased rainfall and diarrhea (Adkins et al. 1987; Chou et al. 2010; Dewan et al. 2013; Seidu et al. 2013; Singh et al. 2001), with some studies reporting stronger associations when heavy rainfall occurred after a dry period (Bush et al. 2014; Carlton et al. 2014; Levy et al. 2016). Rainfall creates a muddy environment, which makes it more difficult to keep hands and household surfaces clean. If the mud contains human or animal feces, as is common in rural agricultural areas with poor sanitation, it could lead to diarrhea pathogen ingestion, especially among infants who exhibit frequent hand-to-mouth behavior (Mattioli et al. 2015; Ngure et al. 2013). If water flows over ground surfaces after a heavy rainfall, pathogens can be washed into watersheds or unprotected water sources (Dorner et al. 2006; Ferguson et al. 2003; Levy et al. 2016). It has been proposed that environmental flushing of feces that have accumulated during dry periods may explain stronger associations between heavy rainfall and diarrhea than when heavy rainfall follows a dry period (Bush et al. 2014; Carlton et al. 2014). The importance of individual transmission pathways likely varies across pathogens, climates, and water, sanitation, and hygiene (WASH) contexts, so prior study findings may not generalize to all regions. Climate change is predicted to increase temperature and the frequency and intensity of tropical cyclones in India, lending particular importance to the association between temperature, rainfall, and child diarrhea in this setting (Knutson et al. 2010; Kumar et al. 2006).
Methods
Study Setting and Design
The study was conducted in 25 rural villages outside the city of Tiruchirappalli in Tamil Nadu, India (Figure 1). The climate is tropical and hot with a rainy monsoon season from August to December, with the most rain occurring from September to November. The region is near sea level, with village elevations ranging from to . The primary water supply for all villages included in the study was groundwater that was pumped into elevated storage tanks. Water from storage tanks was then piped to public stand pipes, with several hundred people using each stand pipe as their primary water source through either public or private taps.
Figure 1.
Map of study region with points marking the location of airport weather station and the intervention and control villages. The map shows the study area in the Tiruchirappalli region in southern India, with study villages plotted as points. The red box in the inset map of India shows where the study region was located within India, and the points are colored dark green for villages receiving the combination of WASH interventions and light yellow for matched control villages. The X-marked point indicates the location of the Tiruchirappalli Airport weather station, where rainfall and temperature were measured over the study period (1 December 2007 to 15 April 2009) and assumed to generalize to all the study villages.
From 2003 to 2007, the non-governmental organizations Water.org and Gramalaya delivered a nonrandomized combination of WASH interventions to the 12 villages. Each village’s WASH conditions were assessed through a participatory rural appraisal, and Gramalaya developed a combined intervention that was responsive to each village’s assessment (Arnold et al. 2010). The main components of the WASH interventions were community mobilization campaigns to build private toilets, village standpipe water distribution improvements, primary school toilet improvements, primary school water tap improvements, subsidized loans for household tap or household latrine construction, and self-help groups to promote WASH and hygiene education. In late 2007, 13 control villages were matched to intervention villages with propensity score matching on pre-intervention characteristics (Arnold et al. 2010).
From 22 January to 16 March 2008, the study enrolled a random sample from each intervention village and matched control villages of 50 households with at least one child living in the household. If a village had eligible households, then the sample included all eligible households. In unannounced visits, field staff visited once per month to assess child diarrheal disease symptoms for up to a total of 12 visits, with final visits occurring in April 2009. Child anthropometry was assessed on the first and last visit.
In the present analysis, we pooled data from intervention and control villages because there were no significant differences in child growth or diarrhea between children living in enrolled households from the intervention and control villages based on the data gathered across the monthly visits (Arnold et al. 2010). The intervention improved village WASH conditions based on measurements collected in the study population. Compared with control villages, intervention villages had higher levels of toilet ownership (57% intervention vs. 26% control) and lower levels of reported open defecation after intervention delivery, although open defecation was common in both (77% intervention vs. 88% control; Arnold et al. 2010).
Ethics
Data collection followed protocols approved by the institutional review boards (IRBs) at the University of California, Berkeley, and Sri Ramachandra Medical College, Chennai, India. Primary caregivers of children were enrolled in the study if they provided informed consent on behalf of their children. Female literacy was sufficiently low in the study area that limiting enrollment to literate caregivers would have threatened the validity of the study. If the child’s primary caregiver was literate, she provided written consent, otherwise caregivers provided oral consent as approved by the local IRB. Oral consent was obtained by field staff by reading out the IRB-approved consent script to eligible participants in the local language (Tamil), and a list of the households that provided oral consent to participate was maintained by the field supervisors.
Outcome Definitions
At enrollment, study staff collected information on household demographics, assets, and WASH conditions. Study fieldworkers conducted up to 12 monthly follow-up visits at each household to query primary caregivers about symptoms of diarrhea in each eligible child during the 7 d before the visit. Eligible children were classified as having had diarrhea during the 7-d recall period if they had three or more loose or watery stools within 24 h or had one or more stools with visible blood (Arnold et al. 2013). For households with multiple eligible children (), fieldworkers assessed diarrhea symptoms separately for each child. Fieldworkers collected samples of stored drinking water from a random, rotating subset of households during monthly survey rounds, starting in the third round (Arnold et al. 2010). Participant households were randomly allocated into four groups. Two of the groups were measured in rounds 3 and 5, and the other two groups were measured in rounds 4 and 6. In survey rounds 7–12, households in one of the four groups were tested. Each household’s drinking water was tested between one and four times over the study period, and 48.9, 48.1, 48.9, 46.6, 25.0, 23.8, 23.8, 22.2, 25.2, and 23.7% of the 900 enrolled households had their drinking water tested during rounds 3–12, respectively. Fieldworkers also collected samples from the primary water sources for each village in each survey round, starting in the third round. Villages had on average 3.1 () primary water sources, which were sampled on average 9.0 () times.
Water samples were tested for the presence of using the test kit (HiMedia K020; HiMedia). Samples were identified as positive for if they turned black within 24 h of incubation with the detection reagents at room temperature.
Exposure Definitions
Daily rainfall and temperature measurements were gathered contemporaneously by study personnel from the Tiruchirappalli International Airport weather station operated by the India Meteorological Department from 1 December 2007 to 15 April 2009 (the study period). The distance from the airport to the 25 study villages ranged from 17 to . The minimum and maximum temperature on each day was recorded by minimum and maximum thermometers housed in a Stevenson screen. The daily mean temperature was defined as the average of the minimum and maximum temperature for each day, and the mean temperature during each 7-d period was defined as the average of the daily mean values during that time period. Associations between temperature and the 7-d prevalence of diarrhea were lagged by 1, 2, or 3 weeks such that the temperature lagged by 1 week was the average daily temperature over the 7 d before the 7-d recall period for diarrhea symptoms (8–14 d before the study visit), whereas 2- and 3-week lagged temperatures were based on average daily temperatures 15–21 d and 22–28 d before the study visit. Lagged weekly mean temperature was evaluated as a continuous variable and as a categorical variable according to quartiles of temperature based on the distribution of 7-d averages (as defined above) from 1 December 2007 to 15 April 2009.
Precipitation during each day was measured with a self-recording rain gauge. Each 7-d period was classified as having heavy rainfall if it included at least 1 d with of precipitation (where is the 80th percentile of daily rainfall on days with of precipitation during 1 December 2007 to 15 April 2009) and as not having heavy rainfall otherwise. Exposure to heavy rainfall was lagged 1, 2, or 3 weeks such that exposure to heavy rainfall lagged by 1 week was based on (vs. 0 d) with rainfall during the 7-d before the start of the 7-d diarrhea recall period (i.e., 8–14 d before the study visit.)
