Abstract
Diabetes mellitus is a significant risk factor for acquired hearing loss and tinnitus. Persons with diabetes (PWD) may present with hearing loss symptoms earlier in life than those without diabetes. Furthermore, diabetes may exacerbate risk for hearing loss related to noise exposure and ototoxic drugs. The purpose of this article is to provide recommendations for the prevention, screening, evaluation, and management of hearing loss in PWD.
Keywords: hearing loss, tinnitus, diabetes, prevention, screening, evaluation, management
The literature overall supports diabetes mellitus as a significant independent risk factor for hearing loss and tinnitus. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Diabetes can contribute to hearing loss affecting cochlear and neural elements 16 17 through numerous mechanisms, including microangiopathy, mitochondrial dysfunction, advance glycation end products/inflammation, and glutamate excitotoxicity. 7 18 19 20 21 22 23 24 25 The net result is increased risk for primarily acquired auditory sensory and neural pathology (i.e., sensorineural hearing loss). Increased risk for infection in persons with diabetes (PWD) also should alert the provider to risk for conductive pathologies of the external and middle ear. 26 In addition, medications for treatment of diabetes and diabetes-related symptoms may have ototoxic side effects including hearing loss and tinnitus (see DiSogra and Meech, 2019 in this edition).
Human and animal evidence suggests potential for damage along the cochlea length from basal to apical regions. 9 The distribution of changes along the cochlea may be related to the relative contributions of the array of mechanisms implicated in diabetes-related hearing loss. For example, microagniopathy may result in direct compromise of vascular supply to the inner ear; the apical region representing the most distal region of this supply may show early pathology. 9 27 28 29 30 On the contrary, elevated risk for noise-induced damage may underlie early changes observed in basal regions of the cochlea. 9 31
Potential for onset of hearing loss earlier in life, increased risk for other determinants of hearing loss (e.g., noise and ototoxic drugs), and risk of diabetes and hearing loss associated with balance dysfunction 32 33 and cognitive decline 34 35 support need for improved preventative approaches and guidelines for evaluation and management of diabetes-related hearing loss. The purpose of this manuscript is to present recommendations to inform prevention, screening, early identification, and management of hearing loss in PWD.
Prevention: Diabetes and Hearing Loss
Prevention includes a range of interventions aimed at reducing risk for compromised health. In general, the three categories of prevention considered include primary, secondary, and tertiary .
Primary prevention refers to prevention of disease or injury prior to onset. For hearing loss, an example of primary prevention would include regulations requiring use of hearing protection in the workplace or installing equipment with lower sound level output. These two examples represent active and passive strategies, respectively, with regard to the worker's behavior. In other words, use of hearing protection requires an active behavioral change among workers, while installation of equipment with lower sound emissions reduces risk without the worker having to make a behavioral choice.
Secondary prevention is aimed at slowing or reducing progression of disease or injury that has occurred. A critical concept in secondary prevention is early detection, which allows for timely intervention. For example, a patient needs to receive a medication with known ototoxic side effects to preserve functional status. Regular monitoring allows early identification of damage to allow implementation of potential otoprotective strategies, such as change in dosage level, change in schedule of drug, or providing hearing-assistive technology to prevent compromised communication. An essential element to secondary prevention of hearing loss is establishing baseline hearing status to allow sensitivity to detect change.
Tertiary prevention is consistent with what we commonly regard as “treatment or management.” The goal of tertiary prevention is to reduce impact of an ongoing disease or injury on function and quality of life. For example, a PWD has acquired hearing loss that is significantly impacting his or her interaction with family and friends leading to isolation and reduced quality of life. Fitting of appropriate amplification and aural rehabilitation recommendations can improve engagement with life and patient's overall well-being.
Primary, secondary, and tertiary prevention-based strategies can be applied at the individual, community, and system level. Furthermore, these concepts should be reinforced across prevention levels and at each patient interaction. Fig. 1 provides an overview of the prevention of hearing loss across categories and levels.
Figure 1.
