Abstract
Background:
Persistent or de novo gastroesophageal reflux disease (GERD) may be a significant clinical issue after gastric/bariatric surgical procedures. We investigated the effect of magnetic sphincter augmentation (MSA) in the treatment of GERD after previous gastric/bariatric surgery.
Database:
We conducted a systematic review according to the Preferred Reporting Items For Systematic Reviews and Meta-analyses statement. We searched multiple databases (PubMed, Cochrane, Embase, Scopus) up to May 2019. We also queried the prospectively collected database of patients who underwent MSA at our tertiary-care hospital and compared postsurgical to naïve patients operated during the same time period.
Results:
Seven studies (3 case series and 4 case reports), for a total of 35 patients, met the inclusion criteria in the systematic review. The most common index operation was a bariatric procedure, either sleeve gastrectomy or Roux-en-Y gastric bypass. After MSA implant, the Gastroesophageal Reflux Disease–Health-Related Quality of Life (GERD-HRQL) score significantly improved compared to baseline (P = .005). Two patients (5.7%) required laparoscopic device removal. In the local institutional cohort series of 67 patients treated by MSA, the prevalence of preoperative grade B esophagitis, operative time, size of MSA, and length of stay were greater in patients with prior gastric surgery compared to naïve patients.
Conclusions:
MSA is a safe, simple, and standardized antireflux procedure. It is also feasible in patients with refractory GERD following gastric/bariatric surgery. Further prospective and comparative studies are needed to validate the preliminary clinical experience in this subset of patients.
Keywords: Gastroesophageal Reflux Disease, Magnetic Sphincter Augmentation, LINX, Bariatric Surgery, Sleeve Gastrectomy
INTRODUCTION
Magnetic sphincter augmentation (MSA) is a new option for the treatment of gastroesophageal reflux disease (GERD). The MSA device (LINX®; Johnson & Johnson, New Brunswick, New Jersey, USA) is a flexible ring made by a series of titanium beads, each containing a magnetic core and linked together with independent titanium arms. The device is implanted laparoscopically around the esophagogastric junction to augment competency of the lower esophageal sphincter.1 The Linx was introduced in 2007, and since then various studies confirmed its safety and efficacy in terms of symptom relief, reduction of esophageal acid exposure and proton-pump inhibitor (PPI) use, and improvement in quality of life.2–4 Moreover, the procedure was associated to less gas-bloat symptoms and increased ability to vomit and belch compared to laparoscopic fundoplication.5
As safety and efficacy of this technique became evident in clinical practice, indications for MSA have gradually expanded after Food & Drug Administration (FDA) approval in 2012 to include patients with large hiatal hernia, Barrett's esophagus, and severe symptoms/complications following previous gastric surgery.6–10 Persistent or de novo GERD after gastric and bariatric surgery may be difficult to manage given the altered anatomy, unavailability of gastric fundus to perform a fundoplication, and the concern of morbidity associated to diversion procedures such as the Roux-en-Y gastric-bypass (RYGB).11–12 Sleeve gastrectomy (SG) has now become the most common surgical procedure for obesity, with the percentage among bariatric operations increasing from 17.8% in 2011 to 53.8% in 2015.13 However, a recent meta-analysis including 46 studies with more than 10,000 patients has shown an increase of de novo GERD in 23%, and a long-term prevalence of esophagitis in 28% and Barrett's esophagus in 8% of patients after SG.14 A population study in Sweden concluded that about 50% of patients undergoing RYGB require continuous PPI therapy and that the efficacy of this procedure in controlling GERD may have been overestimated.15 MSA could be an effective therapeutic modality to control refractory GERD symptoms in these patients. The aim of this study was to perform a systematic review of the literature on the management of persistent or de novo reflux after gastric/bariatric surgery with MSA and to report our personal experience.