Statistical Methods
We plotted exposure–outcome curves to visualize potential nonlinear trends. We fit cubic splines using generalized additive models over the range of observed prior weekly mean temperature and total weekly rainfall accumulation and over observed weekly rainfall accumulation stratified by 60-d rain trends (Hastie and Tibshirani 1986). The degrees of freedom (df) used in each cubic spline were chosen over a range of 1–10 as the degrees of freedom that maximized 10-fold cross-validated prediction accuracy. We estimated Bayesian 95% simultaneous confidence intervals (CIs) around the fitted curves (Nychka 1988). Weekly mean 24-h rainfall accumulation is because although most weeks had average rainfall, there were a few outlier heavy rainfall weeks. We estimated diarrhea prevalence ratios (PRs) between children exposed to quartiles of weekly mean temperature, with the lowest quartile as the reference value, and between children exposed and not exposed to heavy rainfall events prior to the 7-d diarrhea recall period. Weekly rainfall accumulation was natural log-transformed because although most weeks had average daily rainfall, there were a few outlier heavy rainfall weeks. The degrees of freedom used in each cubic spline was chosen over a range of 1–10 as the degrees of freedom that maximized 10-fold cross-validated prediction accuracy.
We estimated both unadjusted and adjusted PRs with binomial regressions (log-link), and models included random effects for village membership to control for potential within-village correlation, and an autoregressive-1 error term on the study week of the household visit to account for potential temporal autocorrelation in diarrhea and weather. Values for the study week ranged from 1 (first study week) for outcomes assessed between 22 and 27 January 2008, the first 7 d of household visits, to 65 for outcomes assessed after 12 April 2009, the final week of household visits. We did not account for the nonindependence of observations for children from the same household because multilevel models including random effects for both village and household membership as well as an autoregressive-1 error term for the study week of data collection did not converge for some estimates, possibly due to the low prevalence of diarrhea. The models were fit using penalized quasi-likelihood using the glmmPQL() function from the “MASS” library in R (Breslow and Clayton 1993; Venables and Ripley 2002). We estimated both unadjusted PRs and PRs adjusted for potential confounders. We repeated this analysis to estimate the associations between heavy rainfall and temperature and presence in stored household drinking water and between heavy rainfall and presence in a village’s primary drinking-water sources. Because the test only detects the presence of in stored water on the day of collection, the lagged weather exposures were offset 1 week later than the corresponding lags for the 7-d diarrhea recall period; that is, 1-week lagged weekly mean temperature was averaged over the 1–7 d prior to the household visit for the outcome but over the 8–14 d prior to the household visit for the child diarrhea outcome.
The following baseline survey variables were selected as potential confounders because they were plausibly associated with diarrhea risk and were missing for of participants: child sex, child age, current breastfeeding status; intervention group; maternal age, literacy, education, and employment status; number of people in household; household water source; reported open defecation from household member; household latrine ownership; indicators for presence of water, soap, ash, towel/cloth, sink, or flies at the household handwashing station; indicators for household participation in a community group, credit finance group, or agricultural work; indicators for if the household had electricity, a bank account, a covered kitchen, a ventilated kitchen, a thatched roof, or a dirt floor; stove type; cooking fuel used; family-owned land; family-owned home; indicator for if family was from a scheduled caste; and separate indicators for ownership of a dog or cat, buffalo, cow, ox, calf, goat, chicken, cell phone, television, motorcycle or scooter, bicycle, or mosquito net; and village-level open defecation rate, which was estimated from the rate of reported open defecation from study household members (Table 1). Mean temperature during the lagged week of heavy rainfall exposure and mean rainfall during the lagged week of average temperature exposure were also considered as potential confounders for associations with rainfall and temperature, respectively. Covariates were selected separately for each adjusted model based on a likelihood ratio for the association between the potential confounder and the outcome, and therefore the set of variables included in each regression model could vary across outcomes and lag periods. In addition, to reduce the risk of over-specification (Arnold et al. 2010; Peduzzi et al. 1996), each model included a maximum of one covariate per 10 outcome events (e.g., no more than six covariates for a model that included 60 cases of diarrhea), with potential covariates eliminated according to p-value rank. Missing covariate data were replaced with the median value for continuous variables and were modeled using a missing indicator term for categorical variables. Mother’s age was the only continuous variable with missingness, with 17 of 900 households missing mother’s age. We also conducted complete-case analyses and report the results in the supplementary material.
Table 1.
Summary of demographic, socioeconomic, and water, sanitation, and hygiene characteristics of the study population at baseline measurement.
| Study characteristics | n (%) or |
|---|---|
| Observations (n) | 14,254 |
| Children (n) | 1,284 |
| Households (n) | 900 |
| Household water samples (n) | 3,025 |
| Villages (n) | 25 |
| Village water samples (n) | 695 |
| Diarrhea cases (n) | 259 |
| Days of weather data (n) | 502 |
| Pre-intervention covariates | |
| Received intervention | 636 (49.5) |
| Did not receive intervention | 648 (50.5) |
| Childa | |
| Sex | |
| Female | 635 (49.5) |
| Male | 645 (50.2) |
| Missing | 4 (0.3) |
| Breastfeedingb | |
| Currently breastfeeding | 2,308 (16.2) |
| Not currently breastfeeding | 11,946 (83.8) |
| Maternalc | |
| Age | |
| Literacy | |
| Yes | 741 (82.3) |
| No | 158 (17.6) |
| Missing | 1 (0.1) |
| Education | |
| None | 131 (14.6) |
| Primary school | 117 (13) |
| Middle school | 246 (27.3) |
| High school | 277 (30.8) |
| Higher secondary | 91 (10.1) |
| College | 28 (3.1) |
| Graduate School | 8 (0.9) |
| Missing | 2 (0.2) |
| Works | |
| Yes | 434 (48.2) |
| No | 451 (50.1) |
| Missing | 15 (1.7) |
| Householdd | |
| People in household | |
| Study children in household | |
| Household sanitation | |
| Reported open defecation | |
| Yes | 745 (82.8) |
| No | 155 (17.2) |
| Own private latrine | |
| Yes | 374 (41.6) |
| No | 526 (58.4) |
| Household water and handwashing | |
| Primary source | |
| Private Tap | 259 (28.8) |
| Public Tap | 574 (63.8) |
| Private Well | 28 (3.1) |
| Public Well | 39 (4.3) |
| Handwashing station | |
| Flies present | 338 (37.6) |
| Flies absent | 562 (62.4) |
| Water present | 564 (62.7) |
| Water absent | 336 (37.3) |
| Soap present | 443 (49.2) |
| Soap absent | 457 (50.8) |
| Ash present | 409 (45.4) |
| Ash absent | 491 (54.6) |
| Towel/cloth present | 168 (18.7) |
| Towel/cloth absent | 732 (81.3) |
| Sink present | 244 (27.1) |
| Sink absent | 656 (72.9) |
| Household characteristics | |
| Community group participation | |
| Yes | 420 (46.7) |
| No | 480 (53.3) |
| Credit finance group participation | |
| Yes | 310 (34.4) |
| No | 590 (65.6) |
| Parent works in agriculture | |
| Yes | 573 (63.7) |
| No | 327 (36.3) |
| Electricity | |
| Yes | 810 (90) |
| No | 90 (10) |
| Bank account | |
| Yes | 195 (21.7) |
| No | 705 (78.3) |
| Scheduled caste | |
| Yes | 117 (13) |
| No | 783 (87) |
| Covered kitchen | |
| Yes | 736 (81.8) |
| No | 162 (18) |
| Missing | 2 (0.2) |
| Ventilated kitchen | |
| Yes | 571 (63.4) |
| No | 37 (4.1) |
| Not known | 292 (32.4) |
| Thatched roof | |
| Yes | 220 (24.4) |
| No | 680 (75.6) |
| Dirt floor | |
| Yes | 617 (68.6) |
| No | 283 (31.4) |
| Owns home | |
| Yes | 836 (92.9) |
| No | 64 (7.1) |
| Owns land | |
| Yes | 853 (94.8) |
| No | 47 (5.2) |
| Stove type | |
| Three stones | 454 (50.4) |
| Kerosene | 295 (32.8) |
| Gas | 145 (16.1) |
| Other | 4 (0.4) |
| Missing | 2 (0.2) |
| Cooking fuel | |
| Wood | 787 (87.4) |
| Liquid petrol gas | 89 (9.9) |
| Kerosene | 24 (2.7) |
| Household assets | |
| Owns buffalo | |
| Yes | 29 (3.2) |
| No | 871 (96.8) |
| Owns cow | |
| Yes | 361 (40.1) |
| No | 539 (59.9) |
| Owns ox | |
| Yes | 37 (4.1) |
| No | 863 (95.9) |
| Owns calf | |
| Yes | 244 (27.1) |
| No | 656 (72.9) |
| Owns goat | |
| Yes | 295 (32.8) |
| No | 605 (67.2) |
| Owns chicken | |
| Yes | 149 (16.6) |
| No | 751 (83.4) |
| Owns dog or cat | |
| Yes | 99 (11) |
| No | 801 (89) |
| Cell phone | |
| Yes | 294 (32.7) |
| No | 606 (67.3) |
| Television | |
| Yes | 588 (65.3) |
| No | 312 (34.7) |
| Motorcycle/scooter | |
| Yes | 228 (25.3) |
| No | 672 (74.7) |
| Bicycle | |
| Yes | 687 (76.3) |
| No | 213 (23.7) |
| Mosquito net | |
| Yes | 120 (13.3) |
| No | 780 (86.7) |
| Villagee | |
| Open defecation ratef |
Out of 1,284 children.
