Abstract
Chronic kidney disease (CKD) universally associates with renal microvascular rarefaction and inflammation, but whether a link exists between these two processes is unclear. We designed a therapeutic construct of vascular endothelial growth factor (VEGF) fused to an elastin-like polypeptide (ELP) carrier and show that it improves renal function in experimental renovascular disease. We test the hypothesis that ELP-VEGF therapy will improve CKD, and that recovery will be driven by decreasing microvascular rarefaction partly via modulation of macrophage phenotype and inflammation.
CKD was induced in 14 pigs, which were observed for 14 weeks. At 6 weeks, renal blood flow and filtration were quantified using multidetector computed tomography, then pigs received single intra-renal ELP-VEGF or placebo (n=7 each). Renal function was quantified again 4 and 8 weeks later. Pigs were euthanized and renal microvascular density, angiogenic and inflammatory markers, fibrosis, macrophage infiltration and phenotype were quantified.
Loss of renal hemodynamics in CKD was progressively recovered by ELP-VEGF therapy, accompanied by improved renal microvascular density, fibrosis, and expression of inflammatory mediators. Although renal macrophage infiltration was similar in both CKD groups, ELP-VEGF therapy distinctly shifted their phenotype from pro-inflammatory M1 to VEGF-expressing M2.
Our study unravels potential mechanisms and feasibility of a new strategy to offset progression of CKD using drug-delivery technologies. The results indicate that renal recovery after ELP-VEGF therapy was largely driven by modulation of renal macrophages towards VEGF-expressing M2 phenotype, restoring VEGF signaling and sustaining improvement of renal function and microvascular integrity in CKD.
Keywords: Chronic kidney disease, microcirculation, angiogenesis, inflammation, macrophages
Summary
Patients with chronic kidney disease (CKD) have higher risk of cardiovascular complications and early mortality than non-CKD patients, irrespective of the etiology. Using a translational swine model of CKD, we showed the therapeutic potential of ELP-VEGF therapy to induce a sustained, progressive renal recovery that outlasted the life of the therapeutic construct. Our results suggest that renoprotective effects of ELP-VEGF therapy are partly through long-term suppression of renal inflammation via a shift in renal macrophages to M2 phenotype, thereby attenuating further MV dysfunction and fibrosis and inducing renal recovery.
Introduction
Chronic kidney disease (CKD) is a progressive condition affecting nearly 15% of the general U.S. population and accounting for almost $100 billion in Medicare costs annually1. Patients with CKD have longer and more frequent hospitalizations and have higher risk of cardiovascular complications and early mortality than non-CKD patients1. The etiology of CKD is diverse, with the most commonly identified causes being hypertension and diabetes (32% and 40% of CKD patients, respectively)1, 2. There is no definitive treatment for CKD and management is focused on mitigation of these and other risk factors to slow its progression3. Hence, development of novel therapeutic strategies to offset the progressive traits of CKD may significantly reduce the burden on the healthcare system and improve patient outcomes.
The pathophysiology of renal injury in CKD involves several factors that contribute to the progressive nature of the disease such as hyperfiltration, hypoperfusion, accumulation of toxins, and autoimmunity, among others4. This heterogeneous group of insults converges on a common pathway which involves deterioration of renal microvascular (MV) integrity and subsequent development of fibrosis towards CKD5, 6. Indeed, progressive damage and loss of the renal microcirculation is a major pathological feature in CKD that correlates with the progression of renal injury7, 8. Furthermore, renal MV endothelial cells are highly susceptible to ischemic insults and have poor regenerative capacity9. We showed in a swine model of chronic renovascular disease that MV rarefaction develops in a context of impaired angiogenic signaling, likely driven by reduced bioavailability of vascular endothelial growth factor (VEGF) in the ischemic kidney10, 11. We also showed that intrarenal administration of VEGF stimulates angiogenesis, MV repair, improves MV density, renal blood flow (RBF), glomerular filtration rate (GFR), fibrosis, and attenuates hypertension in renovascular disease10. These promising results suggest that stimulating repair of the renal microvasculature using pro-angiogenic biologic agents may be a viable strategy in CKD. However, such a therapeutic approach for CKD has never been investigated.
Elastin like polypeptides (ELP) are protein-based carriers composed of a pentapeptide repeat (VPGxG) where x is any amino acid except proline12. They are genetically encoded and may be easily produced by E. coli with control over amino acid sequence and molecular weight13. ELPs show promise as drug delivery vectors for kidney disease as they can be easily conjugated to various peptide or small molecule therapeutics14, 15, and they naturally accumulate in the kidney16. Furthermore, they protect their cargo, acting to enhance half-life and prolong their therapeutic effect13, 15. We recently developed an ELP-VEGF construct and demonstrated the renal targeting characteristics, safety, and therapeutic efficacy of a single administration in a swine model of chronic renovascular disease to be more effective than free VEGF17, 18.
Inflammation is a major pathological feature of CKD that contributes to disease progression and cardiovascular and all-cause mortality19, 20. Macrophages of the innate immune system are key players in both inflammatory and healing responses in the kidney21. As macrophages infiltrate into damaged tissue they undergo polarization to a pro-inflammatory M1 phenotype characterized by increased phagocytic and microbicidal activity22–24, to later re-polarize to an M2 phenotype defined by release of anti-inflammatory mediators, growth factors, and angiogenic cytokines to facilitate the healing process22–24. Tumor studies have shown that VEGF plays a role in attracting and polarizing M2-like macrophages to suppress inflammatory responses and enhance angiogenesis25. Whether modulation of renal macrophage phenotype may be mechanistically related to recovery of renal function after VEGF therapy is unknown. Thus, the current study was designed to determine the efficacy and potential mechanisms of ELP-VEGF therapy in a large animal model of CKD developed in our laboratory26 that mimics the major pathological features of human CKD, including loss of renal function (comparable to CKD stage 2–3a), renal inflammation, microvascular rarefaction, and fibrosis26, which are observed in all forms of CKD irrespective of the etiology. We hypothesize that ELP-VEGF will improve renal hemodynamics and function in CKD, and that this recovery will be driven in part by modulation of renal macrophage phenotype to suppress inflammation and enhance angiogenesis.
