Abstract
Background:
Delayed initiation of adjuvant chemotherapy negatively impacts long-term survival in colorectal cancer patients. Colorectal enhanced recovery protocols result in decreased complications and length of stay; however, the impact of enhanced recovery on the timing of adjuvant chemotherapy remains unknown.
Objective:
This study aimed to identify factors associated with on-time delivery of adjuvant chemotherapy after colorectal cancer surgery, hypothesizing that implementation of an enhanced recovery protocol would result in more patients receiving on-time chemotherapy.
Design:
Retrospective cohort study comparing rate of on-time adjuvant chemotherapy delivery following colorectal cancer resection before and after implementation of an enhanced recovery protocol.
Settings:
Large academic medical center.
Patients:
All patients who underwent non-emergent colorectal cancer resections for curative intent from January 2010-June 2017, excluding patients who had no indication for adjuvant chemotherapy, had received preoperative systemic chemotherapy, or did not have medical oncology records available.
Main Outcome Measures:
Patients before and enhanced recovery were compared, with rate of on-time adjuvant chemotherapy delivery as the primary outcome. Adjuvant chemotherapy delivery was considered on-time if initiated ≤8 weeks postoperatively, and treatment was considered delayed/omitted if initiated >8 weeks postoperatively (delayed) or never received (omitted). Multivariable logistic regression identified predictors of on-time chemotherapy delivery.
Results:
363 patients met inclusion criteria with 189 (52.1%) patients undergoing surgery following enhanced recovery implementation. Groups differed in laparoscopic approach and median procedure duration, both of which were higher following enhanced recovery. Significantly more patients received on-time chemotherapy following enhanced recovery implementation (p=0.007). Enhanced recovery was an independent predictor of on-time adjuvant chemotherapy (p=0.014).
Limitations:
Retrospective and nonrandomized before-and-after design.
Conclusions:
Enhanced recovery was associated with receiving on-time adjuvant chemotherapy. As prompt initiation of adjuvant chemotherapy improves survival in colorectal cancer, future investigation of long-term oncologic outcomes is necessary to evaluate the potential impact of enhanced recovery on survival. See Video Abstract at http://links.lww.com/DCR/Axxx.
Keywords: Adjuvant chemotherapy, Colorectal cancer, Enhanced recovery
Abstract
Antecedentes:
El inicio tardío de la quimioterapia adyuvante afecta negativamente la supervivencia a largo plazo en pacientes con cáncer colorrectal. Los protocolos de recuperación acelerada colorrectales dan lugar a una disminución de las complicaciones y la duración de estancia hospitalaria; sin embargo, el impacto de la recuperación acelerada en el momento de inicio de quimioterapia adyuvante sigue siendo desconocido.
Objetivo:
Este estudio tuvo como objetivo identificar los factores asociados con la administración a tiempo de la quimioterapia adyuvante después de la cirugía de cáncer colorrectal, con la hipótesis de que la implementación de un protocolo de recuperación acelerada daría lugar a que más pacientes reciban quimioterapia a tiempo.
Diseño:
Estudio de cohorte retrospectivo que compara la tasa de administración de quimioterapia adyuvante a tiempo después de la resección del cáncer colorrectal antes y después de la implementación de un protocolo de recuperación acelerada.
Escenario:
Centro médico académico grande.
Pacientes:
Todos los pacientes que se sometieron a resecciones de cáncer colorrectal no emergentes con intención curativa desde enero de 2010 hasta junio de 2017, excluyendo a los pacientes que no tenían indicación de quimioterapia adyuvante, que recibieron quimioterapia sistémica preoperatoria o no tenían registros médicos de oncología disponibles.
Principales medidas de resultados:
Los pacientes se compararon antes y después de la implementación de la recuperación acelerada, con la tasa de administración de quimioterapia adyuvante a tiempo como el resultado primario. La administración de quimioterapia adyuvante se consideró a tiempo si se inició ≤8 semanas después de la operación, y el tratamiento se consideró retrasado / omitido si se inició> 8 semanas después de la operación (retrasado) o nunca fue recibido (omitido). La regresión logística multivariable identificó predictores de administración de quimioterapia a tiempo.
Resultados:
363 pacientes cumplieron con los criterios de inclusión, con 189 (52.1%) pacientes sometidos a cirugía después de la implementación de recuperación acelerada. Los grupos difirieron en el abordaje laparoscópico y la duración media del procedimiento; ambos factores fueron mayores después de la recuperación acelerada. Significativamente más pacientes recibieron quimioterapia a tiempo después de la implementación de recuperación acelerada (p = 0.007). La recuperación acelerada fue un factor predictivo independiente de quimioterapia adyuvante a tiempo (p = 0.014).
Limitaciones:
Diseño retrospectivo, tipo äntes y después¨ no aleatorizado.
Conclusiones:
La recuperación acelerada se asoció con la recepción de quimioterapia adyuvante a tiempo. Debido a que el inicio rápido de la quimioterapia adyuvante mejora la supervivencia en el cáncer colorrectal, en el futuro será necesario investigar los resultados oncológicos a largo plazo para evaluar el impacto potencial de la recuperación acelerada en la supervivencia. Vea el Resumen en video en http://links.lww.com/DCR/Axxx.