Out of 14,254 observations because breastfeeding indicators are time-varying.
Out of 900 mothers or primary caregivers.
Out of 900 households.
Out of 25 villages.
Estimated from rate of reported open defecation from study households.
In a sensitivity analysis, we estimated the association between heavy rainfall and diarrhea prevalence using the 70th percentile () and 90th percentile () of rainfall on days with precipitation during 1 December 2007 to 15 April 2009 as the heavy rain threshold instead of the 80th percentile threshold used in the primary analysis. We also estimated the associations between heavy rainfall and 7-d diarrhea prevalence or presence after stratifying by tertiles of precipitation during the 60 d before the lagged exposure week (i.e., for 1-week lagged heavy rainfall exposure during the 8–14 d before the study visit, we stratified by average rainfall during the 15–74 d before the study visit). Tertile categories for these analyses were based on the distribution of average daily rainfall for all days in the study period.
We conducted all statistical analysis using R (version 3.5.1; R Development Core Team). All data files, analysis R scripts, and figure code are available through Open Science Framework (https://osf.io/2pu3d/).
Results
Study Population
We analyzed 14,254 diarrhea prevalence measurements from 1,284 children from 900 households across the 25 villages (median age in months at enrollment: 30.7, range: 0.9–62.7); (Table 1). There was an average of 11.1 () longitudinal observations per child, and 1,220 children (95%) completed the full 12-month follow-up. We conducted tests on 3,025 stored household drinking-water aliquots randomly sampled across study rounds 3–12, and on 695 aliquots of villages’ primary water sources collected across study rounds 3–12. Households had on average 4.8 () persons and 1.4 () children included in the study, latrines were present at 41.6% of households; open defecation was reportedly practiced in 82.8% of households; 49.2% of households had a handwashing station with soap; and 28.8% of households used a private tap as the primary water source while 63.8% of households used a public tap (standpipe) and the remainder used a well (Table 1).
Outcome and Exposure Measurements
During the study, caregivers reported 259 diarrhea cases. The 7-d prevalence of diarrhea was recorded for 14,259 person-weeks among the 1,284 children included in the analysis, for a 7-d prevalence of 1.8%. Five of the 14,259 measurements collected occurred more than a week after the original study ceased recording weather data and were dropped from the analysis. All 5 measurements were of children who did not have diarrhea in the week prior to measurement.
The study period (1 December 2007 to 15 April 2009) consisted of mostly dry days with periodic spikes in heavy rainfall: 389 of 502 d had no rain, with an overall mean of () of rain per day (Figure 2A). The heaviest rainfall was on 19 December 2007. The study period was rainier than historical averages for the Tiruchirappalli Airport (e.g., rainfall in 2008, the only full year during the study period: , 1971–2000 average: ), but there was a typical seasonal trend, with a less rainy May–August monsoon period (, 1971–2000 average: ) and a rainier September–November monsoon period (, 1971–2000 average: ) (India Meteorological Department; http://www.imd.gov.in/section/climate/extreme/tiruchirapalli2.htm). Fifty-seven percent of the 7-d recall periods were preceded by a 7-d period that included any rainfall, and 18% were preceded by a week that included a heavy rainfall event (at least 1 d with rainfall ) (for the second and third weeks before the 7-d recall period, 60% and 58% had any rain, and 16% and 16% had heavy rain, respectively). There were no missing weather exposure data in the primary analyses, but there was missing information on longer-term (60-d) rainfall trends for 609, 856, and 987 of 14,254 observations for the 1-, 2- and 3-week lagged exposures, respectively, and these observations were dropped from the analysis. The missingness arose because the daily rainfall records began on 1 December 2007, whereas the earliest 60-d rainfall average period began on 26 November 2017, 88 d prior (60-d averaging period, plus 21 d for the 3-week lag, plus the 7-d diarrhea recall period) to the first diarrhea surveillance visit on 22 January 2008.
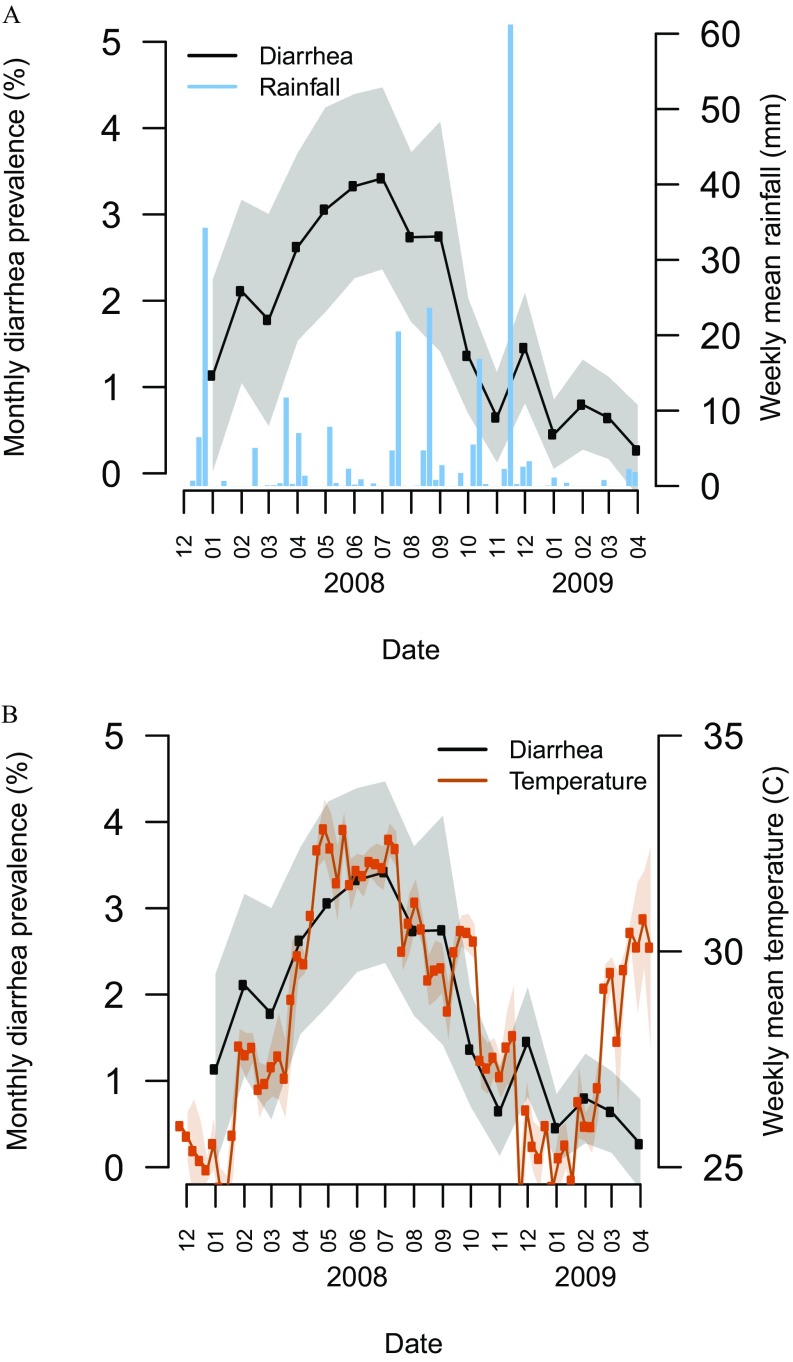
Figure 2.