Concise methods (for additional details, please see supplementary methods): The authors declare that all data and supporting materials have been provided with the published article.
Experimental design and in vivo studies
All studies were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. Twenty-one juvenile pigs (sus scrofa domesticus) were studied for a total of 14 weeks. We induced CKD in 14 pigs through bilateral renal artery stenosis and a high cholesterol diet that was initiated immediately after induction of the stenosis and maintained for 14 weeks, as described26. Blood pressure was measured continuously by telemetry (Data Sciences International, St. Paul, MN), as described17, 27. An additional 7 pigs underwent sham surgery, were fed a normal diet, and served as normal controls.
Six weeks after induction of CKD, pigs were anesthetized with intramuscular telazol and xylazine, intubated, and mechanically ventilated. Anesthesia was maintained with a ketamine/xylazine mixture in normal saline via an ear cannula. Renal hemodynamics were quantified in vivo using contrast-enhanced multidetector computed tomography (MDCT). Time-density curves were plotted to calculate renal blood flow (RBF), glomerular filtration rate (GFR), total and regional perfusion, as previously shown and validated17, 26, 28–30. Cortical and medullary volume (each kidney) and renal vascular resistance were quantified, as shown17, 26, 28–30.
Following quantification of renal function at 6 weeks, a single intra-renal administration of ELP-VEGF (100 μg/kg) or vehicle was injected through a balloon lumen into the kidneys over two minutes. Renal hemodynamics were again quantified at 10 and 14 weeks (4 and 8 weeks post-treatment, respectively).
After 14 weeks of observation, pigs were euthanized (phenobarbital overdose under anesthesia), and kidneys were harvested and prepared for ex vivo morphometric analysis, protein expression (immunohistochemistry, immunoblotting) of inflammatory, fibrotic, and angiogenic factors, and MV density quantification using Micro-CT. Blood and urine were also collected to assess serum creatinine and urinary markers of renal injury neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1).
Ex Vivo Studies
Renal microvascular density and architecture
Following euthanasia, kidneys were perfused with heparinized saline followed by a polymer contrast agent (Microfil MV122), and samples were scanned using a Micro-CT (SkyScan 1076) at a resolution of 9 μm. Analyze™software was used to generate 3D reconstructions and subsequently quantify MV density of microvessels between 0–500 μm in the cortex and medulla, as described10, 17, 26, 31.
Protein expression
Western blotting was performed in renal homogenates, as described17, 18, 26 to quantify expression of VEGF, its receptor Flk-1, phosphorylated nuclear factor kappa B (p-NFkB), IkB, transforming growth factor-β (TGF-β), connective tissue growth factor (CTGF), and tissue inhibitor of metalloproteinase-1 (TIMP-1). Protein expression was quantified relative to beta-actin.
Immunohistochemistry
Paraffin-embedded mid-hilar 5 μm kidney sections from each animal were used to perform immunohistochemistry, as detailed26, for CD68 (1/50), indoleamine-2,3-dioxygenase (IDO, 1/50), and mannose receptor-c type 1 (MRC1, 1/50) to identify M1 and M2 macrophages respectively as their expression is highly conserved between pigs and humans23, 32, 33. Kidney sections were concurrently stained with mouse anti-CD68, rabbit anti-MRC1, and goat anti-IDO followed by species appropriate Alexa Fluor-488, 547, and Cy5 secondary antibodies. Cells were counted using confocal microscopy. Cells positive for both CD68 and IDO or MRC1 were considered M1 or M2 macrophages respectively.
These experiments were extended by additional renal sections co-stained for M1/M2 macrophages and NF-κB (1/100 dilution). Mean fluorescence intensity of NF-κB was quantified using confocal microscopy. Another set of renal sections co-stained for M1 (IDO) or M2 (MRC1) macrophages and VEGF (1/100 dilution) were used to identify macrophages co-expressing IDO/VEGF or MRC1/VEGF. Macrophage VEGF expression was quantified by mean fluorescence intensity of VEGF staining in manually traced M1 and M2 macrophages from CKD and CKD ELP-VEGF pigs.
Renal morphometric analysis
Mid-hilar paraffin-embedded 5 μm kidney sections from each pig were stained with trichrome26, 34, and morphometric analysis was performed to quantify glomerulosclerosis, tubulointerstitial fibrosis, and media-to-lumen ratio, as described11, 35.
In vitro studies: VEGF and macrophage polarization
Human monocytes were differentiated into M1 or naïve M0, exposed to hypoxia, and treated with VEGF to assess phenotype and VEGF expression using quantitative PCR. For details please refer to supplementary methods.
Statistical analysis
Power analysis of prior renal hemodynamics data revealed that a minimal n=6 per group was sufficient to detect a difference of 11% among groups at 80% power with an alpha=0.05 Results are expressed as mean ± SEM. Treatment groups were compared using one-way ANOVA with post-hoc Tukey’s or Fisher (as indicated in Figure legends) for multiple comparisons. Statistical significance was accepted for p≤0.05.
Results
General Characteristics
Body weights of pigs in all groups were similar at conclusion of the study. Mean arterial pressure (MAP) was similarly elevated in CKD and CKD ELP-VEGF pigs prior to treatment at 6 weeks (Table 1A). The degree of renal artery stenosis remained unchanged after 6, 10, and 14 weeks of observation in both CKD groups.
