Abstract
BACKGROUND:
Azithromycin (AZI) is recommended with Ceftriaxone (CRO) for treatment of uncomplicated gonococcal urethritis and cervicitis in the United States (US) and an AZI susceptibility breakpoint is needed. Neither the FDA (Food and Drug Administration) nor the CLSI (Clinical and Laboratory Standards Institute) has set interpretive breakpoints for AZI susceptibility. As a result, AZI antimicrobial susceptibility testing (AST) cannot be interpreted using recognized standards. This has contributed to increasingly unavailable clinical laboratory AST, although gonorrhea is on the rise with over 550,000 US gonorrhea cases reported to the CDC in 2017, the highest number of cases since 1991.
METHODS:
This document summarizes the rationale data reviewed by the CLSI in June 2018.
RESULTS:
CLSI decided to set a susceptible only interpretive breakpoint at the MIC (Minimum Inhibitory Concentration) of and below 1 μg/ml. This is also the epidemiological cut-off value (ECV), i.e., the end of the wild-type susceptibility distribution. This breakpoint presumes that AZI (1 gm single dose) is used in an approved regimen that includes an additional antimicrobial agent (i.e. CRO 250 mg, intramuscular [IM] single dose).
CONCLUSION:
Having a breakpoint can improve patient care and surveillance, and allow future development and FDA regulatory approval of modernized AST to guide treatment. The breakpoint coincides with a EUCAST (European Committee on AST) decision to remove previously established, differing AZI breakpoints and use the ECV as guidance for testing. The CLSI breakpoint is now the recognized standard that defines AZI susceptibility for gonococcal infections.
Keywords: Neisseria gonorrhoeae, Antimicrobial Resistance, Breakpoints, Interpretive Criteria
SUMMARY
This document summarizes the rationale data that led to the recent CLSI decision to set a susceptible only interpretive breakpoint for Neisseria gonorrhoeae and Azithromycin at a MIC (Minimum Inhibitory Concentration) equal to and below 1 μg/ml.
INTRODUCTION
Neisseria gonorrhoeae (herein referred to as GC or gonococcus) can rapidly develop resistance to antimicrobial agents due to innate mechanisms for acquiring resistance genes. As a result, previously recommended treatment options have eventually become ineffective. Currently, CDC recommends dual therapy with CRO (250 mg) and AZI (1 g oral) for the treatment of uncomplicated gonorrhea (1). Dual therapy has been recommended around the world. Its use is thought to preserve the effectiveness of currently available drugs, particularly CRO, because a strain is unlikely to be resistant to both drugs. Strains with resistance to a single agent may be effectively eradicated when immediately treated with two drugs if the strain is susceptible to the second agent.
CDC recommendations for treatment of gonococcal infections with AZI dual or monotherapy have changed over time. Prior to the dual therapy recommendation, AZI monotherapy was highly efficacious for GC treatment and gained regulatory approval but was not CDC recommended because of side effects and cost at the 2 g dose. In 2006 and 2010, CDC recommended or allowed AZI as an alternative monotherapy for cephalosporin allergic patients (2, 3). However, AZI was removed as an alternative monotherapy in the 2015 guidelines after documented treatment failures (1). CDC has recommended a dual therapy GC treatment approach of 1 g AZI with CRO (and initially with other drugs) starting in 2010 to mitigate the threat of emerging CRO resistance mutations (3). An established AZI susceptibility cutoff is critical to guide future dual therapy recommendations, as a drug with GC susceptibility is needed along with CRO to mitigate the emergence of CRO resistance.
In 2017, 555,608 GC cases were reported to the CDC, and an estimated 850,000 cases occurred (4). Gonorrhea remained the second most notifiable condition in the US, behind over 1.7 million reported chlamydia cases. In a recent analysis, CDC reported that 81% of GC cases were treated with the recommended AZI and CRO regimen in a surveillance study setting (5). Using the currently CDC recommended regimen, no confirmed gonorrhea treatment failures have been reported in the US. Furthermore, US isolates with elevated CRO MICs (≥0.125 μg/ml) have been declining after a peak in 2010 and 2011 (4). Globally, recent case reports from the United Kingdom (UK) and Australia identified a novel strain with dual CRO and AZI resistance (6, 7), but such reports remain extremely rare given the scale of the epidemic. Determining the susceptibility of individual strains to currently recommended regimens prior to treatment may become a valuable strategy for patient care. However, those tests would need to be done at the point-of-care to benefit patients, and are currently unavailable.
Gonorrhea is usually empirically treated with no antibiotic susceptibility testing (AST) results available before treatment either based on a clinical syndromic diagnosis and/ or Gram staining while the patient is still in the provider’s office, or after laboratory molecular identification with NAAT (nucleic acid amplification testing) in persons with asymptomatic infection (8). In the US, NAATs have largely replaced diagnostic culture methods because NAATs are more sensitive, faster, and automated to allow cost-effective diagnosis. GC AST requires culture methods and is often only undertaken in cases of suspected treatment failure.