Diarrhea prevalence, rainfall, and temperature over the study period. (A) Mean 7-d prevalence of diarrhea during each month [with 95% confidence interval (CI) band] and weekly rain accumulation over the study period (December 2007–April 2009). (B) Mean 7-d prevalence of diarrhea during each month (with 95% CI band) and weekly mean temperature over the study period.
Weekly average temperature had seasonal trends (Figure 2B), with a 26.8°C () average from October to March, and a 31.0°C () average from April to September, with a maximum of 32.2°C in April and a minimum of 25.4°C in January. The hottest day had a 34°C average and a 40.3°C maximum. The coldest day had a 22°C average and 16.8°C minimum.
Associations between Temperature, Diarrhea, and Water Quality
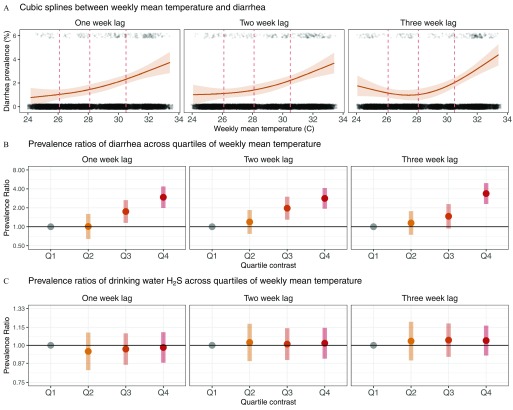
Semiparametric spline fits illustrated a nonlinear, convex increase in 7-d diarrhea prevalence as temperature increased in the 1, 2, and 3 weeks prior to the 7-d diarrhea recall period (Figure 3A). The cutoffs between quartiles of weekly mean temperature were 26.1, 28.1, and 30.5°C. The highest vs. lowest quartile of mean weekly temperature ( vs. ) was positively associated with diarrhea for all lag periods: the adjusted PR was 2.95 (95% CI: 1.99, 4.39) with a 1-week lag, 2.84 (95% CI: 1.95, 4.15) with a 2-week lag, and 3.39 (95% CI: 2.31, 4.98) with a 3-week lag (Figure 3B; see also Table S1). Risk increased by quartile of temperature: Compared with the first quartile, adjusted 1-week lagged PRs increased monotonically for the second [1.01 (95% CI: 0.64, 1.60)], third [1.74 (95% CI: 1.15, 2.64)], and fourth [2.95 (95% CI: 1.99, 4.39)] quartiles (Figure 3B; see also Table S1). There was no difference in inference between estimates from models that only adjusted for village membership and study week (autoregressive term) and models that additionally adjusted for potential confounders, or between models with missing indicator terms and complete-case models (see Table S1).
Figure 3.
Relationships between weekly mean temperature and 7-d prevalence of diarrhea among children in and in stored household drinking water 1, 2, or 3 weeks prior to the 7-d diarrhea recall period, Tamil Nadu, India, 2008–2009. (A) Adjusted associations between weekly temperature and the 7-d prevalence of diarrhea (95% simultaneous confidence bands) estimated with cubic splines, fit with 3 df. The vertical dashed lines in the temperature plots mark the 25th, 50th, and 75th percentiles of temperature over the study period. Observed diarrhea cases are plotted as points at the top of each plot and observed noncases are plotted as points at the bottom of each graph. Points are jittered for visibility. The weekly mean temperature is lagged 1 (left panel), 2 (middle panel), and 3 weeks (right panel) prior to the start of the 7-d diarrhea recall period. (B) Adjusted prevalence ratios (with 95% CI) for diarrhea according to quartiles (Q) of weekly mean temperature lagged 1, 2, and 3 weeks prior to the start of the 7-d diarrhea recall period. Q1–Q4 indicate mean weekly temperature quartiles 1–4, with the first (lowest) quartile of temperature used as the reference level to calculate prevalence ratios, and using 26.1, 28.1, and 30.5°C as the cutoffs between the quartiles. Prevalence ratios were estimated with binomial regressions (log-link) models that included random effects for village membership and an autoregressive-1 error term on the study week of the household visit and potential confounders selected via likelihood ratio tests. The selected covariates were child age; intervention group; primary water source; current breastfeeding status; indicators for household participation in a community group, credit finance group, or agriculture; indicators for if the household had electricity, a thatched roof, a bank account, or a dirt floor; indicators for presence of water, soap, ash, towel/cloth, sink, or flies at the household handwashing station; indicators for ownership of a dog or cat, ox, television, motorcycle or scooter, or mosquito net; and indicator for reported open defecation from a household member. The model with a 3-week lag period was additionally adjusted for mean weekly rainfall during the week of temperature exposure. (See Table S1 for numeric data.) (C) Adjusted prevalence ratios (with 95% CI) for the presence of in household stored drinking-water samples according to quartiles of weekly mean temperature lagged 1, 2, and 3 weeks prior to the start of the 7-d diarrhea recall period. Q1–Q4 indicate mean weekly temperature quartiles 1–4, with the first (lowest) quartile of temperature used as the reference level to calculate prevalence ratios, and using 26.1, 28.1, and 30.5°C as the cutoffs between the quartiles. Prevalence ratios were estimated with binomial regressions (log-link) models that included random effects for village membership and an autoregressive-1 error term on the study week of the household visit and potential confounders selected via likelihood ratio tests. The selected covariates were mean weekly rainfall during the week of temperature exposure; child sex; intervention group; primary water source; maternal age and education; indicators for presence of soap, ash, or sink at the household handwashing station; indicators for if the household has a bank account, a ventilated kitchen, or a latrine; primary cooking fuel used; family-owned land; family-owned home; indicator for if family is from a scheduled caste; indicators for ownership of buffalo, goat, television, or motorcycle or scooter; and village-level open defecation rate, estimated from rate of reported open defecation from study household. (See Table S2 for numeric data.)
There were 3,025 tests of stored household drinking water for , of which 2,551 samples (84.3%) tested positive. No quartile of temperature was significantly different from the lowest quartile and estimated prevalence ratios were close to the null (Figure 3C; see also Table S2).
Association between Heavy Rainfall, Diarrhea, and Water Quality
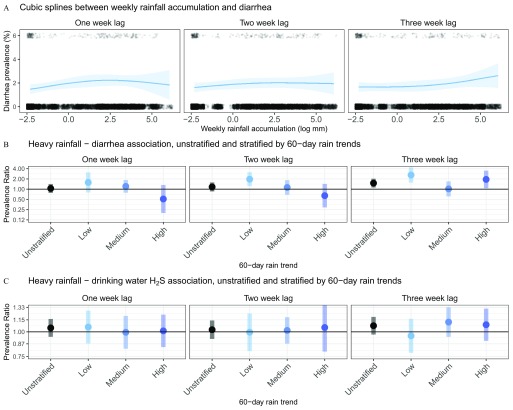
There was no consistent spline shape between increasing weekly mean rainfall and diarrhea prevalence, with an estimated concave shape with a 1- or 2-week lag and a convex shape with a 3-week lag (Figure 4A). The 7-d prevalence of diarrhea increased after a heavy rain event, especially 3 weeks prior to the 7-d recall period, with adjusted PRs of 1.05 (95% CI: 0.78, 1.42) for the 1-week lag, 1.16 (95% CI: 0.86, 1.58) for the 2-week lag, and 1.50 (95% CI: 1.12, 2.02), for the 3-week lag (Figure 4B; see also Table S3). Estimates from unadjusted models and complete-case models were similar to those from models adjusted for covariates with missing data indicators (see Table S3). In the sensitivity analysis of the threshold used to define heavy rainfall events, estimates from unadjusted models using the 70th or 90th percentiles of rainfall across days with of precipitation to define heavy rainfall were consistent with estimates from models using the 80th percentile except that the confidence intervals estimated from the 3-week lag models crossed the null when using the 70th or 90th percentiles (see Figure S1).
