Abstract
Background
Extramammary Paget’s disease is an uncommon intraepidermal adenocarcinoma with poorly defined clinical implications.
Objective
To estimate the risk of second primary neoplasms in patients with extramammary Paget’s disease.
Design
Retrospective analysis of the Surveillance, Epidemiology, and End Results Registry (1973–2014).
Setting
Population-based cancer registries from the United States.
Patients
Patients who were diagnosed with anogenital Paget’s disease.
Main Outcome Measures
Risk of second primary development.
Results
We identified 108 patients with anal Paget’s, 421 patients with male genital (scrotum or penis) Paget’s, and 1,677 patients with female genital (vagina or vulva) Paget’s. Median follow-up time was 5.9 years. The risk of developing colorectal adenocarcinoma was 18.5% for patients with anal Paget’s. Eighty percent of colorectal adenocarcinoma diagnoses were synchronous (within 2 months) to anal Paget’s diagnoses, while metachronous tumors occurred at a median time of 2.4 years. Of patients with anal Paget’s, 8.3% developed an anal adenocarcinoma or non-small cell cancer. In male patients with genital Paget’s, the risk of proximal genitourinary malignancy was 9.7%, scrotal or testicular adenocarcinoma was 0.4%, and penile or scrotal squamous carcinoma was 1.7%. In female patients with genital Paget’s, the risk of proximal genitourinary malignancy was 3.0%, vaginal or vulvar adenocarcinoma was 1.4%, and vaginal or vulvar squamous neoplasm was 1.0%. Five-year overall survival was 59.7%, 73.5%, and 80.7% in patients with anal, male genital, and female genital Paget’s, respectively (p<0.001).
Limitations
The registry did not record surveillance schedule, provider specialty, or non-procedural therapies for extramammary Paget’s disease.
Conclusions
In the largest published cohort of patients with extramammary Paget’s disease, patients with anal Paget’s had a much higher risk of both proximal and local neoplasms as compared to patients with genital Paget’s. Patients with anal Paget’s also experienced worse survival as compared to those with purely genital Paget’s.
See Video Abstract at http://links.lww.com/DCR/Axxx.
Keywords: Anogenital, Extramammary Paget’s disease, Metachronous, Neoplasm, Synchronous
RESUMEN
Antecedentes
la enfermedad de Paget extramamaria es un adenocarcinoma intraepidérmico poco frecuente con implicaciones clínicas poco definidas.
Objetivo
estimar el riesgo de segundas neoplasias primarias en pacientes con enfermedad de Paget extramamaria.
Diseño
Análisis retrospectivo del Registro de Vigilancia, Epidemiología y Resultados Finales (1973–2014).
Lugar
registros de base poblacional en cáncer de los Estados Unidos.
Pacientes
pacientes que fueron diagnosticados con enfermedad de Paget anogenital.
Principales medidas de resultados
Riesgo de desarrollo un cáncer primario adicional.
Resultados
Se identificaron 108 pacientes con Paget anal, 421 pacientes con Paget genital masculino (escroto o pene) y 1677 pacientes con Paget genital femenino (vagina o vulva). Tiempo mediano de seguimiento fue de 5,9 años. El riesgo de desarrollar adenocarcinoma colorrectal fue del 18,5% para los pacientes con Paget anal. El ochenta por ciento de los diagnósticos de adenocarcinoma colorrectal fueron sincrónicos (dentro de los 2 meses) a los diagnósticos de Paget anal, mientras que los tumores metacrónicos ocurrieron en un tiempo promedio de 2,4 años. De los pacientes con Paget anal, el 8.3% desarrolló un adenocarcinoma anal o cáncer de células no pequeñas. En los pacientes masculinos con Paget genital, el riesgo de malignidad genitourinaria proximal fue del 9,7%, el adenocarcinoma escrotal o testicular fue del 0,4% y el carcinoma escamoso del pene o escroto fue del 1,7%. En pacientes femeninas con Paget genital, el riesgo de malignidad genitourinaria proximal fue de 3.0%, el adenocarcinoma vaginal o vulvar fue de 1.4% y la neoplasia escamosa vaginal o vulvar fue de 1.0%. La supervivencia general a cinco años fue del 59.7%, 73.5% y 80.7% en pacientes con anal, genital masculino y genital femenino, respectivamente (p <0.001).
Limitaciones
El registro no señalo el cronograma de vigilancia, la especialidad del proveedor o las terapias sin procedimiento para la enfermedad de Paget extramamaria.
Conclusiones
en la cohorte más grande publicada de pacientes con enfermedad de Paget extramamaria, los pacientes con Paget anal demostraron un riesgo mucho mayor de neoplasias proximales y locales en comparación con los pacientes con Paget genital. Los pacientes con Paget anal además demostraron una peor supervivencia en comparación con aquellos con Paget aislada genital. Vea el resumen del video en http://links.lww.com/DCR/Axxx.
INTRODUCTION
Extramammary Paget’s disease (EMPD) is a rare intraepidermal adenocarcinoma with poorly defined clinical implications. It represents 6.5% of all cutaneous Paget’s disease and involves areas with abundant apocrine glands, such as the anogenital region.1,2 EMPD is divided into two types; primary EMPD is considered carcinoma in situ of apocrine gland ducts, while secondary EMPD is thought to occur from intraepithelial spread of a separate underlying carcinoma.3 EMPD tends to occur in patients aged 50–80 years old, with a female predominance among Caucasians and a male predominance among South-East Asians.4–6 It often presents as pain or pruritis, and appears as a slow-growing, erythematous plaque.7 Diagnosis of EMPD is often delayed due to late presentations and misdiagnoses.8,9
Due to the rarity of the disease, clinical and oncologic outcomes have not been well-defined.2 A seminal report by Chanda et al. in 1985 noted that EMPD lesions were often directly related to underlying malignancies.10 However, the rate of second primary neoplasm development is unclear, with reported rates ranging from 5–86%.2,11–15 Patients diagnosed with EMPD are encouraged to undergo screening for synchronous genitourinary and/or gastrointestinal malignancies, but the appropriate frequency of surveillance is unknown.2
Local excision, often with Mohs micrographic surgery, is considered the mainstay of treatment.16,17 Other options include topical imiquimod, radiation, and photodynamic therapy.7 However, disease recurrence after surgery is not uncommon, and association between surgical excision of EMPD and subsequent development of a malignancy is unclear.2 Due to the clinical uncertainty surrounding EMPD, the primary aim of this study was to query a large national registry to quantify the risk of secondary neoplasms in patients with EMPD.