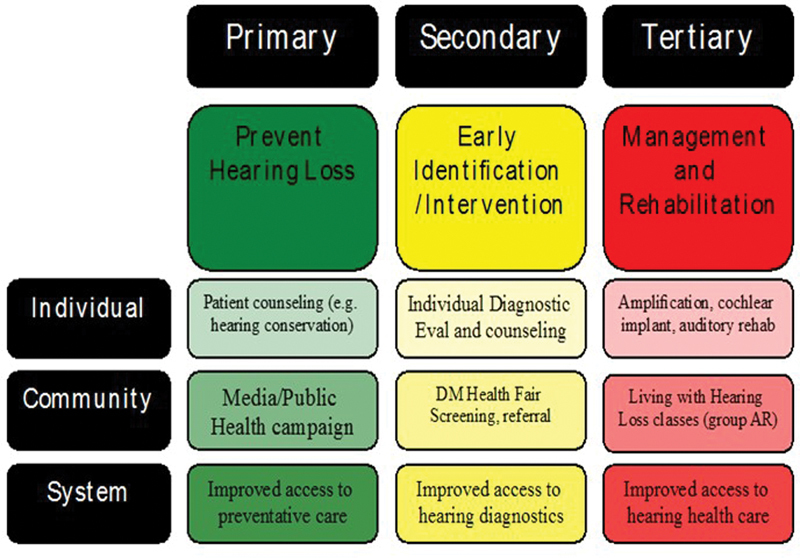
Primary, secondary, and tertiary prevention overview.
Improving Hearing Health Literacy
Health literacy and knowledge of determinants of disease/injury are critical to primary prevention. Primary prevention of hearing loss in PWD includes several strategies. The education of PWD with regard to elevated risk for hearing loss is a first step. Primary prevention strategies for diabetes-related hearing loss include the following:
-
Educating/counseling PWD on risk for hearing loss with diabetes, topics including:
Risk for earlier onset of age-related hearing loss.
Progressive nature of hearing loss due to uncontrolled diabetes.
Increased susceptibility to noise and drug-related hearing loss.
Importance of monitoring and early intervention.
-
Educating/counseling PWD on prevention of hearing loss, topics including:
Limiting noise exposure and use of hearing protection devices.
Awareness of ototoxic medications the patient may require.
-
Education/counseling PWD on early signs of hearing loss, signs including:
Fullness sensation.
Tinnitus.
Reduced ability to understand speech particularly in noisy environments.
Education/counseling PWD on importance of diabetes control (ABCs) and healthy lifestyle.
The ABCs refers to A1c, blood pressure, cholesterol, and smoking as factors the PWD can address to prevent low-risk individuals from moving to higher risk for diabetes-related complications and increased risk for hearing loss. 36 Lifestyle can drastically alter the health status of PWD. Lifestyle factors including diet, physical activity, and smoking are also related to hearing loss. 37 38 39 40 41 42
Recommendations for Screening and Diagnostic Evaluation
Screening Recommendations for Healthcare Providers
Early identification of changes in auditory function is necessary to provide timely intervention strategies. Screening PWD for hearing loss is important to successful prevention strategies. Screening options include pure-tone audiometry screening and otoacoustic emission (OAE) screening. Pure-tone audiometry screening involves presentation of tonal sounds at different frequencies important for speech understanding; the patient then reports sound detection. Testing is typically accomplished with the use of a pure-tone audiometer. Stimuli are presented to calibrated headphones or insert earphones typically at a single level (e.g., 25 dB HL) and at multiple frequencies important for speech understanding. The presence/absence of a detection response (e.g., raise hand) is interpreted as Pass/Refer. There are several online and smartphone-based applications for hearing screening; however, caution is warranted due to the variability of these applications that often lack appropriate calibration.
OAEs are an objective measure of cochlear function. OAE measurement involves administering a sound to the ear via a probe placed in the ear canal; the probe measures distortions and reflections generated in the cochlea that travel back to the external ear. OAEs do not require the patient to make a behavioral response. An OAE screening test involves measurement of responses from the cochlea and determination if the amplitude of the response meets some predetermined criteria for a Pass/Refer interpretation. OAEs are not a measure of hearing, but rather a measure of cochlear function. The presence of OAEs does not rule out mild, neural, or central hearing deficits and the absence of OAEs does not inform on the severity of the hearing loss.
Not all health care providers have access to the necessary equipment and these screening tests provide only limited insight on hearing status; referral to an audiologist may be a necessary step. Other screening tests include the whispered voice test, finger rub test, and watch tick test. The primary limitations of these screening measures are the broad variation in outcomes across examiners and lack of standardized procedures. 43 44 45
Some simple questions with regard to perceived hearing difficulty may provide insight on hearing status and help inform need for further evaluation. The following screening questions are recommended to determine need for further evaluation (these questions are based on those used in the Guide for Pharmacy, Podiatry, Optometry, and Dentistry [PPOD] for Diabetes Management):
Do you or your family perceive any change in your hearing?
