Abstract
The United States is experiencing an opioid crisis imposing enormous fiscal and societal costs, and driving the staggering overdose death rate. While prescription opioid analgesics are essential for treating acute pain, cessation of use in individuals with a physical dependence induces an aversive withdrawal syndrome which promotes continued drug use to alleviate/avoid these symptoms. Additionally, repeated bouts of withdrawal often lead to an increased propensity for relapse. Understanding the neurobiology underlying withdrawal is essential for providing novel treatment options to alleviate physiological and affective components accompanying the cessation of opiate use. Here, we administered morphine, and precipitated withdrawal with naloxone to investigate behavioral and cellular responses in C57BL/6J male and female mice. Following 3 days of administration, both male and female mice demonstrated sensitized withdrawal symptoms. Since the bed nucleus of the stria terminalis (BNST) plays a role in mediating withdrawal-associated behaviors, we examined plastic changes in inhibitory synaptic transmission within this structure 24 hours following the final precipitated withdrawal. In male mice, morphine withdrawal increased spontaneous GABAergic signaling compared to controls. In contrast, morphine withdrawal decreased spontaneous GABAergic signaling in female mice. Intriguingly, these opposing GABAergic effects were contingent upon activity-dependent dynamics within the ex vivo slice. Our findings suggest that male and female mice exhibit some divergent cellular responses in the BNST following morphine withdrawal, and alterations in BNST inhibitory signaling may contribute to the expression of behaviors following opioid withdrawal.
Keywords: bed nucleus of the stria terminalis, GABA, opioids, sex differences, morphine, withdrawal
Introduction
The United States is currently experiencing an opioid epidemic that claims the lives of an estimated 115 Americans every day (Center for Disease Control, 2017). Drug overdoses continue to rise in the U.S., and more than three out of five drug overdose deaths involve an opioid (Hedegaard et al., 2016). While the physiological changes induced by initial opioid use are important for understanding the development of opioid use disorders (OUD), the physiological components of withdrawal play an equally crucial role in the development and maintenance of OUD. The cessation of prescription opiates, which are indeed important for alleviating acute pain, can induce a highly dysphoric withdrawal syndrome marked by negative somatic (vomiting, cold/hot flashes, shaking, diarrhea, etc.) and emotional symptoms (anxiety, dread). Interestingly, the intensity of these withdrawal symptoms is highly correlated with continued opioid use and relapse (Fishman, 2008), and opioid misuse in chronic pain patients is associated with increased distress intolerance (the actual and/or perceived lack of ability to cope with the aversive somatic and/or emotional states associated with withdrawal; McHugh et al., 2016). That is, the anxiety surrounding the mere thought of experiencing withdrawal upon cessation of use can influence whether or not patients will even attempt to discontinue using opiates. Given the magnitude with which withdrawal can both influence the decision to discontinue opiate use, and influence future relapse and continued use, it is imperative to investigate the mechanisms underlying this syndrome. Insight into physiological adaptations in critical nuclei associated with the somatic and affective components of withdrawal may lead to unique treatment strategies targeted to reduce the aversive aspects of withdrawal.
Recent clinical studies highlight the role of biological sex in opioid use and misuse. For example, women have a higher incidence of pain (Tsang et al., 2008) and report higher pain intensity compared with men (Arendt-Nielsen et al., 2004; Eriksen et al., 2003; Greenspan et al., 2007). Women are also more likely to be prescribed opioids and given higher doses for longer periods of time (Simoni-Wastila et al., 2004; Sullivan et al., 2005), and report significantly higher cravings for opioids (Lee and Ho, 2013). Despite these findings, few pre-clinical studies investigate sex as a biological variable in key brain nodes engaged during opioid exposure and withdrawal.
The bed nucleus of the stria terminalis (BNST), a component of the extended amygdala, is a highly sexually dimorphic forebrain structure (Hisasue et al., 2010; Tsuneoka et al., 2017) mainly comprised of gamma-aminobutyric acid (GABA) neurons. The BNST functions as a critical relay center to regulate a range of emotional and motivational processes, via afferents from cortical and limbic structures, and via reciprocal projections with several limbic and hindbrain nuclei (Dong and Swanson, 2004, 2006; McElligott and Winder, 2009; Sun and Cassell, 1993). Recent studies have shown that GABAergic projection neurons from the BNST to the ventral tegmental area (VTA) and lateral hypothalamus (LH) drive motivated behavior (Jennings et al., 2013a,b; Giardino et al., 2018), and regulate fear and anxiety-like responses in the BNST by inhibiting these output neurons (Marcinkiewcz et al., 2016). The BNST plays a critical role in the negative valence associated with opioid withdrawal (Delfs et al., 2000) and inactivation of the BNST has been shown to block morphine withdrawal-potentiated startle (Harris et al., 2006). Previous work investigating sex differences in this region, however, have focused on the posterior division of the BNST where female mice contain roughly half the number of neurons as male mice (Shah et al., 2004; Xu et al., 2012), rather than the anterior portion of the BNST, which is thought to modulate drug seeking and motivational states. To date, there have not been detailed physiological studies investigating cellular responses to opioids in the anterior portion of the BNST in both male and female mice. Thus, this study examined behavior and inhibitory synaptic plasticity in the BNST of male and female mice following repeated bouts of withdrawal from morphine.
Materials and Methods
We performed all experiments in accordance with the University of North Carolina at Chapel Hill (UNC) Institutional Animal Care and Use Committee’s guidelines. C57BL/6J male and female mice (aged 7 weeks; Jackson Laboratories) were group or singly housed within UNC animal facilities on a normal light cycle (lights on at 7 am, lights off at 7 pm) and given food and water ad libitum.
Morphine Withdrawal Paradigm
To model morphine withdrawal, we utilized a naloxone-precipitated morphine withdrawal paradigm (Fig. 1A). Four groups of animals were used: morphine-naloxone males (MN-M), saline-naloxone males (SN-M), morphine-naloxone females (MN-F), and saline-naloxone females (SN-F). Mice were injected daily at approximately 9am with morphine (10 mg/kg) or saline (volume for both injections: 0.1 mL/10g) subcutaneously (s.c.), and immediately returned to their home cages. Two hours later, animals were transported to a separate room where they each received an injection of naloxone (s.c., 1 mg/kg). Following naloxone administration, mice were placed in a clear 58.4 cm X 41.3 cm X 31.4 cm plastic bin (located in the same room in which animals received naloxone injections) and observed for 10 min. Injections and behavioral scoring were repeated across three days. The following individual withdrawal symptoms were tallied and recorded: escape jumps, teeth chattering, wet dog shakes, paw tremors, fecal boli, excessive eye blinking, genital grooming, ptosis, and abnormal posturing (see Supplementary Methods for details on behavioral scoring).
Figure 1:
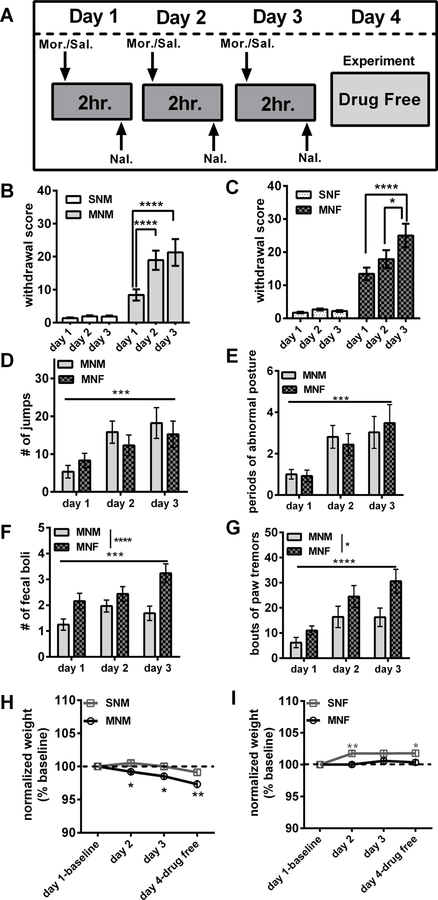
Morphine withdrawal in male and female mice A) Naloxone-precipitated morphine withdrawal paradigm. B) Average withdrawal scores in male mice (SN-M n=22; MN-M n=18). C) Average withdrawal scores in female mice (SN-F n=15; MN-F n=15). D) The average number of jumps between MN-M and MN-F. E) The average number of fecal boli produced between MN-M and MN-F. F) The average number of paw tremors between MN-M and MN-F. F) The average number of paw tremors between MN-M and MN-F. G) The average number of wet dog shakes between MN-M and MN-F. H) Average weight loss in male mice (% baseline). I) Average weight loss in female mice (% baseline). Each bar represents the mean ± SEM, * (p<0.05), ** (p<0.01), *** (p<0.001), **** (p<0.0001).