METHODS
Study Design
We conducted a retrospective study of patients with EMPD diagnosed between 1973–2014 in the Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence – SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases (Nov 2016 submission). The SEER registry, organized by the National Cancer Institute, collects cancer incidence, prevalence, and survival data from population-based cancer registries covering approximately 28% of the United States population. SEER*Stat version 8.3.4 was used to link separate tumors based on patient identification number. Only patients with neoplasms diagnosed between 2004–2014 are entered into the registry; their prior neoplasms diagnosed between 1973–2003 are then recorded. We therefore performed subset analyses of patients diagnosed with EMPD between 2004–2014 to account for this selection bias.
For patients with multiple diagnoses of EMPD, the first recorded diagnosis was used as the index diagnosis for analysis. Likewise, for patients with multiple diagnoses of additional secondary neoplasms, the first recorded diagnosis was used for analysis. Tumors diagnosed within ≤2 months were considered synchronous, while tumors diagnosed >2 months after EMPD diagnosis were considered metachronous. Two months was chosen as a reasonable time in which most patients with a new cancer diagnosis would be evaluated and synchronous lesions could be identified, and is consistent with prior work.18 The Massachusetts General Hospital Institutional Review Board approved this study (Protocol #2017P001719).
Variable Definitions
Diagnoses of EMPD were confirmed based on ICD-O-3 codes of 8542/2 and 8542/3 from histopathologic specimens. Patients with EMPD primary sites of “Anus, Anal Canal and Anorectum” were designated as anal EMPD; those with EMPD primary sites of “Penis” or “Other Male Genital Organs” (scrotum) were designated as male genital EMPD; and those with EMPD sites of “Vulva” and “Vagina” were designated as female genital EMPD. For anal EMPD, secondary tumors of the colon and rectum were considered proximal, while secondary tumors of the anus were considered local. For male genital EMPD, secondary tumors of the kidney, bladder, and prostate were considered proximal, and the penis, scrotum, and testes were considered local. For female genital EMPD, secondary tumors of the kidney, bladder, cervix, and uterus were considered proximal, while the vulva and vagina were considered local. All invasive neoplasms and high-grade squamous lesions were considered secondary tumors, including anal intraepithelial neoplasia III and vulvar/vaginal squamous intraepithelial neoplasia III.
Basic demographic characteristics were obtained, including age, sex, race, marital status, and United States region (based on United States Census bureau designations). Surgery type for anal EMPD was categorized using SEER Anus Surgery Codes (https://seer.cancer.gov/manuals/2016/AppendixC/Surgery_Codes_Anus_2016.pdf) as follows: no procedure, local tumor destruction/excision (local tumor excision, electrocautery, excisional biopsy), abdominoperineal resection (radical resection), and surgery not otherwise specified or unknown. Surgery type for male and female genital EMPD was categorized using SEER All Other Sites Surgery Codes (https://seer.cancer.gov/archive/manuals/2015/AppendixC/all_other_sites/surgery_codes.pdf) as follows: no procedure, local tumor destruction/excision (local tumor destruction/excision, photodynamic therapy, cryosurgery, laser, electrocautery, polypectomy, excisional biopsy), simple/partial surgical removal of the primary site, total surgical removal of primary site, debulking or radical surgery, and surgery not otherwise specified or unknown. Vital status was recorded at the end of the follow-up period. The primary outcome was rate of second primary neoplasm diagnosis.
Statistical Analysis
Variables were summarized as median (interquartile range (IQR)) or count (percentage, 95% confidence interval (CI)) as appropriate. Categorical variables were compared with the Fisher Exact test, and continuous variables were compared with the 2-sample t test. Time-to-event analysis was performed with Kaplan-Meier curves and multivariable Cox proportional hazards models. All statistical analyses were performed using Stata software, version SE 14.0 (StataCorp, College Station, TX, USA). All tests were 2-sided and statistical significance was accepted at the p<0.05 level.
RESULTS
Overall cohort
We identified 2,206 patients with EMPD, of whom 108 patients (4.9%) had anal EMPD, 421 patients (19.1%) had male genital EMPD, and 1,677 patients (76.0%) had female genital EMPD. The cohort was 78.3% female and 80.0% white, with a median (IQR) age of 73 (64–81) years at the time of EMPD diagnosis. The median (IQR) follow-up time was 5.9 (2.5–11.1) years.
Univariate analysis revealed differences in demographics between patients with EMPD of varying primary sites (Table 1). Patients with male genital EMPD tended to be older than those with female genital EMPD (p=0.02), although there were no differences in age between those with anal EMPD and genital EMPD (p=0.38 and p=0.69).
Table 1.
Demographics of patients with anogenital extramammary Paget’s disease, separated by primary site of extramammary Paget’s disease.