Do you have hearing difficulty in quiet or noise?
Have you had your hearing tested in the past 2 years?
Do you know how diabetes can affect your hearing?
Do you know what to do if you perceive a change in hearing?
Do you know how to reduce your risk for hearing loss?
If the patient answers “Yes” to questions 1 or 2 or “No” to questions 3 through 6, it is recommended they be referred for a diagnostic audiological evaluation.
Numerous hearing screening options exist to inform next steps and need for more in-depth evaluation. Yet, screenings may miss subtle forms of hearing loss and limit the ability to establish a baseline assessment early in the diabetic disease process. Therefore, to establish baseline, to enable early identification, and to impart education on prevention strategies, we recommend a comprehensive audiological evaluation for all patients upon diagnosis of diabetes . If the patients report hearing difficulty or if they have not had their hearing tested in the past 2 years, they also should be referred.
Diagnostic Recommendations
A comprehensive audiological evaluation (pure-tone and speech audiometry) is recommended as a minimal test for determining the type and severity of hearing loss in the PWD. Additionally, the professional should take a detailed and thorough case history to identify any associated comorbidities so that the most effective and comprehensive interventions can be provided.
To increase the efficacy of identification, we recommend upon diabetes diagnosis that the PWD be referred to an audiologist for a baseline diagnostic audiological evaluation . Baseline evaluations can establish hearing status to improve ability to monitor change. An audiological evaluation should be performed at least every 2 years or sooner if an individual is experiencing any high-risk characteristics. PWD who are at high risk for hearing and balance problems may have one or more of the following characteristics and require closer monitoring:
Reduced speech understanding (particularly in noisy environments).
Tinnitus perception.
History of high levels of noise exposure.
History of ototoxic drug use.
Sensitivity to loud sounds.
Ear pain or drainage.
Dizziness complaints.
History of falls.
Concern for falling.
Low-risk individuals have none of these characteristics. Assessment of risk status identifies people who need more intensive care and evaluation.
Changes in the auditory system related to diabetes include sensory, neural, and vascular elements. 16 17 These changes can influence hearing along the range of human perception from regions associated with low-frequency and high-frequency hearing. Studies suggest that early indices of pathology may be related to subtle changes in cochlear, 46 auditory neural function, 11 and extended high-frequency hearing. 9 These factors should be considered when determining testing protocols. Based on the outcomes of the comprehensive audiological evaluation and case history, further testing including extended high-frequency threshold audiometry, immittance measures, OAEs, early- and late-auditory evoked potentials, tinnitus assessment, central auditory processing evaluation, and balance assessment may be recommended. The chosen test battery should be based on patient complaints and help determine the most appropriate intervention. Red flags, such as sudden hearing loss or evidence of disease, warrants a referral for medical evaluation.
Case Example
A patient with recent diagnosis of diabetes presents for baseline hearing assessment. The patient's complaints include aural fullness, tinnitus, and reduced speech understanding in noise. The patient denies any balance issues or experience of vertigo and denies any ear pain/drainage or history of ear infections. The patient's medications include metformin, furosemide, and sertraline. Results from the comprehensive audiological evaluation indicated a mild symmetrical high-frequency sensorineural hearing loss with excellent speech understanding in quiet. Based on these results, patient history, and patient's chief complaint, the provider may suggest further testing. For example, a recommendation may be made for OAEs to monitor subtle effects of medications on cochlear function. Speech-in-noise testing may be recommended to determine how noise affects speech understanding compared with understanding in quiet. If the patient's primary complaint is tinnitus, this may lead to recommendation for a tinnitus evaluation.
Continuum of care and counseling is also important. Strategies outlined in the primary prevention section should be reinforced during an evaluation or follow-up visit. PWD should be continually counseled on risk factors and preventative strategies for reducing risk for further hearing loss, including noise exposure, ototoxic medications, healthy lifestyle, and maintenance of ABCs.
A comprehensive audiological evaluation should be performed at least every 2 years or sooner if an individual is experiencing any high-risk characteristics. Based on patient history, complaints, and results of comprehensive audiological evaluation, further testing may be warranted. Sudden hearing loss or evidence of disease should result in a referral for a medical evaluation.