Brain slice preparation whole-cell electrophysiology
All electrophysiology experiments were performed approximately 24 h following the last precipitated withdrawal injection. Mice were anesthetized (isoflurane) and decapitated as previously described (McElligott et al., 2010). Briefly, brains were quickly removed and placed in ice-cold (1–4° C) high-sucrose artificial cerebral spinal fluid (ACSF, in mM: 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10 glucose, 26 NaHCO3) that had been oxygenated (95% O2, 5% CO2) for a minimum of 15 minutes. Coronal slices (300 µm) containing the BNST were prepared using a Leica VT1000 (Leica, Germany), and slices were allowed to equilibrate in oxygenated ACSF (in mM: 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10 glucose, 26 NaHCO3, 34º C) for at least 30 min. Slices were then transferred to a submerged recording chamber (Warner Instruments) and perfused with oxygenated ACSF (28–30 ºC in the bath, inline heater set to 32 ºC) at a rate of 2 ml/min.
Whole-Cell Recordings
BNST neurons were directly visualized with an upright video camera (Hamamatsu). Recording electrodes (3–6 MΩ) were pulled on a P-97 Micropipette Puller (Sutter Instruments) using thin-walled borosilicate glass capillaries. Spontaneous or miniature GABAA-mediated inhibitory postsynaptic currents (sIPSCs or mIPSCs) were acquired in voltage-clamp at −80mV holding potential in 5-minute blocks. Recording electrodes to examine IPSCs were filled with cesium chloride (CsCl) intracellular recording solution (in mM: 130 CsCl, 1 EGTA, 10 HEPES, 2 ATP, 0.2 GTP) in the presence of kynurenic acid (3mM), to block ionotropic glutamatergic receptor currents. mIPSCs were recorded in the presence of kynurenic acid and tetrodotoxin (TTX, 500nM) to block activity dependent synaptic release. Electrically evoked-IPSCs (eIPSCs) were measured by local stimulation (0.1ms duration, two pulses, 50 ms inter-pulse interval) using a bipolar nichrome electrode while neurons were voltage-clamped at −70 mV. Stimulation intensity was adjusted to obtain approximately 80% of the maximum response. Signals were acquired via a Multiclamp 700B amplifier (Molecular Devices), digitized, and analyzed via pClamp 10.6 software (Molecular Devices) and Minianalysis (Synaptosoft; see Supplementary methods for details). Input resistance, holding current, and access resistance were monitored continuously throughout the duration of experiments. Experiments in which changes in access resistance were greater than 20% were not included in the data analysis.
The BNST is a heterogeneous structure containing both interneurons, and neurons that project to nuclei including the VTA, LH, parabrachial nucleus (PBN), and the paraventricular nucleus of the hypothalamus (PVN). The projection neurons targeting the VTA and LH have characteristics of low capacitance, high input resistance, and lack Ih potassium channel current (Kash and Winder, 2006; Dumont and Williams, 2004 Marcinkiewcz et al., 2016). To observe if these characteristics were consistent with other projection targets, we recorded from BNST neurons projecting to the PBN (see Supplementary methods). On average, these cells had a relatively higher capacitance (74.4 ± 7.9 pF) and high resistance (647.8 ± 91.55 MΩ) and also lacked Ih current (n=4 cells dBNST, 2 cells vBNST). Due to the heterogeneous nature of this structure, we analyzed cells that lacked Ih current, and had high input resistance (average 1060 ± 38 MΩ for our recordings). This does not guarantee that we recorded exclusively from projection-like neurons, as it is possible that neurons projecting outside of the BNST may also have within nuclei collaterals. It is also possible that there are additional BNST neurons that project to other targets that do not resemble these electrophysiological characteristics.
Drugs
Morphine sulfate and naloxone HCl were purchased from Sigma Aldrich (www.sigmaaldrich.com). TTX and kynurenic acid were purchased from Abcam (www.abcam.com). We dissolved morphine sulfate and naloxone HCl in sterile saline (0.9%) prior to injection. We injected drugs at a volume of 0.1mL/10g.
Data Analysis
Data are presented as mean ± SEM or cumulative distributions ± SEM. All statistical analyses were performed using GraphPad Prism version 7.03 for Windows (www.graphpad.com). A Student’s unpaired t-test or 2-way ANOVA was performed where appropriate. If a significant interaction was detected in the 2-way ANOVA, a post-hoc Tukey HSD or Holm-Sidak test was performed. Cumulative distributions were analyzed with a Kolmogorov-Smirnov (K-S) test.
Results
Morphine withdrawal in males and females
To model repeated opioid withdrawal, mice were subjected to a morphine withdrawal paradigm (Fig. 1A) modified from studies performed in rats (McElligott et al., 2013; Schulteis et al., 1999). Following administration of naloxone, we observed mice for 10 min and a global withdrawal score (total number of escape jumps + other withdrawal symptoms which were scored as either a 1-present, or 0-absent; McNally and Akil, 2002) was calculated (Fig. 1B & 1C). In both male and female mice, the global withdrawal score was significantly different between morphine and saline groups across days (Treatment x Day interaction; males: F(2,120)=8.054, p<0.001; females: F(2,96)=4.751, p<0.05). MN-M mice (n=32) demonstrated a sensitization of the global withdrawal score, with day 2 (p<0.0001) and day 3 (p<0.0001) significantly increased compared to day 1 (Fig. 1B), while MN-F (n=25) showed day 3 significantly increased compared to day 1 (p<0.0001), and day 2 (p<0.05, Fig 1 B-C)
As recent evidence has demonstrated disparities in behavioral responding between male and female rodents (Becker and Chartoff, 2018, we next compared individual withdrawal behaviors between MN-M and MN-F mice across days of treatment to examine sex differences. Both MN-M and MN-F mice exhibited increased escape jumps and periods of abnormal posture across days (jumps: effect of Day, F(2,110)=9.678, p<0.001; abnormal posture, F(2,110)=10.46, p<0.001; Fig. 1D and E) but there were no differences between males and females (jumps: p=0.722; abnormal posture: p=0.998). In contrast, MN-F mice produced significantly more fecal boli (effect of sex, F(1,165)=18.36, p<0.0001, Fig. 1E), and more paw tremors than MN-M mice (effect of sex F(1,162)=9.34, p<0.01, Fig.1F). While both male and female animals exhibited symptoms of teeth chattering, genital grooming, swallowing movements, and wet dog shakes, we did not observe differences between days or sexes across these measures. While SN-M (n=30) and SN-F (n=25) mice exhibited a few signs of withdrawal following naloxone administration (table 1), most were not to the degree of MN-M or MN-F mice; although we did observe main effects of day in paw tremors (p<0.001), and swallowing movements (p<0.05), and an effect of sex in wet dog shakes (p<0.01). We next evaluated changes in body weight following each bout of withdrawal and on the 4th day prior to euthanasia. MN-M mice exhibited an average of 2.5% loss in body weight across the days of treatment (Treatment x Day interaction, F(3,357)=4.44, p<0.01; Fig. 1H). Post hoc analysis revealed that MN-M mice had significantly decreased body weight on day 2 (p<0.05), day 3, and day 4 (p’s<0.0001), compared to baseline, and between MN-M and SN-M (days 2 and 3, p’s<0.05, day 4 p<0.001). Intriguingly, the SN-F mice demonstrated on average over a 1.5% increase in weight as compared to baseline (Treatment by Day interaction, F(2,297)=3.262, p<0.05; days 2–4 vs. baseline p’s<0.001), and post-hoc analysis revealed significant differences between SN-F and MN-F mice on days 2 (p<0.01) and 4 (p<0.05; Fig. 1I). These results suggest that male and female mice both experience withdrawal under our paradigm, but that the behaviors and physiological changes experienced during this withdrawal are qualitatively different between the sexes.