| Characteristic | Anal EMPD (n=108) | Male Genital EMPD (n=421) | Female Genital EMPD (n=1,677) | P-value |
|---|---|---|---|---|
| Age at diagnosis of EMPD (median, IQR) | 72.5 years (63.5–80.5) | 73.0 years (66.0–80.0) | 72.0 years (63.0–81.0) | 0.02a |
| Sex | <0.001 | |||
| Male | 58 (53.7%) | 421 (100%) | n/a | |
| Female | 50 (46.3%) | n/a | 1,677 (100%) | |
| Race | <0.001 | |||
| White | 90 (83.3%) | 258 (61.3%) | 1,417 (84.5%) | |
| Black | 4 (3.7%) | 0 (0%) | 12 (0.7%) | |
| Asian, American Indian | 14 (13.0%) | 152 (36.1%) | 227 (13.5%) | |
| Unknown | 0 (0%) | 11 (2.6%) | 21 (1.3%) | |
| Marital status at diagnosis of EMPD | <0.001 | |||
| Single (never married) | 13 (12.0%) | 32 (7.6%) | 151 (9.0%) | |
| Married or domestic partner | 64 (59.3%) | 272 (64.6%) | 797 (47.5%) | |
| Divorced, separated, or widowed | 22 (20.4%) | 48 (11.4%) | 560 (33.4%) | |
| Unknown | 9 (8.3%) | 69 (16.4%) | 169 (10.1%) | |
| Region of the United States b | <0.001 | |||
| Northeast | 11 (10.2%) | 39 (9.3%) | 241 (14.4%) | |
| Midwest | 10 (9.3%) | 41 (9.7%) | 204 (12.2%) | |
| South | 12 (11.1%) | 35 (8.3%) | 229 (13.7%) | |
| West | 75 (69.4%) | 306 (72.7%) | 1,003 (59.8%) | |
| Year of EMPD diagnosis | 0.42 | |||
| 1970–1989 | 12 (11.1%) | 32 (7.6%) | 178 (10.6%) | |
| 1990–1999 | 22 (20.4%) | 79 (18.8%) | 275 (16.4%) | |
| 2000–2009 | 47 (43.5%) | 181 (43.0%) | 716 (42.7%) | |
| 2010–2014 | 27 (25.0%) | 129 (30.6%) | 508 (30.3%) | |
| Surgery type for anal EMPD | n/a | |||
| None | 22 (20.4%) | n/a | n/a | |
| Local tumor destruction/excision | 41 (38.0%) | n/a | n/a | |
| Abdominoperineal resection | 13 (12.0%) | n/a | n/a | |
| Surgery NOS or unknown | 32 (29.6%) | n/a | n/a | |
| Surgery type for genital EMPD | n/a | |||
| None | n/a | 66 (15.7%) | 216 (12.9%) | |
| Local tumor destruction/excision | n/a | 150 (35.6%) | 242 (14.4%) | |
| Partial removal of primary site | n/a | 82 (19.5%) | 592 (35.3%) | |
| Total removal of primary site | n/a | 16 (3.8%) | 123 (7.3%) | |
| Debulking or radical surgery | n/a | 5 (1.2%) | 98 (5.8%) | |
| Surgery NOS or unknown | n/a | 102 (24.2%) | 406 (24.2%) | |
P-value is based on a t-test comparing patients with male versus female genital extramammary Paget’s disease.
Based on United States Census Bureau designations.
EMPD, extramammary Paget’s disease; IQR, interquartile range; NOS, not otherwise specified
Patients underwent a variety of procedures for EMPD (Table 1). Of the patients with anal EMPD, 20.4% had no procedure, 38.0% underwent local tumor destruction/excision, and 12.0% underwent abdominoperineal resection. Of the patients with male and female genital EMPD, 15.7% and 12.9% had no procedure, 35.6% and 14.4% underwent local tumor destruction/excision, 19.5% and 35.3% underwent simple/partial resection, and 5.0% and 13.1% underwent complete/radical resection, respectively.
Other primary malignancies
Of the overall cohort, 584 (26.5%, 95% CI 24.7–28.4%) patients manifested at least one other primary neoplasm within the follow-up period.
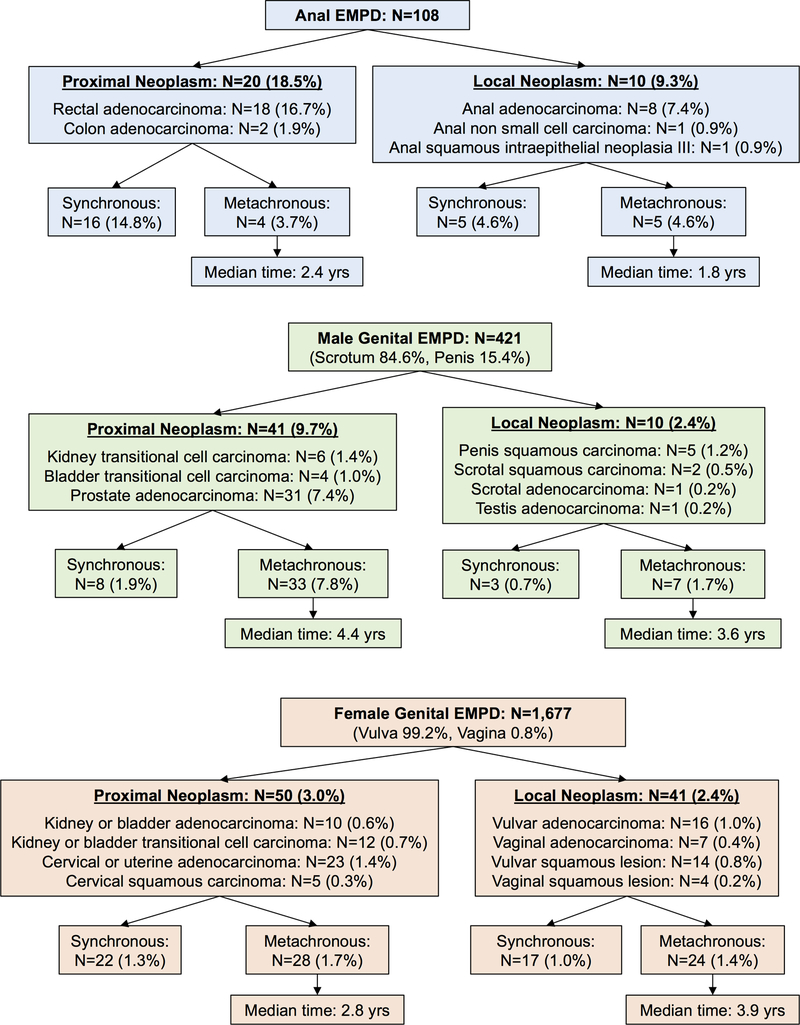
Of the 108 patients with anal EMPD, 20 (18.5%) were diagnosed with proximal neoplasms (colorectal adenocarcinoma) (Figure 1 and Figure 2). Eighty percent of these colorectal cancer diagnoses were synchronous to anal EMPD diagnoses, compared to metachronous colorectal malignancies which occurred at a median time of 2.4 years (range 1.1–4.7 years). Of patients with anal EMPD, 10 (9.3%) were diagnosed with a local, anal neoplasm, including 8.3% who subsequently developed an anal adenocarcinoma or non-small cell carcinoma and 0.9% who developed anal squamous intraepithelial neoplasia III. Half of anal neoplasms were synchronous with anal EMPD, while metachronous tumors occurred at a median time of 1.8 years (range 0.3–8.5 years).
Figure 1.
Numbers and percentages of patients with anogenital Paget’s disease who develop other proximal or local neoplasms. Percentages are based on total number of patients for each primary site of extramammary Paget’s disease (anal, male genital, and female genital).