INTRODUCTION
Colorectal cancer (CRC) is common in the United States, representing the third highest incidence and mortality.1 The primary treatment modality for CRC is en bloc resection of the tumor with regional lymphadenectomy.2 Adjuvant chemotherapy (AC) has consistently been shown to decrease recurrence and improve survival after curative resection of CRC3-5 and is recommended for patients with Stage III-IV CRC, Stage II rectal cancer, and Stage II colon cancer with obstruction, perforation, T4 tumors, or other high-risk features.6-8 Despite these benefits, multiple observational studies have demonstrated delays or omission in initiation of AC leading to worse oncologic outcomes.9-11
Several patient- and disease-related factors—such as age, comorbidities, race, and tumor grade—have been associated with delayed initiation or omission of AC following curative CRC resection.12 While many of these factors are non-modifiable, the prevention of postoperative complications has been identified as an opportunity for improvement efforts with regard to delayed/omitted treatment.13,14
Enhanced recovery protocols (ERP) have been well described in colorectal surgery and consistently associated with decreased morbidity, length of stay (LOS), and hospital costs.15-18 Fundamental tenants of ERPs include a focus on judicious intravenous (IV) fluid management, more liberal use of vasopressors to avoid intravascular overload, and multimodal opioid-sparing analgesia with the overall goal of decreasing the physiologic stress during the perioperative period.19-22
Most studies involving ERPs focus on short-term surgical outcomes. Given the ability of ERP implementation to prevent complications and shorten LOS, we aimed to further investigate the potential impact of a colorectal ERP on the timing and delivery of AC following curative CRC resection.17 We hypothesized that the adoption of a colorectal ERP would be associated with the on-time initiation of AC.
METHODS
Patients
The University of Virginia Institutional Review Board (#19191) approved this retrospective cohort study with waiver of consent. All patients ≥18 years of age who underwent non-emergent CRC surgery prior to (January 1, 2010 to August 5, 2013) and following (August 6, 2013 to June 7, 2017) ERP implementation were identified from a prospectively maintained institutional database. Only patients with CRC undergoing surgery for curative intent meriting treatment with AC qualified for study inclusion. Patients who underwent a surgery for palliative intent were excluded.
Indications for AC included Stage III/IV CRC, Stage II rectal cancer, and Stage II colon cancer with obstruction, perforation, or T4 tumors. For patients with an unclear indication—such as patients with Stage IIA colon cancer—medical oncology notes were reviewed, and the patient was deemed to have an indication for AC if the oncologist recommended it. Patients with no indication for AC were excluded from further review. Patients who underwent a total neoadjuvant approach to chemotherapy were also excluded. If records were unavailable, the patient’s medical oncologist was contacted for missing data. If the necessary data could not be obtained, the patient was excluded.
Data Collection and Outcomes
Patient factors, procedural characteristics, and postoperative outcome data were obtained from a prospectively maintained database supplemented by data from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. ACS NSQIP definitions for all demographic and outcome variables were used and can be found at https://www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide _2015.ashx. In addition, the electronic medical record (EMR) was reviewed for all identified surgical CRC patients to confirm the diagnosis of malignancy and indication for AC.
The characteristics of the AC—including timing and delivery—were determined by chart review of medical and surgical oncology notes. The primary outcome of the study was the percentage of patients who experienced on-time AC initiation. AC delivery was considered on-time if initiated ≤8 weeks postoperatively based on the definition utilized in prior oncologic studies examining timing of AC.9,23 Treatment was considered delayed/omitted if initiated >8 weeks postoperatively (delayed) or never received (omitted). The reasons for omission of AC were recorded.
Secondary outcomes included postoperative complications based on NSQIP definitions [surgical site infection (SSI), wound disruption, postoperative pneumonia, unplanned intubation, pulmonary embolism, postoperative ventilator dependency for >48 hours, urinary tract infection, progressive renal insufficiency, postoperative hemodialysis, cerebrovascular accident, cardiac arrest, myocardial infection, postoperative transfusion within 72 hours, vein thrombosis requiring therapy, postoperative sepsis/septic shock, other postoperative occurrences], LOS, and 30-day readmissions.
Enhanced Recovery Protocol Implementation
Detailed explanations of management strategies for the perioperative care of colorectal surgery patients prior to and following the implementation of the ERP have been previously published.19-22 Prior to the ERP, patients underwent a mechanical bowel preparation the day prior to surgery progressing to nothing by mouth at midnight. Pain management strategies were not protocolized, but typically consisted of a low thoracic epidural with IV opioids as needed for open procedures and patient-controlled analgesia with IV opioids for laparoscopic procedures followed by transition to oral opioids. Intraoperative IV fluid management was left to the discretion of the anesthesia team. Postoperative IV fluids were managed by the surgical team, and decisions involving diet advancement were made by the primary surgeon.17
In order to standardize perioperative care, a colorectal ERP was initiated on August 6, 2013.17 Details of the protocol can be found in Table 1. It should be noted that the same mechanical bowel preparation (PEG-based with oral antibiotics) the day prior to surgery was utilized throughout the study period (prior to and following implementation of the ERP). Data on ERP compliance measures including preoperative carbohydrate loading with Gatorade Thirst Quencher (©PepsiCo), ambulation on postoperative day 1, morphine equivalents, and overall fluid balance were prospectively collected.
Table 1.