Table 1:
General characteristics of each group at 6 weeks (A, prior to treatment, (n=7/group) and at 14 weeks (B, 8 weeks post-treatment, n=6–7/group).
| 1A | Normal | CKD | CKD ELP-VEGF |
| Body weight (kg) | 48.3 ± 5.6 | 49.4 ± 1.7 | 43.3 ± 3.3 |
| Mean arterial pressure (mm Hg) | 101 ± 5.3 | 128.7 ± 9.8* | 137.7 ± 10.8* |
| Renal artery stenosis (%) | 0 | 66.7 ± 2.9* | 70.2 ± 3.6* |
| Tissue perfusion (mL/min/cc tissue) | 4.4 ± 0.2 | 3.7 ± 0.2* | 3.3 ± 0.3* |
| RVR (mm Hg/mL per min) | 0.21 ± 0.03 | 0.29 ± 0.05* | 0.44 ± 0.03* |
| Cortical volume (cc) | 198.1 ± 13.3 | 127.0 ± 6.0* | 145.2 ± 6.9* |
| Medullary volume (cc) | 62.36 ± 1.7 | 46.5 ± 2.6* | 44.2 ± 4.4* |
| 1B | Normal | CKD | CKD ELP-VEGF |
| Body Weight (kg) | 62.3 ± 1.4 | 68 ± 2.1 | 62.2 ± 3.9 |
| Mean arterial pressure (mm Hg) | 106 ± 2.8 | 132.1 ± 1.9* | 124.0 ± 4.3* |
| Tissue perfusion (mL/min/cm3 tissue) | 4.7 ± 0.2 | 4.1 ± 0.2* | 4.4 ± 0.5† |
| RVR (mm Hg/mL per min) | 0.11 ± 0.4 | 0.28 ± 0.02 | 0.23 ± 0.02*† |
| Cortical volume (cm3) | 210.9 ± 6.3 | 137.7 ± 5.2* | 167.6 ± 15.8*†^ |
| Medullary volume (cm3) | 57.5 ± 4.8 | 48.2 ± 3.2* | 55.4 ± 5.1†^ |
Values are expressed as mean ± SEM. Renal volume is presented as the sum of cortical or medullary area for both kidneys. RVR: Renal vascular resistance
p < 0.05 vs normal
p < 0.05 vs untreated CKD at 14 weeks
p < 0.05 vs 6 weeks (Table 1A).
Pre- and post-treatment MDCT-derived quantification of renal hemodynamics in vivo
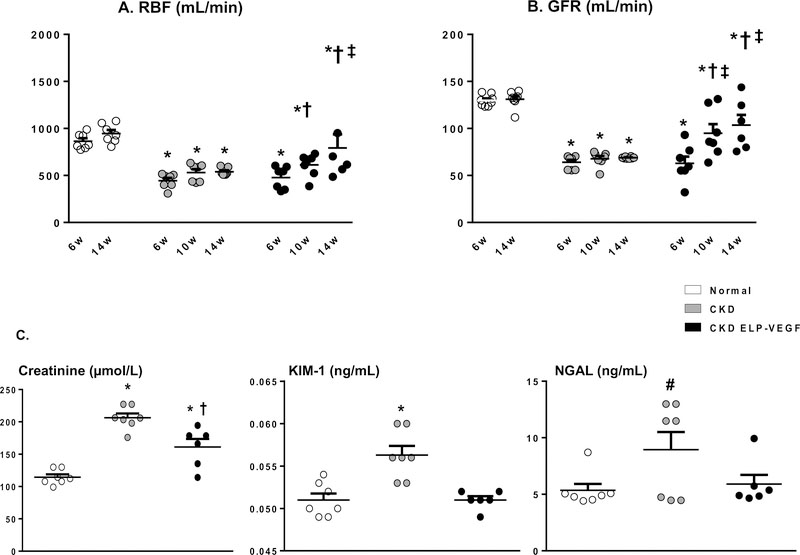
By the time of treatment at 6 weeks, CKD and CKD ELP-VEGF pigs showed a similarly significant loss of RBF (−43.2% and −41.4% respectively, p < 0.05 vs. normal) and GFR (−50.7% and −49.6% respectively, p < 0.05 vs. normal; Figure 1A–B). These changes were associated with significant reductions in renal perfusion and elevated RVR (Table 1A).
Figure 1.
Renal blood flow (A) and glomerular filtration rate (B) in normal, CKD, and CKD ELP-VEGF groups at 6, 10, and 14 weeks (n=6–7/group). Both RBF and GFR were dramatically reduced in all CKD animals at 6 weeks but progressively improved at 10 and 14 weeks (w) in ELP-VEGF treated pigs. C) Serum creatinine and urinary markers of renal injury KIM-1 and NGAL (n=6–7 samples/group) were elevated in untreated CKD and attenuated in ELP-VEGF treated pigs after 14 weeks of observation. *p<0.05 vs. normal; † p<0.05 vs. CKD; ‡ p<0.05 vs. 6 or 10 weeks (Single way ANOVA, Tukey).
# p=0.059 (NGAL, single-way ANOVA).
At 10 weeks (4 weeks after treatment), CKD ELP-VEGF pigs showed significant improvement of RBF (+31.6% vs. 6 weeks, p < 0.05) and GFR (+47.4% vs. 6 weeks, p < 0.05) compared to untreated CKD (p=NS). Interestingly, at 14 weeks, RBF (+65.8% vs 6 weeks, p < 0.05) and GFR (+61.1% vs 6 weeks, p < 0.05) seem to continue their improvement even 8 weeks after treatment (Figure 1A–B), suggesting a long-term and sustained recovery.
On the other hand, RBF and GFR in untreated CKD pigs remained unchanged at 14 weeks (+1.3% and +1.4%, respectively, p=NS vs. 6 weeks), showing persistent loss of renal hemodynamics observed at 6 weeks (−44.4% and −49.4%, respectively, p < 0.05 vs. normal) that implies sustained renal injury. One pig from the CKD ELP-VEGF group died unexpectedly (unrelated to treatment) on week 12, resulting in n=6 at 14 weeks for that group (Figure 1A–B).
Furthermore, ELP-VEGF therapy in CKD was also associated with improved serum creatinine and urinary KIM-1 and NGAL (Figure 1C). Moreover, a significant improvement in renal perfusion and RVR at 14 weeks was followed by a trend towards attenuation of hypertension (Table 1B). On the other hand, no significant changes in any of these parameters were observed in untreated CKD throughout 14 weeks of observation.