Table 1.
Table of somatic withdrawal behaviors in SN-M, SN-F, MN-M, and MN-F
| Jumps | Wet Dog Shakes$$ | Paw Tremors*** | Teeth Chattering | Abnormal Postures | Genital Grooming | Excessive Eye Blinking | Swallowing Movements* | Fecal Boli Count | |
|---|---|---|---|---|---|---|---|---|---|
| Saline Males | |||||||||
| Day 1 | 0±0 | 0.7±0.18 | 0.83±0.25 | 0.03±0.03 | 0.03±0.03 | 0.26±0.16 | 0±0 | 0.3±0.13 | 0.7±0.22 |
| Day 2 | 0±0 | 0.8±0.23 | 4.33±1.56 | 0.16±0.10 | 0.43±0.28 | 0.6±0.22 | 0±0 | 0.63±0.28 | 1.33±0.30 |
| Day 3 | 0±0 | 0.83±0.38 | 5.7±2.16 | 0.33±0.12 | 0.43±0.19 | 0.36±0.14 | 0±0 | 0.26±0.20 | 1.2±0.28 |
| Saline Females | |||||||||
| Day 1 | 0±0 | 1.2±0.27 | 1.20±0.39 | 0.08±0.05 | 0.04±0.0.04 | 0.37±0.1 | 0.04±0.04 | 0.25±0.12 | 1.25±0.21 |
| Day 2 | 0±0 | 1.62±0.38 | 4.0±1.43 | 0.16±0.07 | 0.17±0.10 | 0.75±0.27 | 0±0 | 0.83±0.4 | 1.37±0.27 |
| Day 3 | 0±0 | 1.66±0.29 | 11.75±3.51 | 0.16±0.09 | 0.30±0.13 | 0.45±0.18 | 0±0 | 0.12±0.06 | 1.58±0.26 |
| Jumps*** | Wet Dog Shakes | Paw Tremors****$ | Teeth Chattering | Abnormal Postures*** | Genital Grooming | Excessive Eye Blinking | Swallowing Movements | Fecal Boli Count****$$$ | |
| Morphine Males | |||||||||
| Day 1 | 5.34±1.64 | 0.56±0.13 | 6.19±2.02 | 1.62±0.34 | 1.0±0.23 | 0±0 | 0±0 | 1.03±0.34 | 1.25±0.22 |
| Day 2 | 15.78±2.91 | 0.59±0.24 | 16.38±4.24 | 1.15±0.35 | 2.81±0.55 | 0±0 | 0±0 | 0.78±0.21 | 1.96±0.23 |
| Day 3 | 18.21±4.06 | 0.81±0.37 | 16.29±3.66 | 0.78±0.13 | 3.03±0.77 | 0±0 | 0±0 | 0.53±0.13 | 1.68±0.27 |
| Morphine Females | |||||||||
| Day 1 | 8.32±1.89 | 1.0±0.26 | 11.04±1.77 | 1.44±0.34 | 0.92±0.28 | 0.8±0.8 | 0±0 | 0.52±0.18 | 2.16±0.3 |
| Day 2 | 12.28±2.74 | 1.24±0.21 | 24.52±4.32 | 1.6±0.32 | 2.44±0.53 | 0.4±0.4 | 0±0 | 0.84±0.3 | 2.44±0.28 |
| Day 3 | 15.24±3.49 | 1.08±0.22 | 30.6±4.72 | 0.88±0.21 | 3.48±0.89 | 0±0 | 0±0 | 0.8±0.21 | 3.24±0.36 |
p<0.05
p<0.001
p<0.0001 main effect of day
p<0.05
p<0.01
p<0.001 main effect of sex
Because within slice morphine withdrawal has been shown to enhance GABA transmission (Dumont and Williams, 2004), we hypothesized that withdrawal may engage mechanisms of inhibitory synaptic plasticity. Prior to probing the GABAergic transmission in animals run through our paradigm, we explored the possibility that there may be sex differences in naïve mice. We observed a strong trend for increased sIPSC inter-event-interval (IEI) and amplitude in the BNST neurons in female animals (cumulative distribution (CD): K-S tests p=0.10 and p=0.82 respectively; within cell averages males: 3.89 ± 0.59 Hz, 38.4 ± 6.1 pA, dBNST n= 18, vBNST n= 12; females: 6.56 ± 1.65 Hz, 51.6 ± 6.7 pA, dBNST n=18, vBNST n=11; p=0.16 and p=0.14 respectively, Fig 2). Examining mIPSCs in naïve male and female mice, we found that mIPSC IEI in females was significantly leftward shifted in the CD plot suggesting a greater frequency of GABAergic events in females, although we observed no differences when within cell averages were compared (CD: K-S test p<0.001; within cell averages males: 1.84 ± 0.62 Hz, dBNST n=7, vBNST n=9; females: 1.63 ± 0.38 Hz, dBNST n=7, vBNST n=8, p=0.77, Fig 3C and E). Examining mIPSC amplitude, females showed a rightward shift in the CD plot compared to males, suggesting an increase in the percentage of larger amplitude events, although when within cell averages were compared there was only a trend for larger events (CD: K-S test p<0.01; males: 26.0 ± 3.8 pA; females: 35.9 ± 5.9 pA; p=0.16, Fig 3 D and F). These data suggest that there is greater activity-independent inhibition in BNST neurons in females vs. males. To examine if the presence of TTX affected BNST IPSCs we ran a post-hoc two way-ANOVA. We found a main effect of activity-dependent transmission on both the frequency (p<0.01) and amplitude (p<0.05) of GABAergic events in the BNST, suggesting strong within-slice dynamics regulating inhibition.
