Abstract
Aims:
To examine concurrent validity of inertial sensor (APDM ISway) versus force plate center of pressure (COP) measures of postural sway in cognitively impaired older adults.
Methods:
Participants, mean age 85.6 (SD 4.8), were tested in 4 static standing conditions: (1) eyes open/normal base, (2) eyes open/narrow base, (3) eyes closed/normal base, and (4) eyes closed/narrow base. ISway and COP measures were collected.
Results:
Strong correlations between ISway trunk sway smoothness [ISway JERK, (m2/s5)] and COP path length (r = 0.67–0.85) and COP mean velocity (r = 0.77–0.87); also ISway total sway acceleration path length/trail duration [ISway PATH, (m2/s2)] and COP path length (r = 0.77–0.87) and COP mean velocity (r = 0.77–0.91). Increased sway was detected in narrow versus normal base and eyes closed versus open conditions (P = .001).
Conclusions:
APDM ISway demonstrated concurrent validity to force-plate COP and changes in postural sway were detected between conditions.
Keywords: Cognitive Impairment, Older Adults, Inertial Sensors
1. Introduction
Older adults with cognitive impairment are at increased risk for postural instability,1 and emerging evidence suggests that deficits in postural control associated with increased postural sway are prevalent in persons with mild cognitive impairment (MCI) and early-stage dementia, adversely impacting gait and mobility.1,2 Sensitive and valid measures that can be used in clinical and home care settings are needed to assess postural stability and fall risk in older adults with cognitive impairment. Both age and cognition appear to impact postural sway as demonstrated with greater center of pressure (COP) displacement and sway velocity reported in older compared to younger adults.3, 4 Greater COP velocity has also been reported in older adults with MCI and Alzheimer’s disease (AD) compared to cognitively healthy older adults.1 Even subtle cognitive decline of MCI is reported to have a measurable effect on postural sway in older adults, with effect sizes d=0.29 (95% CI, 0.08–0.45, p< 0.01) reported in a meta-analysis of studies comparing older adults with MCI to those who are cognitively healthy.5 Increased postural sway is predictive of falls in older adults, highlighting the importance of valid measures to detect impairments and monitor change.6,7
Center of pressure (COP) is calculated from ground reaction forces and the amount of torque applied at the support surface to control body-mass acceleration.8 Laboratory-based force plate measures of COP are the gold standard for assessing postural stability. However, portable body-worn inertial sensors may provide an alternative to laboratory-based methods to assess postural control and fall risk in older adults within their natural environment. Inertial sensors have potential advantages over standardized performance-based tests because they do not have the problem of a ceiling effect.9,10 Although specific ISway measures have demonstrated sensitivity and validity in people with Parkinson’s disease (PD),11 older adults with cognitive impairment differ in motor and cognitive strategies. Thus, validation of ISway measures is needed to identify and monitor objective markers of postural instability that can be used by physical and occupational therapists in the care of cognitively impaired older adults within hospital, clinic and home care settings.
The purpose of this study was to (1) examine the concurrent validity of portable inertial postural sway measures (ISway, APDM Wearable Technologies, Portland, OR) in relation to laboratory-based force plate measures of COP (Kistler force plate, Kistler Instrument Corp., Amherst, NY) in older adults with early cognitive decline, and (2) examine changes in postural sway during eyes open versus eyes closed conditions in normal and narrow based stance
2. Methods
2.1. Study design and participants
This was a cross-sectional study that took place in a motion analysis laboratory at University of Washington, Seattle, WA. Participants were recruited for a pilot study of mobility disability in community-dwelling older adults with cognitive impairment, parent grant NIH/NIA P30AG034592-03. Potential participants were first screened by phone to rule out moderate to severe dementia using the Short Blessed Test, a dementia screening interview.12 A Blessed score ≥10 was required for the next stage of screening. In-person neuropsychological assessments were administered to further screen for cognitive study inclusion criteria consistent with MCI or mild dementia inclusion criteria.13, 14 Assessments included: (1) Mini Mental Status Exam (MMSE),15 (2) Clinical Dementia Rating Scale (CDRS),13 and (3) Petersen’s criteria for MCI.14
From an original cohort of 40 community-dwelling participants enrolled in an observational study of mobility disability in older adults with cognitive impairment, 27 completed postural sway measured with both systems, concurrently, in the human motion analysis laboratory. Thus, 27 cognitively impaired older adults (n = 23 MCI, n = 4 mild dementia) were included in this analysis. Inclusion criteria were: (1) age 70 to 95 years, (2) Blessed phone score ≥ 10, (3) self-reported ability to walk at least 100 meters (assistive device allowed), (4) CDRS = 0.5 or 1.0, (5) preserved activities of daily living (ADL), (6) Mini Mental Status Exam (MMSE) >18. Exclusion Criteria were: (1) moderate to severe dementia; (2) not fluent in English; (3) known terminal illness or uncontrolled medical condition; (4) actively suicidal, hallucinating, or delusional; (5) blind or deaf; (6) diagnosis of central nervous system condition other than dementia (e.g., stroke, Parkinson’s disease). The University of Washington institutional review board approved the study procedures, and all participants provided written consent.