Figure 4.
Seven-day prevalence of diarrhea among children in relation to weekly rainfall and heavy rain events, and the prevalence of in stored household drinking water 1, 2, or 3 weeks prior to the 7-d diarrhea recall period in relation to heavy rain events, Tamil Nadu, India, 2008–2009. (A) Adjusted associations between natural log-transformed weekly rainfall accumulation (log-mm) and the 7-d prevalence of diarrhea (95% simultaneous confidence bands) estimated with cubic splines, lagged 1 (left panel), 2 (middle panel), and 3 weeks (right panel) prior to the start of the 7-d diarrhea recall period. Observed diarrhea cases are plotted as points at the top of each plot and observed noncases are plotted as points at the bottom of each graph. Points are jittered for visibility. The splines are fit with 3, 4, and 3 df for the left, center, and right panel, respectively. (B) Adjusted prevalence ratios (with 95% CI) for diarrhea in relation to weeks with heavy rain events ( of rainfall above the 80th percentile of daily accumulation during the study period vs. weeks with no heavy rainfall) for all weeks (unstratified) and stratified by low, medium, and high tertiles of rainfall accumulation during the 60-d prior to the week of exposure, which was lagged 1 (left), 2 (middle), and 3 (right) weeks prior to the start of the 7-d diarrhea recall period. Prevalence ratios were estimated with binomial regressions (log-link) models that included random effects for village membership and an autoregressive-1 error term on the study week of the household visit and potential confounders selected via likelihood ratio tests. (See Table S3 for numeric data and model covariates.) (C) Adjusted prevalence ratios (with 95% CI) for the presence of in household stored drinking-water samples in relation to weeks with heavy rain events ( of rainfall above the 80th percentile of daily accumulation) for all weeks (unstratified) and stratified by low, medium, and high tertiles of rainfall accumulation during the 60-d prior to the week of exposure, which was lagged 1, 2, and 3 weeks prior to the start of the 7-d diarrhea recall period. Prevalence ratios were estimated with binomial regressions (log-link) models that included random effects for village membership and an autoregressive-1 error term on the study week of the household visit and potential confounders selected via likelihood ratio tests. (See Table S4 for numeric data and model covariates.)
A heavy rainfall event before the 7-d recall period (using the 80th percentile as the threshold for heavy rain) was positively associated with evidence of stored household drinking water contamination, but with 95% CIs overlapping the null [adjusted 1-week lag (95% CI: 0.94, 1.16); 2-week lag (95% CI: 0.92, 1.14); and 3-week lag (95% CI: 0.97, 1.19)]; (Figure 4C; see also Table S4). Unadjusted PRs and complete-case PRs were very similar to the adjusted estimates from models with missing data indicators (see Table S4). We tested 695 samples collected from the primary drinking-water source for the study villages, and 407 (58.6%) were positive for , consistent with bacterial contamination. A heavy rainfall event 1–7 d before sample collection was associated with evidence of contamination (adjusted ; 95% CI: 1.10, 1.73), but not heavy rainfall events 8–14 d earlier (, 95% CI: 0.97, 1.34) or 15–21 d earlier (; 95% CI: 0.73, 1.04); (see Table S5).
Modification by Rainfall in the Previous 60 d
There was evidence of effect modification of the heavy rainfall–diarrhea association by longer-term (60-d) rainfall trends (Figure 3B; see also Table S3). When a heavy rainfall event occurred after a dry period (60-d rainfall in the lowest tertile, ), the 7-d prevalence of diarrhea increased, especially with a 2- or 3-week time-lag, with adjusted PRs of 1.57 (95% CI: 0.80, 3.10) for the 1-week lag, 1.96 (95% CI: 1.22, 3.14) for the 2-week lag, and 2.60 (95% CI: 1.55, 4.36) for the 3-week lag (Figure 4B; see also Table S3). In contrast, heavy rainfall events were not consistently associated with the 7-d prevalence of diarrhea after 60-d periods with rainfall in the second or third tertile ( and , respectively). Estimates from unadjusted models and complete-case models were similar to those from models adjusted for covariates with missing data indicators (see Table S3).
Associations between heavy rain events and prevalence ratios in stored household drinking water did not show clear differences when stratified by average rainfall during the previous 60 d (Figure 4C; see also Table S4). Heavy rainfall during the 1–7 d before sample collection was not associated with the presence of in the primary village water source after a 60-d period of low rainfall [ (95% CI: 0.59, 1.30)] or medium rainfall [ (95% CI: 0.64, 1.40)], and was positively, but not significantly, associated evidence of bacterial contamination following 60-d periods with high rainfall [ (95% CI: 0.84, 1.92] (see Table S5).
Discussion
Key Findings
In this cohort of children in rural Tamil Nadu, the prevalence of diarrhea was associated with higher quartiles of average temperature during the first, second, and third weeks before the 7-d diarrhea recall period. The overall association between a heavy rainfall event (vs. no heavy rainfall event) and the 7-d prevalence of diarrhea varied among the three lag periods, but was consistently positive when the heavy rainfall occurred after a 60-d dry period. Fecal contamination of household drinking-water samples, as indicated by a positive test, was not clearly associated with mean temperature or heavy rainfall during the 1–3 weeks before the measurement.
Interpretation
The association between temperature and diarrhea prevalence in this population is consistent with past studies in similar settings. Our results are similar to those from studies in Bangladesh (Ali et al. 2013; Dewan et al. 2013; Hashizume et al. 2008) and other tropical regions (Luque Fernández et al. 2009; Trærup et al. 2011; Zhang et al. 2008) that reported positive associations between diarrhea and increasing ambient temperatures. Our findings for quartiles of average weekly temperatures (based on the average of the daily minimum and maximum temperatures during each 7-d period) are not directly comparable to estimates based on temperature modeled as a continuous variable, but results did suggest a monotonic increase in the prevalence of diarrhea with increasing temperatures in previous weeks.
We found evidence that heavy rainfall events were associated with higher diarrhea risk in the following 1–3 weeks, consistent with findings from prior studies in other populations (Bush et al. 2014; Carlton et al. 2014; Levy et al. 2016). Heavy rainfall events were positively associated with diarrhea risk across 1-, 2-, and 3-week time lags, but only a 3-week lag was statistically significant [adjusted (95% CI: 1.21, 2.02)]; (see Table S3). A study in northern Ecuador reported that diarrhea was associated with heavy rainfall 2 weeks prior, but not 1 week prior (Carlton et al. 2014), and a study in northern Ghana reported that maximum rainfall lagged 2- and 6-weeks, but not 4-weeks prior, was significantly associated with diarrhea (Seidu et al. 2013). Thus, although heavy rainfall has been associated with diarrhea in several studies, the lag between heavy rainfall and diarrhea may be location specific, perhaps because of differences in local transmission dynamics or the incubation periods of the predominant pathogen taxa. Secondary transmission of diarrheal disease could also account for the association with the 3-week lagged heavy rainfall, but secondary transmission also would be expected to increase diarrhea after a 2-week lag (Carlton et al. 2014). The sensitivity analysis of heavy rainfall classification found a consistent increase in diarrhea prevalence regardless of heavy rain classification thresholds or lag period, but variation in estimates across scenarios was small relative to confidence interval width (see Figure S1).
