Abstract
Background:
Only a fraction of red blood cell (RBC) transfusion recipients form alloantibodies, and variables determining responsiveness or non-responsiveness are poorly understood. We and others have previously shown in animal models that pre-treatment with toll-like receptor (TLR) agonists that mimic different types of infections impacts the magnitude or frequency of RBC alloantibody responses. We hypothesized that influenza infection, co-existent with transfusion, would impact responses to transfused RBCs in a manner dependent on type 1(α/β) interferon (IFN) signaling, and tested this in a murine model.
Study Design and Methods:
Wild-type mice or mice lacking the ability to respond to type 1 IFN were infected with influenza prior to the transfusion of transgenic murine RBCs (K1) expressing the human KEL glycoprotein or the triple fusion HOD protein. Alloantibody responses were measured longitudinally post-transfusion by flow cytometric crossmatch, and post-transfusion RBC recovery and survival was evaluated.
Results:
Influenza-infected mice transfused with K1 RBCs developed robust anti-KEL alloantibodies, whereas animals transfused in the absence of infection remained non-responders; influenza-associated RBC alloimmunization was also observed after transfusion of HOD RBCs. Recipient type 1 interferon production was critical to the mechanism of action of influenza-induced RBC alloimmunization, with alloimmunization being significantly decreased in mice unable to sense type 1 IFN (through antibody blockade or genetic approaches).
Conclusion:
These and other data suggest that type 1 IFN responses to TLR agonists or infections regulate RBC alloantibody responses. Studies investigating whether such a correlation exists in humans may be informative.
Keywords: red blood cell, alloimmunization, inflammation, influenza
Introduction:
Red blood cell (RBC) transfusion is a common, often life-saving procedure, with more than 12 million units of RBC transfused annually in the United States1. As ABO and Rh(D) are typically the only antigens matched between donors and recipients, each transfusion recipient is exposed to multiple non-self blood group antigens. As a consequence of this exposure, approximately 3–5% of transfusion recipients develop RBC alloantibodies2. These alloantibodies limit the availability of future compatible RBCs and potentially lead to adverse sequela of transfusions (including acute or delayed hemolytic transfusion reactions) or pregnancy (including hemolytic disease of the newborn).
Aside from antigenic mismatches between donor and recipient, many factors likely contribute to the generation of alloantibodies to transfused RBCs. Using a murine model, we and others have previously demonstrated that the addition of CpG to transfusion RBCs or pretreatment of RBC transfusion recipients with poly(I:C) induces or enhances alloimmunization to transfused blood group antigens3–6. Our recent work has shown that the type 1 interferon (IFN α/β) produced by recipients in response to poly (I:C) administration is critically important to its mechanism of action in enhancing RBC alloimmunization7. In the current studies, we sought to translate past findings involving toll-like receptor agonists into more physiological conditions.
Respiratory infections with influenza A virus cause severe lung inflammation and injury, which results in considerable morbidity and mortality in humans and animals worldwide. The World Health Organization has estimated that there are 3–5 million cases of severe influenza annually, leading to 250,000–500,000 deaths worldwide8. In the United States, the Centers for Disease Control and Prevention estimates that influenza has resulted in 9.2 million to 35.6 million illnesses, with 140,000 to 710,000 hospitalizations annually, since 20109.
Infections may trigger the immune system by several different mechanisms, including an adjuvant effect, molecular mimicry, and bystander activation10. For example, chronic lymphocytic choriomeningitis virus (LCMV) infection has been shown to cause autoimmune anemia in a murine model11. Persistent LCMV infection induces type I IFN production12, suggesting that viruses may be able to promote auto/alloimmunity to RBCs via an IFN pathway. A recent study suggested that disseminated viral infections may increase the risk of red blood cell alloimmunization in humans13. However, the mechanism(s) and pathway(s) that may be responsible remain unclear.
Here we investigated the impact of recipient influenza infection on RBC alloimmunization induction, using an authentic human blood group antigen (K1) as well as a model triple fusion antigen (hen egg lysozyme, ovalbumin, and human Duffy) expressed on transgenic murine RBCs. We report that influenza-associated RBC alloimmunization occurs in both model systems, with the enhanced alloimmunization being dependent on recipient type 1 IFN signaling.
Materials and Methods:
Mice:
C57BL/6 and congenic C57BL/6-Ly5.1 WT mice were purchased from Charles River Laboratories (Wilmington, MA). OT-2 mice (B6.Cg-Tg(TcraTcrb)425Cbn/J) were purchased from Jackson Laboratories (Bar Harbor, ME) and crossed with CD45.1 mice (B6.SJL-PtprcaPepcb/BoyCrl) from the National Cancer Institute (Frederick, MD). IFNAR1−/−14 and IRF3−/−/IRF7−/− mice15 were previously described. Transgenic KEL mice expressing the human Kell glycoprotein (referred to as K1 mice7), as well as HOD mice that have RBC-specific expression of hen egg lysozyme, ovalbumin, and human Duffy, were generated as previously described16. IL-4/IL-21 double reporter mice were generated by crossing C.129-IL4tm1Lky/J (4get) with IL21-IRES-Katuska (Kat) reporter (Il21Kat/+) mice as previously described17 and generously provided by the Joe Craft laboratory, and were then crossed with OT-2 transgenic mice. All mice were 8–12 weeks of age and were backcrossed to the C57BL/6 background for more than 8 generations, except for the OT-2 mice which were crossed on congenic C57BL/6-Ly5.1 WT or a CD90.1 background. Both male and female mice were utilized. All animal protocols were approved by the Yale Institutional Animal Care and Use Committee.