AST availability has become very scarce in the US. Many labs do not offer any AST even for drugs with FDA and CLSI breakpoints (e.g., CRO, cefixime, tetracycline, penicillin) (9, 10). Results would only partially inform alternatives, as AZI is a component of most treatment regimens. The lack of AZI breakpoints has kept manufacturers of user-friendly culture devices (e.g., AZI EtestR, LiofilchemR or other disk tests) from seeking regulatory approval because it is not possible without interpretative criteria. This leaves mainly the time and labor-consuming agar dilution methods where results are too late to inform clinical management. Lastly, the lack of breakpoints has slowed development of molecular susceptibility tests due to the required interpretive criteria for FDA regulatory approval. Such tests could bring substantial improvements in informed patient care because they could be performed together with diagnostic NAAT or as reflex tests. Establishment of an AZI breakpoint may pave the way for modernization of GC diagnostic methods and thereby improve patient care.
METHODS
CLSI met in June 2018 and used its guidelines for development of susceptibility testing criteria (11). They require examination of clinical efficacy, pharmacokinetics and –dynamics (PK/PD), and in-vitro susceptibilities of the previous three years with associated genetic determinants if available (11). EUCAST guidance is similar (12, 13). CLSI previously reviewed susceptibility distributions and established an ECV of 1 μg/ml (Epidemiological Cut-off Value; i.e. the end of the natural wild-type susceptibility distribution presumably without acquired resistance mutations) in January 2017.
RESULTS
GC MIC Distributions
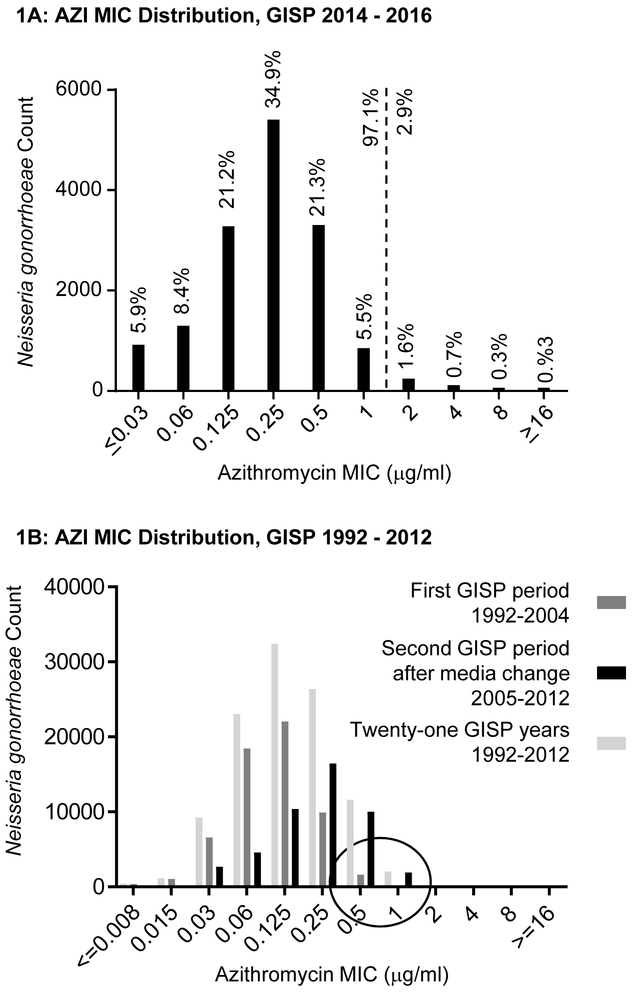
In-vitro susceptibilities came from US national GISP data (Gonococcal Isolate Surveillance Project), 2014– 2016. This CDC-led sentinel site surveillance project existed since 1987. Briefly, isolates are collected from the first 25 men with urethral gonococcal infection (after presumptive identification including gram stain) each month, and in approximately 25–30 STD clinics throughout the US (14). Depending on year, four or five regional laboratories conducted AST by agar dilution method as described by CLSI (15) and further by GISP (16). ATCC 49226 and 2 – 6 additional GC strains were included for quality control, depending on GISP year. Figure 1A shows the AZI MIC distribution of 15,496 isolates from 2014 – 2016. The mode was 0.25 μg/ml. 97.1% of isolates had a MIC of 1 μg/ml or lower, 2.9% had higher MICs. ECV calculations using four methods (17-20) resulted in 1 μg/ml. This confirms previous evaluations and indicates the ECV has not changed.
Fig. 1.
A: AZI susceptibilities of 15,496 GISP GC isolates from the US national surveillance project GISP 2014 – 2016, as determined by the agar dilution method. The dotted line represents previous and current ECV determinations. The x-axis reflects the range of dilutions in the current GISP protocol. B: AZI susceptibilities of GISP GC isolates from 1992 – 2004 (dark grey), 2005 – 2012 (black), and 1992 – 2012 (light grey), as determined by agar dilution and previously reviewed by CLSI. The circle at 0.5 and 1 μg/ml highlights isolates at the CLSI breakpoint before and after 2005 when a media change took place. The shift in MICs by one dilution was likely due to a change in commercial media formulation. It occurred in all participating reference laboratories for AZI only in 2005. This prompted the CDC to change its alert criteria upwards by one dilution as a single event in 2006. Except for this occurrence, no substantial shift in the modal MIC has occurred in GISP.