2.2. Assessment and testing conditions
Prior to the static standing trials, a brief musculoskeletal screening examination was conducted by a licensed physical therapist to identify impairments in lower extremity protective sensation (monofilament), vibration sense, and gross active range of motion. Protective sensation was assessed using a Semmes-Weinstein 5.07 monofilament at 8 sites on the dorsal and plantar surface of each foot.16 Vibration sensation was tested with 128 Hz and 256 Hz tuning forks at the metatarsal phalangeal joint (MTP) and the medial malleolus.16 Participants were also interviewed about their health conditions using the Self-Administered Comorbidity Questionnaire.17
Thirty-second static standing trials were completed in our human motion analysis laboratory. A 30-second duration was selected, based on pre-study procedural testing, to provide a sustained static standing condition without exceeding the participant’s ability to attend to the static standing task. The APDM inertial sensor was secured with an elastic belt at the 5th lumbar vertebral level and participants stood at the center of a laboratory floor-mounted Kistler force plate. APDM ISway and COP data were collected concurrently during 4 standing trials: (1) eyes open/normal base (medial heel-to-medial heel distance = 10 cm), (2) eyes open/narrow base (feet together) (3) eyes closed/normal base, and (4) eyes closed/narrow base. Participants wore walking shoes and were asked to stand still and keep their arms at their sides. During the eyes open standing conditions, participants were instructed to focus on an “x” placed at eye-level on the wall in front of them.
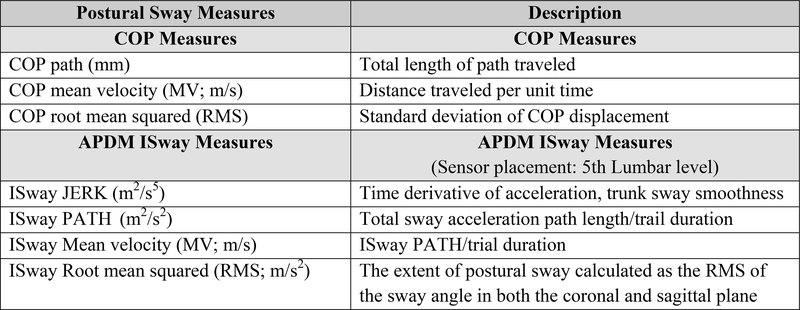
2.3. Center of pressure measures (Figure 1)
Fig. 1.
Postural Sway Measures: Center of Pressure (COP) and APDM Inertial Sensor ISway
The force plate collected ground reaction forces at 120 Hz sampling frequency. COP measures included: (1) COP path length, (2) COP mean velocity (COP MV), and (3) COP root mean squared (COP RMS). Data processing was conducted with a custom LabVIEW program applying a 10-Hz cut-off, zero-phase, low-pass, Butterworth filter.