Intranasal influenza PR8 infection and RBC transfusion:
Influenza virus strain A/PR/8/34 (PR8; H1N1) was propagated for 2–3 days at 35°C in the allantoic cavities of 10-day old fertilized chicken eggs18. Harvested virus was purified by 10–50% sucrose gradient centrifugation, titered by MDCK plaque assay, and stored at −80 °C. For all intranasal inoculations, mice were anesthetized by Ketamine and Xylazine and given live virus in 50 μl of PBS. Weight loss was monitored daily and mice with >25% weight loss or < 2 on the clinical disease scoring system were euthanized. Peripheral blood of K1 or HOD donor mice was collected in 12% Citrate Phosphate Dextrose Adenine (CPDA-1, Jorgensen Labs, Melville, NY), leukoreduced using a Pall syringe filter (East Hills, NY), and washed with saline. Recipient naïve and infected WT, or genetically-deficient or treated mice, were transfused in the lateral tail vein with 75 μL of packed RBCs, the approximate mouse equivalent of 1 unit of human RBCs. In indicated experiments, animals were treated intraperitoneally with 100 mcg of high molecular weight poly (I:C) (InvivoGen, San Diego, CA) 2–4 hours prior to RBC transfusion; in other indicated experiments, RBCs were mixed with recombinant mouse IFN-α (Hycult Biotech, the Netherlands) prior to transfusion.
Detection of RBC alloantibodies:
Antibodies generated against the transgenic K1 RBCs (described in this manuscript as anti-KEL glycoprotein antibodies) were measured by flow-cytometric crossmatch at indicated time points after transfusion and as previously described (24). In brief, K1 or wild type C57BL/6 RBCs were incubated with serum from transfused mice and subsequently stained for RBC-bound IgG (goat anti- mouse IgG APC, Jackson ImmunoResearch, West Grove, PA) or biotinylated IgG subtypes (Bethyl Laboratories, Montgomery, TX) followed by streptavidin conjugated to a fluorophore (BD Biosciences). The adjusted MFI was calculated by subtracting the reactivity of serum with wild type C57BL/6 RBCs from the reactivity of serum with K1 RBCs. Antibodies generated against HOD RBCs were measured in a similar manner, using HOD or antigen negative RBCs as target cells. Flow cytometry was completed using a Miltenyi MACSquant flow cytometer (Germany) and analyzed using FlowJo software (Tree Star, Ashland, OR).
RBC fluorescent labeling and post-transfusion recovery analysis:
K1 or wild type C57BL/6 RBCs were washed 3 times and labeled with chloromethylbenzamido 1,1’-dioctadecyl-3,3,’,3’-tetramethylindocarbocyanine perchlorate (CM-DiI) or 3,3’-dihexadecyloxacarbocyanine perchlorate (DiO) according to the manufacturer’s instructions (Molecular Probes, Eugene, OR) and as previously described19. Experimental and control RBCs were mixed at a 1:1 ratio, and recipient mice were transfused via lateral tail vein. Post-transfusion recovery and survival of the transfused RBCs was determined by comparing the ratio of circulating K1 RBCs to syngeneic wild type C57BL/6 RBCs in recipients at indicated time points post-transfusion. The flow cytometric analysis for RBC recovery was completed using an LSRII flow cytometer (BD Biosciences).
In vivo T-cell differentiation and Tfh cytokine detection:
OVA-specific CD4+ T cells were prepared from spleen and lymph nodes of OT-2 TCR transgenic mice or OT-2 IL4/IL21 reporter mice by negative selection using a CD4+ T Cell Isolation Kit (Stemcell Technologies) according to the manufacturer’s instructions. 104 purified CD4+ OT-2 cells were transferred into mice by retro-orbital injection. The recipient mice were infected with 10 plaque forming units (pfu) of PR8 influenza and transfused 3 days later via lateral tail vein with RBCs. Spleens were harvested 6–7 days post immunization, and single-cell suspensions were prepared, stained, and analyzed on an LSRII flow cytometer (BD Biosciences) or a MACSquant flow cytometer (Miltenyi Biotech, Germany).
IFNAR1 Blockade:
For blocking experiments, mice were i.p. injected with 600ug anti-mouse IFNAR1 blocking antibodies (clone: MAR1–5A3, BioXCell, West Lebanon, NH), isotype control IgG1 (MOPC-21, BioXCell) or PBS 24 hours prior to PR8 infection, 24 hours prior to transfusion, and 3 days after transfusion.
Flow Cytometry:
Single cell suspensions of splenocytes were analyzed following RBC lysis. For analysis, spleens were diced with a razor blade prior to cell filtration with a 70 μM cell strainer. Suspensions were stained with fluorescently conjugated antibodies specific for cell surface proteins, including CD45.2 (104), B220 (RA3–6B2), TCRβ (H57–597), CD4 (GK1.5), CD44 (IM7), Va2 (B20.1), CD90.1 (OX-u) I-A/I-E (MHC II, M5/114.15.2), PD1 (RMP1–30), and GL7(GL7) from Biolegend (San Diego, CA); CD45.1 (A20), CD11c (N418), B220 (RA3–6B2) from Ebioscience (San Diego, CA), CXCR5 (2G8) and Fas/CD95 (Jo2) from BD Biosciences (San Jose, CA). Data was analyzed using FlowJo software (Tree Star, Ashland, OR).