The committee examined previous distributions to determine possible shifts. Potential recent deviations from wildtype could lower the efficacy of drugs evaluated in a different era. Figure 1B shows GISP MIC distributions from 21 years, 1992 −2012. This includes an era in the 1990s when AZI monotherapy was systematically evaluated and found efficacious (see below). The data are separately analyzed for years 1992 – 2004 [dark grey] because a media change occurred in 2005 (see below), 2005– 2012 [black], and these 21 years together [light grey]. For thirteen years, from 1992 until 2004, the mode was 0.125 μg/ml. There was a shift higher by one dilution after 2004. A closer review of available CDC documents revealed that in 2005, an increase in MICs occurred by one dilution when a key manufacturer changed the formulation of commercial media. The change was of unknown nature, but happened simultaneously in the five participating GISP reference laboratories. A slight pH shift has been suspected because AZI is pH dependent while other drugs are not. This prompted GISP to change its criteria for “alerting” CDC from a MIC of 1 to 2 μg/ml in 2006; the only such AZI GISP change. The mode was 0.25 μg/ml from 2005 – 2012 (Fig 1B) and also in 2014 – 2016 (Fig 1A), demonstrating stability. In sum, CLSI concluded the modal MIC is now 0.25 μg/ml and has been stable during GISP if corrections for a documented media change are made.
AZI is a macrolide; the mechanism of antibacterial action is to bind to nucleotides in 23S rRNA, blocking protein synthesis. Mutations in genes coding for 23S rRNA have been associated with monotherapy failure, particularly when all four alleles of GC are impacted (21). Namely, reports by Marita-Ishihara T et al (22) and Gose SO (23) identified treatment failures and isolates with MICs of 4 or ≥ 256 μg/mL, respectively, associated with 23S rRNA C2611T and A2059G mutations, respectively. Other genetic mutations (e.g., meningococcal-like [mosaic] mtrR) can also increase MICs, but often to a lower degree (24, 25). These other mutations have not been associated with treatment failure and were not examined by CLSI.
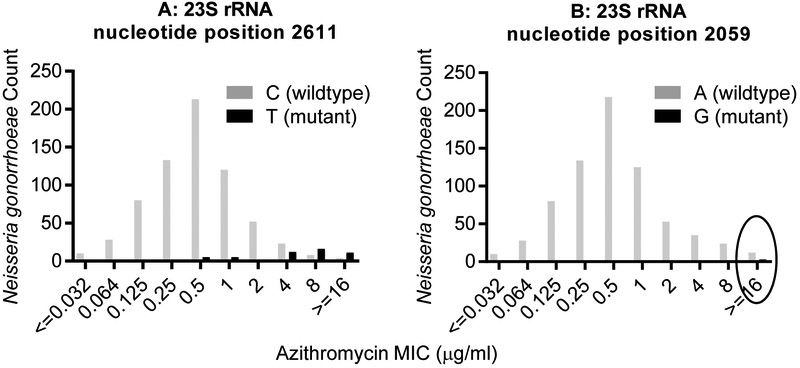
732 GISP isolates from 2013 – 2015 have undergone whole genome sequencing, previously published (24, 26). We now performed genetic marker analysis on this convenience sample (Fig. 2) which consisted of 187 isolates with elevated MICs (AZI ≥ 2, CFX ≥ 0.25, or CRO ≥ 0.125 μg/mL, considered “alert” GISP isolates (16)), and 545 non-alert GISP isolates. Fifty-one isolates had the C2611T mutation; all isolates with four copies of these mutations had MICs of 16 μg/ml or above (Fig 2A). Only three GISP isolates harbored the A2059G mutation, and all had MICs of 16 μg/ml or above (Fig 2B). To our knowledge, these GISP isolates were not associated with treatment failure.
Fig 2:
GC genetic marker association with AZI MICs. A previously sequenced convenience sample of 732 GISP isolates from 2013 – 2015 underwent genetic marker analysis at CDC. Analyses of the 23S rRNA C2611T (A) and A2059G (B) mutations are shown. The wild-type base at position 2611 is C, and is A at position 2059. The circle at ≥16 μg/ml highlights the three isolates with the A2059G mutation.
Clinical data on AZI efficacy for gonococcal urethritis
AZI has many advantageous characteristics. First, it has activity against Chlamydia trachomatis, GC, and Mycoplasma genitalium. These sexually transmitted infections can occur together with overlapping symptoms and are often treated syndromically. Increasingly, antibiotic resistance can occur in some of these infections (summarized in (27)). AZI is generally well tolerated and safe during pregnancy. It works as single dose therapy, is well tolerated in expedited therapy of heterosexual sex partners and cost is acceptable (28).