High test-retest reliability in older adults has been demonstrated, in COP parameters of mean velocity mm/sec (ICC = 0.85 – 0.87) and RMS distance (ICC = 0.80).18 Good to excellent reliability of COP mean velocity and RMS has also been reported during eyes open and eyes closed conditions (ICC ≥ 0.80) in older adults. 18
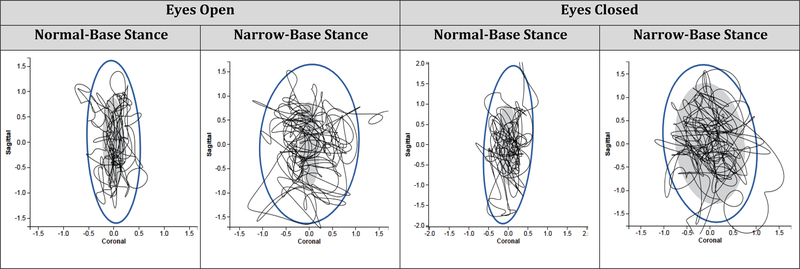
2.4. APDM ISway measures (Figure 2)
Fig. 2.
Representative APDM ISway Diagram from a Single Study Participant during 4 Static Standing Conditions. (gray oval represents normative data from 84 individuals with a mean age of 52)
The APDM instrumented postural sway (ISway) data collection (20–200 Hz sampling rate) during static standing conditions included (1) smoothness of trunk sway [JERK, (m2/s5)]), (2) total sway acceleration path length/trail duration [PATH, (m2/s2)], (3) mean velocity (MV, m/s), and (4) root mean square (RMS). Good to excellent test-retest reliability have been reported for the ISway JERK (ICC = 0.87–0.86), PATH (ICC = 0.89–0.81), MV (ICC = 0.83–0.71), and RMS (ICC = 0.75 – 0.71) in people with Parkinson’s disease (PD) and age-matched healthy adults without PD.19
2.5. Data analysis
Data analysis was completed with SPSS Version 24. Descriptive methods were used for demographic and health data. Pearson correlation coefficients were then used to examine concurrent validity between the ISway and COP measures. ISway and COP parameters that have previously demonstrated reliability and sensitivity in adults with PD were utilized for this analysis.19 Bivariate correlations between ISway measures (ISway JERK, ISway PATH, ISway MV, and ISway RMS) and force plate measures (COP Path length, COP MV, and COP RMS) where matched by anterior-posterior (AP), medial-lateral (ML), and resultant orientations. The strength of Pearson correlation coefficients was interpreted as follows: weak if < 0.10 – 0.29, moderate if 0.30 – 0.49, and strong if ≥ 0.50.20 Repeated measures ANOVA was applied to examine mean differences between the 4 test conditions. This was followed by post-hoc analysis and Bonforroni correction to determine whether there were significant differences in ISway measures between eyes open and eyes closed trials, within the normal-base and the narrow-base stance conditions.
3.0. Results
3.1. Descriptive data (Table 1)
Table 1.
Participant Characteristics (n = 26)
| Mean (SD) | Range | |
|---|---|---|
| Characteristics | ||
| Age (years) | 86.3 (4.9) | 75–93 |
| Sex (female) | 19 (74.1%) | ------ |
| Height (inches) | 63.3 (5.2) | 50–72 |
| Weight (lbs) | 137.8 (26.1) | 98–210 |
| BMI (kg/m2) | 24.8 (3.7) | 18.7–30.4 |
| Global Cognition | ||
| MMSE | 26.8 (2.5) | 23–30 |
| Health Conditions | ||
| % Cardiovascular disease | 52% (14) | ------- |
| % Diabetes | 0.7% (2) | ------- |
| % Osteoarthritis | 59% (16) | -------- |
| % Mild sensory impairment | 22% (6) | -------- |
lbs: pounds; kg/m2: kilograms/meter2; BMI: Body Mass Index (kg/m2); MMSE: Mini Mental Status Exam
Twenty-seven participants completed the normal-base stance conditions, and 26 participants completed the narrow-base stance conditions. One extreme outlier (> 2.0 SD) was removed, leaving 26 normal-base and 25 narrow-base participants for the final analysis. Two participants had mildly impaired protective sensation, but no participants lacked protective sensation. Six participants had reduced vibration sense at the ankle and/or 1st MTP joint, but no participants presented with severe or total loss of ankle or toe vibration. Although 10 participants used a cane or a walker for indoor or outdoor ambulation, they did not need an assistive device for the static standing assessment.
3.2. ISway correlations with COP: Eyes open/normal-base and narrow-base stance: (Table 2)
Table 2.