Statistics:
Statistical analyses were performed using Graph Pad Prism software (San Diego, CA). Statistical significance between two groups was determined using a Mann Whitney U test or an unpaired two-sided Student’s t-test for non-parametric and parametric data, respectively. Significance between three or more groups of non-parametric data was determined using the Kruskal Wallis test with Dunn’s post-test.
Results:
Influenza infection promotes alloantibodies against transfused RBCs
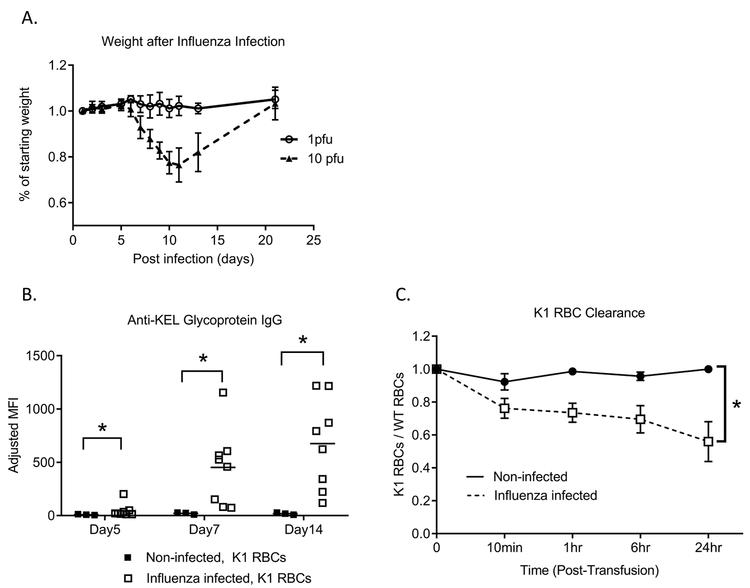
In our previous studies, we have shown that transfused K1 RBCs (expressing the human KEL glycoprotein) do not induce alloimmunity in wild type C57BL/6 recipients in the absence of an adjuvant7. To examine influenza infection-induced alloantibody responses, blood was collected from K1 donors, leukoreduced, and transfused into wild-type recipients that had been infected 3 days earlier with a sublethal dose of influenza PR8. This timing was selected to correlate with a high viral burden and inflammatory cytokines at the time of RBC exposure20,21. Infected mice started losing weight at days 3–5 post-infection, reaching a peak weight loss more than one week after infection, and then started to recover (Figure 1A). Mice transfused in the absence of influenza infection failed to develop detectable anti-KEL glycoprotein IgG alloantibodies. In contrast, all influenza infected mice developed robust anti-KEL alloantibodies (Figure 1B). All tested IgG subtypes were present in influenza infected transfused mice (Supplemental Figure 1).
Figure 1. Influenza infection promotes the generation of clinically significant alloantibodies against K1 RBCs.
(A) Wild type C57BL/6 mice were intranasally infected with 1 PFU (solid line) or 10 PFU (dashed line) of PR8 influenza virus, and weight was monitored daily for up to 3 weeks. (B) 3 days after influenza infection, the infected mice and naïve mice were intravenously transfused with K1 RBCs; anti-KEL glycoprotein IgG responses were measured at indicated time points post-transfusion by flow cytometric crossmatch. (C) 28 days after the initial K1 RBC transfusion, mice were re-transfused with labeled RBCs and K1 RBC post-transfusion recovery and survival of K1 RBCs was evaluated. Data are representative of 2–3 independent experiments, *p<0.05.
Not all antibodies are equally efficient in neutralizing target antigens22. To determine the significance of the influenza-induced anti-KEL alloantibodies, a secondary transfusion of a mixture of lipophilically labeled K1 and wild type C57BL/6 RBCs was administered to the animals transfused initially in the presence or absence of influenza infection. Premature clearance of K1 over C57BL/6 RBCs was observed in alloimmunized but not non-alloimmunized animals (Figure 1C), suggesting clinically detrimental role of anti-KEL glycoprotein alloantibodies induced during influenza infection.
Influenza infection enhances germinal center B cell responses
To determine whether influenza infection-associated RBC alloantibody responses correlated with germinal center B cell generation, the percentage of germinal center B cells in influenza-infected mice was quantitated 6 days after a 2nd RBC transfusion, which occurred 1 month after the initial transfusion. Mice infected with influenza and transfused with K1 RBCs had significantly higher germinal center B-cell numbers than those transfused with K1 RBCs alone (Supplemental Figure 2). However, it is notable that infection alone, in the absence of RBC transfusion, also significantly increased germinal center B-cell responses, with 0.5–2.6% of B cells being germinal center B cells. Thus, although influenza infection enhances germinal center B cell responses it is not apparent that K1 RBC transfusion enhances this response any further.