Key elements of the enhanced recovery protocol
| Day prior to surgery |
| Regular diet until 1800 |
| Mechanical bowel prep with oral antibiotics |
| Chlorohexidine shower |
| Day of surgery |
| Clears until 2 hours prior to surgery |
| 20 ounces of Gatorade Thirst Quencher (©PepsiCo) 2 hours prior to induction |
| Alvimopan 12 mg PO |
| Multimodal analgesia: |
| Celecoxib 200 mg PO |
| Gabapentin 600 mg PO |
| Acetaminophen 975 mg PO |
| Intraoperative care |
| Intrathecal morphine (250 μg) prior to induction |
| No intravenous opioids |
| IV analgesia: |
| Lidocaine 40 μg/kg/min |
| Ketamine 0.6 mg/kg/min |
| Goal-directed fluid algorithm based on the Pleth Variability Index |
| Postoperative care |
| Diet: clear liquids, solid food postoperative day 1 |
| Analgesia: |
| Acetaminophen 975 mg IV every 6 hours |
| Lidocaine infusion 0.5-1 mg/min until postoperative day 2 |
| Oxycodone 5 mg PO every 4 hours as needed |
| Ambulation: begins evening of postoperative day 0 |
| Other medications: |
| Alvimopan 12 mg BID for 7 days |
| Magnesium oxide 400 mg PO daily |
| Fluids: lactated ringers 40 mL/h for 24 hours |
PO=By mouth; IV=Intravenous; BID=Twice daily
Statistical Analysis
Categorical variables are reported as frequencies with percentages and continuous variables as median with interquartile range. Univariate analyses were conducted to evaluate for differences between patients treated before and after ERP implementation as well as for patients who did and did not receive on-time AC. Differences were compared using the Chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables. Given an estimated baseline rate of on-time AC initiation of 30% in the pre-ERP population and a predicted increase in the rate to 45% in the ERP group, 352 patients were required to achieve statistical power (1-β) of 0.80.
A multivariable logistic regression model was fit to identify factors independently associated with on-time initiation of AC. Dependent variables were chosen a priori based on clinical factors previously shown to affect the timing and delivery of AC following CRC resection. An alpha value of 0.05 was used as a threshold for statistical significance. All of the statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
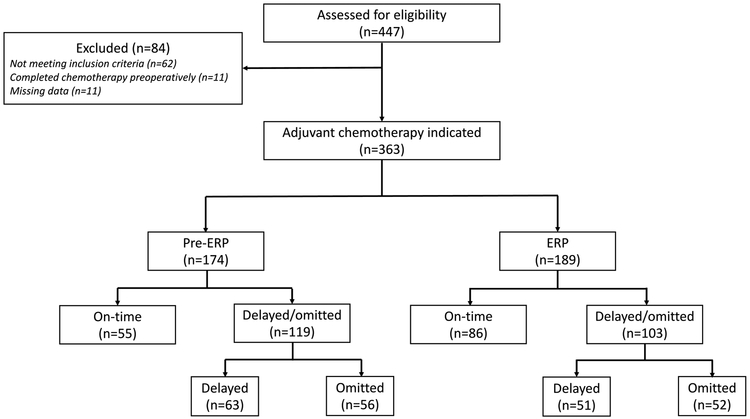
Four hundred forty-seven patients underwent surgery for CRC between January 2010 and June 2017 with exclusion of 84 patients as described in Figure 1. Ultimately, 363 patients were identified as meeting study inclusion criteria, meaning that AC was indicated. One hundred seventy-four patients (47.9%) underwent surgery prior to ERP implementation, and 189 patients (52.1%) underwent surgery following implementation. Compliance to the key ERP elements was similar to previously published data from our institution (Supplemental Table).17
Figure 1.
Flow diagram of included study patients
Demographic variables, cancer characteristics, and procedural details for the 2 groups are reported in Table 2. The majority of patients had rectal cancer and received neoadjuvant chemoradiation. Patient characteristics in each group were similar with the exception of a higher rate of dyspnea and malnutrition in the pre-ERP group. More patients in the ERP group received neoadjuvant chemotherapy. Additionally, the ERP group had a higher rate of laparoscopic procedures and a longer median operating room time. Thirty-day postoperative outcomes between patients undergoing surgery prior to and following ERP implementation are also reported in Table 2. SSI occurred less frequently in the ERP group; however, the rate of overall complications did not differ between groups. Median LOS was significantly shorter in the ERP group at 4.0 days compared to 7.0 days prior to implementation of the ERP (p<0.001).
Table 2.
Patient characteristics, oncologic factors, procedural details, and postoperative outcomes before and after implementation of an enhanced recovery protocol
| Pre-ERP n = 174 |
ERP n = 189 |
p value | |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 61.1 (53.1-68.7) | 62.4 (53.6-72.2) | 0.262 |
| BMI (kg/m2) | 28.3 (24.3-32.0) | 28.3 (24.1-32.4) | 0.922 |
| Male | 93 (53.5%) | 110 (58.2%) | 0.362 |
| Race | 0.659 | ||