When heavy rainfall followed a 60-d period of low average rainfall, the adjusted PR for diarrhea following a 7-d period with (vs. without) a heavy rain event ranged between 1.57 (95% CI: 0.80, 3.10) for a 1-week lag and 2.60 (95% CI: 1.55, 4.36) for a 3-week lag, whereas PRs were inconsistent when heavy rainfall occurred after periods of medium or high average rainfall (Figure 4B). A possible explanation for this finding is that human and animal feces that accumulate in the environment during dry periods may be flushed by heavy rainfall into contact with children. These findings are consistent with a study in Chennai, India, of hospital admissions for diarrhea (Bush et al. 2014) and a study in Ecuador of child diarrhea ascertained based on caregiver recall (Carlton et al. 2014), which both reported increased diarrhea when heavy rainfall occurred after dry periods compared with when heavy rainfall occurred after wet periods.
Our findings on the associations between heavy rainfall and all-cause diarrhea are consistent with prior studies; however, there is limited information on the environmental pathways through which heavy rain might increase diarrhea risk, and, to our knowledge, our analysis of prevalence in village source water and household drinking water is novel. Heavy rainfall can flush feces directly into surface water, but villages in the study area stored groundwater in overhead tanks that should theoretically have been protected from contamination through this route. However, heavy rainfall can directly contaminate groundwater, and studies in Nigeria have reported more frequent microbial contamination of borehole groundwater samples in the rainy season and of samples collected near poor sanitation facilities (Auld et al. 2004; Fong et al. 2007; Gelting et al. 2005; Kumpel et al. 2017). Poorly constructed or maintained boreholes with cracks in the cement aprons or linings are potentially vulnerable to the intrusion of fecal contamination from the surface during rainfall, but we believe that it is more likely that other mechanisms contributed to the association between heavy rainfall and diarrhea in our study population.
Stored household drinking water in study households had more frequent detection of than village source standpipes [2,551 of 3,025 positive household samples (84%) versus 407 of 695 village samples (59%)], which suggests that drinking water was contaminated between the source and point-of-use, possibly by dirty hands dipped into the stored water (Khush et al. 2013). Heavy rainfall 3 weeks prior to sample collection was associated with a higher prevalence of detection in stored drinking water (89.2% vs. 83.3% positive), although the prevalence ratio confidence interval overlapped the null and there was a larger difference in the prevalence of in village source water after heavy rainfall 1-week prior (69.2% vs. 56.6% positive), but neither association had a clear pattern of modification by 60-d rain trends. It is possible that in our study population, heavy rainfall increased the risk of child diarrhea by contaminating drinking water at the village source, whereas the larger increase in diarrhea when a heavy rain event followed a dry period may have been caused by the flushing of accumulated pathogens into contact with children. Children could ingest feces washed into mud through hand-to-mouth behavior with muddy hands, or muddy conditions could lead to more food contamination.
Limitations
Temperature and rainfall measurements taken at the Tiruchirappalli Airport were assumed to apply to all villages because weather data were not gathered at the village level. This assumption may be more reasonable for temperature than for rainfall, which can be more heterogeneous than temperature at provincial scales. The uncertainty in the true weather at each village is a limitation of this study, and exposure misclassification may have led to bias. Low temporal resolution in both the exposures and outcomes used in analyses may have led to bias and reduced precision.
There is potential for residual confounding, particularly if there were unmeasured time-varying confounders with seasonal patterns that matched rainfall and influenced diarrheal disease or prevalence. In addition, although included covariates had low or no missingness (), the indicator method of handling missing categorical data can lead to bias, even if the missingness is random (Greenland and Finkle 1995). We also did not model multiple children living in the same household with a household-level random effect in a multi-level model, which could affect the precision of our estimates.
This study was based on diarrhea data collected over a 15-month period from January 2008 to April 2009, which is a shorter period than used in similar studies; therefore, we cannot examine interannual variability of the observed patterns (Bush et al. 2014; Carlton et al. 2014; Curriero et al. 2001; Thomas et al. 2006). The study period was also rainier than historical averages for the study area.
Reported diarrhea was not confirmed in a laboratory, where causal pathogens could have been identified. The higher diarrhea prevalence during hotter periods suggests that many of the diarrhea cases were caused by bacterial, protozoal, or parasitic infections rather than viral pathogens. Findings from past studies suggest that lower temperatures increase the transmission of viral diarrhea (D’Souza et al. 2008; Konno et al. 1983), whereas higher temperatures are associated with bacterial diarrhea (Ali et al. 2013; Dewan et al. 2013; D’Souza et al. 2004; Luque Fernández et al. 2009; Trærup et al. 2011; Zhang et al. 2008). A meta-analysis of factors influencing rotavirus infections, a common viral diarrhea pathogen, found evidence of a protective effect of high temperature, but the investigators did not identify any studies that examined associations between rainfall and rotavirus diarrhea (Levy et al. 2016). Therefore, information on specific pathogens involved in individual cases would help clarify the effects of temperature and rainfall on diarrhea in future studies.
Without laboratory confirmation, reported diarrhea is subject to reporting bias. We would not expect diarrhea reporting to be differential by temperature or rainfall, but it is possible that the lower diarrhea prevalence in January–April 2009 compared with January–April 2008 could have resulted from caregivers underreporting illness as the study progressed (Schmidt et al. 2011). Overall, the child diarrhea prevalence of 1.8% was low compared with a previous trial in the region that found a 10% prevalence of chronic diarrhea (Rahmathullah et al. 1991), and the relatively small numbers of cases reduced the precision of our estimates and limited power to detect associations, especially in the subgroup analysis of heavy rainfall stratified by 60-d rain trends.
The test only detects the presence of in water, so it does not capture any increase in fecal contamination in already-contaminated water sources. As a binary measure, the test may not be adequately powered to detect small changes in concentration due to rainfall, and most stored household drinking-water samples were positive across all sampling visits (), so an increase in after heavy rain would have been difficult to detect. Therefore, the observed null association between heavy rainfall and household stored drinking-water prevalence may only provide evidence that there was no major increase in fecal contamination after heavy rain, rather than that there was no increase in fecal contamination. However, a previous analysis of this cohort found a strong relationship between increased prevalence of in water samples and the concentration of coliform bacteria in the water samples (Khush et al. 2013). In addition, a key limitation of the test is that it is not a direct measure of pathogen presence, only of fecal contamination. Thus, the test does not indicate whether the fecal contamination contains bacterial pathogens, viral pathogens, or no pathogens. However, we would expect a stronger association between heavy rainfall and prevalence of in stored household drinking water if heavy rain was flushing appreciable amounts of feces from the environment and into drinking water, and if heavy rainfall increased fecal contamination of drinking water, there would be an increased likelihood of contamination with diarrhea pathogens.
A potential driver of the patterns observed in rainfall, water contamination, and diarrheal disease that warrants further research could be time-varying levels of host immunity. Children who were infected and became ill after a heavy rain event may still be infected but not experience clinical illness after exposure to the same pathogens during subsequent heavy rain events. This hypothesis could also explain higher diarrheal disease risk after the low tertile of 60-d rain because the children may have been less recently exposed to pathogens transported by rainfall and, therefore, have waning immunity. The higher risk of contamination in drinking water after heavy rainfall was modest, with 95% CIs overlapping the null, but the association was consistently positive and did not vary significantly over the strata of 60-d rain trends. Potentially, heavy rain events that follow higher levels of rain do not increase diarrheal disease risk due to host immunity; nevertheless, asymptomatic individuals may still shed pathogens into the environment and contaminate drinking-water sources after heavy rain.
Generalizability
Our findings require confirmation, but if they are valid, they might be generalizable to other rural populations in southern India with similar weather patterns and WASH conditions. Regions with low open defecation could have a smaller pathogen reservoir in the environment, and regions without monsoon deluges may not receive enough precipitation to cause the surface flow that washes enteric pathogens into human contact. However, the WASH characteristics of study villages are similar to much of rural India (Kumar and Das 2014), a region with a very high population and burden of diarrhea, so our findings might be relevant to a large portion of the children at risk of weather-driven diarrheal disease. An interesting future study could explore the geography, WASH conditions, or predominant pathogens of low-income countries where high temperature and heavy rainfall are not associated with increased diarrhea.