Correlations: ISway vs. force plate COP in eyes open/normal-base & eyes open/narrow-base stance
| Eyes Open/Normal-base | Eyes Open/Narrow-base | |||||||
|---|---|---|---|---|---|---|---|---|
| ISway JERK (m2/s5) | ISway PATH (m2/s2) | ISway MV (m/s) | ISway RMS | ISway JERK (m2/s5) | ISway PATH (m2/s2) | ISway MV (m/s) | ISway RMS | |
| COP Path Length | ||||||||
| AP | 0.70** | 0.68** | −0.7 | 0.18 | 0.85** | 0.84** | −0.01 | 0.38 |
| ML | 0.66** | 0.72** | 0.36 | 0.68** | 0.57* | 0.71** | −0.07 | 0.70** |
| Resultant | 0.68** | 0.77** | 0.19 | 0.38 | 0.72** | 0.78** | −0.21 | 0.49* |
| COP MV | ||||||||
| AP | 0.70** | 0.68** | 0.36 | 0.18 | 0.85** | 0.84** | −0.01 | 0.38 |
| ML | 0.65** | 0.72** | −0.07 | 0.68** | 0.57** | 0.70** | −0.07 | 0.70** |
| Resultant | 0.67** | 0.77** | 0.19 | 0.37 | 0.73** | 0.78** | −0.02 | 0.49* |
| COP RMS | ||||||||
| AP | 0.56* | 0.56* | 0.08 | 0.30 | 0.57** | 0.33 | −0.02 | 0.18 |
| ML | 0.42* | 0.42* | 0.32 | 0.66** | 0.22 | 0.73** | −0.01 | 0.45* |
Abbreviations and definitions: COP, center of pressure; COP Path Length, center of pressure path length (mm); RMS, root mean squared; JERK, ISway JERK, smoothness of trunk sway (m2/s5); ISway PATH, acceleration path length (m2/s2); ISway MV, mean velocity (m/S); ISway RMS, root mean squared. AP, Anterior-Posterior; ML, Medial-Lateral;
Correlation is significant at the 0.05 level (2-tailed);
Correlation is significant at the 0.01 level (2-tailed)
ISway JERK (m2/s5)
During the eyes open/normal-base and eyes open/narrow-base stance conditions, moderate to strong correlations were demonstrated between ISway JERK and COP measures. In normal-base stance, JERK was strongly correlated with COP path length (resultant r = 0.68) and COP MV (resultant r = 0.67), and moderately correlated with COP AP RMS (r = 0.56) and ML RMS (r = 0.42). In narrow-base stance, JERK was moderately to strongly correlated with COP path length (resultant r = 0.72) and COP MV (resultant r = 0.73), moderately correlated with COP AP RMS (r = 0.57) and weakly correlated with COP ML RMS (r = 0.22).
ISway PATH (m2/s2)
During the eyes open/normal-base and eyes open/narrow-base stance conditions, moderate to strong correlations were demonstrated between ISway PATH and COP measures. In normal-base stance, ISway PATH was strongly correlated in COP path length (resultant r = 0.77), COP MV (resultant r = 0.77), and moderately correlated with COP RMS (AP r = 56; ML r = 42). In eyes open/narrow-base stance, ISway PATH was strongly correlated with COP path length (resultant r = 0.78), COP MV (resultant r = 0.78), and COP ML RMS (r = 0.73) but weakly correlated with COP AP RMS (r = 0.33).
ISway MV (m/s)
Correlations between ISway MV and COP path length (resultant r = 0.19), MV (resultant r = 0.19), and COP RMS (AP r = 0.08; ML r = 0.32) were weak to moderate during the eyes open/normal-base condition and very weak in the eyes open/narrow-base condition.
ISway RMS (m/s2)
In the eyes open/normal-base condition, correlations between ISway RMS and COP path length (resultant r = 0.38), COP MV (resultant r = 0.37), and COP RMS (ML r = 0.66) were moderate to strong. Strong correlations between ISway RMS and all COP ML measures (r = 0.66–0.68) were observed, but there were relatively weak relationships between ISway RMS COP AP measures (0.18–0.30) in normal-base stance. A similar pattern of correlations was present in the eyes open/narrow-base stance condition.