Influenza infection promotes antigen-specific Tfh generation and cytokine production
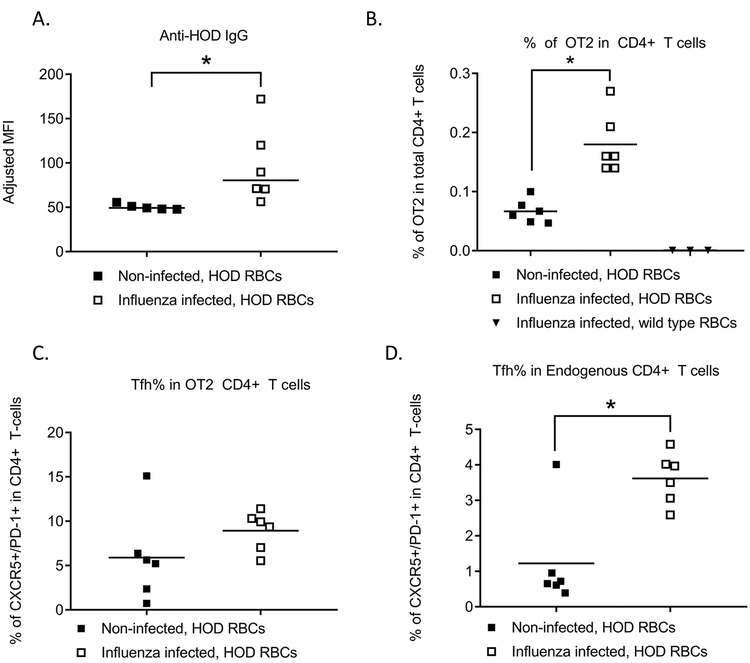
Follicular helper T cell responses (Tfh) are important for maintaining a strong germinal center B cell response. Given the inability to analyze antigen specific Tfh responses to transfused K1 RBCs, we utilized a model system in which transfused RBCs express the triple fusion HOD antigen (hen egg lysozyme, ovalbumin, and Duffy) for this line of investigation; anti-HOD alloantibody responses have previously been confirmed to be T-cell dependent23. We adoptively transferred ova-specific OT-2 CD4+ T-cells into wild type C57BL/6 mice, with some mice being infected with influenza prior to transfusion with HOD RBCs. Following transfusion, influenza infected mice produced a significantly elevated level of anti-HOD alloantibodies, compared to uninfected mice (Figure 2A). Recipient CD4+ T-cells, both endogenous and OT-2, were evaluated by flow cytometry. Expansion of antigen specific CD4+ T-cells was observed in mice transfused with HOD RBCs as has previously been reported24, and concomitant influenza infection further increased the percentage of antigen specific CD4+ T-cells.(Figure 2B). Influenza infection prior to transfusion also led to a significant increase in the percentage of endogenous Tfh CD4+ T-cells (CXCR5+PD1+), as well as a non-statistically significant increase in the percentage of antigen-specific OT-2 Tfh cells (Supplemental Figure 3 and Figures 2C, 2D).
Figure 2. Influenza infection promotes antigen-specific Tfh generation and dual IL4/IL21 production.
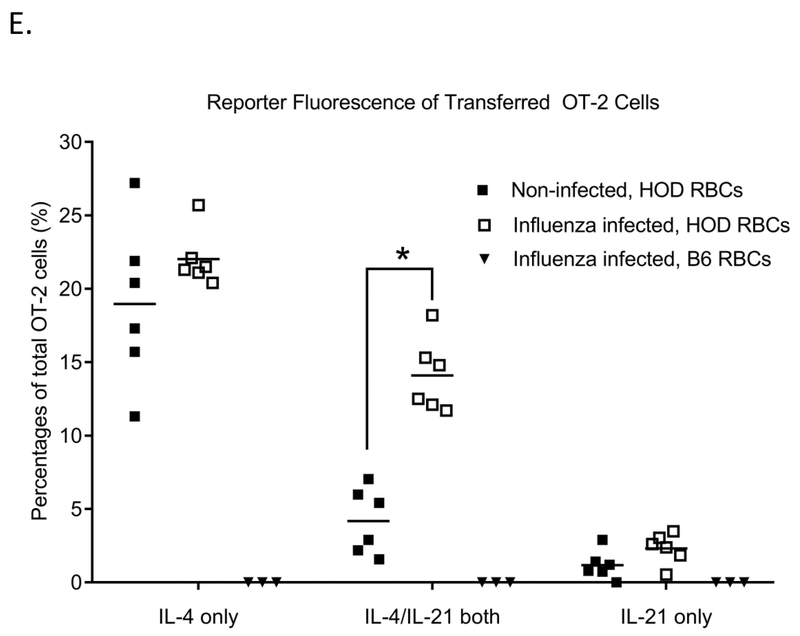
104 OT-2 CD4+ T-cells were adoptively transferred into naïve wild type C57BL/6 mice or mice infected with 10 PFU of PR8 influenza virus, followed by a HOD RBC transfusion. (A) Anti-HOD IgG alloantibody responses as measured by flow cytometry. (B) Percentage of OT-2 cells in total CD4+ T-cells, (C) percentage of endogenous CD4+ T-cells that were CXCR5+/PD-1+, and (D) percentage of OT-2 CD4+ T-cells that were CXCR5+/PD-1+ were evaluated by flow cytometry 6 days post-RBC transfusion. For (E), 104 cells CD4+ T-cells from C57BL/6 (B6) IL21Kat/+IL4GFP/+ double reporter mice crossed with OT-2 mice were adoptively transferred into naïve wild type C57BL/6 mice or mice infected with 10 PFU of PR8 influenza virus, followed by a HOD RBC transfusion. Fluorescence of IL21 and IL4 producing cells was evaluated 6 days post-transfusion, after gating on ova-specific CD4+ T-cells. For B-E, pre-gating included inclusion of TCRβ+ cells, exclusion of B220+ cells, and inclusion of CD4+, CD44+ cells. OT-2 cells were identified by CD45.1 or CD90.1 positivity. Data shown are representative of 3 independent experiments, *p<0.05.