METHODS
We conducted a systematic review according to the Preferred Reporting Items For Systematic Reviews and Meta-analyses (PRISMA) statement. An extensive literature search was conducted by four independent authors (CGR, EA, VL, KA) to identify all clinical reports on MSA published between 2008 and May 2019. PubMed, Cochrane, Embase, and Scopus databases were queried using the following terms: “Magnetic sphincter augmentation,” “MSA,” “LINX,” “Gastric surgery,” “Bariatric surgery,” “Gastro-esophageal reflux disease,” “GERD,” and every possible combination with AND/OR. The search was restricted to studies published in English and was completed by consulting the listed references of each article. Studies were included if outcomes of patients receiving MSA implants after any type of gastric surgery were reported. Abstracts were excluded. Disagreements among authors were resolved by consensus; if no agreement could be reached, the senior author (LB) made the decision. For each selected study, data extracted included first author name, year of publication, number of patients, age, sex, body mass index, index surgical procedure, indication for surgery, Gastroesophageal Reflux Disease–Health-Related Quality of Life (GERD-HRQL) score, DeMeester score, time to reoperation, operative time, size of MSA device, crural repair, length of hospital stay, persistent symptoms, device explants, followup, and postoperative GERD-HRQL score. The methodological quality of the studies was assessed, according to Murad et al.16 based on the most critical factors that increase the risk of bias in this specific clinical context.
In addition to the systematic literature review, after Institutional Review Board (IRB) approval (protocol 00311, February 25, 2019) we analyzed the prospectively collected database at our tertiary-care hospital to identify all patients who underwent MSA for GERD. As inclusion criteria, we considered patients who were implanted following gastric surgery and naïve patients who received a MSA implant for primary GERD during the same time period. A comparative analysis of clinical characteristics and outcomes in the two patient groups was performed. Routine preoperative workup included upper gastrointestinal endoscopy, barium swallow study, standard or high-resolution manometry, and 48-hour esophageal pH monitoring with Bravo capsule. The GERD-HRQL questionnaire was administered preoperatively and during the follow-up visits to all patients. Demographic characteristics, body mass index, duration of GERD symptoms, frequency of typical/atypical symptoms, dose and duration of proton pump inhibitor (PPI) therapy, GERD-HRQL score, size of hiatal hernia measured endoscopically, grade of esophagitis, presence of Barrett's esophagus, time and type of index surgical procedure, operative time, size of MSA, crura repair, and postoperative outcomes including GERD-HRQL score were collected. The technique of MSA implantation has been described elsewhere.1 In the presence of large hiatal hernia, full hiatal dissection with mobilization of the thoracic esophagus was performed to provide an adequate length of intra-abdominal esophagus; the crura were repaired with synthetic nonabsorbable sutures. Patients were discharged home on postoperative day 1 and were encouraged to eat a semisolid diet at least 6 times daily during the first 2 postoperative months. Follow-up visits and testing were scheduled between 6 and 12 months postoperatively.
Statistical analysis was performed using the SPSS software version 23 (SPSS, Inc, Chicago, Illinois, USA). Continuous and categorical variables were compared using the Wilcoxon and the Fisher's exact test, respectively. Statistical significance was established at the 0.05 level.