Figure 2:
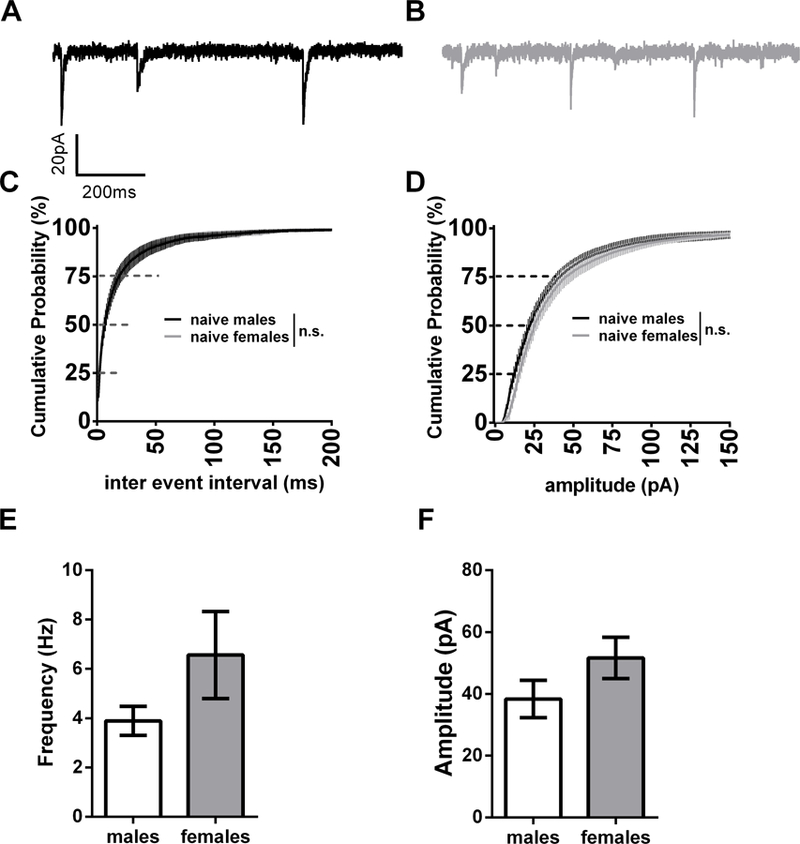
sIPSCs in naïve mice A) Representative trace of sIPSC in male B) Representative trace of sIPSCs in female C) Cumulative distribution plot comparing naïve male and female inter event interval D) Cumulative distribution plot comparing naïve male and female amplitude E) Bar graph of male vs. female frequency from cell averages F) Bar graph of male vs. female amplitude from cell averages
Figure 3:
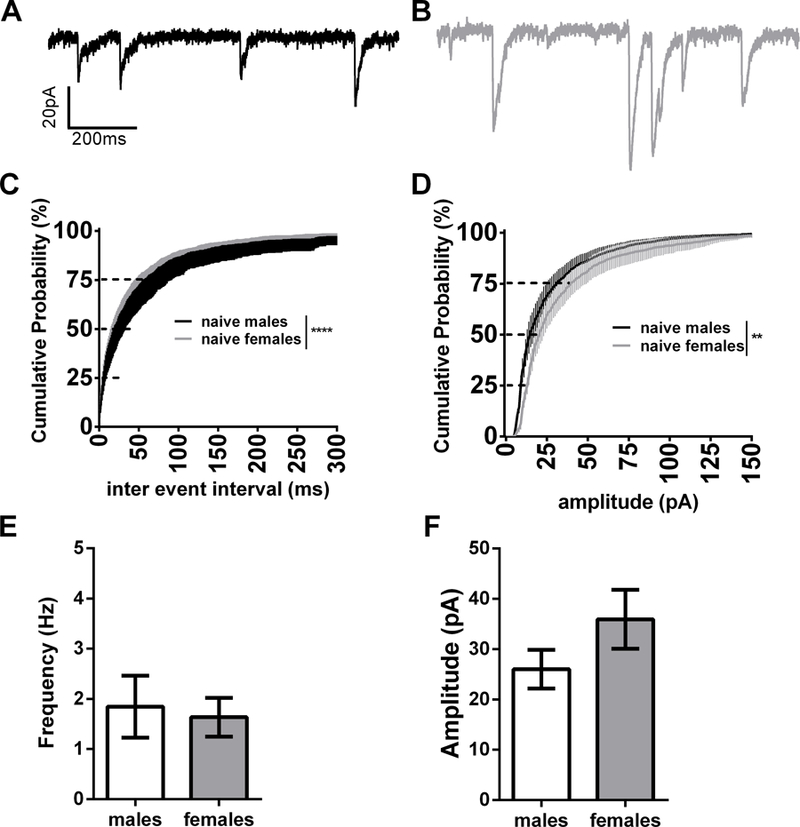
mIPSCs in naïve mice A) Representative trace of sIPSC in male B) Representative trace of sIPSCs in female C) Cumulative distribution plot comparing naïve male and female inter event interval D) Cumulative distribution plot comparing naïve male and female amplitude E) Bar graph of male vs. female frequency from cell averages F) Bar graph of male vs. female amplitude from cell averages. **p<0.01, ****p<0.0001
Morphine withdrawal alters BNST GABAergic signaling in males and females
Next, we investigated the ability of the in vivo withdrawal paradigm to induce plastic changes in synaptic inhibition onto BNST neurons 24 hours into withdrawal. In males, morphine withdrawal resulted in a significant leftward shift on the CD curve for spontaneous-IPSC inter event interval (MN-M vs. SN-M, K-S test p<0.0001, Fig 3A). Surprisingly, morphine withdrawal in females resulted in a rightward shift on the CD curve for sIPSC inter event interval (MN-F vs. SN-F K-S test p<0.0001, Fig. 3C). Furthermore, when we examined the within cell averages for frequency there was a significant difference between morphine and saline groups, across males and females (Treatment x Sex interaction; F(1,68)=10.26, p<0.01; 3.14 ± 0.51 Hz for SN-M, n= dBNST 7, vBNST 7; 6.39 ± 1.06 Hz for MN-M, n= dBNST 11, vBNST 11; p<0.05, Cohen’s D=0.859; 6.98 ± 0.93 Hz for SN-F, n= dBNST 8, vBNST 9; 4.31 ± 0.77 Hz for MN-F, n= dBNST 10, vBNST 9, p<0.05, Cohen’s D 0.735; Fig.4C,D,G). In both males and females, we observed a trending decrease in sIPSC amplitude (CD, K-S test, p=0.072) following morphine withdrawal (Fig. 4D and 4F). Examining cell averages, there was a main effect of sex (p<0.01), but no interaction (55.75 ± 8.14 pA for SN-M, 47.42 ± 5.45 pA for MN-M; 37.88 ± 3.68 pA for SN-F, 35.76 ± 3.50 pA for MN-F, Fig. 4F.) These data suggest repeated morphine withdrawal engages a bidirectional plasticity in GABAergic tone on BNST neurons in male and female mice, increasing GABA release in male mice and decreasing GABA release in female mice.
Figure 4:
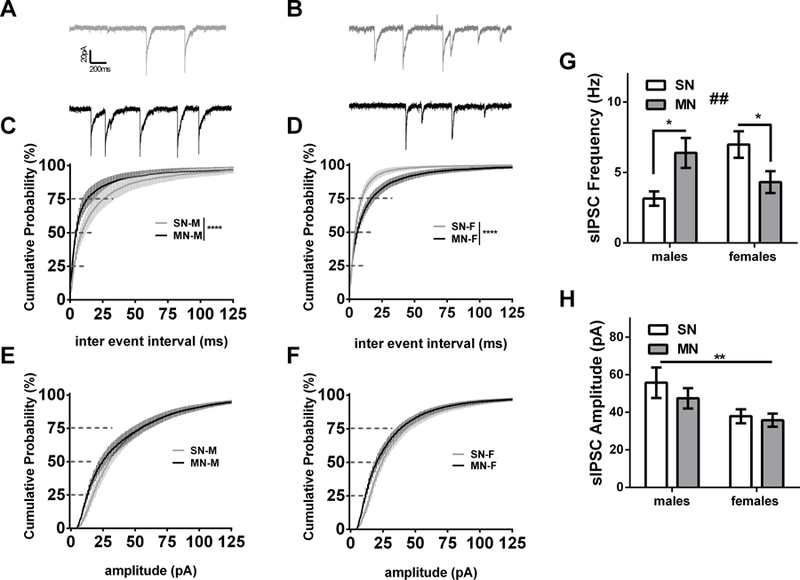
Morphine withdrawal differentially alters BNST spontaneous GABAergic transmission in male and female mice. A) Representative currents (SN-M gray, MN-M black) B) Representative currents (SN-F gray, MN-F black) C) Cumulative distribution plot comparing sIPSC inter event interval in males (**** p<0.0001, SN-M n=14; MN-M n=22). D) Cumulative distribution plot comparing sIPSC inter event interval in females (**** p<0.0001.SN-F n=17; MN-F n=19). F) Cumulative distribution plot comparing sIPSC amplitude in females from same cells as D. E) Cumulative distribution plot comparing sIPSC amplitude in males from the same cells as C. Bar graph of sIPSC frequency cell averages (## drug by sex interaction p<0.01, * P<0.05) F) Bar graph of sIPSC amplitude cell averages had main effect of sex (** p<0.01).