To investigate the role of OT-2 Tfh cells during alloimmunization, we utilized OT-2 mice that express IL-21 and IL-4 reporter genes (OT-2 IL21Kat/+IL4GFP/+ double reporter mice). 10,000 CD4+ T cells from the OT-2 double reporter mice were adoptively transferred prior to influenza infection. Mice were subsequently transfused with HOD RBCs 3 days after infection, and IL-4/IL-21 production by Tfh cells was longitudinally evaluated by flow cytometry. Within 6 days following the transfusion, a significantly higher percentage of OT-2 cells in influenza-infected compared to non-infected mice demonstrated dual fluorescence of IL-4 and IL-21 producing cells (Supplemental Figure 4 and Figure 2E). Collectively, these results indicate that influenza infection enhances the function of Tfh cells that are antigen specific for the transfused HOD RBCs.
Infection-induced alloimmune responses to transfused RBCs are Type I IFN dependent
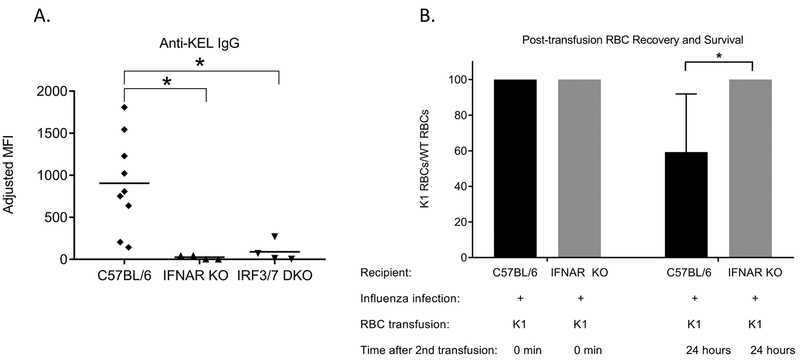
Influenza infection induces Type I interferons (IFN α/β) and NFkB-inducible cytokines, including TNFα and IL-620,21, and influenza has also been shown to activate inflammasomes25. Given our recent reports suggesting the importance of IFNα/β on RBC alloimmunization7,26, we hypothesized that the mechanism through which influenza regulates RBC alloimmunization in this murine model is at least in part dependent on recipient IFNα/β signaling. To investigate this hypothesis, we infected wild type C57BL/6 mice or mice lacking IFNAR1 receptors (IFNAR KO) with influenza and transfused them 3 days later with K1 RBCs. In contrast to the high level of anti-KEL glycoprotein IgG alloantibodies detected in infected wild type C57BL/6 mice, the alloantibody level in infected IFNAR KO mice was significantly reduced (Figure 3A).
Figure 3. Infection-induced alloimmune responses to transfused RBCs are Type I IFN dependent.
(A)Wild type C57BL/6 mice, mice lacking type 1 IFN receptors (IFNAR KO mice), or mice lacking the interferon regulatory factor (IFR) 3 and 7 pathways (IRF 3/7 double KO mice) were intranasally infected with 10 PFU of PR8 influenza virus and transfused 3 days later with K1 RBCs; anti-KEL glycoprotein IgG responses were measured by flow cytometric crossmatch. (B) 28 days after the initial K1 RBC transfusion, wild type or IFNAR KO mice were re-transfused with labeled RBCs and K1 RBC post-transfusion recovery and survival was evaluated. Data shown are representative of 2–3 independent experiments, * p<0.05; error bars depict standard deviation.
To determine whether the IFNa/b signaling pathway was required for influenza-induced alloimmunization, we utilized mice genetically lacking the interferon regulatory factor (IRF) 3 and 7 (IRF 3/7 double KO mice). Similar to results from IFNAR KO mice, IRF 3/7 double KO recipients infected with influenza prior to K1 RBC transfusion also generated minimal anti-KEL glycoprotein alloantibodies (Figure 3A). Collectively, these data in IFNAR KO and IRF 3/7 double KO recipients show the importance of IFNα/β production as well as type 1 IFN sensing in influenza-associated alloimmune responses to transfused K1 RBCs. Additional experiments were also completed to show the relevance of IFNα/β signaling in alloantibody responses to transfused HOD RBCs (Supplemental Figure 5).
To track the K1 RBC post-transfusion recovery and survival, one month after the primary transfusion we re-transfused wild type or IFNAR KO recipients with a mixture of lipophilically labeled K1 and C57BL/6 RBCs. As predicted, preferential clearance of K1 RBCs was observed in alloimmunized C57BL/6 recipient mice (Figure 1C shows full time course and Figure 3B shows two time points) but not in non-alloimmunized IFNAR KO recipients (Figure 3B).