RESULTS
Systematic Review10,17–22
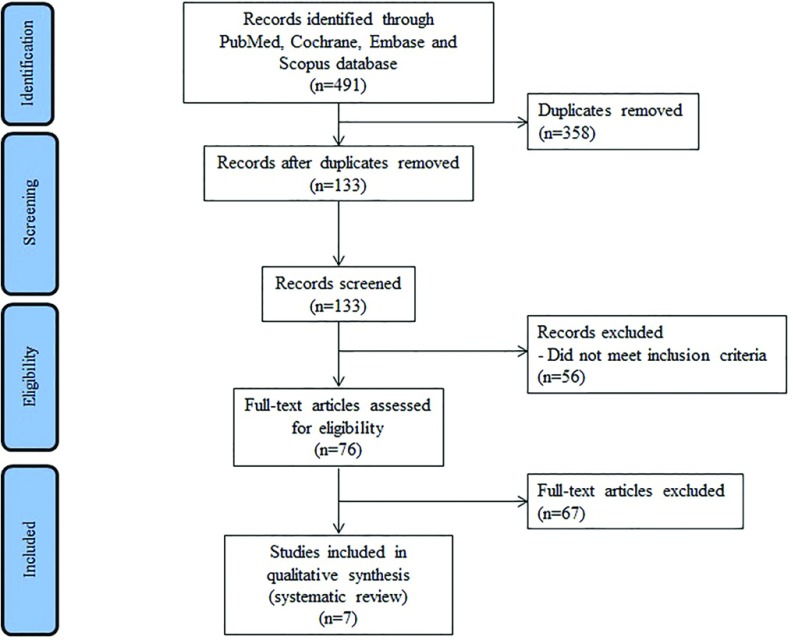
The PRISMA diagram is presented in Figure 1. Out of 491 studies screened, 7 matched the inclusion criteria. In total, there were 35 patients who received a MSA implant after gastric surgery; the sample size of the individual studies ranged from 1 to 13 patients. All studies were observational, and only one provided a comparative analysis of postbariatric and standard procedures.22 Demographic, clinical, and operative variables of the patient sample are shown in Table 1. The mean age of patients ranged from 25 to 60 years, and the majority (69%) were females. The mean body mass index ranged from 27.9 to 39kg/m2. Before MSA implant, all patients were on PPI therapy. Six studies reported esophageal pH monitoring data, and the DeMeester score ranged from 27.7 to 66.6.
Figure 1.
PRISMA diagram.
Table 1.
Systematic Review of the Literature
| Author (Reference) | No. Patients | Index Operation | Indication for Surgery | GERD-HRQL Score | DeMeester Score | Time to Reoperation, Months | Operative Time Minutes | Persistent Symptoms (n) | Explants (n) | F.U. Months | GERD-HRQL Score Post-MSA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Desart17 | 7 | SG | Obesity | 17.1 | 56.6 | 18.1 | — | 0 | 0 | 1 | 5.1 |
| Muñoz-Largacha18 | 2 | RYGB | Recurrent GERD (1) Obesity (1) | 30 | 14–55.4 | NR | 130.5 | 0 | 0 | 8.5 | 3 |
| Hawasli19 | 1 | RYGB | Obesity | 21 | 27.7 | 96 | 71 | 0 | 0 | 1.5 | 0 |
| Hawasli20 | 1 | SG | Obesity | 64 | 66.6 | 30 | 47 | 0 | 0 | 12 | 7 |
| Hawasli21 | 13 | SG | Obesity | 47 | 46 | 43 | 79 | 7 (6 acid reflux, 1 dysphagia) | 1 | 26 | 12 |
| Kuckelman22 | 10 | SG (8) RYGB (2) | Obesity | 41 | — | 75 | 114 | 5 (4 dysphagia, 1 acid reflux) | 1 | 15 | 10 |
| Melloni10 | 1 | B-II | Perforated peptic ulcer | 26 | 38.2 | 60 | 80 | 0 | 0 | 12 | 2 |
B-II, Billroth II; GERD-HRQL, Gastroesophageal reflux disease–Health-related quality of life; MSA, Magnetic Sphincter Augmentation; NR, Not Reported; RYGB, Roux-en-Y Gastric Bypass; SG, sleeve gastrectomy.