3.3. Eyes closed/normal-base and eyes closed/narrow-base stance (Table 3)
Table 3.
Correlations: ISway vs. force plate COP in eyes closed/normal-base & eyes closed/narrow-base stance
| Eyes Closed/Normal-Base | Eyes Closed/Narrow-Base | |||||||
|---|---|---|---|---|---|---|---|---|
| ISway JERK (m2/s5) | ISway PATH (m2/s2) | ISway MV (m/s) | ISway RMS | ISway JERK (m2/s5) | ISway PATH (m2/s2) | ISway MV (m/s) | ISway RMS | |
| COP Path Length | ||||||||
| AP | 0.81** | 0.80** | 0.20 | 0.38 | 0.89** | 0.91** | 0.37 | 0.68** |
| ML | 0.77** | 0.85** | 0.16 | 0.70** | 0.66** | 0.81** | 0.40* | 0.25 |
| Resultant | 0.75** | 0.82** | 0.29 | 0.49* | 0.85** | 0.87** | 0.32 | 0.75** |
| COP MV | ||||||||
| AP | 0.81** | 0.85** | 0.20 | 0.38 | 0.87** | 0.91** | 0.36 | 0.74** |
| ML | 0.76** | 0.78** | 0.16 | 0.70** | 0.83** | 0.91** | 0.46* | 0.24 |
| Resultant | 0.75** | 0.82** | 0.29 | 0.49* | 0.87** | 0.91** | 0.41* | 0.83** |
| COP RMS | ||||||||
| AP | 0.55** | 0.60** | 0.30 | 0.48* | 0.69** | 0.75** | 0.40* | 0.77** |
| ML | 0.22 | 0.35 | 0.41* | 0.64** | 0.56** | 0.65** | 0.71* | 0.35 |
Abbreviations and definitions: COP, center of pressure; COP Path Length, center of pressure path length (mm); RMS, root mean squared; JERK, ISway JERK, smoothness of trunk sway (m2/s5); ISway PATH, acceleration path length (m2/s2); ISway MV, mean velocity (m/S); ISway RMS, root mean squared. AP, Anterior-Posterior; ML, Medial-Lateral;
Correlation is significant at the 0.05 level (2-tailed);
Correlation is significant at the 0.01 level (2-tailed)
ISway JERK (m2/s5)
During the eyes closed/normal-base and eyes closed/narrow-base stance conditions, moderate to strong correlations were demonstrated between ISway JERK and COP. In normal-base stance, JERK was moderately to strongly correlated with COP path length (resultant r = 0.75), COP velocity (resultant r = 0.75), and COP RMS (AP r = 0.55; ML r = 0.22). In the eyes closed/narrow-base condition, JERK was also moderately to strongly correlated with COP path length (resultant r = 0.85), COP velocity (resultant r = 0.87), and COP RMS (AP 0. 69; ML r = 0.56).
ISway PATH (m2/s2)
During the eyes closed/normal-base and eyes closed/narrow-base stance conditions, moderate to strong correlations were demonstrated between ISway PATH and COP measures. In normal-base stance, ISway PATH was strongly correlated with COP path length (resultant r = 0.82), COP MV (r = 0.82), and COP RMS (AP r = 0.60; ML r = 0.35). In the eyes closed/narrow-base condition, ISway PATH was also strongly correlated with COP path (resultant r = 0.87), COP velocity (r = 0.91), and COP RMS (AP r = 0.75; ML r = 0.65).
3.4. Differences in ISway measures between 4 static standing conditions
Repeated measures analysis revealed significant differences in sway between the 4 testing conditions for ISway JERK [F(3,29) = 10.4, P < .001] and ISway PATH [F(3.29) = 26.101, P = < .001]. As expected ISway measures were significantly increased in narrow-base compared to normal-base stance in the eyes open conditions for ISway JERK [mean diff =2.65 (SE 0.53), P < .001] and ISway PATH [mean diff = 4.48 (SE 0.55), P <.001] and also the eyes closed conditions for ISway JERK [mean diff =3.78 (1.23), P < .03] and ISway PATH [mean diff =5.59 (1.02), P <.001]. ISway measures were also greater in eyes closed compared to eyes open conditions. In normal-base stance, ISway parameters were significantly increased with eyes closed compared to eyes open for ISway JERK [mean diff =1.79 (0.41), P = .001] and ISway PATH [mean diff =2.53 (0.45), P = .001]. In narrow-base stance conditions, sway parameters were significantly increased with eyes closed compared to eyes open for ISway PATH[mean diff = 3.63 (1.11), P = .01], but not in ISway JERK [mean diff = 2.92 (1.36), P = 0.2]. [Table 4].