FDA approved an AZI indication for gonococcal urethritis and cervicitis after monotherapy was systematically evaluated in the 1980s and 1990s (29). CLSI reviewed the largest multicenter, open, randomized control trial by Handsfield et al, conducted in ten US public STD clinics and enrolling 724 men and women (30). The authors reported that monotherapy with a single oral 2 g AZI dose was 99.9% effective against uncomplicated urogenital gonorrhea (95% confidence interval [95%CI] 97.9%−100%). Another study arm with CRO monotherapy had 97.7% efficacy (95%CI 95.5%−99.9%). The report included no AST or PK/PD data. The committee considered the availability of GISP data from the time period relevant. As shown in Fig 1B, in 1992 – 2004, 3.1% (n=1,849) of the 60,298 GISP isolates had MICs of 0.5 μg/mL and above; this cutoff is equivalent to today’s MIC of 1 μg/mL due to the media change. This suggests that GC with this MIC was likely present and AZI susceptible during the Handsfield trial. GC cultures without AST were used to demonstrate treatment efficacy of 99.6% (male urethra), 80% (male rectum), 97.8% (cervix or female urethra), 100% (female rectum), 100% (all pharynx) (30). Of note, defining microbiologic cure on the basis of negative GC cultures rather than NAAT remains the preferred method, as recently discussed for the evaluation of the drug Zoliflodacin (31).
Handsfield et al reported gastrointestinal side effects (30). Several smaller European studies evaluated 1 g AZI due to fewer side effects and lower cost (e.g., (32, 33)). In 2010, Bignell and Garley conducted a systematic review of all available clinical data from 1990 – 2006 (34). They identified nine prospective studies of 1g AZI with an aggregate cure rate of 520/539 cases (96.5%; 95% CI 94.3% - 97.6%). When retrospective studies by Habib and Fernando (35) were included, the aggregate rate was 688/ 709 (97.0%; 95% CI 95.2 – 97.9%) (34). Post-or pre-treatment in-vitro AST was rarely performed in those studies (34). There are no contemporary US data on monotherapy efficacy including microbiologic treatment outcomes; the committee noted a need for further comprehensive investigation. A 2014 trial evaluated a single 2 g oral dose with extended release formulation in 130 Japanese men (36). The overall efficacy was lower at 93.8%, with 100% eradication associated with urethral isolates with MICs of ≤ 0.25 μg/mL (n=57), 96.9% eradication associated with MICs of 0.5 (n=31/32), 58.3% eradication with MICs of 1 (n=7/12), and persistent infection associated with isolates with MICs of 2 or greater (n=2). However, microbiological cure was evaluated with NAAT at 7 to 14 days post treatment, which is known to detect remaining DNA from non-viable organisms ((37), reviewed and commented on by Zenilman (38)). Non-random DNA persistence associates with higher MICs to the provided treatment (37); nevertheless, it subsides over time and is below the threshold for culture (37). It results in over-estimation of clinical failure (37, 38), hence, regulatory agencies prefer culture as method for microbiologic cure as recently discussed for the efficacy trial of Zoliflodacin by Taylor et al (31). Another limitation was that it was a single study conducted in Japan, with associated isolates of significantly higher MICs than seen in GISP ((36); statistical comparison of GISP and study isolates not shown), limiting impact on global susceptibility definitions.
CLSI also reviewed limited clinical data from case reports of treatment failure of monotherapy (39-43), and dual therapy (6, 7, 44). A recent US surveillance study by Weston et al found that 3.1% of gonorrhea infections were treated with AZI monotherapy (3); nevertheless, reports of treatment failure after monotherapy are rare. A notable case of confirmed AZI monotherapy failure occurred in Oregon (40) when the initial strain had a MIC of 1 μg/ml, but the re-tested MIC was 8 μg/ml after treatment in a patient without sexual re-exposure. This suggests resistance mutations were acquired or the patient had a “mixed infection”, i.e., infection with at least two strains of different MICs (45). Cases of dual therapy failure with very high AZI MICs (>256 μg/mL) have not occurred in the US to our knowledge.
Pharmacokinetic and –dynamic (PK/PD) evaluations of AZI
CLSI largely focused on the ZithromaxR packet insert (Pfizer AZI brand name) for PK/PD data review (46). The packet insert relied on evaluations prior to FDA approval in the 1970s through 1990s (reviewed in (47, 48)), including animal studies showing higher drug availability in tissues than in serum (49, 50). In particular, after oral administration, AZI rapidly leaves the circulation to enter tissues achieving high and prolonged drug concentrations in peripheral sites such as skin and mucosal tissues including genital sites. According to the ZithromaxR packet insert, oral administration of a single 500 mg dose to healthy adult volunteers results in a mean Tmax in blood of only 2.2 hours, with a Cmax of 0.5 μg/mL. Nineteen hours later, drug concentration in the cervix (the only genital tissue with detailed drug measurements given) is 2.9 μg/g, 70-fold higher than in blood. Other genital tissues were examined and had extensive drug distribution but exact levels were not reported (46). At 10 – 12 and 9 – 18 hours, sputum and tonsils had AZI concentrations of 2.9 μg/mL and 4.5 μg/g, 30 and >100 fold greater than in blood, respectively (29). This may reflect levels in the overall oral cavity, a potentially very important site for GC persistence. GC is a facultative intracellular bacteria and can survive in polymorphonuclear leukocytes (PMNs). The package insert reports that median AZI exposure (AUC 0–288) in PMNs was 800-fold greater than in serum following a three day regimen (46).