Ex Vivo Studies
Renal microvascular architecture
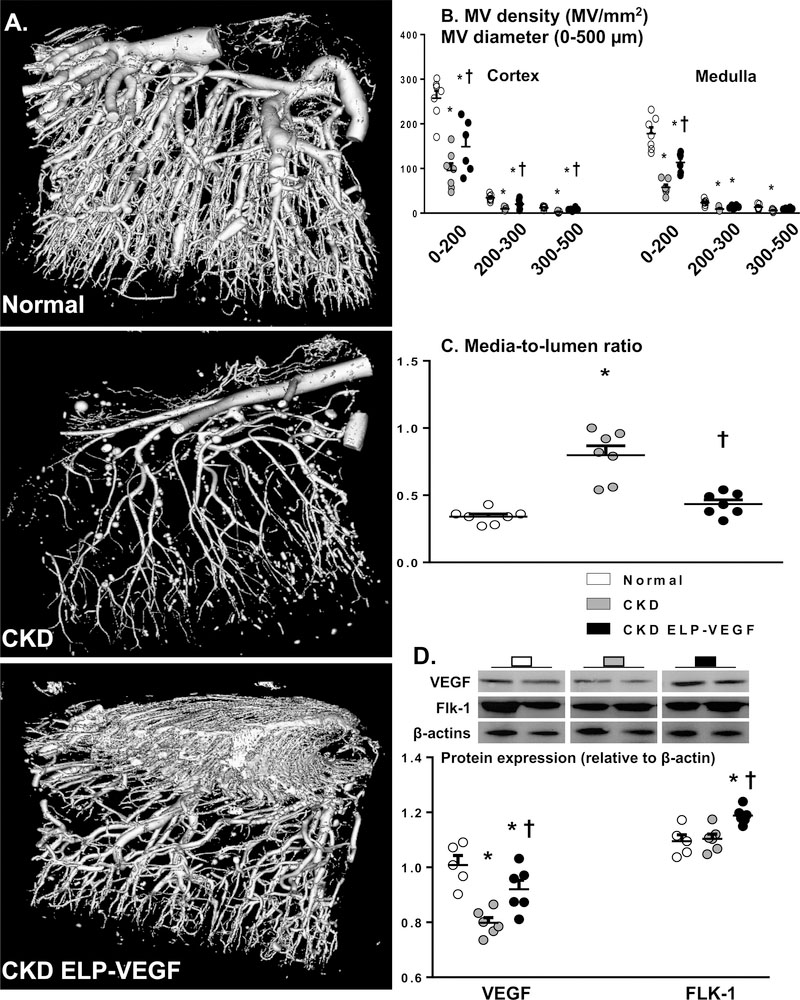
Renal MV density (vessels < 500 μm in diameter) was reduced in both cortex and medulla of CKD kidneys compared to normal (Figure 2A and B), associated with increased media-to-lumen ratio and decreased protein expression of VEGF but not its receptor Flk-1 (Figure 2C and D). On the other hand, ELP-VEGF therapy in CKD significantly improved renal MV density compared to untreated CKD (more evident on vessels < 200 μm in diameter, but also with a strong positive trend on vessels between 200–500 μm), accompanied by improved media-to-lumen ratio and expression of VEGF and Flk-1 (Figure 2A–D).
Figure 2.
Representative Micro-CT images of renal microvasculature, quantification of cortical and medullary microvascular density, and media-to-lumen ratio (A-C, n=6–7/group; single way ANOVA, Fisher), and renal protein expression of VEGF/Flk-1 (D, 2 representative bands per group, n=5–6/group; single way ANOVA, Tukey) from normal, CKD, and CKD ELP-VEGF pigs after 14 weeks of observation. MV density and media-to-lumen ratio in CKD were significantly improved by ELP-VEGF therapy compared to placebo, accompanied by augmented expression of renal VEGF and the Flk-1 receptor. *p<0.05 vs. normal; † p<0.05 vs. CKD.
Renal Inflammation and characterization of macrophages
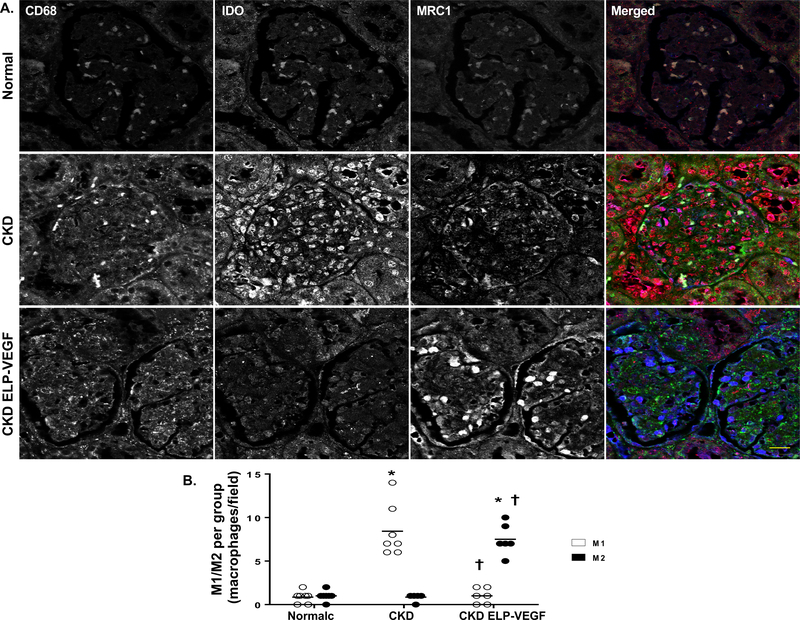
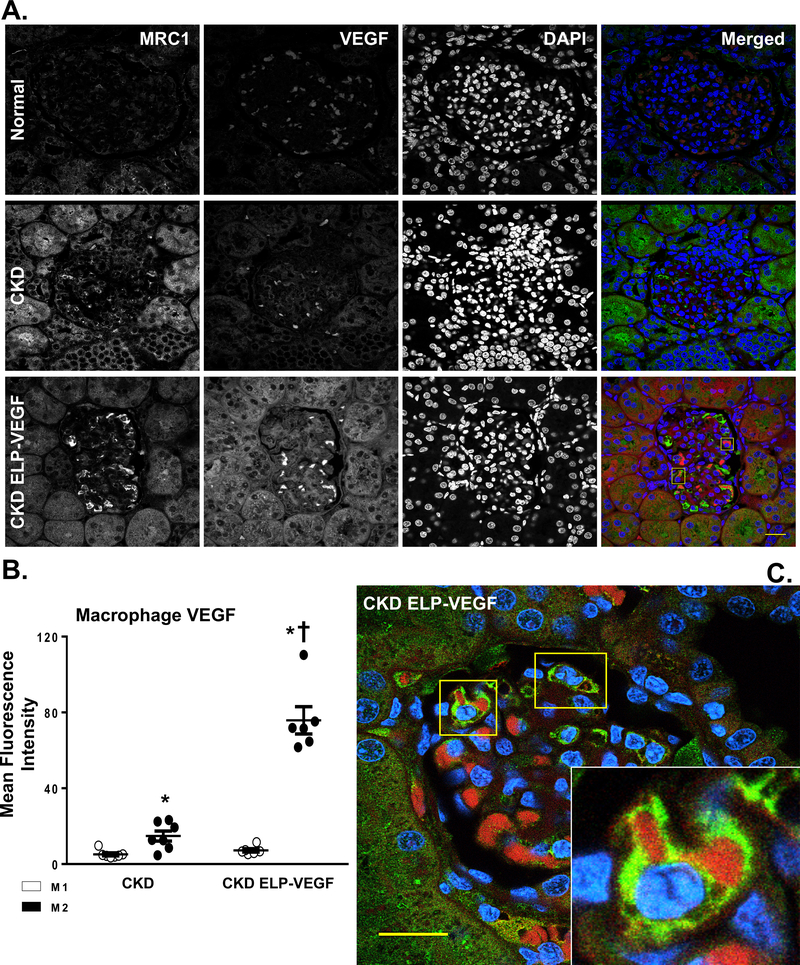
Abundant macrophages were observed in all CKD kidneys, independent of the treatment/placebo. As shown in Figure 3, the total number of renal macrophages in CKD and CKD ELP-VEGF was similar. Interestingly, CD68+ macrophage infiltration in CKD was predominantly polarized to an M1 phenotype (IDO+, MRC1-, Figure 3A–B) densely expressing NF-κB (Supplementary Figure S1A–C), whereas in the ELP-VEGF treated kidneys, the macrophages were clearly polarized to a M2 (IDO-, MRC1+) phenotype (Figure 3A–B). These M2 macrophages had significantly reduced NF-κB expression (Supplementary Figure S1A–C) and enhanced VEGF immunoreactivity (Figure 4A–C), as clearly observed in the 3D full Z-stack (Supplementary video SV1). Accumulation of VEGF-expressing M2 macrophages after ELP-VEGF therapy were observed in glomeruli and peritubular spaces.
Figure 3.