Antibody blockade of type 1 IFN receptors prevents influenza-induced alloimmunization to transfused K1 RBCs
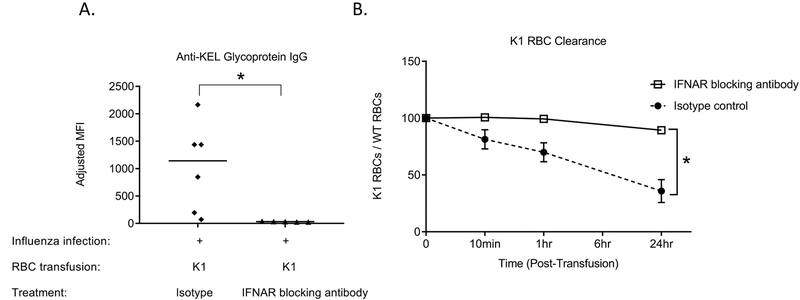
Given that mice genetically lacking IFNα/β receptors or genetically lacking IRF3 and IRF7 had a reduced alloantibody response to transfused K1 RBCs in the presence of influenza infection, we next examined whether blockade of IFNα/β receptors in wild type mice could reduce alloimmunization. For these studies, C57BL/6 mice were treated with IFNAR1 blocking antibody (MAR1–5A3) or an isotype matched IgG1 control 24 hours prior to influenza infection, 24 hours prior to K1 RBC transfusion, and 3 days after K1 RBC transfusion. Anti-KEL IgG antibodies were evaluated post-transfusion. Mice treated with control isotype antibody (or a saline control) had robust anti-KEL glycoprotein IgG levels after K1 RBC transfusion, whereas those treated with the IFNAR blocking antibody failed to produce anti-KEL IgG (Figure 4A). One month after the primary transfusion, we re-transfused the recipients with a mixture of lipophilically labeled K1 and C57BL/6 RBCs to track K1 RBC post-transfusion recovery and survival. K1 RBC clearance was significantly decreased in mice transfused initially in the presence of the IFNAR1 blocking antibody, compared to mice transfused initially in the presence of the isotype control antibody (Figure 4B). These data further demonstrate the relevance of IFNα/β signaling in influenza-associated RBC alloimmunization.
Figure 4. Type I IFN receptor blockade prevents influenza-induced alloimmune responses to transfused K1 RBCs.
(A) Wild type C57BL/6 mice were treated with IFNAR1 blocking antibody (MAR1–5A3) or an isotype matched IgG1 control 24 hours prior to influenza infection, 24 hours prior to K1 RBC transfusion, and 3 days after K1 RBC transfusion and anti-KEL IgG antibodies were evaluated by flow cytometry post-transfusion. (B) 28 days after the initial K1 RBC transfusion, these same mice were re-transfused with labeled RBCs K1 RBC post-transfusion recovery and survival was evaluated. Data shown are representative of 2 independent experiments, *p<0.05.
Discussion:
We have shown for the first time that recipient infection with a viral infection (influenza) increases the magnitude and frequency of clinically significant RBC alloimmunization to transfused RBCs. This was observed in two different RBC antigen systems, one with transfused murine RBCs expressing the human KEL (K1) glycoprotein and the other with transfused murine RBCs expressing a model triple fusion HOD antigen. Further, we have shown differences in RBC antigen-specific CD4+ T-cell function in the presence or absence of influenza infection. Last, using knockout mice and antibody-mediated receptor blockade, we have demonstrated a role for recipient IFNα/β production and sensing in influenza-induced RBC alloimmunization.
Only a fraction of human RBC transfusion recipients will ever form alloantibodies after transfusion or pregnancy2. In fact, fewer than 3–5% of all transfused patients will become alloimmunized, with more than 10% of transfused patients with myelodysplastic syndrome27 and up to 30–50% of transfused patients with sickle cell disease becoming alloimmunized. The reasons for the differences in alloantibody prevalence rates are not fully understood, but recipient variables likely contribute. RBC alloantibodies are clinically significant, leading to difficulties in locating compatible RBCs for future transfusions, increased risk of acute or delayed hemolytic transfusion reactions, and a risk of hemolytic disease of the newborn in pregnancy. The genetic predisposition of individuals likely plays some role in RBC alloantibody formation, as do environmental factors. Among environmental factors, infections have been implicated to play a role in the initiation of autoimmune diseases and are also thought to play a role in alloimmunity.
An enlarging body of literature associates RBC exposure during “inflammatory” conditions with an increased likelihood of becoming RBC alloimmunized6,13. This has been shown in multiple animal models, with poly (I:C) or CpG having been shown to increase the frequency and magnitude of alloimmunization in some models4,5,7,28,29. Similar results have been shown in human studies. Elevated alloimmunization rates have been reported for RBC transfusion recipients with sickle cell disease and acute chest syndrome30, and patients with autoimmune diseases, including inflammatory bowel disease31,32,33. However, not all inflammatory stimuli enhance alloimmunization. For example, endotoxemia decreased the likelihood of alloimmunization34,35 in multiple animal models and in humans13.