Only a limited number of PK/PD studies have been done since then, e.g., evaluations of 1 g for extra-genital C. trachomatis infections in Australia (51, 52). Slow-release and regular AZI formulations were compared in blood but not genital tissues (53), finding their bioavailability comparable in serum of Japanese men with gonococcal urethritis (54).
DISCUSSION
The following summarizes CLSI’s rationale and public health considerations supportive of setting the susceptibility breakpoint at the MIC of 1 μg/ml. It is justified by the end of the wild-type susceptibility distribution, i.e., the stable and confirmed ECV of 1 μg/ml. There is evidence of clinical susceptibility to AZI monotherapy from rigorous clinical efficacy evaluation in the 1990s through early 2000s (reviewed in (34)) when an estimated 3.1% of US GISP isolates had an equivalent MIC or higher (Fig 1B). In addition, there have been no reports of dual therapy failures in the US. In instances of suspected treatment failures, specimens can be submitted to CDC for AST (instructions online (55)). CDC is regularly contacted for suspected failures, however upon further evaluation these infections have been due to sexual re-infection when partners are not effectively treated and when patients did not abstain from sexual activity until their partner has completed treatment, as is recommended.
Another line of reasoning is a public health concern related to protecting currently recommended drugs such as CRO, until progressive resistance is demonstrated through surveillance. If a lower AZI susceptibility breakpoint were set, more gonorrhea strains would appear closer to the limit of susceptibility. It is possible that providers could increasingly digress from recommended dual therapy in order to increase perceived likely treatment success for their patient. This may include the use of higher AZI doses - which could lead to an increased risk of gastrointestinal side effects and higher cost- or the use of other broad spectrum antimicrobials. Importantly, these changes are urgently needed for highly resistant cases and for the preservation of future options should new effective GC drugs fail to materialize.
In international reports of gonorrhea treatment failures with high in-vitro AZI and CRO MICs, ertapenem was used for treatment (4, 5). There is currently no evidence of a clinical benefit of this drug for cases with AZI MIC of 1 μg/ml. To illustrate, as shown in Figure 1A, 1,298 cases from sentinel surveillance in 2014 – 2016 had isolates with MIC of 1 μg/ml or above. To our knowledge, all of the patients responded to dual therapy and resolved their infections. This suggests that the use of broad spectrum antibiotics, potentially associated with costly hospital-supervised infusion, would be neither necessary nor beneficial for the patient. If CRO or ertapenem monotherapy use increases, it is possible that resistance will emerge, further threatening the few remaining options for treatment.
The CDC STD treatment recommendations will undergo review in the near future, after systematic expert review of available evidence including GISP data. When resistance to recommended treatment reached 5% in the past, a change in treatment recommendations is considered (2, 3). A change may eventually be recommended due to emerging isolates with AZI MICs well above 1 μg/mL and if co-occurring with CRO decreased susceptibility. There are limited current alternatives and uncertainty for drugs in the pipeline. Having an AZI susceptibility breakpoint can be beneficial because it can allow AST- guided treatment in individual patients.
These deliberations left open the question of a resistance breakpoint. Treatment failures with some recent strains with presumably very high-level resistance and MICs >256 μg/ml have emerged (e.g., (6, 7, 23), others), mostly associated with the discussed 23S rRNA mutations. While there is agreement on their association with treatment failure, there is still not clear and sufficient clinical efficacy data available on infections caused by isolates with MICs of 2, 4 μg/ml, and so forth. CLSI established a “susceptible only” breakpoint and deferred a decision to set an intermediate or resistant breakpoint pending the availability or review of additional data. Setting a disk diffusion breakpoint was also deferred pending acquisition of correlative AST data.
Finally, to reflect lingering uncertainty of today’s clinical AZI efficacy given high dual therapy uptake, the committee recommended to follow a CLSI precedent for Helicobacter pylori infection (9) and added the comment “This breakpoint presumes that AZI (1 gm single dose) is used in an approved regimen that includes an additional antimicrobial agent (i.e. CRO 250 mg IM single dose)”.