Management
Early diagnosis and proper intervention can reduce risk for further acquired hearing loss and facilitate early treatment. The most common type of damage to the auditory system related to diabetes is sensory, neural, and vascular in nature, that is, sensorineural hearing loss, in contrast to increased risk for external and middle ear pathologies, where supporting data are limited. 26 Sensorineural hearing loss is commonly addressed by use of devices that amplify sound and/or aural rehabilitation strategies. PWD in early stages could have hearing loss in the range of mild to moderately severe in degree. 12 Considering their hearing demands, amplification and compensatory strategies can be advised. Surgical or pharmaceutical treatment of hearing loss currently has limited options in individuals with sensorineural hearing loss. Implantable devices such as a cochlear implant may be an option, but are reserved for patients with severe to profound hearing deficits and who derive limited benefit from hearing aids.
The maintenance of access to sound, communication, and quality of life with hearing loss is not different for the PWD as compared with a patient without diabetes and with hearing loss. Management options include communication strategies, aural rehabilitation, auditory training, assistive listening devices, hearing aids, and implantable devices. The recommended treatment is dependent on the type and degree of hearing loss and personal goals for functionality. There is currently no research on specific protocols for hearing loss management in PWD. Chartrand 47 provides an overview of some potential considerations including sensitivity to earmold materials, increased risk of ear canal infection/bleeding, higher odds of abnormal loudness perception, balance issues, and consideration of central auditory involvement. The provider should be cognizant of these concerns and exercise appropriate precautions.
PWD with hearing loss may choose to defer recommendations for amplification. The reasons may include lack of perceived deficit, stigma of hearing aids, or cost of devices. The patient should be counseled on communication strategies, alternative solutions for hearing loss (e.g., personal sound amplification products), other assistive listening devices, and relationship of untreated hearing with negative consequences.
The recommended treatment of hearing loss in PWD is always dependent on the impact of hearing loss impairment and personal goals for functionality.
Conclusion
Diabetes elevates risk for acquired forms of hearing loss. Here we have outlined primary, secondary, and tertiary prevention and management strategies for PWD. Health care providers including physicians, primary care providers, diabetes educators, and PPOD members should be cognizant of the relationship between diabetes and hearing loss. We recommend upon diagnosis that PWD undergo a baseline comprehensive audiological evaluation and follow-up at least every 2 years or sooner if high-risk characteristics are indicated. Furthermore, the PWD should be counseled on ways to reduce risk for acquired hearing loss including reducing noise exposure, adaption of healthy lifestyle, and maintaining ABCs. Management of hearing loss in PWD is comparable to traditional approaches for persons without diabetes; however, the provider should be aware of potential increased sensitivity to earmold materials and ear canal infection/bleeding.
Footnotes
Conflict of Interest The authors have nothing to disclose.
References
- 1.Akinpelu O V, Mujica-Mota M, Daniel S J. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. Laryngoscope. 2014;124(03):767–776. doi: 10.1002/lary.24354. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Eavey R D, Wang M, Curhan S G, Curhan G C. Type 2 diabetes and the risk of incident hearing loss. Diabetologia. 2019;62(02):281–285. doi: 10.1007/s00125-018-4766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M B. Diabetes mellitus and the incidence of hearing loss: a cohort study. Int J Epidemiol. 2017;46(02):727. doi: 10.1093/ije/dyw342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruickshanks K J, Nondahl D M, Dalton D S et al. Smoking, central adiposity, and poor glycemic control increase risk of hearing impairment. J Am Geriatr Soc. 2015;63(05):918–924. doi: 10.1111/jgs.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somogyi A, Rosta K, Vaszi T. [Hearing impairment and tinnitus in patients with type 2 diabetes] Orv Hetil. 2013;154(10):363–368. doi: 10.1556/OH.2013.29562. [DOI] [PubMed] [Google Scholar]