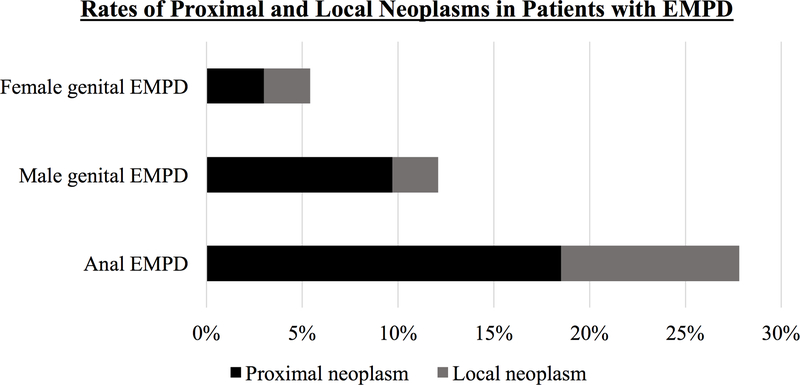
Figure 2.
Rates of proximal and local neoplasms in patients with anogenital Paget’s disease, based on primary site of disease.
For patients with male genital EMPD, the primary site of Paget’s disease was scrotal in 84.6% of patients and penile in 15.4% of patients. The risk of proximal genitourinary (GU) malignancy was 9.7%, including 7.4% who developed prostate adenocarcinoma. Of these proximal GU malignancies, only 19.5% were synchronous with genital EMPD, while the remainder were metachronous, occurring at a median time of 4.4 years (range 0.3–23.2 years). In terms of local neoplasms, only 0.4% developed an incident scrotal or testicular adenocarcinoma, and 1.7% developed a penile or scrotal squamous carcinoma. Thirty percent of these local neoplasms were synchronous with male genital EMPD diagnosis, while metachronous tumors occurred at a median time of 3.6 years (range 0.3–13.0 years).
For female patients with genital EMPD, nearly all sites of Paget’s disease were vulvar (99.2%), and the remainder were vaginal (0.8%). The risk of proximal GU malignancy was 3.0%, including 1.4% who developed cervical or uterine adenocarcinoma. Nearly half (44.0%) of these proximal neoplasms were synchronous with EMPD diagnosis, while the remainder were metachronous, being diagnosed in a median time of 2.8 years (range 0.3–24.3 years). In terms of local neoplasms, only 1.4% developed an incident vaginal/vulvar adenocarcinoma, and only 1.0% developed a vulvar/vaginal squamous neoplasm. Of the local neoplasms, 41.5% were synchronous with genital EMPD diagnosis, and 58.5% were metachronous, diagnosed in a median time of 3.9 years (range 0.3–17.6 years).
Subset analysis of patients who were diagnosed with anogenital EMPD between 2004–2014 included 1,265 patients (59 anal EMPD, 247 male genital EMPD, 959 female genital EMPD). In this group, patients with anal EMPD continued to have higher rates of proximal and local neoplasms (15.3% and 8.5%) compared to patients with male (5.7% and 2.8%) and female (2.2% and 2.5%) genital EMPD.
Predictors of subsequent proximal or local neoplasms in patients with EMPD
In patients with anal EMPD, univariate analysis revealed no significant differences in demographics between patients who did or did not develop a colorectal or other anal neoplasm (Table 2). Surgical treatment for anal EMPD was not associated with development of subsequent neoplasms (p=0.2), nor was vital status at the end of the follow-up period (p=0.9). Univariate analysis of patients with genital EMPD also revealed no correlations between age, race, and procedure type for EMPD and risk of subsequent neoplasm (Table 3).
Table 2.
Univariate analysis of patients with anal Paget’s disease, based on diagnosis of colorectal or other anal neoplasms.
| Characteristic | No colorectal or other anal neoplasm (n=78) | Colorectal neoplasm (n=20) | Other anal neoplasm (n=10) | Colorectal or other anal neoplasm (n=30) | P-value a |
|---|---|---|---|---|---|
| Age at diagnosis of EMPD (median, IQR) | 73.0 years (64.0–82.0) | 71.5 years (63.0–77.0) | 71.0 years (63.0–82.0) | 71.5 years (63.0–77.0) | 0.29 |
| Sex | 0.42 | ||||
| Male | 40 (51.3%) | 11 (55.0%) | 7 (70.0%) | 18 (60.0%) | |
| Female | 38 (48.7%) | 9 (45.0%) | 3 (30.0%) | 12 (40.0%) | |
| Race | 0.59 | ||||
| White | 66 (84.6%) | 15 (75.0%) | 9 (90.0%) | 24 (80.0%) | |
| Black | 2 (2.6%) | 2 (10.0%) | 0 (0.0%) | 2 (6.7%) | |
| Asian, American Indian | 10 (12.8%) | 3 (15.0%) | 1 (10.0%) | 4 (13.3%) | |
| Marital status at diagnosis of EMPD | 0.20 | ||||
| Single (never married) | 7 (9.0%) | 5 (25.0%) | 1 (10.0%) | 6 (20.0%) | |
| Married or domestic partner | 45 (57.7%) | 13 (65.0%) | 6 (60.0%) | 19 (63.3%) | |
| Divorced, separated, or widowed | 19 (24.4%) | 2 (10.0%) | 1 (10.0%) | 3 (10.0%) | |
| Unknown | 7 (9.0%) | 0 (0.0%) | 2 (20.0%) | 2 (6.7%) | |
| Region of the United States b | 0.67 | ||||
| Northeast | 7 (9.0%) | 3 (15.0%) | 1 (10.0%) | 4 (13.3%) | |
| Midwest | 8 (10.3%) | 1 (5.0%) | 1 (10.0%) | 2 (6.7%) | |
| South | 10 (12.8%) | 1 (5.0%) | 1 (10.0%) | 2 (6.7%) | |
| West | 53 (68.0%) | 15 (75.0%) | 7 (70.0%) | 22 (73.3%) | |
| Year of EMPD diagnosis | 0.49 | ||||
| 1970–1989 | 7 (9.0%) | 4 (20.0%) | 1 (10.0%) | 5 (16.7%) | |
| 1990–1999 | 16 (20.5%) | 4 (20.0%) | 2 (20.0%) | 6 (20.0%) | |
| 2000–2009 | 33 (42.3%) | 9 (45.0%) | 5 (50.0%) | 14 (46.7%) | |
| 2010–2014 | 22 (28.2%) | 3 (15.0%) | 2 (20.0%) | 5 (16.7%) | |
| Surgery type for EMPD | 0.15 | ||||
| None | 20 (25.6%) | 1 (5.0%) | 1 (10.0%) | 2 (6.7%) | |
| Local tumor destruction/excision | 27 (34.6%) | 9 (45.0%) | 5 (50.0%) | 14 (46.7%) | |
| Abdominoperineal resection | 8 (10.3%) | 4 (20.0%) | 1 (10.0%) | 5 (16.7%) | |
| Surgery NOS or unknown | 23 (29.5%) | 6 (30.0%) | 3 (30.0%) | 9 (30.0%) | |
| Vital status at end of follow-up period | 0.92 | ||||
| Alive | 32 (41.0%) | 8 (40.0%) | 4 (40.0%) | 12 (40.0%) | |
| Dead | 46 (59.0%) | 12 (60.0%) | 6 (60.0%) | 18 (60.0%) | |
P-values are based on t-tests and Fisher Exact tests comparing patients with no colorectal or other anal neoplasm versus patients with a colorectal or other anal neoplasm.