Representative immunostaining for markers of total (CD68, green), M1 (IDO, red), and M2 (MRC1, blue) macrophages (A) and quantification of M1/M2 staining (B) from normal, CKD, and CKD ELP-VEGF pigs after 14 weeks of observation (n=6–7/group). Dense renal infiltration of macrophages was observed after 14 weeks of CKD, with a predominance of M1 macrophages over M2. Notably, although the number of renal macrophages remained elevated, there was a marked shift towards an M2 phenotype after ELP-VEGF therapy. Scale bar represents 20 μm. *p<0.05 vs. normal; † p<0.05 vs. CKD (Single way ANOVA, Tukey).
Figure 4.
A-B) Representative quantification (A) of renal cross sections showing co-immunoreactivity for M2 macrophages (MRC1, green), VEGFA (red), and DAPI (blue) from normal, CKD, and CKD ELP-VEGF pigs after 14 weeks of observation (n=6–7/group). B) Quantification of mean fluorescence intensity of VEGF staining in M1 and M2 macrophages. C) Representative magnified renal cross section showing glomerular cells displaying co-immunoreactivity of MRC1, VEGFA and DAPI (n=6–7/group) treated with ELP-VEGF therapy. Normal kidneys showed dispersed VEGF staining, which was attenuated in CKD. After ELP-VEGF therapy in CKD, significantly increased VEGF expression was observed predominantly inside of M2 macrophages. Although representative glomeruli are shown here, accumulation of VEGF-expressing M2 macrophages after ELP-VEGF therapy was also observed in the peritubular spaces. Scale bar: 20 μm. *p<0.05 vs. CKD M1 and CKD ELP VEGF M1; † p<0.05 vs. CKD M2 (Single way ANOVA, Tukey).
VEGF and Macrophages polarization in vitro
VEGF exposure under hypoxia significantly increased expression of the M2 macrophage marker MRC1 only in naïve M0 but not in M1, which was associated with increased VEGF expression, suggesting a direct effect of VEGF on M0 to M2 differentiation (Supplementary Figure S2).
Renal expression of fibrogenic factors and fibrosis
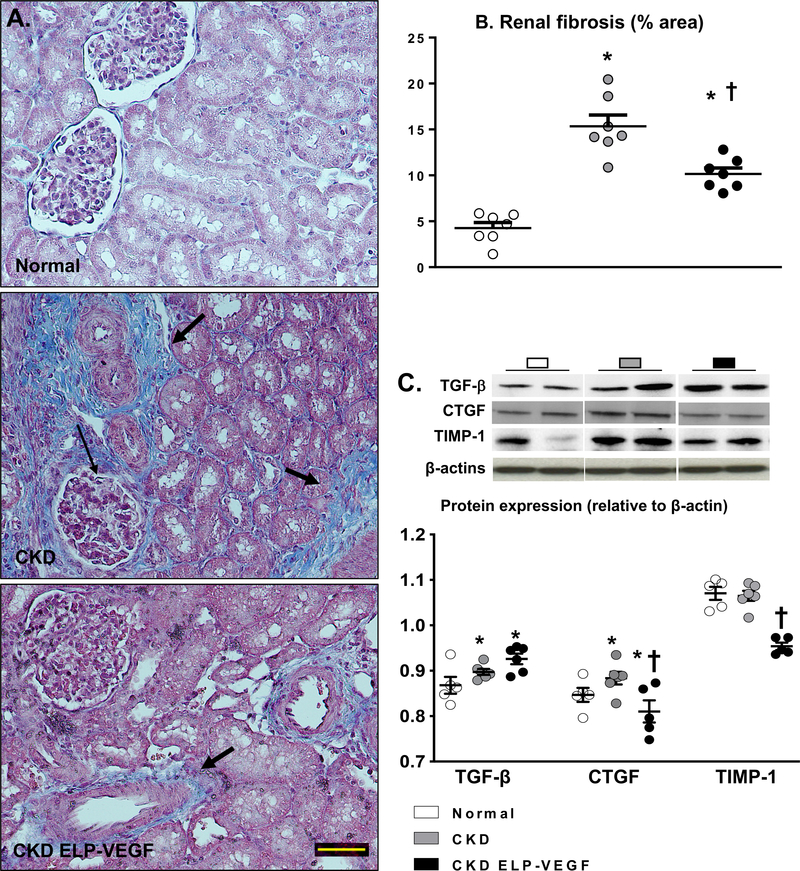
Significant renal fibrosis was observed throughout renal compartments in untreated CKD (Figure 5A–B), but was improved in ELP-VEGF treated pigs, predominantly in the tubulointerstitial and glomerular compartments. Concomitantly, renal expression of pro-fibrotic factors CTGF and TIMP-1 were also improved after ELP-VEGF therapy in CKD, whereas TGF-β remained elevated (Figure 5C).