Recent work by Gibb et al. suggest that IFNα/β sensing by murine transfusion recipients is critically important in the steps leading to RBC alloantibody formation in the presence of the adjuvant poly(I:C)7. Thus, we hypothesized that influenza infection20,21 promotes RBC alloimmunization in a IFNα/β manner. The innate sensors TLR7, RIG I, and Nlrp3 can sense influenza infection36. For example, sRNA from influenza is known to induce IFNα/β by binding endosomal TLR7, which utilizes the adaptor protein, MyD88, to mediate downstream signaling. MDA5 and RIG-I in the cytosol can also recognize viral genome RNAs and can utilize the signaling protein, mitochondrial antiviral signaling protein 13 (MAVS), to induce IFNα/β. Both pathways converge upon the canonical transcription factors in the nucleus, IRF 3 and IRF7. Data obtained from experiments in IFNAR KO as well as IRF 3/7 DKO recipient mice show that IFNα/β sensing is critically important in influenza-associated RBC alloimmunization. Additional experiments in wild type C57BL/6 recipients, known to be capable of anti-KEL glycoprotein IgG formation under other conditions7, were completed to ensure that the findings in IFNAR KO or IRF3/7 DKO mice were not due to a unique humoral immune defect of those animals.
Limitations of these studies should be mentioned. First, the cell type(s) specifically producing type 1 IFN could not be identified. Studies were attempted using reporter mice expressing YFP at the time of IFNβ production; prior studies using poly (I:C) as an adjuvant consistently demonstrated IFNα/β production by CD8α+ dendritic cells7. However, influenza infection did not consistently lead to detectable IFNα/β production in any one specific cell type using this YFP model likely due to the lack of assay sensitivity. Another weakness of the current studies is a lack of determination of which upstream pathways of IRF 3/7 are most critical and which cells are most critical in sensing type 1 IFN; future studies using mice lacking pathways such as MAVS or RIG1 may be informative, as may studies in mice with select cell populations lacking type 1 IFN receptors. Last, the inclusion of more animals per group or the use of internal controls such that data from all reproductions of a particular experiment could have been shown on a single graph would have increased statistical power and may have strengthened the conclusions drawn.
In sum, this is the first study to investigate the role of influenza infection in promoting clinically significant alloimmune responses to transfused RBCs in a murine model. With recipient influenza infection, RBC antigen-specific Tfh cells were induced, generating IL-4 and IL-21. The recipient IFNα/β generated as a result of influenza infection was necessary for the observed infection-enhanced RBC alloimmunization, as mice genetically lacking IFNα/β signaling (IFNAR KO recipients) and wild type C57BL/6 mice treated with IFNα/β receptor blocking antibodies did not form RBC alloantibodies. Based on these and other murine studies, human studies investigating a potential correlation between IFNα/β production and RBC alloantibody “responders” may be informative. Such investigations may eventually lead to development of translational, targeted therapies to minimize RBC alloimmunization in patients at highest risk of this complication.
Supplementary Material
Acknowledgments:
DRG and JEH would like to thank Akiko Iwasaki for her support and helpful conversations.
This work was supported by grants from the NIH (HL126076 and HL132951 to JEH and K08HL141446 to DRG), and from the American Society of Hematology (Scholar Award to DRG).
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.US Department of Health and Human Services. 2011. National Blood Collection and Utilization Survey Report, http://www.hhs.gov/sites/default/files/ash/bloodsafety/2011-nbcus.pdf. Accessed on 1/3/2019.
- 2.Kormoczi GF, Mayr WR. Responder individuality in red blood cell alloimmunization. Transfus Med Hemother 2014;41: 446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendrickson JE, Desmarets M, Deshpande SS, Chadwick TE, Hillyer CD, Roback JD, Zimring JC. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion 2006;46: 1526–36. [DOI] [PubMed] [Google Scholar]
- 4.Bao W, Yu J, Heck S, Yazdanbakhsh K. Regulatory T-cell status in red cell alloimmunized responder and nonresponder mice. Blood 2009;113: 5624–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Heck S, Yazdanbakhsh K. Prevention of red cell alloimmunization by CD25 regulatory T cells in mouse models. American Journal of Hematology 2007;82: 691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryder AB, Zimring JC, Hendrickson JE. Factors Influencing RBC Alloimmunization: Lessons Learned from Murine Models. Transfus Med Hemother 2014;41: 406–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibb DR, Liu J, Natarajan P, Santhanakrishnan M, Madrid DJ, Eisenbarth SC, Zimring JC, Iwasaki A, Hendrickson JE. Type I IFN Is Necessary and Sufficient for Inflammation-Induced Red Blood Cell Alloimmunization in Mice. J Immunol 2017;199: 1041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization, Influenza Disease Burden. Https://www.who.int/influenza/surveillance_monitoring/en. Accessed on 1/3/2019
- 9.Centers for Disease Control and Prevention. Disease Burden of Seasonal Influenza. Https://www.cdc.gov/flu/about/burden/index.html. Accessed on 1/3/2019.