In 33 (94.3%) patients, the MSA procedure was performed after prior bariatric surgery. The most common index operation causing GERD was SG (n = 29), followed by RYGB (n = 4). A MSA device was implanted laparoscopically in all patients and there were no conversions to open surgery. The operative time ranged from 47 to 184 minutes. A concomitant crural repair was reported in 15 (43%) patients.20,21 The mean hospital length of stay was 1 day. All patients reported improvement of the GERD-HRQL score at the time of the last follow-up visit (5.6 ± 4.4 vs 35.2 ± 16.5, P = .005). However, 12 (34.3%) patients complained of persistent symptoms after MSA. Seven (20.0%) of them reported recurrent reflux symptoms but only 3 (8.6%) resumed daily PPI therapy. Overall, 5 (14.3%) patients reported persistent dysphagia; 3 of them underwent successful endoscopic dilatation, and two (5.7%) required laparoscopic device removal 18 days and 4 months after implant, respectively. There was no mortality and the postoperative followup ranged from 1 to 38 months (Table 1). All the included studies had a moderate risk for bias based on a global assessment of the methodological quality.16
Local Institutional Case Series
Between March 2007 and May 2019, 300 patients underwent MSA at our institution. Four of them (1.3%) had previous gastric/bariatric surgery in other hospitals and were referred for GERD. One of these patients, Case 1 is included in the systematic review.10 Demographic and clinical characteristics of patients who underwent MSA implant for primary (n = 63) or persistent/de novo (n = 4) GERD after gastric surgery during the same time period (October 2017 to December 2018) were compared. Patient characteristics and clinical outcomes were similar except for a statistically significant greater prevalence of grade-B esophagitis (P = .014), operative time (P = .000, CI = 67.6–87.9), size of MSA (P = .046, CI = 14.8–15.2), and length of stay (P = .038, CI = 2.3–2.7) in patients with prior gastric surgery (Table 2).
Table 2.
Comparative Analysis of Patients Who Underwent MSA Implant for Primary GERD and for Persistent/de novo GERD During the Same Time Period at a Single Institution
| Demographic and Clinical Characteristics | Standard (n = 63) | Post-Surgical (n = 4) | P |
|---|---|---|---|
| Age, years, mean ± SD | 51.4 ± 13.6 | 54.3 ± 11.6 | .723 |
| Male, no. (%) | 45 (71.4) | 2 (50) | .216 |
| BMI, mean ± SD | 25.5 ± 4.2 | 29.0 ± 2.6 | .108 |
| Symptoms duration, years, mean ± SD | 9.3 ± 7.2 | 4 ± 2.6 | .997 |
| Daily PPI dose (mg), mean ± SD | 20.6 ± 20.6 | 50 ± 26.4 | .073 |
| Duration PPI therapy, years, mean ± SD | 4.9 ± 6.3 | 12 ± 15.7 | .270 |
| GERD-HRQL, mean ± SD | 19.3 ± 6.3 | 18.7 ± 9.3 | .756 |
| Hiatus hernia, cm, mean ± SD | 2.1 ± 1.5 | 3 ± 1 | .246 |
| DeMeester score, mean ± SD | 31.2 ± 23.6 | 24 ± 2.7 | .695 |
| Los Angeles Grade, no. (%) | |||
| A | 5 (7.9) | 1 (25) | .252 |
| B | 3 (4.8) | 3 (75) | <.005 |
| C | 2 (3.2) | 0 (0.0) | 1.000 |
| D | 1 (1.6) | 0 (0.0) | 1.000 |
| Barrett's esophagus, no. (%) | 9 (14.2) | 1 (25) | 1.000 |
| Operative characteristics | |||
| Operative time, minutes, mean ± SD | 71.4 ± 27 | 206.7 ± 24.7 | <.005 |
| MSA device, no. beads, median (IQR) | 15 (2) | 16 (0) | <.05 |
| Full mediastinal dissection, no. (%) | 31 (49.2) | 4 (100) | .239 |
| Postoperative characteristics | |||
| Complications, no. (%) | 1 (1.6) | 1 (25) | .090 |
| In-hospital length of stay, days, mean ± SD | 2.5 ± 0.7 | 3.5 ± 0.7 | <.05 |
| Followup, months, median (IQR) | 11 (8) | 13 (3.5) | .199 |
| Explants, no. (%) | 0 (0.0) | 0 (0.0) | — |
| GERD-HRQL, mean ± SD | 2.3 ± 1.9 | 3 ± 1 | .444 |
B-II, Billroth II; BMI, body mass index; GERD-HRQL, gastroesophageal reflux disease–health-related quality of life, MSA, magnetic sphincter augmentation; PPI, proton-pump inhibitor; RYGB, Roux-en-Y Gastric bypass; SG, sleeve gastrectomy.