| White | 148 (85.1%) | 163 (86.2%) | |
| Black | 23 (13.2%) | 20 (10.6%) | |
| Asian | 1 (0.6%) | 1 (0.5%) | |
| Not reported | 2 (1.2%) | 5 (2.7%) | |
| Hypertension | 96 (55.2%) | 96 (50.8%) | 0.404 |
| Diabetes mellitus | 38 (21.8%) | 32 (16.9%) | 0.236 |
| ESRD | 1 (0.6%) | 1 (0.5%) | 0.953 |
| COPD | 8 (4.6%) | 10 (5.3%) | 0.761 |
| Dyspnea | 33 (19.0%) | 12 (6.4%) | <0.001 |
| Ascites | 3 (1.7%) | 2 (1.1%) | 0.587 |
| Bleeding disorder | 3 (1.7%) | 3 (1.6%) | 0.919 |
| Current smoker | 37 (21.3%) | 31 (16.4%) | 0.236 |
| 10% body weight loss | 23 (13.2%) | 11 (5.8%) | 0.016 |
| Preoperative transfusion | 1 (0.6%) | 2 (1.1%) | 0.611 |
| Chronic steroid use | 4 (2.3%) | 6 (3.2%) | 0.611 |
| Functional health status | 0.172 | ||
| Independent | 167 (96.0%) | 187 (98.9%) | |
| Partially dependent | 6 (3.5%) | 2 (1.1%) | |
| Totally dependent | 1 (0.6%) | 0 (0.0%) | |
| ASA classification | 0.755 | ||
| ASA 1 | 0 (0.0%) | 1 (0.5%) | |
| ASA 2 | 75 (43.1%) | 76 (40.2%) | |
| ASA 3 | 93 (53.5%) | 105 (55.6%) | |
| ASA 4 | 6 (3.5%) | 7 (3.7%) | |
| Oncologic factors | |||
| Site | 0.399 | ||
| Colon | 67 (38.5%) | 81 (42.9%) | |
| Rectum | 107 (61.5%) | 108 (57.1%) | |
| Neoadjuvant chemoradiation | 97 (55.8%) | 102 (54.0%) | 0.734 |
| Neoadjuvant chemotherapy | 7 (4.0%) | 19 (10.1%) | 0.026 |
| High grade | 58 (33.3%) | 58 (30.7%) | 0.589 |
| Final stage | 0.890 | ||
| 2 | 55 (31.6%) | 58 (30.7%) | |
| 3 | 91 (52.3%) | 97 (51.3%) | |
| 4 | 28 (16.1%) | 34 (18.0%) | |
| Adjuvant chemotherapy | 0.022 | ||
| On-time | 55 (31.6%) | 86 (45.5%) | |
| Delayed | 63 (36.2%) | 51 (27.0%) | |
| Omitted | 56 (32.2%) | 52 (27.5%) | |
| Procedural details | |||
| Laparoscopic/Robotic | 16 (9.2%) | 73 (38.6%) | <0.001 |
| Wound classification | 0.790 | ||
| Clean | 2 (1.2%) | 2 (1.1%) | |
| Clean/contaminated | 160 (92.0%) | 170 (90.0%) | |
| Contaminated | 11 (6.3%) | 14 (7.4%) | |
| Dirty/infected | 1 (0.6%) | 3 (1.6%) | |
| Ileostomy | 77 (53.1%) | 68 (46.9%) | 0.108 |
| Procedure duration (minutes) | 221.5 (164.0-284.0) | 260.0 (205.0-336.0) | <0.001 |
| Postoperative Complications | |||
| Unplanned intubation | 4 (2.3%) | 4 (2.1%) | 0.906 |
| Superficial incisional SSI | 22 (12.6%) | 5 (2.7%) | <0.001 |
| Deep incisional SSI | 2 (1.2%) | 3 (1.6%) | 0.721 |
| Organ space SSI | 12 (6.9%) | 12 (6.4%) | 0.834 |
| Composite SSI | 33 (19.0%) | 20 (10.6%) | 0.024 |
| Wound disruption | 4 (2.3%) | 0 (0.0%) | 0.036 |
| UTI | 5 (2.9%) | 4 (2.1%) | 0.643 |
| Sepsis | 4 (2.3%) | 5 (2.7%) | 0.832 |
| Intraoperative/postoperative transfusion | 26 (14.9%) | 20 (10.6%) | 0.212 |
| Progressive renal insufficiency | 2 (1.2%) | 8 (4.2%) | 0.073 |
| Acute renal failure | 3 (1.7%) | 1 (0.5%) | 0.276 |
| Myocardial infarction | 3 (1.7%) | 2 (1.1%) | 0.587 |
| Cerebrovascular accident | 2 (1.2%) | 1 (0.5%) | 0.514 |
| Vein thrombosis requiring therapy | 4 (2.3%) | 2 (1.1%) | 0.354 |
| Pulmonary embolism | 2 (1.2%) | 0 (0.0%) | 0.139 |
| Total Complication(s) | 61 (35.1%) | 52 (27.5%) | 0.121 |
| Return to OR | 9 (5.2%) | 10 (5.3%) | 0.960 |
| Length of stay (days) | 7.0 (5.0-10.0) | 4.0 (3.0-7.0) | <0.001 |
| 30-day readmissions | 30 (17.2%) | 29 (15.3%) | 0.625 |
Categorical variables listed as N (%) and continuous variables listed as median (interquartile range)
ERP=Enhanced recovery protocol; BMI=Body mass index; ESRD=End stage renal disease; COPD=Chronic obstructive pulmonary disease; ASA Classification=American Society of Anesthesiologists physical status classification, SSI=Surgical site infection; UTI=Urinary tract infection; OR=Operating room
Adjuvant Chemotherapy Delivery
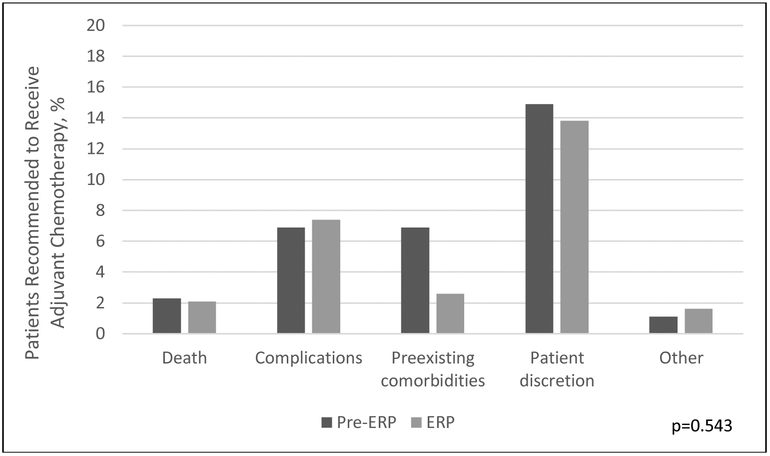
A total of 255 patients out of 363 received AC, with an overall AC delivery rate of 70.2% [118 patients in the pre-ERP group (67.8%) and 137 patients in the ERP group (72.5%); p=0.359]. One hundred eight total patients never received AC, 56 of the 174 (32.2%) patients prior to and 52 of the 189 patients (27.5%) following ERP implementation (p=0.022) (Figure 1; Table 2). Patients who underwent surgery pre- and post-ERP implementation had similar reasons for omitting therapy with patient discretion representing the highest proportion in both groups (p=0.543) (Figure 2).