- 10.Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol 2009;9: 246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutelier JP, Johnston SJ, El Idrissi Me-A, Pfau CJ. Involvement of CD4+ cells in lymphocytic choriomeningitis virus-induced autoimmune anaemia and hypergammaglobulinaemia. J Autoimmun 1994;7: 589–99. [DOI] [PubMed] [Google Scholar]
- 12.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 2013;340: 202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evers D, van der Bom JG, Tijmensen J, Middelburg RA, de Haas M, Zalpuri S, de Vooght KM, van de Kerkhof D, Visser O, Pequeriaux NC, Hudig F, Zwaginga JJ. Red cell alloimmunisation in patients with different types of infections. Br J Haematol 2016;175: 956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science 1994;264: 1918–21. [DOI] [PubMed] [Google Scholar]
- 15.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 2000;13: 539–48. [DOI] [PubMed] [Google Scholar]
- 16.Desmarets M, Cadwell CM, Peterson KR, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood 2009;114: 2315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, Craft J. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 2016;17: 1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8(+) T cell responses to influenza A virus. Nat Immunol 2013;14: 246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimring JC, Hair GA, Chadwick TE, Deshpande SS, Anderson KM, Hillyer CD, Roback JD. Nonhemolytic antibody-induced loss of erythrocyte surface antigen. Blood 2005;106: 1105–12. [DOI] [PubMed] [Google Scholar]
- 20.Al-Garawi AA, Fattouh R, Walker TD, Jamula EB, Botelho F, Goncharova S, Reed J, Stampfli MR, O’Byrne PM, Coyle AJ, Jordana M. Acute, but not resolved, influenza A infection enhances susceptibility to house dust mite-induced allergic disease. J Immunol 2009;182: 3095–104. [DOI] [PubMed] [Google Scholar]
- 21.Pommerenke C, Wilk E, Srivastava B, Schulze A, Novoselova N, Geffers R, Schughart K. Global transcriptome analysis in influenza-infected mouse lungs reveals the kinetics of innate and adaptive host immune responses. PLoS One 2012;7: e41169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014;5: 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natarajan P, Liu D, Patel SR, Santhanakrishnan M, Beitler D, Liu J, Gibb DR, Liepkalns JS, Madrid DJ, Eisenbarth SC, Stowell SR, Hendrickson JE. CD4 Depletion or CD40 L Blockade Results in Antigen-Specific Tolerance in a Red Blood Cell Alloimmunization Model. Front Immunol 2017;8: 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arneja A, Salazar JE, Jiang W, Hendrickson JE, Zimring JC, Luckey CJ. Interleukin-6 receptor-alpha signaling drives anti-RBC alloantibody production and T-follicular helper cell differentiation in a murine model of red blood cell alloimmunization. Haematologica 2016;101: e440–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang IK, Iwasaki A. Inflammasomes as mediators of immunity against influenza virus. Trends Immunol 2011;32: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibb DR, Liu J, Santhanakrishnan M, Natarajan P, Madrid DJ, Patel S, Eisenbarth SC, Tormey CA, Stowell SR, Iwasaki A, Hendrickson JE. B cells require Type 1 interferon to produce alloantibodies to transfused KEL-expressing red blood cells in mice. Transfusion 2017;57: 2595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singhal D, Kutyna MM, Chhetri R, Wee LYA, Hague S, Nath L, Nath SV, Sinha R, Wickham N, Lewis ID, Ross DM, Bardy PG, To LB, Reynolds J, Wood EM, Roxby DJ, Hiwase DK. Red cell alloimmunization is associated with development of autoantibodies and increased red cell transfusion requirements in myelodysplastic syndrome. Haematologica 2017;102: 2021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stowell SR, Girard-Pierce KR, Smith NH, Henry KL, Arthur CM, Zimring JC, Hendrickson JE. Transfusion of murine red blood cells expressing the human KEL glycoprotein induces clinically significant alloantibodies. Transfusion 2014;54: 179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith NH, Hod EA, Spitalnik SL, Zimring JC, Hendrickson JE. Transfusion in the absence of inflammation induces antigen-specific tolerance to murine RBCs. Blood 2012;119: 1566–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fasano RM, Booth GS, Miles M, Du L, Koyama T, Meier ER, Luban NL. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br J Haematol 2015;168: 291–300. [DOI] [PubMed] [Google Scholar]
- 31.Papay P, Hackner K, Vogelsang H, Novacek G, Primas C, Reinisch W, Eser A, Mikulits A, Mayr WR, Kormoczi GF. High Risk of Transfusion-induced Alloimmunization of Patients with Inflammatory Bowel Disease. Am J Med 2012;125: 717 e1–8. [DOI] [PubMed] [Google Scholar]
- 32.Ryder AB, Hendrickson JE, Tormey CA. Chronic inflammatory autoimmune disorders are a risk factor for red blood cell alloimmunization. Br J Haematol 2016;174: 483–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karafin MS, Westlake M, Hauser RG, Tormey CA, Norris PJ, Roubinian NH, Wu Y, Triulzi DJ, Kleinman S, Hendrickson JE. Risk factors for red blood cell alloimmunization in the Recipient Epidemiology and Donor Evaluation Study (REDS-III) database. Br J Haematol 2018;181: 672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabro S, Gallman A, Gowthaman U, Liu D, Chen P, Liu J, Krishnaswamy JK, Nascimento MS, Xu L, Patel SR, Williams A, Tormey CA, Hod EA, Spitalnik SL, Zimring JC, Hendrickson JE, Stowell SR, Eisenbarth SC. Bridging channel dendritic cells induce immunity to transfused red blood cells. J Exp Med 2016;213: 887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendrickson J, Roback, JD, Hillyer, CD, Easley, KA, Zimring, JC. Discrete toll like receptor agonists have differential effects on alloimmunization to red blood cells. Transfusion 2008: 1869–77. [DOI] [PubMed] [Google Scholar]
- 36.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol 2014;14: 315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.