Previous research has shown that withdrawal can activate certain cell populations within the BNST (Dumont and Williams, 2004; Hamlin et al., 2004), and as we note above, within-slice activity modulates GABAergic transmission in male and female naïve animals. Therefore, we next investigated mIPSCs 24 hours after the final naloxone injection. Similar to the sIPSCs, in males, we observed a leftward shift on the CD curve for mIPSC IEI (K-S test p<0.0001, Fig 5A). In contrast to sIPSCs, the effect of withdrawal on mIPSCs in female mice also resulted in a leftward shift of the CD curve (K-S test p<0.0001, Fig. 5C). Comparing within cell frequency averages, we observed a strong trend towards differences between males and females (effect of Sex, p=0.0825) but not a significant interaction (1.26 ± 0.22 Hz for SN-M, n= dBNST 10, vBNST 9; 1.84 ± 0.25 Hz for MN-M, n= dBNST 7, vBNST 5; 2.02 ± 0.58 Hz for SN-F, n= dBNST 7, vBNST 6; 2.76 ± 0.68 Hz for MN-F, n= dBNST 8, vBNST 7). Morphine withdrawal induced a rightward shift on the CD curve for mIPSC amplitude in males, yet, similar to sIPSCs, maintained a leftward shift in females (K-S test p<0.0001, Fig. 5B and 5D respectively). Within cell amplitude averages, however, did not result in a significant interaction (22.33 ± 2.20 pA for SN-M, n= dBNST 10, vBNST 9; 24.79 ± 2.47 pA for MN-M; 26.59 ± 1.85 pA for SN-F; 26.54 ± 3.58 pA for MN-F). The CDs show that in the absence of activity-dependent synaptic transmission, morphine withdrawal increases the frequency of GABA release, but reveals opposite mIPSC amplitude profiles. These data suggest that sexually dimorphic within-slice network dynamics may partially account for the divergent sIPSC profile observed between BNST neurons in male and female mice following morphine withdrawal.
Figure 5:
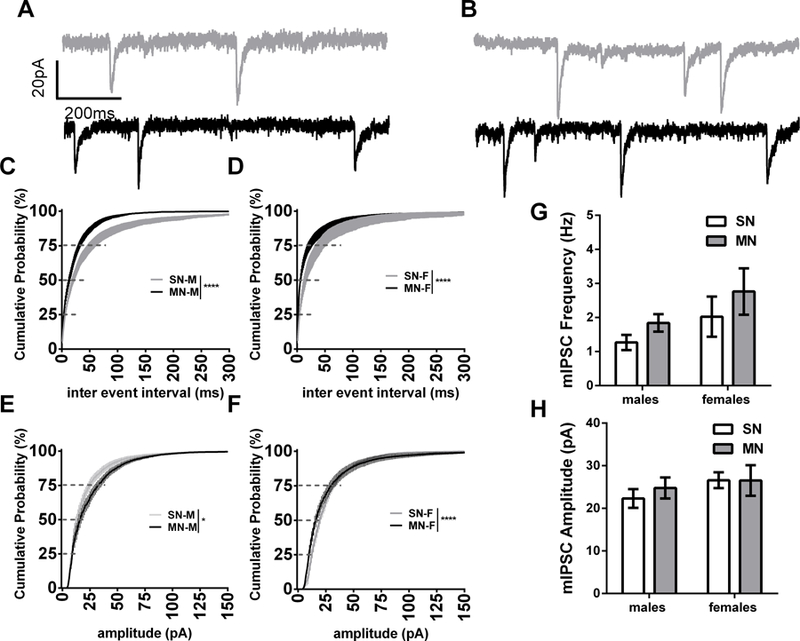
Morphine withdrawal similarly altered mIPSCs in male and female mice. A) Representative traces of SN-M and MN-M mIPSCs. B) Representative tracels of SN-F and MN-F mIPSCs. C) Cumulative distribution plot comparing miniature-IPSC inter event interval in male mice (SN-M n=14; MN-M n=22). D) Cumulative distribution plot comparing miniature-IPSC inter event interval in female mice (SN-F n=17; MN-F n=19). E) Cumulative distribution plot comparing miniature-IPSC amplitude in male mice from the same cells as C F) Cumulative distribution plot comparing miniature-IPSC amplitude in female mice from the same cells as D. G) Bar graph of mIPSC frequency in male and female mice H) Bar graph of mIPSC amplitude in male and female mice**** (p<0.0001).
Previously we have observed that norepinephrine in the BNST differentially modulates spontaneous and miniature events because it depolarizes certain populations of BNST neurons and alters the release of neuropeptides (Dumont and Williams, 2004; McElligott et al., 2010). Furthermore, we recently demonstrated that endogenous release of the neuropeptides dynorphin and corticotropin releasing factor (CRF) differentially regulate GABA transmission within the BNST (Normandeau et al., 2018). We therefore predicted that driving activity-dependent neurotransmission within the slice via electrical stimulation may promote the release of signaling factors that could impact BNST inhibitory transmission following morphine withdrawal. To test this hypothesis, we used a paired-pulse electrical stimulation (50 ms inter-stimulus interval) and recorded evoked IPSCs. Examining the paired-pulse ratio (PPR) in both male and female mice, we found a significant effect of drug treatment (p<0.01). Furthermore, planned t-tests showed morphine withdrawal significantly increased the PPR in MN-M compared to SN-M (1.06 ± 0.08 PPR for SN-M, n= dBNST 6, vBNST 6; 1.39 ± 0.1 PPR for MN-M, n= dBNST 8, vBNST 12, p<0.05, Cohen’s D 0.866, Fig. 5C), and MN-F compared to SN-F (1.08 ± 0.07 PPR for SN-F, n= dBNST 10, vBNST 12; 1.42 ± 0.12 PPR for MN-F, n= dBNST 8, vBNST 11, p<0.05, Cohen’s D 0.737, Fig. 5C). These data demonstrate that electrically evoking neurotransmission within the slice reduces GABAergic synaptic efficacy in both sexes following morphine withdrawal.
Discussion
In this study, we aimed to model repeated cycles of opioid withdrawal and examine synaptic changes that may contribute to the development and maintenance of OUDs (Koob and Volkow, 2016). Recent data suggests that 4 of 5 new heroin users previously used a prescription opioid (Cicero et al., 2014). Therefore, it is likely that the transition to an OUD occurs in patients who experience withdrawal from clinically prescribed opioids, and may potentially seek illicit avenues to continue usage. Indeed, the intensity of somatic withdrawal symptoms strongly correlates with poor treatment compliance for OUD, as patients resume opioid use as a negative reinforcer to alleviate withdrawal (Fishman, 2008). Furthermore, elevated distress intolerance to opioid withdrawal promotes opioid misuse in chronic pain patients (McHugh et al., 2016), suggesting that both increased sensitivity to this dysphoric state, and anxiety surrounding a potential future withdrawal experience may contribute to OUDs. Previously, we and others have shown that repeated precipitated withdrawal sensitizes withdrawal behavior (Schulteis et al. 1999, McElligott et al. 2013). These data in rodents are similar to clinical observations in which repeated bouts of precipitated withdrawal sensitize withdrawal responses in both opioid naïve individuals and those with a prior use history (Azolosa et al., 1994; Kirby and Stitzer, 1993). It has been demonstrated that repeated morphine (e.g. 2 or 3 days of morphine administration followed by 1 injection of naloxone on day 3), but not repeated naloxone (e.g. daily injections of morphine and naloxone) is an important factor in the presentation of affective and somatic withdrawal symptoms (Schulteis et al., 1999, Schulteis et al., 1997). Therefore it is possible that we may find similar plastic changes induced by repeated exposure to morphine followed by a single precipitated withdrawal, or even to morphine treatment alone followed by spontaneous withdrawal. Additional studies, however, suggest otherwise. For example, Rothwell and colleagues demonstrated that 7 days of repeated morphine/naloxone pairings enhanced psychomotor sensitization to a challenge dose of morphine, as compared to 7 days of morphine/saline pairings (Rothwell et al., 2010). Furthermore, others have found that repeated precipitated withdrawal engages stress mechanisms including activation of the HPA axis that are not observed with spontaneous withdrawal in “drug-dependent” mice (Zelena et al., 2005). Indeed, salivary and serum cortisol levels are elevated in humans experiencing opioid detoxification, as well as in protracted withdrawal (Bearn et al., 2001; Camí et al., 1992; Li et al., 2008; Wisniewski et al., 2006). Collectively, these findings suggest that repeated precipitated withdrawal using naloxone can significantly affect both behavior and physiology compared to morphine alone. In the future, we will investigate whether the effects we observe here are due to the repeated naloxone administration, or if they will manifest as a result of a single precipitated withdrawal, or spontaneous withdrawal.