Figure 5.
Representative renal cross-sections stained with trichrome and morphometric analysis (A-B, n=6–7/group), and renal protein expression of TGF-β, CTGF, and TIMP-1 (C, 2 representative bands per group, n=5–6/group) from normal, CKD, and CKD ELP-VEGF pigs after 14 weeks of observation. Dense peritubular and glomerular fibrosis in CKD was ameliorated by ELP-VEGF. This was associated with improved renal expression of pro-fibrotic growth factors. *p<0.05 vs. normal; † p<0.05 vs. CKD (Single way ANOVA, Tukey).
Discussion
The damage of the renal microcirculation is a universal pathological feature in CKD that plays a pivotal role in the pathogenesis of CKD, yet there are no available treatments which target MV dysfunction and loss. The present study supports the potential and shows the efficacy of a new therapeutic strategy using drug-delivery technology to recuperate renal function in CKD. We tested this approach using our translational swine model of CKD26 that displays major common pathological attributes observed in CKD regardless of the etiology. A single dose of ELP-VEGF induced a sustained, progressive renal recovery which far outlasted the life of the injected construct. Our study thus shows for the first time that ELP-VEGF-driven MV healing may be a feasible strategy in CKD and that effects of VEGF on renal macrophage phenotype and inflammation may be a distinct underlying mechanism of renal recovery, MV protection, and reduction of renal injury.
Prior to treatment, all CKD pigs showed a significant loss of RBF and GFR (comparable to human CKD stage 236) which associated with increased MAP and serum creatinine. We observed, 4 weeks after treatment, that ELP-VEGF improved RBF and GFR, which is consistent with previous studies using ELP-VEGF in a model of unilateral renovascular disease17, 18. Recovery of GFR in CKD patients is uncommon (observed in about 25% of patients under nephrology care) and occurs more frequently at earlier stages37. This is the first study using this model in which renal function has been sequentially quantified with a longitudinal follow-up 4 and 8 weeks after VEGF administration. Notably, we observed that improvement of RBF and GFR occurred in a stepwise fashion 4 and 8 weeks after ELP-VEGF therapy and was associated with attenuated hypertension and reduced creatinine. Renal recovery was further evidenced by improved urinary concentration of markers of renal injury KIM-1 and NGAL38, 39. If compared to human CKD, the ELP-VEGF treated pigs had improved to CKD stage 1 while untreated pigs remained stage 2 by the completion of the 14-week study. Given that loss of nephrons is irreversible, it is possible that recovery of renal function occurs via regaining of “hibernating” renal parenchyma (i.e. damaged but recoverable nephrons)40, 41. We propose that by protecting the renal microvasculature, ELP-VEGF improves blood flow to recoverable parenchyma, leading to the regaining of function and subsequent regression of CKD stage observed in our model.
We showed that the ELP-VEGF construct has a prolonged half-life compared to free VEGF18 and that a single ELP-VEGF treatment should be fully cleared from the body within three days. Interestingly, our study shows that RBF and GFR continued to improve 4 and even 8 weeks after treatment in CKD, long after the ELP-VEGF had been administered and likely cleared. VEGF is a potent angiogenic cytokine which plays a pivotal role in maintenance of MV integrity. Signaling through the Flk-1 receptor, VEGF induces endothelial proliferation and tube formation to stimulate neovascularization and acts as a pro-survival factor to maintain existing MV networks42. We observed a considerable improvement in cortical and medullary MV density after intra-renal ELP-VEGF therapy compared to placebo, which was most pronounced among small diameter vessels (under 200μm) but also showed improvements in larger cortical microvessels as well (200–500 μm). This more pronounced effect on MV density of smaller vessels first agrees with previous studies indicating that VEGF induces MV sprouting from pre-existing vessels (angiogenesis) rather than de novo vasculogenesis10, 17. Supported by the micro-CT resolution, the effects ELP-VEGF therapy on the renal MV network may have likely included, at least, interlobular, afferent and efferent arterioles, glomerular and peritubular capillaries. Notably, we also observed a reduced intra-renal MV media-to-lumen ratio after ELP-VEGF therapy, suggesting attenuated MV remodeling and protection of the existing vasculature as well. Paired with the significant improvement in renal hemodynamics after ELP-VEGF, these findings support a prominent role of the renal MV integrity on both CKD pathophysiology and recovery prospects.
A recent clinical study in 1,444 patients undergoing cardiac surgery shows that higher levels of circulating VEGF associates with lower cardiovascular and renal (e.g. acute kidney injury) mortality risk43. In line with those provocative findings, our study support the notion that single ELP-VEGF therapy may activate a long-term endogenous mechanism of MV repair that contributes to the progressive functional recovery and that reversal of MV rarefaction in CKD has a distinct functional consequence. A potential underlying mechanism of this prolonged recovery may involve an effect of VEGF on macrophage phenotype and inflammation. Inflammation is closely linked to renal function and predicts further decline in CKD19, 20. NF-κB is a transcription factor with a central role in controlling inflammation, cell proliferation and survival44. NF-κB is normally found in the cytoplasm bound by its inhibitor IκB. In events of cellular stress, IkB becomes ubiquitinated and is subsequently targeted to the proteasome for degradation, thereby allowing NF-κB to translocate to the nucleus and stimulate transcription of pro-inflammatory genes45. We showed that diet-induced dyslipidemia favors renal inflammation by increasing NF-κB activity via stimulating proteasome degradation of IκB46, 47 and that increased renal inflammation and NF-κB expression are present in renovascular disease48 and in the CKD model26. We observed upregulation of the renal NF-κB /IκB pathway in untreated CKD, accompanied by dense staining of NF-κB in renal macrophages. Given that NF-κB is known to induce M1 macrophage differentiation49, 50, up-regulation of NF-κB in our model26 may likely be a major force stimulating differentiation of macrophages to an injurious M1 phenotype.