Table 4.
ISway Change in Eyes Open vs. Eyes Closed Conditions
| Normal Base Stance (n = 26) | |||||
| ISway Measures | EO Mean (SE) | EC Mean (SE) | Mean Change EO vs. EC (SE) | Significance (P-value)€ | 95% CI |
| ISway JERK | 2.134 (0.32) | 3.925 (0.57) | 1.791 (0.41) | .001** | 0.646, 2.937 |
| JERK AP | 2.459 (0.28) | 5.063 (0.77) | 2.604 (0.61) | .001** | 0.865, 4.343 |
| JERK ML | 1.047 (0.18) | 1.457 (0.31) | 0.411 (0.22) | .44 | −0.222, 1.043 |
| ISway PATH | 8.081 (0.57) | 10.606 (0.81) | 2.526 (0.45) | < .001** | 1.269, 3.783 |
| PATH AP | 6.357 (0.38) | 8.810 (0.72) | 2.453 (0.49) | < .001** | 1.056, 3.85 |
| PATH ML | 3.874 (0.30) | 4.656 (0.42) | 0.782 (.25) | .03* | 0.06, 1.504 |
| Narrow Base Stance (n = 25) | |||||
| ISway Measures | EO Mean (SE) | EC Mean (SE) | Mean Change EO vs. EC (SE) | Significance (P-value) € | 95% CI |
| ISway JERK | 4.787 (0.77) | 7.705 (1.57) | 2.918 (1.36) | .24 | −0.914, 6.750 |
| JERK AP | 4.126 (0.60) | 7.921 (1.98) | 3.795 (1.79) | .27 | −1.362, 8.952 |
| JERK ML | 4.871 (0.94) | 9.374 (3.23) | 4.502 (3.17) | .21 | −4.649, 13.653 |
| ISway PATH | 12.559 (1.01) | 16.192 (1.54) | 3.633 (1.1) | .01* | 0.512, 6.754 |
| PATH AP | 7.802 (0.57) | 10.641 (1.15) | 2.839 (0.87) | .02* | 0.347, 5.332 |
| PATH ML | 7.899 (0.67) | 10.304 (1.11) | 2.405 (0.92) | .09 | −0.252, 5.062, |
Abbreviations and definitions: EO, Eyes Open; EC, Eyes Closed; ISway JERK: ISway JERK (m2/s5); ISway; PATH: Sway Acceleration Path Length* (m2/s2); AP, Anterior-posterior; ML, Medial-lateral;
,Bonferronni corrected P-value;
Significant at the 0.05 level;
Significant at the 0.01 level (2-tailed)
4.0. Discussion
The results of this study demonstrate strong concurrent validity between portable APDM ISway measures, JERK and PATH, in relation to gold-standard laboratory-based force plate measures of COP in older adults with cognitive impairment. In relation to ISway JERK and PATH, strong correlations with COP measures were demonstrated across all 4 static standing conditions, with the strongest correlations observed in the eyes-closed trials during both normal- and narrow-base stance. In addition, significant increases in ISway measures were detected in the eyes closed compared to eyes open conditions.
In previous studies, ISway JERK has been reported to discriminate between adults with untreated PD compared to age-matched controls.21 ISway JERK has also been reported to be sensitive to changes over 1 year in people with PD, suggesting that accelerometer-based technology could be useful for monitoring disease progression.11 Similar applications may be appropriate for older adults with cognitive impairment who are at risk for falling, but mechanisms underlying the instability likely differ.