Figure 2.
Reasons for omission of adjuvant chemotherapy before and after implementation of enhanced recovery
Of the 255 patients that received AC, 141 of those patients received their AC on-time. More patients received delayed AC in the pre-ERP group as compared to the ERP group [63 (36.2%) vs 51 (27.0%)]. The proportion of patients receiving on-time initiation of AC was significantly higher in the ERP group [55 (31.6%) vs 86 (45.5%)] (p=0.022) (Table 2).
Demographic variables, cancer characteristics, and procedural details for patients who received on-time versus delayed/omitted AC are reported in Table 3. Patients receiving on-time AC were younger [59.1 (51.2-66.2) vs 63.9 (55.4-73.5) years; p<0.001] with higher stage disease as compared to patients with delayed/omitted AC. Groups also differed in American Society of Anesthesiologists physical status classification (ASA). Additionally, more patients receiving on-time AC underwent laparoscopic surgery; however, procedure duration did not differ. Thirty-day postoperative outcomes between patients who did and did not receive on-time AC initiation are reported in Table 3. The rates of both SSIs and overall complications were significantly decreased in the group receiving on-time AC. LOS was shorter in patients who received on-time AC, and these patients also had decreased rates of reoperation and 30-day readmission.
Table 3.
Patient characteristics, oncologic factors, procedural details, and postoperative outcomes in patients with on-time compared to delayed/omitted delivery of adjuvant chemotherapy
| On-time Chemotherapy n = 141 |
Delayed/Omitted Chemotherapy n = 222 |
p value |
|
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 59.1 (51.2-66.2) | 63.9 (55.4-73.5) | <0.001 |
| BMI (kg/m2) | 28.3 (24.0-32.2) | 28.1 (24.1-32.3) | 0.761 |
| Male | 78 (55.3%) | 125 (56.3%) | 0.854 |
| Race | 0.296 | ||
| White | 126 (89.4%) | 185 (83.3%) | |
| Black | 11 (7.8%) | 32 (14.4%) | |
| Asian | 1 (0.7%) | 1 (0.5%) | |
| Not reported | 3 (2.1%) | 4 (1.8%) | |
| Hypertension | 62 (44.0%) | 130 (58.6%) | 0.007 |
| Diabetes mellitus | 23 (16.3%) | 47 (21.2%) | 0.253 |
| ESRD | 0 (0.0%) | 2 (0.9%) | 0.258 |
| COPD | 9 (6.4%) | 9 (4.1%) | 0.319 |
| Dyspnea | 17 (12.1%) | 28 (12.6%) | 0.876 |
| Ascites | 1 (0.7%) | 4 (1.8%) | 0.384 |
| Bleeding disorder | 1 (0.7%) | 5 (2.3%) | 0.261 |
| Current smoker | 25 (17.7%) | 43 (19.4%) | 0.700 |
| 10% body weight loss | 14 (9.9%) | 20 (9.0%) | 0.769 |
| Preoperative transfusion | 1 (0.7%) | 2 (0.9%) | 0.844 |
| Chronic steroid use | 5 (3.6%) | 5 (2.3%) | 0.463 |
| Functional health status | 0.218 | ||
| Independent | 140 (99.3%) | 214 (96.4%) | |
| Partially independent | 1 (0.7%) | 7 (3.2%) | |
| Totally dependent | 0 (0.0%) | 1 (0.5%) | |
| ASA classification | 0.007 | ||
| ASA 1 | 1 (0.7%) | 0 (0.0%) | |
| ASA 2 | 73 (51.8%) | 78 (35.1%) | |
| ASA 3 | 64 (45.4%) | 134 (60.4%) | |
| ASA 4 | 3 (2.1%) | 10 (4.5%) | |
| Oncologic factors | |||
| Colon cancer | 62 (44.0%) | 86 (38.7%) | 0.323 |
| Neoadjuvant chemoradiation | 71 (50.4%) | 128 (57.7%) | 0.173 |
| Neoadjuvant chemotherapy | 14 (9.9%) | 12 (5.4%) | 0.103 |
| High grade | 49 (34.8%) | 67 (30.2%) | 0.363 |
| Final stage | 0.001 | ||
| 2 | 32 (22.7%) | 81 (36.5%) | |
| 3 | 74 (52.5%) | 114 (51.4%) | |
| 4 | 35 (24.8%) | 27 (12.2%) | |
| Procedural details | |||
| ERP | 86 (61.0%) | 103 (46.4%) | 0.007 |
| Laparoscopic/Robotic | 45 (31.9%) | 44 (19.8%) | 0.009 |
| Wound classification | 0.206 | ||
| Clean | 3 (2.1%) | 1 (0.5%) | |
| Clean/contaminated | 123 (33.9%) | 207 (93.2%) | |
| Contaminated | 13 (9.2%) | 12 (5.4%) | |
| Dirty/infected | 2 (1.4%) | 2 (0.9%) | |
| Ileostomy | 63 (44.5%) | 82 (36.9%) | 0.142 |
| Procedure duration (minutes) | 236.0 (179.0-304.0) | 241.0 (187.0-326.0) | 0.600 |
| Postoperative Outcomes | |||
| Unplanned intubation | 0 (0.0%) | 8 (3.6%) | 0.023 |