M1 macrophages are often considered deleterious as they sustain the pro-inflammatory environment, leading to progression of renal injury and development of fibrosis51–53. M1 macrophages in the CKD kidney may release inflammatory cytokines and worsen vascular dysfunction51, further stimulating M1 polarization in a feed-back mechanism49. In contrast, M2 macrophages are frequently involved in renal repair and functional recovery23, 54. An abundance of pro-inflammatory M1 macrophage infiltration is characteristic of CKD, and failure of these cells to switch to an M2 phenotype is associated with further deterioration of renal function26, 51. Based on the observed effect of VEGF on macrophage polarization in tumor studies, it is possible that ELP-VEGF interaction with Flt-1 on macrophages directly stimulates M2 differentiation25. Indeed, VEGF has been shown to facilitate development of tumor-associated macrophages (closely analogous to M2s), which have been subsequently shown to enhance local vascularization and suppress immune activation25, 55. These effects, while deleterious in cancer, could prove to be beneficial in kidneys afflicted by MV injury and inflammation as observed in CKD. Furthermore, a recent study shows that VEGF may exert a negative regulation of NF-κB signaling56. Thus, a transient burst of VEGF as used in our study may have provided sufficient anti-inflammatory stimulus to break a likely vicious cycle of M1 polarization and inflammation in CKD. Supporting this, the results of our in vitro study of macrophage polarization demonstrate a direct effect of VEGF to polarize macrophages to an M2 phenotype and increase angiogenic potential (Supplementary Figure S2). However, given the transient nature of the construct, the administered ELP-VEGF and its short-term effects on renal VEGF concentration and downstream signaling are unlikely to be reflected in the ex vivo analysis of tissues collected 8 weeks after treatment (Figure 2D). As such, the relatively modest increase in Flk-1 and VEGF expression by western blot possibly reflects an increased overall number of endothelial cells expressing the Flk-1 receptor and improved endogenous VEGF production, as the administered VEGF has been long since cleared. The shift of renal macrophages to an M2 phenotype following ELP-VEGF therapy was associated with significant M2 immunoreactivity of VEGF and recovery of renal VEGF expression, implying that M2 macrophages may help to restore angiogenic signaling and MV integrity in CKD. This potential role of VEGF in development of a trophic macrophage phenotype supports a novel mechanism of renal MV recovery.
Our results indicate that therapeutic effects of ELP-VEGF in CKD may be partly mediated by polarization of macrophages to an M2 phenotype, thereby suppressing renal inflammation, contributing to MV proliferation and repair, and consequently enhancing functional recovery. Nevertheless, it is interesting that the total number of renal macrophages was similar but the phenotype was different after ELP-VEGF therapy, suggesting that VEGF did not interfere with macrophage recruitment. Hence, it is possible that the change in macrophage phenotype after ELP-VEGF therapy may reflect salutary consequences of improved renal perfusion, resulting in a healthier, less pro-inflammatory renal parenchyma. We cannot exclude the possibility that effects of VEGF on macrophage polarization may possibly be concomitant to (or mediated by) direct effects of VEGF on neovascularization.
Development of renal fibrosis is likely an irreversible step in the progression of kidney disease. M2 macrophages are suggested to be a source of pro-fibrotic cytokines like TGFβ and have been proposed to significantly contribute to renal fibrosis57, 58. The increase in renal M2 macrophages may explain the unchanged renal expression of TGFβ. Nevertheless, M2 predominance after ELP-VEGF therapy was associated with attenuation of fibrosis and improved renal expression of other pro-fibrotic factors such as CTGF, and TIMP-1. Thus, our findings may align with the conclusions of Lech et al51, that persistent tubular inflammation and atrophy plays a prominent role in the development of renal fibrosis and that the beneficial immunomodulatory effects of M2 macrophages likely outweigh any direct pro-fibrotic effects.
Limitations and Opportunities
In humans, CKD develops gradually over many years, with most CKD patients being over 651. This makes it especially challenging to create a translational model that is physiologically accurate without using very long studies. Domestic pigs may continue to grow to over 300 kg in time, making long term studies difficult. The animals used in this study were juvenile pigs which grew from 30 kg to about 70 kg (roughly the size of an adult human) after 14 weeks but were still sexually immature. We showed the translational traits of our model, demonstrating major common pathological features of CKD that are present regardless of the initial insult26, but we believe that studies using older animals and later timepoints would be ideal to address potential roles of confounding biological variables such as aging. Similarly, given that the etiology of CKD is variable, future studies inducing additional insults that may exacerbate the progression of renal injury in CKD (e.g. diabetes, obesity59–62) will expand our current findings and may contribute to determine the extent and timely application of this novel therapeutic strategy and to move it towards potential clinical applications.
Perspectives
The results of this study support the feasibility of targeted VEGF therapy as a novel strategy for CKD. This study demonstrates the therapeutic potential of ELP-VEGF to recover renal MV integrity, hemodynamics, and function in a highly translational model of CKD and shows that ELP-VEGF therapy led to sustained, progressive recovery outlasting the life of the therapeutic construct. Our results also suggest that renoprotective effects are partly through suppression of renal inflammation by inducing a sustained shift in renal macrophages to an anti-inflammatory M2 phenotype, thereby attenuating further MV dysfunction and fibrosis and inducing renal recovery.
CKD awareness in patients is generally low and can advance undetected. Earlier recognition and intervention could slow progression, prevent complications, and reduce cardiovascular risk63, 64. Thus, the data presented in this study support the translational potential of ELP-VEGF therapy to slow or partially reverse the progressive nature of CKD and to improve the prospects of renal recovery.