Based on United States Census Bureau designations.
EMPD, extramammary Paget’s disease; IQR, interquartile range; NOS, not otherwise specified
Table 3.
Univariate analysis of patients with male genital EMPD and female genital EMPD, based on diagnosis of proximal or local neoplasms.
| MALE GENITAL EMPD (n=421) | FEMALE GENITAL EMPD (n=1,677) | |||||
|---|---|---|---|---|---|---|
| Characteristic | No proximal or local neoplasm (n=370) | Proximal or local neoplasm (n=51) | P-value | No proximal or local neoplasm (n=1,586) | Proximal or local neoplasm (n=91) | P-value |
| Age at diagnosis of EMPD (median, IQR) | 73.0 years (66.0–80.0) | 73.0 years (66.0–80.0) | 0.95 | 72.0 years (63.0–81.0) | 70.0 years (62.0–79.0) | 0.34 |
| Race | 0.08 | 0.56 | ||||
| White | 220 (59.5%) | 38 (74.5%) | 1,342 (84.6%) | 75 (82.4%) | ||
| Black | 0 (0.0%) | 0 (0.0%) | 11 (0.7%) | 1 (1.1%) | ||
| Asian, American Indian | 139 (37.6%) | 13 (25.5%) | 212 (13.4%) | 15 (16.5%) | ||
| Unknown | 11 (3.0%) | 0 (0.0%) | 21 (1.3%) | 0 (0.0%) | ||
| Marital status at diagnosis of EMPD | 0.89 | 0.36 | ||||
| Single (never married) | 29 (7.8%) | 3 (5.9%) | 143 (9.0%) | 8 (8.8%) | ||
| Married or domestic partner | 240 (64.9%) | 32 (62.8%) | 759 (47.9%) | 38 (41.8%) | ||
| Divorced, separated, or widowed | 42 (11.4%) | 6 (11.8%) | 522 (32.9%) | 38 (41.8%) | ||
| Unknown | 59 (16.0%) | 10 (19.6%) | 162 (10.2%) | 7 (7.7%) | ||
| Region of the United States a | 0.67 | 0.01 | ||||
| Northeast | 33 (8.9%) | 6 (11.8%) | 234 (14.8%) | 7 (7.7%) | ||
| Midwest | 37 (10.0%) | 4 (7.8%) | 184 (11.6%) | 20 (22.0%) | ||
| South | 29 (7.8%) | 6 (11.8%) | 218 (13.8%) | 11 (12.1%) | ||
| West | 271 (73.2%) | 35 (68.6%) | 950 (59.9%) | 53 (58.2%) | ||
| Year of EMPD diagnosis | 0.019 | 0.11 | ||||
| 1970–1989 | 24 (6.5%) | 8 (15.7%) | 170 (10.7%) | 8 (8.8%) | ||
| 1990–1999 | 67 (18.1%) | 12 (23.5%) | 252 (15.9%) | 23 (25.3%) | ||
| 2000–2009 | 158 (42.7%) | 23 (45.1%) | 678 (42.8%) | 38 (41.8%) | ||
| 2010–2014 | 121 (32.7%) | 8 (15.7%) | 486 (30.6%) | 22 (24.2%) | ||
| Surgery type for EMPD | 0.27 | 0.45 | ||||
| None | 58 (15.7%) | 8 (15.7%) | 204 (12.9%) | 12 (13.2%) | ||
| Local tumor destruction/excision | 135 (36.5%) | 15 (29.4%) | 230 (14.5%) | 12 (13.2%) | ||
| Partial removal of primary site | 75 (20.3%) | 7 (13.7%) | 567 (35.8%) | 25 (27.5%) | ||
| Total removal of primary site | 14 (3.8%) | 2 (3.9%) | 113 (7.1%) | 10 (11.0%) | ||
| Debulking or radical surgery | 5 (1.4%) | 0 (0.0%) | 93 (5.9%) | 5 (5.5%) | ||
| Surgery NOS or unknown | 83 (22.4%) | 19 (37.3%) | 379 (23.9%) | 27 (29.7%) | ||
| Vital status at end of follow-up period | 0.35 | 0.007 | ||||
| Alive | 207 (56.0%) | 25 (49.0%) | 957 (60.3%) | 42 (46.2%) | ||
| Dead | 163 (44.1%) | 26 (51.0%) | 629 (39.7%) | 49 (53.9%) | ||
Based on United States Census Bureau designations.