| Superficial incision SSI | 5 (3.6%) | 22 (9.9%) | 0.024 |
| Deep incisional SSI | 0 (0.0%) | 5 (2.3%) | 0.073 |
| Organ space SSI | 2 (1.4%) | 22 (9.9%) | 0.002 |
| Composite SSI | 7 (5.0%) | 46 (20.7%) | <0.001 |
| Wound disruption | 0 (0.0%) | 4 (1.8%) | 0.109 |
| UTI | 1 (0.7%) | 8 (3.6%) | 0.084 |
| Sepsis | 1 (0.7%) | 8 (3.6%) | 0.084 |
| Intraoperative/postoperative transfusion | 14 (9.9%) | 32 (14.4%) | 0.211 |
| Progressive renal insufficiency | 1 (0.7%) | 9 (4.1%) | 0.058 |
| Acute renal failure | 1 (0.7%) | 3 (1.4%) | 0.568 |
| Myocardial infarction | 0 (0.0%) | 5 (2.3%) | 0.073 |
| Cerebrovascular accident | 0 (0.0%) | 3 (1.4%) | 0.166 |
| Vein thrombosis requiring therapy | 1 (0.7%) | 5 (2.3%) | 0.261 |
| Pulmonary embolism | 0 (0.0%) | 2 (0.9%) | 0.258 |
| Complication(s) | 23 (16.3%) | 90 (40.5%) | <0.001 |
| Return to OR | 3 (2.1%) | 16 (7.2%) | 0.034 |
| Length of stay (days) | 5.0 (3.0-6.0) | 6.0 (4.0-10.0) | <0.001 |
| 30-day readmissions | 12 (8.5%) | 47 (21.2%) | 0.001 |
Categorical variables listed as N (%) and continuous variables listed as median (interquartile range)
BMI=Body mass index; ESRD=End stage renal disease; COPD=Chronic obstructive pulmonary disease; ASA Classification=American Society of Anesthesiologists physical status classification; ERP=Enhanced recovery protocol, SSI=Surgical site infection; UTI=Urinary tract infection; OR=Operating room
The multivariable logistic regression model identified younger age (p=0.001), white race (0.038), higher stage disease (p=0.002), ileostomy (p=0.030), and shorter LOS (p=0.025) as independent predictors of on-time delivery of therapy (Table 4). Importantly, the ERP was independently associated with on-time delivery of AC (p=0.014). Laparoscopy was not independently associated with on-time delivery of therapy (0.697). The C-statistic was 0.794 indicating a good ability to discriminate between patients with and without on-time AC delivery.
Table 4.
Multivariable logistic regression results and predictors of on-time delivery of adjuvant chemotherapy following colorectal cancer resection
| Variable | OR (95% CI) | p value |
|---|---|---|
| Age (years) | 0.96 (0.94-0.98) | 0.001 |
| BMI (kg/m2) | 1.01 (0.97-1.05) | 0.703 |
| Male | 0.97 (0.56-1.64) | 0.894 |
| White | 2.23 (1.05-4.75) | 0.038 |
| Diabetes mellitus | 1.09 (0.54-2.19) | 0.810 |
| Current smoker | 0.88 (0.44-1.75) | 0.709 |
| 10% body weight loss | 1.79 (0.74-4.33) | 0.194 |
| Chronic steroid use | 1.77 (0.36-8.64) | 0.478 |
| Independent functional status | 0.54 (0.05-5.62) | 0.608 |
| ASA class ≥3 | 0.65 (0.38-1.12) | 0.124 |
| Colon cancer | 0.82 (0.25-2.77) | 0.761 |
| Neoadjuvant treatment | 0.62 (0.20-1.88) | 0.396 |
| Final stage | 1.93 (1.28-2.91) | 0.002 |
| ERP | 2.05 (1.16-3.63) | 0.014 |
| Laparoscopic | 1.19 (0.61-2.33) | 0.697 |
| Wound classification (ref=Clean/contaminated) | 0.071 | |
| Clean | 6.46 (0.54-77.90) | |
| Contaminated | 2.95 (1.10-7.93) | |
| Dirty/infected | 3.05 (0.22-42.81) | |
| Ileostomy | 2.03 (1.07-3.84) | 0.030 |
| Procedure duration (minutes) | 1.00 (0.99-1.00) | 0.076 |
| Complication(s) | 0.64 (0.39-1.03) | 0.067 |
| Return to OR | 1.29 (0.25-6.78) | 0.762 |
| Length of stay (days) | 0.92 (0.86-0.99) | 0.025 |
| 30-day readmission | 0.46 (0.20-1.07) | 0.071 |
BMI=Body mass index; ASA Classification=American Society of Anesthesiologists physical status classification; ERP=Enhanced recovery protocol; OR=Operating room
DISCUSSION
To our knowledge, this study is the first to date evaluating the effect of enhanced recovery on timing and delivery of AC following CRC resection. Following the implementation of a highly standardized colorectal ERP, a higher percentage of patients received on-time AC compared to patients undergoing resection prior to ERP implementation, supporting our initial hypothesis.