In the current study, ISway JERK and PATH were more strongly associated with the COP measures than ISway MV or RMS. Weaker relationships observed between COP and ISway RMS and ISway MV may be due to differences in methods of calculating acceleration (an integrated acceleration signal) versus COP (differentiated COP displacement).19 Lower concurrent validity in ISway and COP velocity measures has also been reported in people with PD.19
Our results demonstrate that APDM ISway measures are sensitive to changes in base of support and visual conditions in older adults with cognitive impairment. For example, in normal-base stance, ISway JERK and PATH increased during the eyes-closed/normal-base trials and PATH significantly increased during the eyes-closed/narrow-base trials. Increased sway with the eyes closed suggests reduced postural control without visual input. This finding is consistent with COP parameter changes previously reported in relation to varied visual conditions.1,2
Vision stabilization plays a key role in postural control but lower extremity proprioception is also important. In a cohort of healthy adults (mean age 71, range = 27–93), wearing an inertial sensor, Anson (2017) reported that ankle proprioceptive sensitivity was the dominant sensory predictor of increased postural sway during narrow-base eyes-open and eyes-closed stance on a solid surface, exceeding vision and vestibular input.22 With aging, there is increased reliance on proprioception that contributes to changes in postural control,23 and reduced attention associated the cognitive impairment may further contribute to instability.
Leandri (2009) reported that increased AP sway was associated with reduced cognitive function in patients with AD and discriminated between amnestic MCI and cognitively normal older adults.2 Measuring sway AP path length with a body-worn sensor while altering visual and somatosensory input, Leandri et al (2015) implicated reduced vestibular system function as a key contributing factor of increased postural sway (during eyes closed stance) in relation to increased cognitive impairment.24 In older adults with MCI and AD, Leandri et. al. (2009) have also suggested that the vestibular system is functionally linked to the hippocampus in memory function.2
Body-worn sensor technology has demonstrated validity with sensory integration tests of postural sway in people with PD, 25 but static postural sway measures do not transfer to predicting dynamic instability during gait and other functional tasks.26 Horak et al. (2016) suggests that postural sway and gait represent independent domains of mobility in adults with PD.26 Similar to studies in PD, future research in cognitively impaired older adults would be beneficial in characterizing postural control strategies.27 In the current study, ISway parameter changes were detected during 4 static conditions, but the clinical meaning and predictive validity of these postural sway changes needs further study. In particular, research is needed to understand the role of postural sway measures in predicting falls in older adult with cognitive impairment.
This study was not without limitations. The sample size was relatively small and a single trial, rather than multiple trials, of each condition was conducted. However, we were able to evaluate the utility of APDM ISway measures in cognitively impaired older adults by examining concurrent validity of this portable and user-friendly technology in relation to laboratory-based measures of postural sway. We did not examine test-retest reliability, however, good to excellent reliability of the included ISway measures has previously been reported in other older adults populations. A longitudinal observational study is needed to examine predictive validity of ISway measures for falls and mobility disability in older adults with cognitive impairment.
5.0. Conclusion
The results of this study demonstrated strong concurrent validity between APMD ISway measures (JERK and PATH) in relation to gold-standard laboratory-based force plate measures of COP in older adults with cognitive impairment. The APMD ISway measures detected increased postural sway with changes in base of support and visual conditions. These findings are clinically relevant for rehabilitation professionals evaluating and treating postural instability in cognitively impaired older adult within clinical or home care settings.
Funding Acknowledgements:
This work was supported by the National Institute of Health/NIA under Grant P30 AG034592–03, P.I. Linda Teri, PhD. Improving Healthcare for Cognitively Impaired Elders and Their Caregivers.