Naloxone is often used as a means of rapidly alleviating respiratory depression in cases of opioid overdose, and it is becoming increasingly available in many states for over the counter use by laypersons (Wheeler et al., 2015). Interestingly, the rates of patients receiving multiple does of naloxone from emergency medical service providers is on the rise (up 26% from 2012–2015, Faul et al., 2017), and numbers surely will increase as more states implement OTC availability. This is intriguing, as naloxone increases vulnerability to relapse and continued drug use (Hyman et al., 2007). Additionally, naloxone is used in the treatment of OUD in combination with burprinorphine (Suboxone). While the intent of this combined drug replacement therapy is to prevent abuse liability, naloxone is present in sublingual films at bioavailable doses that have been demonstrated to reverse analgesia in opioid dependent patients (Liu and Wittbrodt, 2002). While the administration of naloxone has been critical in saving the lives of many individuals, the acute physiological and emotional effects of this drug are not well understood. Thus, we believe that the present set of experiments are of crucial relevance to the current opioid crisis, as repeated administration of naloxone in individuals may alter underlying mechanisms that may influence further drug-seeking and relapse.
Although sex differences in the analgesic potency of opioids have been examined previously (Kest et al., 1999), both male and female mice develop conditioned place preference to the same dose (10 mg/kg) of morphine (Le Merrer et al., 2012; Neelakantan et al., 2016), which suggests that the rewarding and analgesic effects of opioids may be mediated through distinct circuitries. Few studies, however, have investigated withdrawal characteristics in male and female rodents (Blum et al., 1976; Cicero et al., 2002). Both morphine-naloxone male (MN-M) and morphine-naloxone female (MN-F) mice experienced significant somatic signs of withdrawal and robust sensitization of withdrawal across the 3-day paradigm, however, on average, females exhibited more somatic symptoms (Fig. 1B & 1C, table 1). This was surprising, given the aforementioned decreased sensitivity to the analgesic properties of morphine observed in females and the relatively low dose of morphine administered. Additionally, weight loss has previously been described as a characteristic of morphine withdrawal (Goeldner et al., 2013; McElligott et al., 2013). Consistent with these findings, we found that MN-M exhibited a 2.5% loss in body weight; however, MN-F did not lose weight across the paradigm (Fig. 1H and 1I). This decrease in body weight is not likely due to increased gut motility, because MN-F produced significantly more fecal boli (Fig. 1E), but it may be attributed instead to a decrease in food consumption or an increase in energy expenditure. Although there are differences in the analgesic properties of morphine between male and female mice, these data demonstrate that such analgesic differences do not necessarily correlate with the nature of withdrawal.
Evidence suggests that the BNST acts as a central hub in the acute responses involved in opioid withdrawal, however, whether these changes lead to persistent alterations was previously unknown. Altering noradrenergic signaling within the BNST modulates both the somatic and affective components of morphine withdrawal (Delfs et al., 2000). Furthermore, the BNST is known to regulate feeding, fear, anxiety- and reward-like behaviors via projections to the VTA and LH (Jennings et al., 2013a,b; Marcinkiewcz et al., 2016). In male rodents, spontaneous and naloxone-precipitated withdrawal inhibits activity of VTA dopamine neurons (Diana et al., 1995; Georges et al., 2006) and hence, reduces dopamine release in the nucleus accumbens (Acquas et al., 1991; Kalivas, 1993). Additionally, studies have found that orexin neurons in the LH contribute to somatic withdrawal behaviors (Georgescu et al., 2003; Sharf et al., 2008), and inputs from the BNST onto these neurons drive antagonistic emotional valence behaviors (Giardino et al., 2018). In the BNST, within slice morphine withdrawal increases GABAergic cell firing, enhancing the release of GABA (Dumont and Williams, 2004). It was unknown however, if withdrawal would induce plastic adaptations in inhibitory synapses that would persist 24 hours after withdrawal. Using a posthoc analysis, we focused our electrophysiology experiments on neurons with a physiological profile consistent with neurons projecting to the VTA, LH, and PBN (exhibiting high input resistance, and lacking Ih current; see methods). Interestingly, our results suggest that there is likely an enhanced inhibitory tone on this population of BNST neurons in naïve female animals, compared with naïve males. This is intriguing as fMRI data suggests that women have stronger structural connectivity between the BNST and other brain regions as compared to men (Avery et al., 2014). We did not observe appreciable differences in GABAergic transmission between naïve and SN control animals suggesting naloxone by itself does not produce long-term effects on GABAergic plasticity. In male mice, morphine withdrawal increased the frequency of sIPSCs and mIPSCs onto these BNST neurons suggesting an enhancement in presynaptic GABA release onto this circuit. Similarly, female mice that experienced morphine withdrawal had an enhancement in inhibition onto BNST neurons, evidenced by mIPSCs; however, they exhibited decreased sIPSCs in the absence of TTX. These data suggest that within-slice activity dependent network dynamics in the BNST of female mice following morphine withdrawal drive the opposing s/mIPSC results. This suggests the possibility that female mice that underwent morphine withdrawal have increased activity dependent release of a signaling molecule. To test this hypothesis, we electrically stimulated the slice. While electrical stimulation releases the GABA that we actively measure, it will also potentially release a host of other signaling factors within the slice such as monoamines and neuropeptides (Karkhanis et al., 2016; Stella et al., 1997). We found that morphine withdrawal increased the PPR of evoked GABAergic transmission onto BNST neurons in both male and female mice. An increase in PPR is typically associated with a decrease in neurotransmitter release, similar to the observed decrease in sIPSC frequency in female mice. These data suggest that unknown factors within the slice are released in an activity dependent fashion, which may regulate GABAergic tone onto BNST neurons. Furthermore, these data suggest that the release and/or abundance of these to-be-identified factors may be sexually dimorphic. Likely candidates for mediating this difference in GABAergic tone could include neuropeptides, such as CRF, which are known to be sexually dimorphic. Indeed, several neuropeptides investigated by our lab and others have shown differential effects on both pre- and post-synaptic GABAergic function (Kash and Winder, 2006; Krawczyk et al., 2011; Li et al., 2012; Normandeau et al., 2018; Pleil et al., 2015).
Both male and female mice that experienced morphine withdrawal had a decrease in mIPSC amplitude on BNST neurons, while also exhibiting an increase in mIPSC frequency. A decrease in mIPSC amplitude is typically consistent with a postsynaptic effect on GABA receptor function, while an increase in frequency is typically associated with a pre-synaptic mechanism. Interestingly, we observed these differences in the cumulative distribution analysis (p<0.001) but only found a trend for an increase in mIPSC frequency in the cell average analysis (p=0.17). This may suggest that distinct GABA inputs are differentially modulated following morphine withdrawal. The BNST contains a dense population of GABAergic interneurons (Kudo et al., 2012; Lebow and Chen, 2016) and receives GABAergic input largely through projections from the central nucleus of the amygdala (CeA, Herman et al., 2013). Although we observe a number of changes in inhibition onto BNST neurons, whether the observed alterations in GABAergic signaling stem from local BNST interneurons or through afferents is unknown. Previous studies show that morphine withdrawal increases cFos expression, a marker of neuronal activation, in the BNST and CeA (Hamlin et al., 2004) suggesting that the CeA→BNST circuit contributes to the affective component of withdrawal (Nakagawa et al., 2005). Future experiments will investigate inhibitory circuits from afferents to the BNST, as well as microcircuits within the BNST, and how they impinge onto neurons projecting to areas such as the VTA, LH, PBN and PVN.