The optimal delivery of AC necessitates prompt commencement of therapy following surgery to achieve maximal effect.10,23 Multiple observational studies support the benefits of early AC initiation, revealing improvements in both disease-free and overall survival.10,24 AC is thought to favorably impact survival in cancer patients through the eradication of circulating tumor cells and micro-metastases in patients who would otherwise be destined to recur.10 Animal models have suggested that the improved effectiveness of early AC is additionally related to physiologic changes associated with surgery. The insult of a major operation may result in an increased number of metastatic cells leading to the accelerated growth of distant metastases. Additionally, the increase in pro-inflammatory and oncogenic growth factors—including tumor necrosis factor α—seen following surgery makes avoiding delays in AC paramount.10
The overall rate and timing of AC initiation reported in our study falls within published rates of AC delivery in large population-based studies, which range widely from 30-75%.12,13,25 However, while our overall rate of AC delivery was 70.2%, only 55.0% of those who received AC initiated therapy on time. Several studies have sought to identify risk factors for delayed initiation and omission of AC following CRC resection. Many of the identified risk factors in the current study exhibit overlap with previously published literature including increasing age, race, disease stage, and prolonged LOS.9,13,25,26
Most notable for the current analysis is identification of the ERP as a risk-adjusted predictor of on-time delivery of AC following CRC resection. There is an ever-growing body of literature supporting ERPs; however, a paucity of research remains regarding their potential effect on oncologic outcomes. ERPs have been widely recognized as a major advancement in perioperative care, particularly in the field of colorectal surgery, due to resulting improvements in clinical outcomes including reductions in postoperative complications, hospital LOS, and health system costs.17,27,28
There are several possible relationships driving the association between the colorectal ERP and on-time delivery of AC identified in this study. Several retrospective analyses have associated longer LOS and postoperative complications with increased rates of delayed and omitted AC. As ERPs have been shown to decrease both LOS and the rate of postoperative complications, it is possible that these improvements are driving the effect of our institutional ERP on improved AC delivery.17 While we did identify a reduction in overall number of complications, there were significantly fewer SSIs in the ERP group [33 (19.0%) vs 20 (10.6%; p=0.024]. Surgical site infections have been previously shown to impact rates of both delayed and omitted AC and thus likely contributed to our increased rate of on-time AC delivery in the ERP group.14,29 Similar to the current analysis, multiple prior studies have also demonstrated improvements in SSI rates following ERP implementation.17,30
Minimally invasive surgery was also far more common among patients undergoing resection following ERP implementation. This increased utilization is attributable to the expansion of robot-assisted rectal surgery at our institution, particularly during the first two years following ERP implementation.31 While other observational studies have linked laparoscopic surgery to shorter LOS and fewer postoperative complications, minimally invasive surgery was not significantly associated with the on-time delivery of AC in this analysis.
Ultimately, the inclusion of the aforementioned factors in the logistic regression indicates that the positive effect of the ERP on AC delivery is independent of these outcome improvements. Tanzer et al. was able to show reductions in hospital LOS within an ERP for total hip arthroplasty simply by reducing the expected LOS communicated to patients.32 Similarly, a systematic review suggested that positive patient expectations can drive better health outcomes.33 This type of expectation management could also be playing a role in the ERP’s positive effect on the timing and delivery of AC, given the increase in structured perioperative patient counseling and improved methods of communication between health care providers and patients that occurs within the protocol. It is possible that patients may be more prepared to undergo AC—even after a complicated postoperative course—if they are better prepared preoperatively from a psychological standpoint.
In both colon and rectal adenocarcinoma, the approach to AC is rapidly evolving. Results from the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) collaboration will likely result in shorter and less toxic courses of AC for moderate-risk patients, potentially making the prospect of AC less daunting.34 With the clear benefit of anti-PD-1 targeting immunotherapy for microsatellite unstable patients with metastatic disease, the ongoing Alliance A021502 study will evaluate the benefit of anti-PD-L1 in the adjuvant setting.35 In rectal cancer, increasing use of total neoadjuvant therapy may also reduce the necessity or duration of AC.36 Given these practice changes, ERPs will be increasingly important to ensure patients who receive less AC benefit from starting on time.
There are several notable limitations to the current study. The retrospective before-and-after study design inherently imposes the potential for selection bias, though all patients undergoing surgery after implementation of the ERP were treated on protocol without exception. In addition, the potential for confounding factors is mitigated by the inclusion of potential confounders in the regression model and the well-matched baseline demographics of the pre- and post-implementation groups. This study included patients with both colon and rectal cancer. While there are fundamental differences in the oncologic treatment of these diseases, the inclusion of patients whose final staging merited adjuvant chemotherapy according to National Comprehensive Cancer Network (NCCN) guidelines minimizes those differences in the present study. The before-and-after design also precludes a survival analysis, given that a significant proportion of the ERP patients have not yet accrued meaningful postoperative follow up time. Long-term oncologic outcomes will be assessed once long-term follow-up data become available for all patients. While we are able to demonstrate only association and not causation, there were no institutional or accreditation efforts targeting the timing and delivery of AC for CRC during the study period. Finally, given the institutional variability in ERPs, results from this single institution study may not be broadly generalizable.
CONCLUSION
This study identified a higher rate of on-time AC delivery following the implementation of an institutional ERP. Several risk-adjusted predictors of the on-time delivery of AC following CRC resection were also identified. As the prompt initiation of AC has been shown to decrease recurrence and improve survival in CRC patients, further investigation of long-term oncologic outcomes will be necessary to evaluate the potential impact of ERPs on overall and disease-free survival.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was provided by National Institutes of Health surgical oncology grant T32 CA163177.
Footnotes
Financial Disclosures: None reported.
Presented as a poster presentation at the American Society of Colon and Rectal Surgeons Annual Scientific Meeting, Nashville, Tennessee, May 19-23, 2018.