Since the CLSI decision, USCAST (US Committee on AST) adopted CLSI’s susceptibility breakpoint (56). In January 2019, EUCAST replaced previously established differing GC AZI breakpoints with a note “AZI is always used in conjunction with another effective agent. For testing purposes with the aim of detecting acquired resistance mechanisms, the ECOFF is 1 mg/L.” (57) (ECOFF = Epidemiologic cut-off value, equivalent to ECV). Thus, these standard setting institutes similarly noted the need for dual therapy and similarly concluded the ECV of 1 ug/mL marks the end of wild-type susceptibility. CLSI adopted the EVC as susceptible breakpoint while EUCAST currently has no AZI breakpoints. This leaves the CLSI susceptible only AZI GC breakpoint as main interpretative criteria established by a recognized standard setting institute. The breakpoint gives much-needed guidance for laboratory-supported clinical gonorrhea care. It can also be used by researchers and public health officials in communications to precisely define GC antimicrobial susceptibility.
ACKNOWLEDGEMENTS
The authors wish to thank current and previous members of the ad-hoc CLSI GC AST working group for their contributions to ECV determinations and critical data reviews. Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING
This work was supported by CDC (Centers for Disease Control and Prevention) and in part made possible through support from CDC’s Advanced Molecular Detection (AMD) and Combating Antibiotic Resistant Bacteria (CARB) programs (ENK, MS, SSC, HW, JP); by APHL (Association of Public Health Laboratories) (ER), and by the Massachusetts General Hospital (MJF).
Footnotes
DISCLOSURES
MJF is a member of the Board of Directors, CLSI; she is a consultant for SeLux Diagnostics and a stockholder, Nabriva Therapeutics.
REFERENCES
- 1.Workowski KA, Bolan GA, Centers for Disease C, Prevention. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease C, Prevention. 2007. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 56:332–6. [PubMed] [Google Scholar]
- 3.Centers for Disease C, Prevention. 2012. Update to CDC’s Sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 61:590–4. [PubMed] [Google Scholar]
- 4.CDC. 2018. Sexually Transmitted Disease Surveillance 2017. https://www.cdc.gov/std/stats17/default.htm. Accessed Sept 26, 2018.
- 5.Weston EJ, Workowski K, Torrone E, Weinstock H, Stenger MR. 2018. Adherence to CDC Recommendations for the Treatment of Uncomplicated Gonorrhea - STD Surveillance Network, United States, 2016. MMWR Morb Mortal Wkly Rep 67:473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Public health England, 2018, UK case of Neisseria gonorrhoeae with high-level resistance to azithromycin and resistance to ceftriaxone aquired abroad. https://www.gov.uk/government/publications/multi-drug-resistant-gonorrhoea-in-england-2018. Accessed July 31, 2018.
- 7.Health AGDo. 2018. Multi-drug resistant gonorrhoea. http://www.health.gov.au/internet/main/publishing.nsf/Content/mr-yr18-dept-dept004.htm. Accessed July 31, 2018.
- 8.Centers for Disease C, Prevention. 2014. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae−−2014. MMWR Recomm Rep 63:1–19. [PMC free article] [PubMed] [Google Scholar]
- 9.CLSI. 2018. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 10.FDA. 2018. FDA-Recognized Antimicrobial Susceptibility Test Interpretive Criteria. Accessed 09/13/2018.
- 11.CLSI. Development of In Vitro Susceptibility Testing criteria and Quality Control Parameters; Approved Guideline, 3rd Edition; CLSI Document M23 – A3; Wayne, PA, CLSI; 2008. [Google Scholar]
- 12.EUCAST. 2016. Setting breakpoints for new antimicrobial agents. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_SOPs/EUCAST_SOP_1.2_Setting_breakpoints_new_agents_20161121.pdf. Accessed accessed 2/7/2019.
- 13.EUCAST. 2018. Breakpoint review and revision. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_SOPs/2018/EUCAST_SOP_3.2_Breakpoint_Review_and_Revision_20180123.pdf. Accessed accessed 2/7/2019.
- 14.Kirkcaldy RD, Harvey A, Papp JR, Del Rio C, Soge OO, Holmes KK, Hook EW 3rd, Kubin G, Riedel S, Zenilman J, Pettus K, Sanders T, Sharpe S, Torrone E. 2016. Neisseria gonorrhoeae Antimicrobial Susceptibility Surveillance - The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014. MMWR Surveill Summ 65:1–19. [DOI] [PubMed] [Google Scholar]
- 15.CLSI. 2018. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. CLSI standard M07. [Google Scholar]
- 16.CDC. 2016. Gonococcal Isolate Surveillance Project (GISP) Protocol. https://www.cdc.gov/std/gisp/GISP-Protocol-May-2016.pdf. Accessed 1/22/2019.