Conclusions
We found positive associations between high temperature, heavy rainfall, and all-cause diarrhea in children and evidence suggesting that drinking water contamination may not be the primary infection pathway in rural Tamil Nadu. The association between heavy rain events and diarrhea was stronger when heavy rain followed a 60-d dry period, which suggests an infection mechanism whereby rain flushes accumulated environmental contaminants into human contact. This occurred despite the population’s reliance on groundwater and standpipe distribution systems, which should be more protected from flushed contamination than surface water. Heavy rain had a weak association with a higher prevalence of bacteria, an indicator of fecal contamination, in the village water sources and household drinking water, but there was no effect modification of either association by longer-term rain trends. This suggests that in southern India heavy rainfall may influence diarrhea pathogen ingestion by the child through other routes in addition to contaminated drinking water. As climate change models project increasing temperature and extreme rainfall events in India, child morbidity from diarrhea could worsen in the absence of interventions that reduce enteric pathogen transmission through multiple pathways.
Supplementary Material
Acknowledgments
The original cohort study was funded by a grant from the Open Square Foundation to the Aquaya Institute.
References
- Adkins HJ, Escamilla J, Santiago LT, Rañoa C, Echeverria P, Cross JH. 1987. Two-year survey of etiologic agents of diarrheal disease at San Lazaro Hospital, Manila, Republic of the Philippines. J Clin Microbiol 25(7):1143–1147, PMID: 3038946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Kim DR, Yunus M, Emch M. 2013. Time series analysis of cholera in Matlab, Bangladesh, during 1988–2001. J Health Popul Nutr 31(1):11–19, PMID: 23617200, 10.3329/jhpn.v31i1.14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold BF, Galiani S, Ram PK, Hubbard AE, Briceño B, Gertler PJ, et al. 2013. Optimal recall period for caregiver-reported illness in risk factor and intervention studies: a multicountry study. Am J Epidemiol 177(4):361–370, PMID: 23364878, 10.1093/aje/kws281. [DOI] [PubMed] [Google Scholar]
- Arnold BF, Khush RS, Ramaswamy P, London AG, Rajkumar P, Ramaprabha P, et al. 2010. Causal inference methods to study nonrandomized, preexisting development interventions. Proc Natl Acad Sci U S A 107(52):22605–22610, PMID: 21149699, 10.1073/pnas.1008944107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld H, MacIver D, Klaassen J. 2004. Heavy rainfall and waterborne disease outbreaks: the Walkerton example. J Toxicol Environ Health A 67(20–22):1879–1887, PMID: 15371222, 10.1080/15287390490493475. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Clayton DG. 1993. Approximate inference in generalized linear mixed models. J Am Stat Assoc 88(421):9–25, 10.1080/01621459.1993.10594284. [DOI] [Google Scholar]
- Bush KF, O’Neill MS, Li S, Mukherjee B, Hu H, Ghosh S, et al. 2014. Associations between extreme precipitation and gastrointestinal-related hospital admissions in Chennai, India. Environ Health Perspect 122(3):249–254, PMID: 24345350, 10.1289/ehp.1306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton EJ, Eisenberg JNS, Goldstick J, Cevallos W, Trostle J, Levy K. 2014. Heavy rainfall events and diarrhea incidence: the role of social and environmental factors. Am J Epidemiol 179(3):344–352, PMID: 24256618, 10.1093/aje/kwt279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton EJ, Woster AP, DeWitt P, Goldstein RS, Levy K. 2016. A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases. Int J Epidemiol 45(1):117–130, PMID: 26567313, 10.1093/ije/dyv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. 1998. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol 148(5):497–506, PMID: 9737562, 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- Checkley W, Epstein LD, Gilman RH, Figueroa D, Cama RI, Patz JA, et al. 2000. Effect of El Niño and ambient temperature on hospital admissions for diarrhoeal diseases in Peruvian children. Lancet 355(9202):442–450, PMID: 10841124, 10.1016/S0140-6736(00)82010-3. [DOI] [PubMed] [Google Scholar]
- Chou W-C, Wu J-L, Wang Y-C, Huang H, Sung F-C, Chuang C-Y. 2010. Modeling the impact of climate variability on diarrhea-associated diseases in Taiwan (1996–2007). Sci Total Environ 409(1):43–51, PMID: 20947136, 10.1016/j.scitotenv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Curriero FC, Patz JA, Rose JB, Lele S. 2001. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am J Public Health 91(8):1194–1199, PMID: 11499103, 10.2105/AJPH.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza RM, Becker NG, Hall G, Moodie KBA. 2004. Does ambient temperature affect foodborne disease? Epidemiology 15(1):86–92, PMID: 14712151, 10.1097/01.ede.0000101021.03453.3e. [DOI] [PubMed] [Google Scholar]
- D’Souza RM, Hall G, Becker NG. 2008. Climatic factors associated with hospitalizations for rotavirus diarrhoea in children under 5 years of age. Epidemiol Infect 136:(1):56–64, PMID: 17352836, 10.1017/S0950268807008229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan AM, Corner R, Hashizume M, Ongee ET. 2013. Typhoid fever and its association with environmental factors in the Dhaka Metropolitan Area of Bangladesh: a spatial and time-series approach. PLoS Negl Trop Dis 7(1):e1998, PMID: 23359825, 10.1371/journal.pntd.0001998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner SM, Anderson WB, Slawson RM, Kouwen N, Huck PM. 2006. Hydrologic modeling of pathogen fate and transport. Environ Sci Technol 40(15):4746–4753, PMID: 16913133, 10.1021/es060426z. [DOI] [PubMed] [Google Scholar]
- Ferguson C, Husman A. M d R, Altavilla N, Deere D, Ashbolt N. 2003. Fate and transport of surface water pathogens in watersheds. Crit Rev Environ Sci Technol 33(3):299–361, 10.1080/10643380390814497. [DOI] [Google Scholar]
- Fong T-T, Mansfield LS, Wilson DL, Schwab DJ, Molloy SL, Rose JB. 2007. Massive microbiological groundwater contamination associated with a waterborne outbreak in Lake Erie, South Bass Island, Ohio. Environ Health Perspect 115(6):856–864, PMID: 17589591, 10.1289/ehp.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelting R, Sarisky J, Selman C, Otto C, Higgins C, Bohan PO, et al. 2005. Use of a systems-based approach to an environmental health assessment for a waterborne disease outbreak investigation at a snowmobile lodge in Wyoming. Int J Hyg Environ Health 208(1–2):67–73, PMID: 15881980, 10.1016/j.ijheh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Greenland S, Finkle WD. 1995. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol 142(12):1255–1264, PMID: 7503045, 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AAM. 2013. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 10(4):220–229, PMID: 23229327, 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales S, Kovats S, Lloyd S, Campbell-Lendrum D. 2014. Quantitative Risk Assessment of the Effects of Climate Change on Selected Causes of Death, 2030s and 2050s. Geneva, Switzerland:World Health Organization. [Google Scholar]
- Hashizume M, Armstrong B, Wagatsuma Y, Faruque ASG, Hayashi T, Sack DA. 2008. Rotavirus infections and climate variability in Dhaka, Bangladesh: a time-series analysis. Epidemiol Infect 136(9):1281–1289, PMID: 17988426, 10.1017/S0950268807009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. 1986. Generalized additive models. Stat Sci 1(3):297–310, 10.1214/ss/1177013604. [DOI] [PubMed] [Google Scholar]
- Interagency Working Group on Climate Change and Health (U.S.). 2010. A human Health Perspective on Climate Change: A Report Outlining the Research Needs on the Human Health Effects of Climate Change. https://www.niehs.nih.gov/health/materials/a_human_health_perspective_on_climate_change_full_report_508.pdf [accessed 15 March 2018].