DISCUSSION
This study shows that MSA is feasible, safe, and effective in patients presenting with refractory GERD after gastric surgery, and that bariatric procedures represent the most common index operation in these patients. The widespread rise of bariatric surgery, mainly SG,23 in the western world, has been associated with an increased incidence of either pre-existing or de novo GERD.14 Elimination of the angle of His, resection of the sling fibers, decreased lower esophageal sphincter pressure/length, decreased gastric volume and emptying, increased intragastric pressure and transdiaphragmatic pressure gradient are the main putative factors promoting gastroesophageal reflux after SG.24 Refractory GERD and volume regurgitation is common in these patients despite daily therapy with PPI and dose escalation.25–26 Currently, severe GERD is the most common reason for reoperation after SG,27–28 and conversion to RYGB is the most common remedial procedure although symptoms persist in up to 20%–30% of these patients.29 Due to the increased prevalence of de novo reflux (23%), the alarming long-term rate of Barrett's esophagus (8%), and the potential for esophageal adenocarcinoma after SG,14 this procedure should not be offered to patients who are deemed at risk to aggravate pre-existing symptoms or to develop de novo GERD based on preoperative esophageal function testing. This is consistent with the recommendations of the fifth International Consensus Conference for SG where 80% of expert surgeons felt that Barrett's esophagus was an absolute contraindication to SG.30
Since revisional bariatric surgery carries a fair rate of complications, such as anastomotic leak, and mortality,12,28,31 minimally invasive alternatives for the treatment of postoperative GERD have recently emerged. MSA is a standardized and reproducible laparoscopic procedure that has proven safe and effective in the treatment of primary GERD.2–4 As shown in the present review, experience with MSA in patients with postsurgical GERD is still limited, but the safety profile and the short-term results appear comparable to those of naïve patients except for a higher removal rate after bariatric surgery (5.7%) compared to the general population (3.4%)32 It has also been suggested that MSA may represent a valid prophylactic measure during SG, but this hypothesis should be tested in randomized clinical trials.33
The endoscopic Stretta procedure and the laparoscopic electrical lower esophageal sphincter stimulation have also been proposed in postbariatric GERD patients. Short-term (6-month) improvement of GERD symptoms was noted in a small cohort of patients with previous RYGB who underwent the Stretta procedure,34 whereas one third of patients undergoing Stretta after SG were dissatisfied with the outcomes.35 Interestingly, an international multicenter registry study including 17 patients followed for a median of 12 months after SG showed that laparoscopic electrical stimulation significantly reduced GERD symptoms, PPI use, GERD-HRQL scores, and esophageal acid exposure.36
The major limitations of the present study are the small sample size, the short patient followup, the heterogeneity of preoperative patient workup, and the lack of postoperative pH studies that limit broad generalizable conclusions. However, pre- and postoperative quality of life data were reported in all studies, and there were two comparative cohorts, one included in the systematic review and the other representing our institutional case series. Finally, despite this review has included only case series and case reports, their methodological quality and risk of bias were assessed according to the criteria of Murad et al.16
CONCLUSIONS
MSA combined with crural repair appears to be an encouraging therapeutic option in patients with persistent or de novo GERD after gastric/bariatric surgery due to the relatively easy procedure, the safety profile, and the satisfactory short-term outcomes. Further prospective and comparative studies are needed to validate the application of MSA in the postbariatric patient population and its potential to replace revisional RYGB.
Contributor Information
Carlo Galdino Riva, Department of Biomedical Sciences for Health, Division of General and Foregut Surgery, IRCCS Policlinico San Donato, University of Milano, Milano, Italy..
Emanuele Asti, Department of Biomedical Sciences for Health, Division of General and Foregut Surgery, IRCCS Policlinico San Donato, University of Milano, Milano, Italy..
Veronica Lazzari, Department of Biomedical Sciences for Health, Division of General and Foregut Surgery, IRCCS Policlinico San Donato, University of Milano, Milano, Italy..
Krizia Aquilino, Department of Biomedical Sciences for Health, Division of General and Foregut Surgery, IRCCS Policlinico San Donato, University of Milano, Milano, Italy..