- 17.Pfaller MA, Diekema DJ, Ghannoum MA, Rex JH, Alexander BD, Andes D, Brown SD, Chaturvedi V, Espinel-Ingroff A, Fowler CL, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Sheehan DJ, Walsh TJ, Clinical, Laboratory Standards Institute Antifungal Testing S. 2009. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J Clin Microbiol 47:3142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Osterlund A, Rodloff A, Steinbakk M, Urbaskova P, Vatopoulos A. 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J Antimicrob Chemother 52:145–8. [DOI] [PubMed] [Google Scholar]
- 19.Arendrup MC, Garcia-Effron G, Lass-Florl C, Lopez AG, Rodriguez-Tudela JL, Cuenca-Estrella M, Perlin DS. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and isosensitest media. Antimicrob Agents Chemother 54:426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CLSI. Accessed on 7/11/2018 ECOFFinder. https://clsi.org/education/microbiology/ecoffinder/. Accessed July 31, 2018.
- 21.Jacobsson S, Golparian D, Cole M, Spiteri G, Martin I, Bergheim T, Borrego MJ, Crowley B, Crucitti T, Van Dam AP, Hoffmann S, Jeverica S, Kohl P, Mlynarczyk-Bonikowska B, Pakarna G, Stary A, Stefanelli P, Pavlik P, Tzelepi E, Abad R, Harris SR, Unemo M. 2016. WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014. J Antimicrob Chemother 71:3109–3116. [DOI] [PubMed] [Google Scholar]
- 22.Morita-Ishihara T, Unemo M, Furubayashi K, Kawahata T, Shimuta K, Nakayama S, Ohnishi M. 2014. Treatment failure with 2 g of azithromycin (extended-release formulation) in gonorrhoea in Japan caused by the international multidrug-resistant ST1407 strain of Neisseria gonorrhoeae. J Antimicrob Chemother 69:2086–90. [DOI] [PubMed] [Google Scholar]
- 23.Gose SO, Soge OO, Beebe JL, Nguyen D, Stoltey JE, Bauer HM. 2015. Failure of azithromycin 2.0 g in the treatment of gonococcal urethritis caused by high-level resistance in California. Sex Transm Dis 42:279–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grad YH, Harris SR, Kirkcaldy RD, Green AG, Marks DS, Bentley SD, Trees D, Lipsitch M. 2016. Genomic Epidemiology of Gonococcal Resistance to Extended-Spectrum Cephalosporins, Macrolides, and Fluoroquinolones in the United States, 2000–2013. J Infect Dis 214:1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shafer WM. 2018. Mosaic Drug Efflux Gene Sequences from Commensal Neisseria Can Lead to Low-Level Azithromycin Resistance Expressed by Neisseria gonorrhoeae Clinical Isolates. MBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grad YH, Kirkcaldy RD, Trees D, Dordel J, Harris SR, Goldstein E, Weinstock H, Parkhill J, Hanage WP, Bentley S, Lipsitch M. 2014. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect Dis 14:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, Marrazzo JM, Sonder GJB, Schwebke JR, Hoornenborg E, Peeling RW, Philip SS, Low N, Fairley CK. 2017. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 17:e235–e279. [DOI] [PubMed] [Google Scholar]
- 28.Golden MR, Whittington WL, Handsfield HH, Hughes JP, Stamm WE, Hogben M, Clark A, Malinski C, Helmers JR, Thomas KK, Holmes KK. 2005. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med 352:676–85. [DOI] [PubMed] [Google Scholar]
- 29.Waugh MA. 1996. Azithromycin in gonorrhoea. Int J STD AIDS 7 Suppl 1:2–4. [DOI] [PubMed] [Google Scholar]
- 30.Handsfield HH, Dalu ZA, Martin DH, Douglas JM Jr., McCarty JM, Schlossberg D. 1994. Multicenter trial of single-dose azithromycin vs. ceftriaxone in the treatment of uncomplicated gonorrhea. Azithromycin Gonorrhea Study Group. Sex Transm Dis 21:107–11. [DOI] [PubMed] [Google Scholar]
- 31.Taylor SN, Marrazzo J, Batteiger BE, Hook EW 3rd, Sena AC, Long J, Wierzbicki MR, Kwak H, Johnson SM, Lawrence K, Mueller J. 2018. Single-Dose Zoliflodacin (ETX0914) for Treatment of Urogenital Gonorrhea. N Engl J Med 379:1835–1845. [DOI] [PubMed] [Google Scholar]
- 32.Waugh MA. 1993. Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women. J Antimicrob Chemother 31 Suppl E:193–8. [DOI] [PubMed] [Google Scholar]
- 33.Steingrimsson O, Olafsson JH, Thorarinsson H, Ryan RW, Johnson RB, Tilton RC. 1990. Azithromycin in the treatment of sexually transmitted disease. J Antimicrob Chemother 25 Suppl A:109–14. [DOI] [PubMed] [Google Scholar]
- 34.Bignell C, Garley J. 2010. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex Transm Infect 86:422–6. [DOI] [PubMed] [Google Scholar]