- 6.Spankovich C, Le Prell C G, Lobarinas E, Hood L J. Noise history and auditory function in young adults with and without type 1 diabetes mellitus. Ear Hear. 2017;38(06):724–735. doi: 10.1097/AUD.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 7.Frisina S T, Mapes F, Kim S, Frisina D R, Frisina R D.Characterization of hearing loss in aged type II diabetics Hear Res 2006211(1-2):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okhovat S A, Moaddab M H, Okhovat S H et al. Evaluation of hearing loss in juvenile insulin dependent patients with diabetes mellitus. J Res Med Sci. 2011;16(02):179–183. [PMC free article] [PubMed] [Google Scholar]
- 9.Austin D F, Konrad-Martin D, Griest S, McMillan G P, McDermott D, Fausti S. Diabetes-related changes in hearing. Laryngoscope. 2009;119(09):1788–1796. doi: 10.1002/lary.20570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalton D S, Cruickshanks K J, Klein R, Klein B E, Wiley T L. Association of NIDDM and hearing loss. Diabetes Care. 1998;21(09):1540–1544. doi: 10.2337/diacare.21.9.1540. [DOI] [PubMed] [Google Scholar]
- 11.Konrad-Martin D, Austin D F, Griest S, McMillan G P, McDermott D, Fausti S. Diabetes-related changes in auditory brainstem responses. Laryngoscope. 2010;120(01):150–158. doi: 10.1002/lary.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bainbridge K E, Hoffman H J, Cowie C C. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med. 2008;149(01):1–10. doi: 10.7326/0003-4819-149-1-200807010-00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bainbridge K E, Cheng Y J, Cowie C C. Potential mediators of diabetes-related hearing impairment in the U.S. population: National Health and Nutrition Examination Survey 1999-2004. Diabetes Care. 2010;33(04):811–816. doi: 10.2337/dc09-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bainbridge K E, Hoffman H J, Cowie C C. Risk factors for hearing impairment among U.S. adults with diabetes: National Health and Nutrition Examination Survey 1999-2004. Diabetes Care. 2011;34(07):1540–1545. doi: 10.2337/dc10-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bainbridge K E, Cowie C C, Gonzalez F, II et al. Risk factors for hearing impairment among adults with diabetes: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) J Clin Transl Endocrinol. 2016;6:15–22. doi: 10.1016/j.jcte.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukushima H, Cureoglu S, Schachern P A, Paparella M M, Harada T, Oktay M F. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg. 2006;132(09):934–938. doi: 10.1001/archotol.132.9.934. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima H, Cureoglu S, Schachern P A et al. Cochlear changes in patients with type 1 diabetes mellitus. Otolaryngol Head Neck Surg. 2005;133(01):100–106. doi: 10.1016/j.otohns.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Canlon B, Schacht J. Acoustic stimulation alters deoxyglucose uptake in the mouse cochlea and inferior colliculus. Hear Res. 1983;10(02):217–226. doi: 10.1016/0378-5955(83)90055-2. [DOI] [PubMed] [Google Scholar]
- 19.Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11(12):3071–3109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 20.Lisowska G, Namysłowski G, Morawski K, Strojek K. Cochlear dysfunction and diabetic microangiopathy. Scand Audiol Suppl. 2001;(52):199–203. doi: 10.1080/010503901300007524. [DOI] [PubMed] [Google Scholar]
- 21.McQueen C T, Baxter A, Smith T L et al. Non-insulin-dependent diabetic microangiopathy in the inner ear. J Laryngol Otol. 1999;113(01):13–18. doi: 10.1017/s0022215100143051. [DOI] [PubMed] [Google Scholar]
- 22.Smith T L, Raynor E, Prazma J, Buenting J E, Pillsbury H C.Insulin-dependent diabetic microangiopathy in the inner ear Laryngoscope 1995105(3, Pt 1):236–240. [DOI] [PubMed] [Google Scholar]
- 23.Wackym P A, Linthicum F H., Jr Diabetes mellitus and hearing loss: clinical and histopathologic relationships. Am J Otol. 1986;7(03):176–182. [PubMed] [Google Scholar]
- 24.Anjaneyulu M, Berent-Spillson A, Russell J W. Metabotropic glutamate receptors (mGluRs) and diabetic neuropathy. Curr Drug Targets. 2008;9(01):85–93. doi: 10.2174/138945008783431772. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Xia M, Xu A. Expression of VEGF, iNOS, and eNOS is increased in cochlea of diabetic rat. Acta Otolaryngol. 2008;128(11):1178–1186. doi: 10.1080/00016480801901774. [DOI] [PubMed] [Google Scholar]
- 26.Chung J H, Lee S H, Park C W, Kim C, Park J K, Shin J H. Clinical significance of arterial stiffness in idiopathic sudden sensorineural hearing loss. Laryngoscope. 2016;126(08):1918–1922. doi: 10.1002/lary.25853. [DOI] [PubMed] [Google Scholar]
- 27.Susmano A, Rosenbush S W. Hearing loss and ischemic heart disease. Am J Otol. 1988;9(05):403–408. [PubMed] [Google Scholar]