EMPD, extramammary Paget’s disease; IQR, interquartile range; NOS, not otherwise specified
Time between diagnoses of EMPD and second primary neoplasms
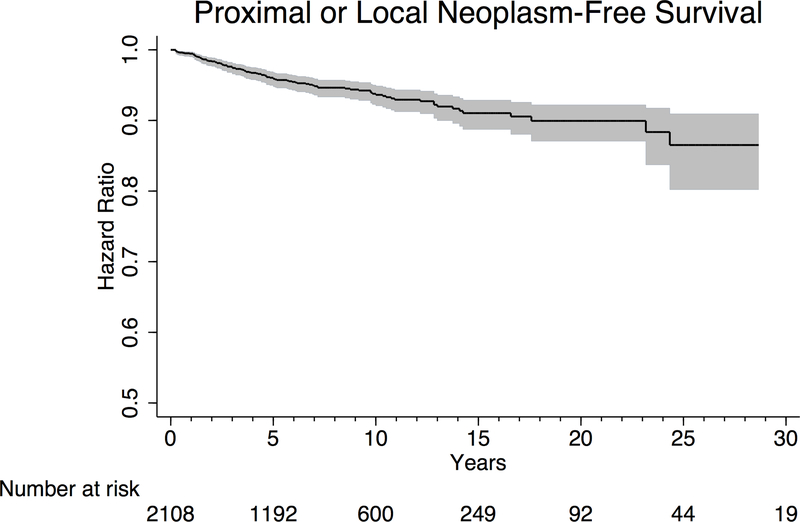
In time-to-event analysis, we examined “proximal or local neoplasm-free survival”, with diagnosis of a subsequent metachronous neoplasm as the primary endpoint. All synchronous lesions were designated as having “failed” at time zero. Approximately 4.1% (95% CI 3.2–5.2%) of patients developed a subsequent proximal or local neoplasm within 5 years of EMPD diagnosis (Figure 3A). Separating patients by primary site of EMPD, 13.3% (95% CI 6.8–25.2%) of patients with anal EMPD, 8.1% (95% CI 5.5–12.0%) of patients with male genital EMPD, and 2.8% (95% CI 2.0–3.8%) of patients with female genital EMPD were diagnosed with a subsequent proximal or local neoplasm within 5 years (p<0.001) (Figure 3B). On subset analysis of patients diagnosed with EMPD between 2004–2014, 15.1% (95% CI 6.4–33.2%) of patients with anal EMPD, 9.1% (95% CI 5.2–15.5%) of patients with male genital EMPD, and 3.2% (95% CI 2.0–4.9%) of patients with female genital EMPD were diagnosed with a secondary neoplasm within 5 years (p<0.001).
Figure 3.
Kaplan Meier curves depicting time to development of metachronous proximal or local neoplasms in (A) all patients with anogenital Paget’s disease, and (B) all patients with anogenital Paget’s disease, separated by primary site of disease. Grey shaded area represents 95% confidence interval.
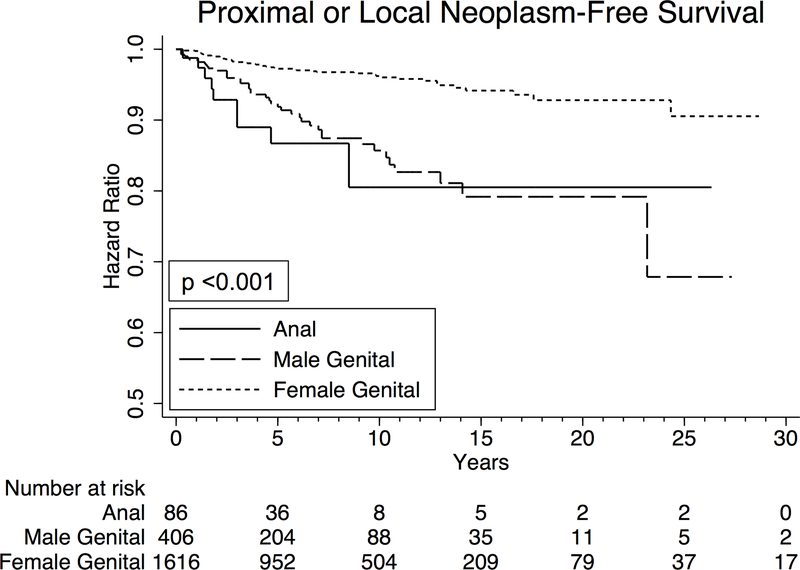
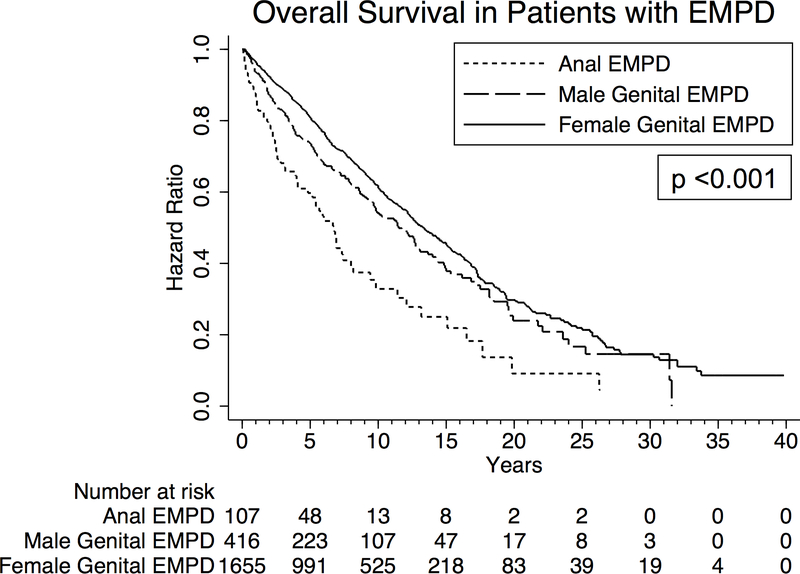
Analysis of overall survival in patients with EMPD
Among the entire cohort, 1-year and 5-year overall survival were 95.3% and 78.3%, respectively. Five-year overall survival was 59.7%, 73.5%, and 80.7% in patients with anal, male genital, and female genital EMPD, respectively (p<0.001) (Figure 4). Focusing on only patients with anal EMPD, there was no correlation between survival and development of colorectal or anal neoplasms (p=0.8).
Figure 4.
Kaplan Meier curves depicting overall survival in all patients with anogenital Paget’s disease, separated by primary site of disease.
On multivariable Cox proportional hazards analysis, male and female genital EMPD were both associated with improved survival compared to anal EMPD (hazard ratio (HR) 0.62, 95% CI 0.41–0.94, p=0.03, and HR 0.39, 95% CI 0.27–0.56, p<0.001), even after adjusting for multiple possible confounders (Table 4). Being diagnosed with a second primary neoplasm was not associated with overall survival (p=0.95). Undergoing local excision for EMPD or simple removal of the primary site of EMPD were both associated with improved survival compared to no procedure (HR 0.71, 95% CI 0.55–0.92, p=0.01, and HR 0.68, 95% CI 0.53–0.87, p=0.002, respectively). Radical resection of EMPD was not associated with improved survival (p=0.96).
Table 4.