- 35.Habib AR, Fernando R. 2004. Efficacy of azithromycin 1g single dose in the management of uncomplicated gonorrhoea. Int J STD AIDS 15:240–2. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda M, Ito S, Kido A, Hamano K, Uchijima Y, Uwatoko N, Kusuyama H, Watanabe A, Miyamura R, Miyata K, Deguchi T. 2014. A single 2 g oral dose of extended-release azithromycin for treatment of gonococcal urethritis. J Antimicrob Chemother 69:3116–8. [DOI] [PubMed] [Google Scholar]
- 37.Bissessor M, Whiley DM, Fairley CK, Bradshaw CS, Lee DM, Snow AS, Lahra MM, Hocking JS, Chen MY. 2015. Persistence of Neisseria gonorrhoeae DNA following treatment for pharyngeal and rectal gonorrhea is influenced by antibiotic susceptibility and reinfection. Clin Infect Dis 60:557–63. [DOI] [PubMed] [Google Scholar]
- 38.Zenilman JM. 2015. Editorial commentary: persistent gonococcal DNA: artifact or real? Further insights into the biology of a remarkable pathogen. Clin Infect Dis 60:564–5. [DOI] [PubMed] [Google Scholar]
- 39.Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. 2011. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill 16. [PubMed] [Google Scholar]
- 40.Soge OO, Harger D, Schafer S, Toevs K, Raisler KA, Venator K, Holmes KK, Kirkcaldy RD. 2012. Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011). Sex Transm Dis 39:877–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waters LJ, Boag FC, Betournay R. 2005. Efficacy of azithromycin 1 g single dose in the management of uncomplicated gonorrhoea. Int J STD AIDS 16:84. [DOI] [PubMed] [Google Scholar]
- 42.McLean CA, Wang SA, Hoff GL, Dennis LY, Trees DL, Knapp JS, Markowitz LE, Levine WC. 2004. The emergence of Neisseria gonorrhoeae with decreased susceptibility to Azithromycin in Kansas City, Missouri, 1999 to 2000. Sex Transm Dis 31:73–8. [DOI] [PubMed] [Google Scholar]
- 43.Chisholm SA, Neal TJ, Alawattegama AB, Birley HD, Howe RA, Ison CA. 2009. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J Antimicrob Chemother 64:353–8. [DOI] [PubMed] [Google Scholar]
- 44.Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, Unemo M. 2016. Failure of Dual Antimicrobial Therapy in Treatment of Gonorrhea. N Engl J Med 374:2504–6. [DOI] [PubMed] [Google Scholar]
- 45.Martin IM, Ison CA. 2003. Detection of mixed infection of Neisseria gonorrhoeae. Sex Transm Infect 79:56–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfizer. Revised January 2013. ZithromaxR (azithromycin tablets) and (azithromycin for oral suspension). https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf. Accessed July 24, 2018
- 47.Foulds G, Shepard RM, Johnson RB. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother 25 Suppl A:73–82. [DOI] [PubMed] [Google Scholar]
- 48.Lode H 1991. The pharmacokinetics of azithromycin and their clinical significance. Eur J Clin Microbiol Infect Dis 10:807–12. [DOI] [PubMed] [Google Scholar]
- 49.Davila D, Kolacny-Babic L, Plavsic F. 1991. Pharmacokinetics of azithromycin after single oral dosing of experimental animals. Biopharm Drug Dispos 12:505–14. [DOI] [PubMed] [Google Scholar]
- 50.Shepard RM, Falkner FC. 1990. Pharmacokinetics of azithromycin in rats and dogs. J Antimicrob Chemother 25 Suppl A:49–60. [DOI] [PubMed] [Google Scholar]
- 51.Kong FY, Tabrizi SN, Fairley CK, Vodstrcil LA, Huston WM, Chen M, Bradshaw C, Hocking JS. 2015. The efficacy of azithromycin and doxycycline for the treatment of rectal chlamydia infection: a systematic review and meta-analysis. J Antimicrob Chemother 70:1290–7. [DOI] [PubMed] [Google Scholar]
- 52.Kong FY, Rupasinghe TW, Simpson JA, Vodstrcil LA, Fairley CK, McConville MJ, Hocking JS. 2017. Pharmacokinetics of a single 1g dose of azithromycin in rectal tissue in men. PLoS One 12:e0174372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu P, Allaudeen H, Chandra R, Phillips K, Jungnik A, Breen JD, Sharma A. 2007. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob Agents Chemother 51:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soda M, Ito S, Matsumaru N, Nakamura S, Nagase I, Takahashi H, Ohno Y, Yasuda M, Yamamoto M, Tsukamoto K, Itoh Y, Deguchi T, Kitaichi K. 2018. Evaluation of the Microbiological Efficacy of a Single 2-Gram Dose of Extended-Release Azithromycin by Population Pharmacokinetics and Simulation in Japanese Patients with Gonococcal Urethritis. Antimicrob Agents Chemother 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. Antibiotic-resistant gonorrhea basic information. 2018. Available at: https://www.cdc.gov/std/gonorrhea/arg/basic.htm Accessed 31 July 2018.
- 56.USCAST. 2018. Breakpoint tables for interpretation of MICs and zone diameters. http://www.uscast.org. Accessed Feb 7, 2019
- 57.EUCAST. 2019. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters http://www.eucast.org. Accessed 1/21/2019.