- Jagai JS, Sarkar R, Castronovo D, Kattula D, McEntee J, Ward H, et al. 2012. Seasonality of rotavirus in South Asia: a meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PLoS One 7(5):e38168, PMID: 22693594, 10.1371/journal.pone.0038168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khush RS, Arnold BF, Srikanth P, Sudharsanam S, Ramaswamy P, Durairaj N, et al. 2013. H2S as an indicator of water supply vulnerability and health risk in low-resource settings: a prospective cohort study. Am J Trop Med Hyg 89(2):251–259, PMID: 23716404, 10.4269/ajtmh.13-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson TR, McBride JL, Chan J, Emanuel K, Holland G, Landsea C, et al. 2010. Tropical cyclones and climate change. Nat Geosci 3:157–163, 10.1038/ngeo779. [DOI] [Google Scholar]
- Konno T, Suzuki H, Katsushima N, Imai A, Tazawa F, Kutsuzawa T, et al. 1983. Influence of temperature and relative humidity on human rotavirus infection in Japan. J Infect Dis 147(1):125–128, PMID: 6822748, 10.1093/infdis/147.1.125. [DOI] [PubMed] [Google Scholar]
- Kulinkina AV, Mohan VR, Francis MR, Kattula D, Sarkar R, Plummer JD, et al. 2016. Seasonality of water quality and diarrheal disease counts in urban and rural settings in south India. Sci Rep 6:20521, PMID: 26867519, 10.1038/srep20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Das KC. 2014. Drinking water and sanitation facility in India and its linkages with diarrhoea among children under five: evidences from recent data. Int J Humanit Soc Sci Invent 3(4):50–60. [Google Scholar]
- Kumar KR, Sahai AK, Kumar KK, Patwardhan SK, Mishra PK, Revadekar JV, et al. 2006. High-resolution climate change scenarios for India for the 21st century. Curr Sci 90:(3):334–345, 10.4269/ajtmh.16-0175. [DOI] [Google Scholar]
- Kumpel E, Cock-Esteb A, Duret M, Waal D d, Khush R. 2017. Seasonal variation in drinking and domestic water sources and quality in Port Harcourt, Nigeria. Am J Trop Med Hyg 96(2):437–445, PMID: 27821689, 10.4269/ajtmh.16-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama JR, Seas CR, León-Barúa R, Gotuzzo E, Sack RB. 2004. Environmental temperature, cholera, and acute diarrhoea in adults in Lima, Peru. J Health Popul Nutr 22(4):399–403, PMID: 15663172. [PubMed] [Google Scholar]
- Levy K, Hubbard AE, Eisenberg JN. 2009. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol 38(6):1487–1496, PMID: 19056806, 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy K, Woster AP, Goldstein RS, Carlton EJ. 2016. Untangling the impacts of climate change on waterborne diseases: a systematic review of relationships between diarrheal diseases and temperature, rainfall, flooding, and drought. Environ Sci Technol 50(10):4905–4922, PMID: 27058059, 10.1021/acs.est.5b06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379(9832):2151–2161, PMID: 22579125, 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- Luque Fernández MÁ, Bauernfeind A, Jiménez JD, Gil CL, Omeiri NE, Guibert DH. 2009. Influence of temperature and rainfall on the evolution of cholera epidemics in Lusaka, Zambia, 2003–2006: analysis of a time series. Trans R Soc Trop Med Hyg 103(2):137–143, PMID: 18783808, 10.1016/j.trstmh.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Mattioli MCM, Davis J, Boehm AB. 2015. Hand-to-mouth contacts result in greater ingestion of feces than dietary water consumption in Tanzania: a quantitative fecal exposure assessment model. Environ Sci Technol 49(3):1912–1920, PMID: 25559008, 10.1021/es505555f. [DOI] [PubMed] [Google Scholar]
- McCabe-Sellers BJ, Beattie SE. 2004. Food safety: emerging trends in foodborne illness surveillance and prevention. J Am Diet Assoc 104(11):1708–1717, PMID: 15499359, 10.1016/j.jada.2004.08.028. [DOI] [PubMed] [Google Scholar]
- McMahan L, Devine AA, Grunden AM, Sobsey MD. 2011. Validation of the H2S method to detect bacteria of fecal origin by cultured and molecular methods. Appl Microbiol Biotechnol 92(6):1287–1295, PMID: 22038242, 10.1007/s00253-011-3520-z. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2197–2223, PMID: 23245608, 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Ngure FM, Humphrey JH, Mbuya MNN, Majo F, Mutasa K, Govha M, et al. 2013. Formative research on hygiene behaviors and geophagy among infants and young children and implications of exposure to fecal bacteria. Am J Trop Med Hyg 89(4):709–716, PMID: 24002485, 10.4269/ajtmh.12-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nychka D. 1988. Bayesian confidence intervals for smoothing splines. J Am Stat Assoc 83(404):1134–1143, 10.1080/01621459.1988.10478711. [DOI] [Google Scholar]
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. 1996. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49(12):1373–1379, PMID: 8970487, 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC. 1991. Diarrhea, respiratory infections, and growth are not affected by a weekly low-dose vitamin A supplement: a masked, controlled field trial in children in southern India. Am J Clin Nutr 54(3):568–577, PMID: 1877512, 10.1093/ajcn/54.3.568. [DOI] [PubMed] [Google Scholar]
- Schmidt W-P, Arnold BF, Boisson S, Genser B, Luby SP, Barreto ML, et al. 2011. Epidemiological methods in diarrhoea studies—an update. Int J Epidemiol 40(6):1678–1692, PMID: 22268237, 10.1093/ije/dyr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidu R, Stenström TA, Löfman O. 2013. A comparative cohort study of the effect of rainfall and temperature on diarrhoeal disease in faecal sludge and non-faecal sludge applying communities, Northern Ghana. J Water Clim Change 4(2):90–102, 10.2166/wcc.2013.032. [DOI] [Google Scholar]
- Singh RBK, Hales S, de Wet N, Raj R, Hearnden M, Weinstein P. 2001. The influence of climate variation and change on diarrheal disease in the Pacific Islands. Environ Health Perspect 109(2):155–159, PMID: 11266326, 10.1289/ehp.01109155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HV, Patterson WJ, Hardie R, Greene LA, Benton C, Tulloch W, et al. 1989. An outbreak of waterborne cryptosporidiosis caused by post-treatment contamination. Epidemiol Infect 103(3):703–715, PMID: 2606168, 10.1017/S0950268800031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Charron DF, Waltner-Toews D, Schuster C, Maarouf AR, Holt JD. 2006. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975–2001. Int J Environ Health Res 16(3):167–180, PMID: 16611562, 10.1080/09603120600641326. [DOI] [PubMed] [Google Scholar]
- Trærup SLM, Ortiz RA, Markandya A. 2011. The costs of climate change: a study of cholera in Tanzania. Int J Environ Res Public Health 8(12):4386–4405, PMID: 22408580, 10.3390/ijerph8124386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. 2002. Modern Applied Statistics with S. Fourth Edition. New York:Springer. [Google Scholar]
- WHO (World Health Organization), UNICEF (U.N. International Children’s Fund). 2017. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines. Geneva, Switzerland:World Health Organization and the United Nations Children’s Fund. [Google Scholar]
- Willocks L, Crampin A, Milne L, Seng C, Susman M, Gair R, et al. 1998. A large outbreak of cryptosporidiosis associated with a public water supply from a deep chalk borehole. Outbreak Investigation Team. Commun Dis Public Health 1(4):239–243, PMID: 9854881. [PubMed] [Google Scholar]
- Yamamoto N, Urabe K, Takaoka M, Nakazawa K, Gotoh A, Haga M, et al. 2000. Outbreak of cryptosporidiosis after contamination of the public water supply in Saitama Prefecture, Japan, in 1996. Kansenshogaku Zasshi 74(6):518–526, PMID: 10916342, 10.11150/kansenshogakuzasshi1970.74.518. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bi P, Hiller JE. 2008. Weather and the transmission of bacillary dysentery in Jinan, northern China: a time-series analysis. Public Health Rep 123(1):61–66, PMID: 18348481, 10.1177/003335490812300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.