Finally, this study focused on the impact of repeated morphine withdrawal on GABAergic synapses in the BNST. The BNST receives substantial innervation from various glutamatergic regions (McElligott and Winder, 2009) and multiple neuromodulators that are liable to the effects of stress and drugs of abuse, including norepinephrine and CRF. Indeed, our previous studies have demonstrated that a similar morphine withdrawal paradigm potentiates NE synapses in the BNST (McElligott et al., 2013), and stress alters glutamatergic transmission via α1-adrenergic receptor mediated long-term depression (McElligott and Winder, 2008; McElligott et al. 2010). Future studies will determine if the paradigm utilized here also promotes synaptic plasticity within noradrenergic and glutamatergic synapses.
Here we have presented evidence that both male and female mice exhibit significant withdrawal from a low dose of morphine, and that withdrawal symptoms exacerbate over our 3-day paradigm. While female mice are less sensitive to the analgesic properties of morphine in general, they appear to have enhanced sensitivity to somatic symptoms of withdrawal, but a decreased sensitivity to the weight loss associated with morphine withdrawal. Additionally, repeated morphine withdrawal differentially regulates spontaneous GABAergic tone onto BNST neurons in male and female mice by within slice activity-dependent transmission. Thus, our data highlight both behavioral and physiological sex differences resulting from opioid withdrawal, and underscore the importance of conducting experiments in both sexes. Our data demonstrate synaptic plasticity induced by withdrawal in the BNST, a critical nucleus mediating both somatic and affective withdrawal behaviors. This is significant, as individuals are less likely to discontinue opioid use due to anxiety surrounding a potential future experience of a withdrawal syndrome. Furthermore, the changes that occur as a result of withdrawal may induce long-term adaptations, promoting susceptibility to relapse via negative affect, and increased instances/sensitivity to the frequency and onset of drug cravings in abstinent individuals. Future studies will investigate and compare the acute and protracted behavioral and neurobiological changes induced by naloxone-precipitated withdrawal with the hopes of identifying mechanisms leading to more effective therapeutic targets for alleviating the highly dysphoric symptoms that accompany withdrawal.
Supplementary Material
Figure 6:
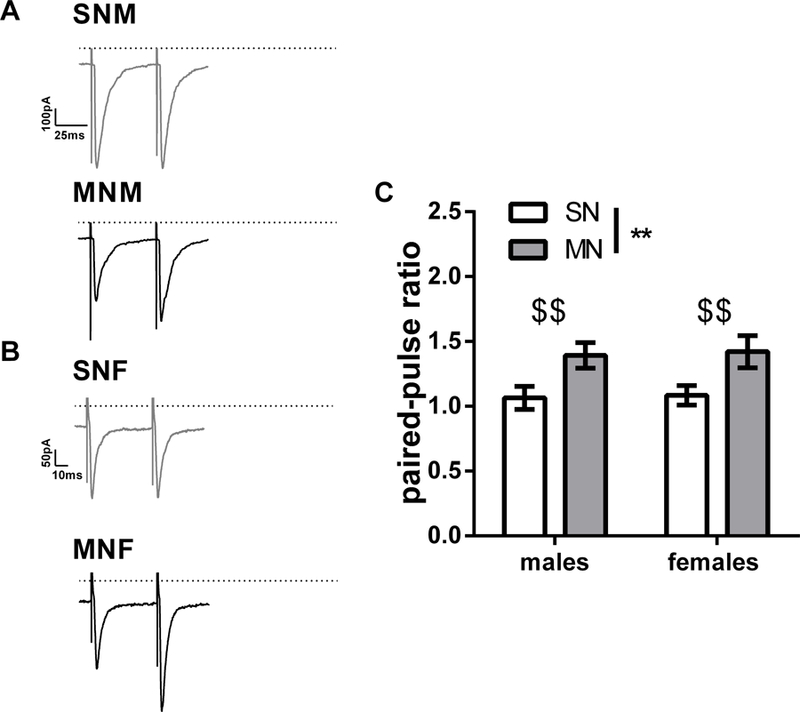
Morphine withdrawal increases the paired-pulse ratio in male and female mice. A) Representative paired-pulse traces from BNST neurons in male mice (SN-M top; MN-M bottom, dashed line 0 pA, scale bar 100 pA x 25 ms). B) Representative paired-pulse traces from BNST neurons in female mice (SN-F, top; MN-F bottom, scale bar 50 pA x 10 ms). C). Bar graph of paired-pulse ratios (main effect of drug treatment, ** p<0.01; $ $ planned t-tests p<0.01, SN-M n=12; MN-M n=20, SN-F n=22; MN-F n=19). Each bar represents the mean ± SEM.
Acknowledgements
The authors would like to thank Dr. Patrick Rothwell and Dr. Thomas Kash for helpful discussions regarding this manuscript, and Dr. Joyce Besheer for edits and feedback on a previous version of the manuscript.
Funding
This work was supported by NIH awards 4T32MH093315–05 and KO1AA02355502.
Footnotes
Disclosure
The authors have no conflicts of interest.
References
- Acquas E, Carboni E, Di Chiara G (1991) Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. Eur J Pharmacol 193:133–134. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Bajaj P, Drewes AM (2004) Visceral pain: Gender differences in response to experimental and clinical pain. European Journal of Pain 8:465–472. [DOI] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, and Blackford JU (2014). BNST neurocircuitry in humans. NeuroImage 91, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azolosa JL, Stitzer ML, and Greenwald MK (1994). Opioid physical dependence development: effects of single versus repeated morphine pretreatments and of subjects’ opioid exposure history. Psychopharmacology (Berl.) 114, 71–80. [DOI] [PubMed] [Google Scholar]
- Bearn J, Buntwal N, Papadopoulos A, and Checkley S (2001). Salivary cortisol during opiate dependence and withdrawal. Addict. Biol 6, 157–162. [DOI] [PubMed] [Google Scholar]
- Becker JB and Chartoff E (2018) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 0:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Eubanks JD, Wiggins B, Wallace JE (1976) Morphine withdrawal reactions in male and female mice. Am J Drug Alcohol Abuse 3:363–368. [DOI] [PubMed] [Google Scholar]
- Camí J, Gilabert M, San L, and de la Torre R (1992). Hypercortisolism after opioid discontinuation in rapid detoxification of heroin addicts. Br. J. Addict 87, 1145–1151. [DOI] [PubMed] [Google Scholar]
- CDC, National Center for Health Statistics (2017) Wide-ranging online data for epidemiologic research (WONDER)
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP (2014) The changing face of heroin use in the United States a retrospective analysis of the past 50 years. JAMA Psychiatry 71:821–826. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER (2002) Gender-linked differences in the expression of physical dependence in the rat. Pharmacology Biochemistry and Behavior 72:691–697. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G (2000) Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403:430–434. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni A, Gessa G (1995) Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. The Journal of Pharmacology and Experimental Therapeutics 272:781–785. [PubMed] [Google Scholar]
- Dong HW and Swanson LW (2004) Projections from Bed Nuclei of the Stria Terminalis, Posterior Division: Implications for Cerebral Hemisphere Regulation of Defensive and Reproductive Behaviors. Journal of Comparative Neurology 471:396–433. [DOI] [PubMed] [Google Scholar]
- Dong HW and Swanson LW (2006) Projections from bed nuclei of the stria terminalis, anteromedial area: Cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. Journal of Comparative Neurology 494:142–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC and Williams JT (2004) Noradrenaline Triggers GABAA Inhibition of Bed Nucleus of the Stria Terminalis Neurons Projecting to the Ventral Tegmental Area. Journal of Neuroscience 24:8198–8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Jensen MK, Sjøgren P, Ekholm O, Rasmussen NK (2003) Epidemiology of chronic non-malignant pain in Denmark. Pain 106:221–228. [DOI] [PubMed] [Google Scholar]
- Faul M, Lurie P, Kinsman JM, Dailey MW, Crabaugh C, & Sasser SM (2017). Multiple naloxone administrations among emergency medical service providers is increasing. Prehospital Emergency Care, 21(4), 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M (2008) Precipitated withdrawal during maintenance opioid blockade with extended release naltrexone. Addiction 103:1399–1401. [DOI] [PubMed] [Google Scholar]
- Georges F, LeMoine C, Aston-Jones G (2006) No effect of morphine on ventral tegmental dopamine neurons during withdrawal. Translational Research 26:5720–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, DiLeone RJ (2003) Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. Journal of Neuroscience 23:3106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li SB, Malenka RC, de Lecea L (2018) Parallel circuits from the bed nucleus of the stria terminalis to the lateral hypothalamus drive opposing emotional states. Nature Neuroscience 21(8):1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL (2011) Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biological Psychiatry 69:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, Leresche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Traub RJ (2010) Studying sex and gender differences in pain and analgesia: A consensus report. Pain 132:26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Buller KM, Day TA, Osborne PB (2004) Effect of naloxone-precipitated morphine withdrawal on c-fos expression in rat corticotropin-releasing hormone neurons in the paraventricular hypothalamus and extended amygdala. Neuroscience Letters 362:39–43. [DOI] [PubMed] [Google Scholar]