- 28.Bertrand R A, Huang Z. Association between audiometric patterns and probabilities of cardiovascular diseases. Laryngoscope Investig Otolaryngol. 2018;3(06):478–485. doi: 10.1002/lio2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wattamwar K, Qian Z J, Otter J et al. Association of cardiovascular comorbidities with hearing loss in the older old. JAMA Otolaryngol Head Neck Surg. 2018;144(07):623–629. doi: 10.1001/jamaoto.2018.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tay H L, Ray N, Ohri R, Frootko N J. Diabetes mellitus and hearing loss. Clin Otolaryngol Allied Sci. 1995;20(02):130–134. doi: 10.1111/j.1365-2273.1995.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 31.Bohne B A, Harding G W. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21(04):505–509. [PubMed] [Google Scholar]
- 32.Kukidome D, Nishikawa T, Sato M et al. Impaired balance is related to the progression of diabetic complications in both young and older adults. J Diabetes Complications. 2017;31(08):1275–1282. doi: 10.1016/j.jdiacomp.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Jiam N T, Li C, Agrawal Y. Hearing loss and falls: a systematic review and meta-analysis. Laryngoscope. 2016;126(11):2587–2596. doi: 10.1002/lary.25927. [DOI] [PubMed] [Google Scholar]
- 34.Palta P, Carlson M C, Crum R M et al. Diabetes and cognitive decline in older adults: the Ginkgo Evaluation of Memory Study. J Gerontol A Biol Sci Med Sci. 2017;73(01):123–130. doi: 10.1093/gerona/glx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deal J A, Betz J, Yaffe K et al. Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC study. J Gerontol A Biol Sci Med Sci. 2017;72(05):703–709. doi: 10.1093/gerona/glw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konrad-Martin D, Reavis K M, Austin D et al. Hearing impairment in relation to severity of diabetes in a veteran cohort. Ear Hear. 2015;36(04):381–394. doi: 10.1097/AUD.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curhan S G, Wang M, Eavey R D, Stampfer M J, Curhan G C. Adherence to healthful dietary patterns is associated with lower risk of hearing loss in women. J Nutr. 2018;148(06):944–951. doi: 10.1093/jn/nxy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spankovich C, Le Prell C G. Associations between dietary quality, noise, and hearing: data from the National Health and Nutrition Examination Survey, 1999-2002. Int J Audiol. 2014;53(11):796–809. doi: 10.3109/14992027.2014.921340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan W, Cruickshanks K J, Klein B Eet al. Modifiable determinants of hearing impairment in adults Prev Med 201153(4-5):338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loprinzi P D, Cardinal B J, Gilham B. Association between cardiorespiratory fitness and hearing sensitivity. Am J Audiol. 2012;21(01):33–40. doi: 10.1044/1059-0889(2011/11-0024). [DOI] [PubMed] [Google Scholar]
- 41.Loprinzi P D, Lee H, Gilham B, Cardinal B J. Association between accelerometer-assessed physical activity and tinnitus, NHANES 2005-2006. Res Q Exerc Sport. 2013;84(02):177–185. doi: 10.1080/02701367.2013.784840. [DOI] [PubMed] [Google Scholar]
- 42.Loprinzi P D, Joyner C. Relationship between objectively measured physical activity, cardiovascular disease biomarkers, and hearing sensitivity using data from the National Health and Nutrition Examination Survey 2003-2006. Am J Audiol. 2017;26(02):163–169. doi: 10.1044/2017_AJA-16-0057. [DOI] [PubMed] [Google Scholar]
- 43.Pirozzo S, Papinczak T, Glasziou P.Whispered voice test for screening for hearing impairment in adults and children: systematic review BMJ 2003327(7421):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eekhof J A, de Bock G H, de Laat J A, Dap R, Schaapveld K, Springer M P. The whispered voice: the best test for screening for hearing impairment in general practice? Br J Gen Pract. 1996;46(409):473–474. [PMC free article] [PubMed] [Google Scholar]
- 45.Strawbridge W J, Wallhagen M I. Simple tests compare well with a hand-held audiometer for hearing loss screening in primary care. J Am Geriatr Soc. 2017;65(10):2282–2284. doi: 10.1111/jgs.15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spankovich C, Long G R, Hood L J. Early indices of reduced cochlear function in young adults with type-1 diabetes revealed by DPOAE fine structure. J Am Acad Audiol. 2019;30(06):459–471. doi: 10.3766/jaaa.17113. [DOI] [PubMed] [Google Scholar]
- 47.Chartrand M S.Diabetes Mellitus and Hearing 2003. Available at:https://www.audiologyonline.com/articles/diabetes-mellitus-and-hearing-1120. Accessed September 25, 2019 [Google Scholar]