Multivariable Cox proportional hazards analysis of predictors of mortality in patients with anogenital Paget’s disease.
| Variable | Hazard Ratio | 95% Confidence Interval | P-value | |
|---|---|---|---|---|
| Age at diagnosis of EMPD | ||||
| ≤ 60 years old | Reference | |||
| 61–70 years old | 2.45 | 1.84 | 3.25 | <0.001 |
| 71–80 years old | 5.28 | 4.00 | 6.98 | <0.001 |
| >80 years old | 12.78 | 9.58 | 17.05 | <0.001 |
| Sex | ||||
| Female | Reference | |||
| Male | 2.17 | 1.29 | 3.65 | 0.004 |
| Race | ||||
| White | Reference | |||
| Black | 1.15 | 0.53 | 2.51 | 0.72 |
| Asian, American Indian | 0.75 | 0.62 | 0.91 | 0.003 |
| Unknown | 0.13 | 0.02 | 0.92 | 0.04 |
| Marital status at diagnosis of EMPD | ||||
| Single (never married) | Reference | |||
| Married or domestic partner | 0.91 | 0.70 | 1.18 | 0.47 |
| Divorced, separated, or widowed | 1.14 | 0.87 | 1.49 | 0.33 |
| Unknown | 1.01 | 0.73 | 1.40 | 0.94 |
| Primary site of EMPD | ||||
| Anal | Reference | |||
| Male genital | 0.62 | 0.41 | 0.94 | 0.03 |
| Female genital | 0.39 | 0.27 | 0.56 | <0.001 |
| Diagnosis of other proximal or local neoplasm | 1.01 | 0.80 | 1.26 | 0.95 |
| Surgery type for EMPD | ||||
| None | Reference | |||
| Local tumor destruction/excision | 0.71 | 0.55 | 0.92 | 0.01 |
| Partial removal of primary site | 0.68 | 0.53 | 0.87 | 0.002 |
| Total removal of primary site, or debulking, or radical surgery | 1.01 | 0.76 | 1.33 | 0.96 |
| Surgery NOS or unknown | 1.02 | 0.82 | 1.28 | 0.86 |
EMPD, extramammary Paget’s disease; NOS, not otherwise specified
On subset analysis of patients diagnosed with EMPD between 2004–2014, there was no significant difference in 5-year survival between patients with anal (71.7%) and male genital EMPD (71.7%) (p=0.43), but significantly improved survival in patients with female genital EMPD (82.3%) compared to anal EMPD (p=0.01) and male genital EMPD (p=0.04). Multivariable Cox regression demonstrated no difference in survival between anal and male genital EMPD patients (p=0.38), but significantly decreased mortality in female genital EMPD patients compared to anal EMPD patients (HR 0.48, 95% CI 0.24–0.96, p=0.04). Diagnosis of a secondary neoplasm continued to have no significant association with survival (p=0.31).
DISCUSSION
This study represents the largest published cohort of patients with anogenital EMPD to date. In this group of 2,206 patients, rates of proximal and local neoplasms were higher in patients with anal EMPD (18.5% and 9.3%, respectively) than for patients with male or female genital EMPD (9.7% and 2.4%, and 3.0% and 2.4%, respectively). Risk of a synchronous neoplasm was 19.4%, 2.6%, and 2.3% in patients with anal, male genital, and female genital EMPD, respectively. The 5-year incidence of a second primary neoplasm after EMPD diagnosis was also higher in patients with anal EMPD (13.3%) compared to patients with male or female genital EMPD (8.1% and 2.8%) (p<0.001).
It is generally accepted that patients with EMPD are at higher risk of proximal malignancies than the overall population. Our data not only confirm this risk of proximal disease, but also demonstrate a substantially higher risk of local neoplasms in anal EMPD patients as compared to genital EMPD patients. Nearly one in ten patients with anal EMPD will develop an anal neoplasm (mostly anal adenocarcinoma). This stark finding has not been well-characterized previously, and demonstrates that malignant transformation of anal EMPD at the local site must be considered during surveillance of these lesions. Untreated and non-responding anal EMPD lesions should be followed closely and re-biopsied when concerning characteristics appear. The higher rate of local progression in anal Paget’s may be related to the anatomic challenges of performing excisions in the perianal region compared to the genitalia, as well as the substantial pain associated with removal of anal lesions, therefore leading to lower rates of optimal management of anal EMPD. This higher rate of local malignancy may be a contributing factor to why anal EMPD is associated with significantly higher mortality than genital EMPD.
Our data also reveal a substantial risk of metachronous neoplasms in patients with anogenital EMPD, which may be diagnosed many years after EMPD. This is consistent with other reports, including the 30-year experience at the Cleveland Clinic, which describes 25 patients with perianal EMPD, 16% of whom had synchronous malignancies and 16% developed metachronous malignancies during a median follow-up time of 5 years.17
These data emphasize the need for thorough surveillance of patients with EMPD for other malignancies during evaluation and follow-up, particularly patients with anal EMPD. A Mayo Clinic study found that screening practices vary widely, demonstrating the need for increased awareness of the risk of malignancy.11 Our data did not identify any clinical characteristic associated with increased risk of neoplasm, suggesting that all patients with EMPD should be screened empirically. Clinicians should be vigilant for both local and proximal neoplasms. Of note, the development of metachronous lesions appears to taper off 8 years after EMPD diagnosis (Figure 3B), therefore it may be reasonable to reduce the frequency of surveillance at that time.
Local excision of EMPD is considered the gold standard,7,19 yet our data show that 20.4% of patients with anal EMPD received no procedural treatment, a higher percentage than that of patients with genital EMPD. This could be due to other factors not captured in the database, such as comorbidities or goals of care. Nevertheless, our data also demonstrate significant improvement in survival associated with excision of EMPD compared to no procedure, emphasizing the importance of surgical management when feasible. Nevertheless, given the numerous treatment options for EMPD, multidisciplinary management (by dermatology, radiation oncology, medical oncology, and surgery) is warranted.
With this study, our hope is to increase awareness of this rare condition and its substantial risk of associated malignancy. EMPD is often misdiagnosed, leading to delays in management.8,9 EMPD should be considered in cases of chronic anogenital dermatoses, and clinicians should biopsy lesions that do not respond to initial treatments.2 This is particularly true for white and Asian patients older than 50 years of age, who are at higher risk of developing EMPD.20,21 Our findings also support careful screening of patients with EMPD for synchronous malignancies, surveillance for metachronous lesions, and close monitoring of anal EMPD patients for local progression.