- Harris AC, Atkinson DM, Aase DM, Gewirtz JC (2006) Double dissociation in the neural substrates of acute opiate dependence as measured by withdrawal-potentiated startle. Neuroscience 139:1201–1210. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, Minino AM (2017) Drug Overdose Deaths in the United States, 1999–2016. NCHS Data Brief 294:1–8. [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M (2013) Novel Subunit-Specific Tonic GABA Currents and Differential Effects of Ethanol in the Central Amygdala of CRF Receptor-1 Reporter Mice. Journal of Neuroscience 33:3284–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisasue S, Seney ML, Immerman E, Forger NG (2010) Control of cell number in the bed nucleus of stria terminalis of mice: role of testosterone metabolites and estrogen receptors subtypes. J Sex Med 7:1401–1409. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong K-IA, Doebrick C, & Sinha R (2007). Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Experimental and Clinical Psychopharmacology, 15(2), 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD (2013a) The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341:1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD (2013b) Distinct extended amygdala circuits for divergent motivational states. Nature 496:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (1993) Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev 18:75–113. [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Weiner JL, Jones SR (2016) Early-life social isolation stress increases kappa opioid receptor responsiveness and downregulates the dopamine system. Neuropsychopharmacology 41:2263–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL and Winder DG (2006) Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology 51:1013–1022. [DOI] [PubMed] [Google Scholar]
- Kest B, Wilson SG, Mogil JS (1999) Sex differences in supraspinal morphine analgesia are dependent on genotype. The Journal of Pharmacology and Experimental Therapeutics 289:1370–1375. [PubMed] [Google Scholar]
- Kirby KC, and Stitzer ML (1993). Opioid physical dependence development in humans: effect of time between agonist pretreatments. Psychopharmacology (Berl.) 112, 511–517. [DOI] [PubMed] [Google Scholar]
- Koob GF and Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Georges FF, Sharma R, Mason X, Berthet A, Bézard E, Dumont EC (2011) Double-Dissociation of the Catecholaminergic Modulation of Synaptic Transmission in the Oval Bed Nucleus of the Stria Terminalis. Journal of Neurophysiology 105:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Watanabe M (2012) Three Types of Neurochemical Projection from the Bed Nucleus of the Stria Terminalis to the Ventral Tegmental Area in Adult Mice. Journal of Neuroscience 32:18035–18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA and Chen A (2016) Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry 21:450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CWS and Ho IK (2013) Sex differences in opioid analgesia and addiction: Interactions among opioid receptors and estrogen receptors. Molecular Pain 9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li J, Epstein DH, Zhang XY, Kosten TR, and Lu L (2008). Serum cortisol secretion during heroin abstinence is elevated only nocturnally. Am. J. Drug Alcohol Abuse 34, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, Stuber GD, Kash TL (2012) Presynaptic inhibition of GABA release in the BNST by kappa opioid receptor signaling. Biol Psychiatry 71:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, and Wittbrodt E (2002). Low-dose oral naloxone reverses opioid-induced constipation and analgesia. J. Pain Symptom Manage 23, 48–53. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Tipton GJ, Ramakrishnan C, Kozicz T, Deisseroth K, Thiele TE, McElligott ZA, Holmes A, Heisler LK, Kash TL (2016) Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott Z and Winder D (2009) Modulation of Glutamatergic Synaptic Transmission in the Bed Nucleus of the Stria Terminalis. Prog in NeuroPsychopharmacol Biol Psychiatry 615:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, Winder DG (2010) Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proceedings of the National Academy of Sciences 107: 2271–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Fox ME, Walsh PL, Urban DJ, Ferrel MS, Roth BL, Wightman MR (2013) Noradrenergic synaptic function in the bed nucleus of the stria terminalis varies in animal models of anxiety and addiction. Neuropsychopharmacology 38:1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Weiss RD, Cornelius M, Martel MO, Jamison RN, Edwards RR (2016) Distress intolerance and prescription opioid misuse among patients with chronic pain. J Pain 17:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP and Akil H (2002) Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience, 112:605–617. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S (2005) Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience 134:9–19. [DOI] [PubMed] [Google Scholar]
- Normandeau CP, Torruella-Suarez ML, Sarret P, McElligott ZA, Dumont EC (2018) Neurotensin and dynorphin bi-directionally modulate CeA inhibition of oval BNST neurons in male mice Neuropharmacology 143:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, Olson DP, Lowell BB, Grant KA, Thiele TE, Kash TL (2015) NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci 18:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Gewirtz JC, and Thomas MJ (2010). Episodic withdrawal promotes psychomotor sensitization to morphine. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 35, 2579–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF (1999) Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol 10:235–242. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R (2004) Visualizing sexual dimorphism in the brain. Neuron 43:313–319. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Dileone RJ (2009) Orexin mediates morphine place preference, but not morphine-induced hyperactivity or sensitization. Brain Res 1317:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni-Wastila L, Ritter G, Strickler G (2004) Gender and other factors associated with the nonmedical use of abusable prescription drugs. Subst Use Misuse 39:1–23. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Plomelli D (1997) A second endogenous’ cannabinoid that modulates long-term potentiation. Nature 388:773–778. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Steffick D, Unützer J (2005) Regular use of prescribed opioids: Association with common psychiatric disorders. Pain 119:95–103. [DOI] [PubMed] [Google Scholar]
- Sun N and Cassell MD (1993) Intrinsic GABAergic neurons in the rat central extended amygdala. The Journal of Comparative Neurology 330:381–404. [DOI] [PubMed] [Google Scholar]
- Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Watanabe M (2008) Common Chronic Pain Conditions in Developed and Developing Countries: Gender and Age Differences and Comorbidity With Depression-Anxiety Disorders. Journal of Pain 9:883–891. [DOI] [PubMed] [Google Scholar]
- Tsuneoka Y, Tsukahara S, Yoshida S, Takase K, Oda S, Kuroda M, Funato H (2017) Moxd1 Is a Marker for Sexual Dimorphism in the Medial Preoptic Area, Bed Nucleus of the Stria Terminalis and Medial Amygdala. Frontiers in Neuroanatomy 11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E, Jones TS, Gilbert MK, & Davidson PJ (2015). Opioid overdose prevention programs providing naloxone to laypersons-United States, 2014. MMWR. Morbidity and mortality weekly report, 64(23), 631–635. [PMC free article] [PubMed] [Google Scholar]
- Wisniewski AB, Brown TT, John M, Cofranceso J, Golub ET, Ricketts EP, Wand G, and Dobs AS (2006). Cortisol levels and depression in men and women using heroin and cocaine. Psychoneuroendocrinology 31, 250–255. [DOI] [PubMed] [Google Scholar]
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Shah NM (2012) Modular genetic control of sexually dimorphic behaviors. Cell 148:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelena D, Barna I, Mlynarik M, Gupta OP, Jezova D, and Makara GB (2005). Stress symptoms induced by repeated morphine withdrawal in comparison to other chronic stress models in mice. Neuroendocrinology 81, 205–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.