Footnotes
Financial Disclosure/Conflict of Interest: None
References
- 1.Deschamps T, Beauchet O, Annweiler C, et al. Postural control and cognitive decline in older adults: position versus velocity implicit motor strategy. Gait Posture 2014;39(1):628–30. doi: 10.1016/j.gaitpost.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 2.Leandri M, Cammisuli S, Cammarata S, et al. Balance features in Alzheimer’s disease and amnestic mild cognitive impairment. J Alzheimers Dis 2009;16(1):113–20. doi: 10.3233/JAD-2009-0928 [DOI] [PubMed] [Google Scholar]
- 3.Degani AM, Leonard CT, Danna-Dos-Santos A. The effects of early stages of aging on postural sway: A multiple domain balance assessment using a force platform. J Biomech 2017;64:8–15. doi: 10.1016/j.jbiomech.2017.08.029 [DOI] [PubMed] [Google Scholar]
- 4.Tavares JT, Biasotto-Gonzalez DA, Boa Sorte Silva NC, et al. Age-Related Changes in Postural Control in Physically Inactive Older Women. J Geriatr Phys Ther 2017. doi: 10.1519/JPT.0000000000000169 [DOI] [PubMed] [Google Scholar]
- 5.Bahureksa L, Najafi B, Saleh A, et al. The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment. Gerontology 2017;63(1):67–83. doi: 10.1159/000445831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piirtola M, Era P. Force platform measurements as predictors of falls among older people - a review. Gerontology 2006;52(1):1–16. doi: 10.1159/000089820 [DOI] [PubMed] [Google Scholar]
- 7.Howcroft J, Kofman J, Lemaire ED. Review of fall risk assessment in geriatric populations using inertial sensors. J Neuroeng Rehabil 2013;10(1):91. doi: 10.1186/1743-0003-10-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seimetz C, Tan D, Katayama R, et al. A comparison between methods of measuring postrual stability: force plates versus accelerometers. Biomed Sci Instrum 2012;48:386–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Howcroft J, Lemaire ED, Kofman J, et al. Elderly fall risk prediction using static posturography. PLoS One 2017;12(2):e0172398. doi: 10.1371/journal.pone.0172398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howcroft J, Kofman J, Lemaire ED. Feature selection for elderly faller classification based on wearable sensors. J Neuroeng Rehabil 2017;14(1):47. doi: 10.1186/s12984-017-0255-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancini M, Carlson-Kuhta P, Zampieri C, et al. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture 2012;36(3):471–6. doi: 10.1016/j.gaitpost.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katzman R, Brown T, Fuld P, et al. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry 1983;140(6):734–9. doi: 10.1176/ajp.140.6.734 [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997;9 Suppl 1:173–6; [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56(3):303–8. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 16.Smieja M, Hunt DL, Edelman D, et al. Clinical examination for the detection of protective sensation in the feet of diabetic patients. International Cooperative Group for Clinical Examination Research. J Gen Intern Med 1999;14(7):418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sangha O, Stucki G, Liang MH, et al. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49(2):156–63. doi: 10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- 18.Lin D, Seol H, Nussbaum MA, et al. Reliability of COP-based postural sway measures and age-related differences. Gait Posture 2008;28(2):337–42. doi: 10.1016/j.gaitpost.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 19.Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil 2012;9:59. doi: 10.1186/1743-0003-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, N.J: Lawrence Erlbaum 1988. [Google Scholar]
- 21.Mancini M, Horak FB, Zampieri C, et al. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Parkinsonism Relat Disord 2011;17(7):557–62. doi: 10.1016/j.parkreldis.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anson E, Bigelow RT, Swenor B, et al. Loss of Peripheral Sensory Function Explains Much of the Increase in Postural Sway in Healthy Older Adults. Front Aging Neurosci 2017;9:202. doi: 10.3389/fnagi.2017.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiesmeier IK, Dalin D, Maurer C. Elderly Use Proprioception Rather than Visual and Vestibular Cues for Postural Motor Control. Front Aging Neurosci 2015;7:97. doi: 10.3389/fnagi.2015.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leandri M, Campbell J, Molfetta L, et al. Relationship between balance and cognitive performance in older people. J Alzheimers Dis 2015;45(3):705–7. doi: 10.3233/JAD-142883 [DOI] [PubMed] [Google Scholar]
- 25.Freeman L, Gera G, Horak FB, et al. Instrumented Test of Sensory Integration for Balance: A Validation Study. J Geriatr Phys Ther 2016. doi: 10.1519/JPT.0000000000000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horak FB, Mancini M, Carlson-Kuhta P, et al. Balance and Gait Represent Independent Domains of Mobility in Parkinson Disease. Phys Ther 2016;96(9):1364–71. doi: 10.2522/ptj.20150580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baston C, Mancini M, Schoneburg B, et al. Postural strategies assessed with inertial sensors in healthy and parkinsonian subjects. Gait Posture 2014;40(1):70–5. doi: 10.1016/j.gaitpost.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]