Stefano Siboni, Department of Biomedical Sciences for Health, Division of General and Foregut Surgery, IRCCS Policlinico San Donato, University of Milano, Milano, Italy..
Luigi Bonavina, Department of Biomedical Sciences for Health, Division of General and Foregut Surgery, IRCCS Policlinico San Donato, University of Milano, Milano, Italy..
References:
- 1. Bonavina L, Saino GI, Bona D, et al. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg. 2008;12:2133–40. [DOI] [PubMed] [Google Scholar]
- 2. Bonavina L, DeMeester T, Fockens P, et al. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg. 2010;252:857–62. [DOI] [PubMed] [Google Scholar]
- 3. Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med. 2013;368:719–27. [DOI] [PubMed] [Google Scholar]
- 4. Bonavina L, Saino G, Bona D, Sironi A, Lazzari V. One hundred consecutive patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease: 6 years of clinical experience from a single center. J Am Coll Surg. 2013;217:577–85. [DOI] [PubMed] [Google Scholar]
- 5. Aiolfi A, Asti E, Bernardi D, et al. Early results of magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: Systematic review and meta-analysis. Int J Surg. 2018;52:82–8. [DOI] [PubMed] [Google Scholar]
- 6. Kuckelman JP, Phillips CJ, Hardin MO, Martin MJ. Standard vs expanded indications for esophageal magnetic sphincter augmentation for reflux disease. JAMA Surg. 2017;152:890–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rona KA, Reynolds J, Schwameis K, et al. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc. 2017;31:2096–102. [DOI] [PubMed] [Google Scholar]
- 8. Buckley FP, 3rd, Bell RCW, Freeman K, Doggett S, Heidrick R. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg Endosc. 2018;32:1762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwameis K, Nikolic M, Castellano DGM, et al. Crural closure improves outcomes of magnetic sphincter augmentation in GERD patients with hiatal hernia. Sci Rep. 2018;9:8:7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Melloni M, Lazzari V, Asti E, Bonavina L. Magnetic sphincter augmentation is an effective option for refractory duodeno-gastro-oesophageal reflux following Billroth II gastrectomy. BMJ Case Rep. 2018;2018 pii: bcr-2018-225364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Treitl D, Nieber D, Ben-David K. Operative treatments for reflux after bariatric surgery: current and emerging management options. J Gastrointest Surg. 2017;21:577–82. [DOI] [PubMed] [Google Scholar]
- 12. Fulton C, Sheppard C, Birch D, Karmali S, de Gara C. A comparison of revisional and primary bariatric surgery. Can J Surg. 2017;60:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponce J, DeMaria EJ, Nguyen NT, Hutter M, Sudan R, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in 2015 and surgeon workforce in the United States. Surg Obes Relat Dis. 2016;12:1637–9. [DOI] [PubMed] [Google Scholar]
- 14. Yeung KTD, Penney N, Ashrafian L, Darzi A, Ashrafian H. Does sleeve gastrectomy expose the distal esophagus to severe reflux? A systematic review and meta-analysis. Ann Surg. 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Holmberg D, Santoni G, Xie S, Lagergren J. Gastric bypass surgery in the treatment of gastro-oesophageal reflux symptoms. Aliment Pharmacol Ther. 2019;50:159–66. [DOI] [PubMed] [Google Scholar]
- 16. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Medicine. 2018;23:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desart K, Rossidis G, Michel M, Lux T, Ben-David K. Gastroesophageal reflux management with the LINX® system for gastroesophageal reflux disease following laparoscopic sleeve gastrectomy. J Gastrointest Surg. 2015;19:1782–6. [DOI] [PubMed] [Google Scholar]