REFERENCES
- 1.Lamanna A, Sheaffer H, Guerra C, Kochman M. colorectal cancer screening navigation for the underserved: experience of an urban program. Gastroenterol Hepatol (N Y). 2016;12:547–551. [PMC free article] [PubMed] [Google Scholar]
- 2.Figueredo A, Rumble RB, Maroun J, et al. ; Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer. 2003;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.[No authors listed.] Adjuvant therapy for patients with colon and rectum cancer. Consens Statement. 1990;8:1–25. [PubMed] [Google Scholar]
- 4.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage iii colon cancer. N Engl J Med. 2018;378:1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel JD, Eskicioglu C, Weiser MR, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Colon Cancer. Dis Colon Rectum. 2017;60:999–1017. [DOI] [PubMed] [Google Scholar]
- 7.Benson AB, Venook AP, Al-Hawary MM, et al. NCCN Guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monson JR, Weiser MR, Buie WD, et al. ; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56:535–550. [DOI] [PubMed] [Google Scholar]
- 9.Kim IY, Kim BR, Kim YW. Factors affecting use and delay (≥8 weeks) of adjuvant chemotherapy after colorectal cancer surgery and the impact of chemotherapy-use and delay on oncologic outcomes. PLoS One. 2015;10:e0138720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–2342. [DOI] [PubMed] [Google Scholar]
- 11.Gatta G, Zigon G, Aareleid T, et al. Patterns of care for European colorectal cancer patients diagnosed 1996-1998: a EUROCARE high resolution study. Acta Oncol. 2010;49:776–783. [DOI] [PubMed] [Google Scholar]
- 12.Etzioni DA, El-Khoueiry AB, Beart RW Jr. Rates and predictors of chemotherapy use for stage III colon cancer: a systematic review. Cancer. 2008;113:3279–3289. [DOI] [PubMed] [Google Scholar]
- 13.van der Geest LG, Portielje JE, Wouters MW, et al. ; All Nine Hospitals in the Leiden Region of the Comprehensive Cancer Centre The Netherlands. Complicated postoperative recovery increases omission, delay and discontinuation of adjuvant chemotherapy in patients with Stage III colon cancer. Colorectal Dis. 2013;15:e582–e591. [DOI] [PubMed] [Google Scholar]
- 14.Kim IY, Kim BR, Kim YW. The impact of anastomotic leakage on oncologic outcomes and the receipt and timing of adjuvant chemotherapy after colorectal cancer surgery. Int J Surg. 2015;22:3–9. [DOI] [PubMed] [Google Scholar]
- 15.Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531–1541. [DOI] [PubMed] [Google Scholar]
- 16.Group EC; ERAS Compliance Group. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg. 2015;261:1153–1159. [DOI] [PubMed] [Google Scholar]
- 17.Thiele RH, Rea KM, Turrentine FE, et al. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015;220:430–443. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56:667–678. [DOI] [PubMed] [Google Scholar]
- 19.Thiele RH, Raghunathan K, Brudney CS, et al. ; Perioperative Quality Initiative (POQI) I Workgroup. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper Med (Lond). 2016;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holubar SD, Hedrick T, Gupta R, et al. ; Perioperative Quality Initiative (POQI) I Workgroup. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond). 2017;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEvoy MD, Scott MJ, Gordon DB, et al. ; Perioperative Quality Initiative (POQI) I Workgroup. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on optimal analgesia within an enhanced recovery pathway for colorectal surgery: part 1-from the preoperative period to PACU. Perioper Med (Lond). 2017;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott MJ, McEvoy MD, Gordon DB, et al. ; Perioperative Quality Initiative (POQI) I Workgroup. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) Joint Consensus Statement on Optimal Analgesia within an Enhanced Recovery Pathway for Colorectal Surgery: Part 2-From PACU to the Transition Home. Perioper Med (Lond). 2017;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010;46:1049–1055. [DOI] [PubMed] [Google Scholar]
- 24.Day AR, Middleton G, Smith RV, Jourdan IC, Rockall TA. Time to adjuvant chemotherapy following colorectal cancer resection is associated with an improved survival. Colorectal Dis. 2014;16:368–372. [DOI] [PubMed] [Google Scholar]
- 25.Ha GS, Kim YW, Choi EH, Kim IY. Factors associated with the lack of adjuvant chemotherapy following curative surgery for stage II and III colon cancer: a Korean national cohort study. Anticancer Res. 2017;37:915–922. [DOI] [PubMed] [Google Scholar]
- 26.Hendren S, Birkmeyer JD, Yin H, Banerjee M, Sonnenday C, Morris AM. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53:1587–1593. [DOI] [PubMed] [Google Scholar]
- 27.Miller TE, Thacker JK, White WD, et al. ; Enhanced Recovery Study Group. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118:1052–1061. [DOI] [PubMed] [Google Scholar]
- 28.Zhao JH, Sun JX, Gao P, et al. Fast-track surgery versus traditional perioperative care in laparoscopic colorectal cancer surgery: a meta-analysis. BMC Cancer. 2014;14:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merkow RP, Bentrem DJ, Mulcahy MF, et al. Effect of postoperative complications on adjuvant chemotherapy use for stage III colon cancer. Ann Surg. 2013;258:847–853. [DOI] [PubMed] [Google Scholar]
- 30.Hawkins AT, Geiger TM, King AB, et al. An enhanced recovery program in colorectal surgery is associated with decreased organ level rates of complications: a difference-in-differences analysis. Surg Endosc. 2019;33:2222–2230 [DOI] [PubMed] [Google Scholar]
- 31.Martin AN, Berry PS, Friel CM, Hedrick TL. Impact of minimally invasive surgery on short-term outcomes after rectal resection for neoplasm within the setting of an enhanced recovery program. Surg Endosc. 2018;32:2517–2524. [DOI] [PubMed] [Google Scholar]
- 32.Tanzer D, Smith K, Tanzer M. Changing patient expectations decreases length of stay in an enhanced recovery program for THA. Clin Orthop Relat Res. 2018;476:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondloch MV, Cole DC, Frank JW. Does how you do depend on how you think you’ll do? A systematic review of the evidence for a relation between patients’ recovery expectations and health outcomes. CMAJ. 2001;165:174–179. [PMC free article] [PubMed] [Google Scholar]
- 34.André T, Iveson T, Labianca R, et al. ; for the IDEA Steering Committee. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) Collaboration: Prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with Stage III colon cancer: trial design and current status. Curr Colorectal Cancer Rep. 2013;9:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boland PM, Ma WW. Immunotherapy for colorectal cancer. Cancers (Basel). 2017;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33:1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.