Our study has a number of limitations. The SEER registry does not document non-procedural treatments, frequency/type of surveillance, how EMPD was detected, or specialty of the provider performing the procedure for EMPD. It also does not record whether the EMPD lesion was primary or secondary (based on immunohistologic staining of CD7 and CD20), which is clinically relevant as secondary EMPD lesions tend to have higher rates of other malignancies.22 Stage and tumor size were also missing for 75% of EMPD lesions in the database. Only patients with neoplasms diagnosed between 2004–2014 are entered into this registry and their prior neoplasms are then recorded, leading to potential for selection bias. We attempted to address this by performing subset analyses of patients diagnosed with EMPD between 2004–2014, which demonstrated similar results to the overall cohort. Additionally, the SEER registry may not be fully representative of the United States population, as it captures data from specific states and intentionally over-represents ethnic minorities and underserved populations.
CONCLUSION
This study is the largest published cohort of patients with anogenital EMPD, a rare disease with poorly-defined clinical outcomes. It highlights the high risk of proximal and local, synchronous and metachronous malignancies associated with EMPD, particularly in patients with anal EMPD, who have an 18.5% risk of colorectal malignancy and a 9.3% risk of anal neoplasia. Excision of EMPD was associated with improved overall survival. Patients with anal EMPD experienced worse survival compared to those with purely genital EMPD. Clinicians must have a low threshold to biopsy anogenital skin lesions that do not respond to initial therapies, particularly in white and Asian patients older than 50 years of age. Clinicians must also carefully screen all patients with EMPD for distant and local neoplasms, during evaluation and follow-up, particularly in patients with anal EMPD.
ACKNOWLEDGMENTS
GCL was supported by the NIH T32 Research Training in Alimentary Tract Surgery grant DK007754-13.
Funding/Support: GCL is currently receiving support from an NIH T32 grant (Research Training in Alimentary Tract Surgery, DK007754-13).
The authors acknowledge that if this manuscript is provisionally accepted, a video abstract will be required prior to final acceptance.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Financial Disclosures: None report.
REFERENCES
- 1.Lopes Filho LL, Lopes IM, Lopes LR, Enokihara MM, Michalany AO, Matsunaga N. Mammary and extramammary Paget’s disease. An Bras Dermatol. 2015;90:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28:807–826. [DOI] [PubMed] [Google Scholar]
- 3.Wagner G, Sachse MM. Extramammary Paget disease - clinical appearance, pathogenesis, management. J Dtsch Dermatol Ges. 2011;9:448–454. [DOI] [PubMed] [Google Scholar]
- 4.Funaro D, Krasny M, Lam C, Desy D, Sauthier P, Bouffard D. Extramammary Paget disease: epidemiology and association to cancer in a Quebec-based population. J Low Genit Tract Dis. 2013;17:167–174. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Choe YS, Jung HD, et al. ; Korean Society for Skin Cancer and Korean Dermatopathology Research Group. A multicenter study on extramammary Paget’s disease in Korea. Int J Dermatol. 2011;50:508–515. [DOI] [PubMed] [Google Scholar]
- 6.Lian P, Gu WL, Zhang Z, et al. Retrospective analysis of perianal Paget’s disease with underlying anorectal carcinoma. World J Gastroenterol. 2010;16:2943–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wollina U, Goldman A, Bieneck A, Abdel-Naser MB, Petersen S. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27. [DOI] [PubMed] [Google Scholar]
- 8.Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625–1630. [DOI] [PubMed] [Google Scholar]
- 9.Hatta N, Yamada M, Hirano T, Fujimoto A, Morita R. Extramammary Paget’s disease: treatment, prognostic factors and outcome in 76 patients. Br J Dermatol. 2008;158:313–318. [DOI] [PubMed] [Google Scholar]
- 10.Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13:1009–1014. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt AR, Long BJ, Weaver AL, et al. Evidence-based screening recommendations for occult cancers in the setting of newly diagnosed extramammary Paget disease. Mayo Clin Proc. 2018;93:877–883. [DOI] [PubMed] [Google Scholar]
- 12.Mammary Kanitakis J. and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581–590. [DOI] [PubMed] [Google Scholar]
- 13.Kyriazanos ID, Stamos NP, Miliadis L, Noussis G, Stoidis CN. Extra-mammary Paget’s disease of the perianal region: a review of the literature emphasizing the operative management technique. Surg Oncol. 2011;20:e61–e71. [DOI] [PubMed] [Google Scholar]
- 14.Minicozzi A, Borzellino G, Momo R, Steccanella F, Pitoni F, de Manzoni G. Perianal Paget’s disease: presentation of six cases and literature review. Int J Colorectal Dis. 2010;25:1–7. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Grossfeld GD, McAninch JW, Santucci R. Extramammary Paget’s disease of the penis and scrotum: excision, reconstruction and evaluation of occult malignancy. J Urol. 2001;166:2112–2116. [DOI] [PubMed] [Google Scholar]
- 16.Hendi A, Brodland DG, Zitelli JA. Extramammary Paget’s disease: surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2004;51:767–773. [DOI] [PubMed] [Google Scholar]
- 17.Isik O, Aytac E, Brainard J, Valente MA, Abbas MA, Gorgun E. Perianal Paget’s disease: three decades experience of a single institution. Int J Colorectal Dis. 2016;31:29–34. [DOI] [PubMed] [Google Scholar]
- 18.Lee GC, Kunitake H, Milch H, et al. What is the risk of anal carcinoma in patients with anal intraepithelial neoplasia III? Dis Colon Rectum. 2018;61:1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mengjun B, Zheng-Qiang W, Tasleem MM. Extramammary Paget’s disease of the perianal region: a review of the literature emphasizing management. Dermatol Surg. 2013;39:69–75. [DOI] [PubMed] [Google Scholar]
- 20.Yang WJ, Kim DS, Im YJ, et al. Extramammary Paget’s disease of penis and scrotum. Urology. 2005;65:972–975. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Lu M, Dong GQ, et al. Penile and scrotal Paget’s disease: 130 Chinese patients with long-term follow-up. BJU Int. 2008;102:485–488. [DOI] [PubMed] [Google Scholar]
- 22.van der Linden M, Schuurman MS, Bulten J, et al. Stop routine screening for associated malignancies in cutaneous noninvasive vulvar Paget disease? Br J Dermatol. 2018;179:1315–1321. 10.1111/bjd.16894 [DOI] [PubMed] [Google Scholar]