- 18. Muñoz-Largacha JA, Hess DT, Litle VR, Fernando HC. Lower esophageal magnetic sphincter augmentation for persistent reflux after Roux-en-Y gastric bypass. Obes Surg. 2016;26:464–6. [DOI] [PubMed] [Google Scholar]
- 19. Hawasli A, Phillips A, Tarboush M. Laparoscopic management of reflux after Roux-en-Y gastric bypass using the LINX system and repair of hiatal hernia: a case report. Surg Obes Relat Dis. 2016;12:e51–4. [DOI] [PubMed] [Google Scholar]
- 20. Hawasli A, Tarakji M, Tarboush M. Laparoscopic management of severe reflux after sleeve gastrectomy using the LINX® system: technique and one year follow up case report. Int J Surg Case Rep. 2017;30:148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hawasli A, Sadoun M, Meguid A, Dean M, Sahly M, Hawasli B. Laparoscopic placement of the LINX® system in management of severe reflux after sleeve gastrectomy. Am J Surg. 2019;217:496–9. [DOI] [PubMed] [Google Scholar]
- 22. Kuckelman JP, Phillips CJ, Derickson MJ, Faler BJ, Martin MJ. Esophageal magnetic sphincter augmentation as a novel approach to post-bariatric surgery gastroesophageal reflux disease. Obes Surg. 2018;28:3080–6. [DOI] [PubMed] [Google Scholar]
- 23. English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14:259–63. [DOI] [PubMed] [Google Scholar]
- 24. Nadaleto BF, Herbella FA, Patti MG. Gastroesophageal reflux disease in the obese: pathophysiology and treatment. Surgery. 2016;159:475–86. [DOI] [PubMed] [Google Scholar]
- 25. Sheu BS, Cheng HC, Chang WL, Chen WY, Kao AW. The impact of body mass index on the application of on-demand therapy for Los Angeles grades A and B reflux esophagitis. Am J Gastroenterol. 2007;102:2387–94. [DOI] [PubMed] [Google Scholar]
- 26. El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32:720–37. [DOI] [PubMed] [Google Scholar]
- 27. Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Madalosso CA, Gurski RR, Callegari-Jacques SM, Navarini D, Mazzini G, Pereira M. The impact of gastric bypass on gastroesophageal reflux disease in morbidly obese patients. Ann Surg. 2016;263:110–6. [DOI] [PubMed] [Google Scholar]
- 30. Gagner M, Hutchinson C, Rosenthal R. Fifth international consensus conference: current status of sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:750–6. [DOI] [PubMed] [Google Scholar]
- 31. Cheung D, Schwitzer NJ, Gill RS, Shi X, Karmali S. Revisional bariatric surgery following failed primary laparoscopic sleeve gastrectomy: a systematic review. Obes Surg. 2014;1757–63. [DOI] [PubMed] [Google Scholar]
- 32. Lipham JC, Taiganides PA, Louie BE, Ganz RA, DeMeester TR. Safety analysis of first 1000 patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease. Dis Esophagus. 2015;28:305–11. [DOI] [PubMed] [Google Scholar]
- 33. Altieri MS, Pryor AD. Gastroesophageal reflux disease after bariatric procedures. Surg Clin North Am. 2015;95:579–91. [DOI] [PubMed] [Google Scholar]
- 34. Mattar SG, Qureshi F, Taylor D, Schauer PR. Treatment of refractory gastroesophageal reflux disease with radiofrequency energy (Stretta) in patients after Roux-en-Y gastric bypass. Surg Endosc. 2006;20:850–54. [DOI] [PubMed] [Google Scholar]
- 35. Khidir N, Angrisani L, Al-Qahtani J, Abayazeed S, Bashah M. Initial experience of endoscopic radiofrequency waves delivery to the lower esophageal sphincter (Stretta procedure) on symptomatic gastroesophageal reflux disease post-sleeve gastrectomy. Obes Surg. 2018;28:3125–30. [DOI] [PubMed] [Google Scholar]
- 36. Borbély Y, Bouvy N, Schulz HG, Rodriguez LA, Ortiz C, Nieponice A. Electrical stimulation of the lower esophageal sphincter to address gastroesophageal reflux disease after sleeve gastrectomy. Surg Obes Relat Dis. 2018;14:611–5. [DOI] [PubMed] [Google Scholar]