Supplementary Material
Novelty and Significance.
1). What Is New
Our results support the feasibility of VEGF therapy to offset the progression or renal injury using a novel therapeutic construct of a drug-delivery vector (elastin-like polypeptide, ELP) fused to VEGF that was tested in a translational model of CKD.
Our study provides mechanistic evidence that modulation of macrophage phenotype and renal inflammation by ELP-VEGF therapy plays a role in the sustained renal recovery.
2). What Is Relevant?
Renal inflammation and microvascular (MV) rarefaction are major pathological features of CKD that are reproduced in the swine model used in this study. Targeting these pathways may thus present a novel therapeutic strategy for CKD.
ELPs confer a distinctly higher renal accumulation and can be conjugated to nearly any peptide or small molecule therapeutics, offering the possibility of developing targeted therapies for renal pathologies beyond the application described herein.
Acknowledgements and sources of funding
This work was supported by grants R01HL095638, P01HL51971, P20GM104357, and R01HL121527 from the National Institute of Health and grants IPA34170267, PRE34380314, and PRE34380274 from the American Heart Association.
Footnotes
Disclosures: GLB is owner of Leflore Technologies LLC, a private company working to develop and commercialize ELP-based technologies in several disease areas. GLB and ARC are inventors on patents related to ELP technology.
References
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. American Journal of Kidney Diseases. 2018;71:A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horowitz B, Miskulin D, Zager P. Epidemiology of hypertension in ckd. Advances in chronic kidney disease. 2015;22:88–95 [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg M Overview of the management of chronic kidney disease in adults. In: Forman JP, ed. Uptodate; 2018. [Google Scholar]
- 4.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: Global dimension and perspectives. Lancet (London, England). 2013;382:260–272 [DOI] [PubMed] [Google Scholar]
- 5.Nangaku M Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. Journal of the American Society of Nephrology : JASN. 2006;17:17–25 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi J, Tanaka T, Nangaku M. Recent advances in understanding of chronic kidney disease. F1000Research. 2015;4:F1000 Faculty Rev-1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015;87:297–307 [DOI] [PubMed] [Google Scholar]
- 8.Xavier S, Vasko R, Matsumoto K, Zullo JA, Chen R, Maizel J, Chander PN, Goligorsky MS. Curtailing endothelial tgf-beta signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in ckd. Journal of the American Society of Nephrology : JASN. 2015;26:817–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ligresti G, Nagao RJ, Xue J, Choi YJ, Xu J, Ren S, Aburatani T, Anderson SK, MacDonald JW, Bammler TK, Schwartz SM, Muczynski KA, Duffield JS, Himmelfarb J, Zheng Y. A novel three-dimensional human peritubular microvascular system. Journal of the American Society of Nephrology : JASN. 2016;27:2370–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chade AR, Kelsen S. Reversal of renal dysfunction by targeted administration of vegf into the stenotic kidney: A novel potential therapeutic approach. American journal of physiology. Renal physiology. 2012;302:F1342–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chade AR, Kelsen S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circulation. Cardiovascular interventions. 2010;3:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urry DW, Trapane TL, Wood SA, Walker JT, Harris RD, Prasad KU. D-ala5 analog of the elastin polypentapeptide. Physical characterization. Int J Pept Protein Res. 1983;22:164–175 [DOI] [PubMed] [Google Scholar]
- 13.George EM, Liu H, Robinson GG, Mahdi F, Perkins E, Bidwell GL 3rd. Growth factor purification and delivery systems (pads) for therapeutic angiogenesis. Vascular cell. 2015;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moktan S, Ryppa C, Kratz F, Raucher D. A thermally responsive biopolymer conjugated to an acid-sensitive derivative of paclitaxel stabilizes microtubules, arrests cell cycle, and induces apoptosis. Invest New Drugs. 2012;30:236–248 [DOI] [PubMed] [Google Scholar]
- 15.Bidwell GL 3rd, Fokt I, Priebe W, Raucher D. Development of elastin-like polypeptide for thermally targeted delivery of doxorubicin. Biochem Pharmacol. 2007;73:620–631 [DOI] [PubMed] [Google Scholar]
- 16.Bidwell GL 3rd, Mahdi F, Shao Q, Logue OC, Waller JP, Reese C, Chade AR. A kidney-selective biopolymer for targeted drug delivery. American journal of physiology. Renal physiology. 2017;312:F54–f64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chade AR, Tullos NA, Harvey TW, Mahdi F, Bidwell GL 3rd. Renal therapeutic angiogenesis using a bioengineered polymer-stabilized vascular endothelial growth factor construct. Journal of the American Society of Nephrology : JASN. 2016;27:1741–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chade AR, Williams ML, Guise E, Vincent LJ, Harvey TW, Kuna M, Mahdi F, Bidwell GL 3rd. Systemic biopolymer-delivered vascular endothelial growth factor promotes therapeutic angiogenesis in experimental renovascular disease. Kidney Int. 2018;93:842–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911 [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658 [DOI] [PubMed] [Google Scholar]
- 21.Smigiel KS, Parks WC. Macrophages, wound healing, and fibrosis: Recent insights. Current rheumatology reports. 2018;20:17. [DOI] [PubMed] [Google Scholar]
- 22.Kon V, Linton MF, Fazio S. Atherosclerosis in chronic kidney disease: The role of macrophages. Nature Reviews Nephrology. 2010;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng XM, Tang PMK, Li J, Lan HY. Macrophage phenotype in kidney injury and repair. Kidney Diseases. 2015;1:138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. The Journal of Clinical Investigation. 2008;118:3522–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muramatsu M, Yamamoto S, Osawa T, Shibuya M. Vascular endothelial growth factor receptor-1 signaling promotes mobilization of macrophage lineage cells from bone marrow and stimulates solid tumor growth. Cancer research. 2010;70:8211–8221 [DOI] [PubMed] [Google Scholar]
- 26.Chade AR, Williams ML, Engel J, Guise E, Harvey TW. A translational model of chronic kidney disease in swine. American journal of physiology. Renal physiology. 2018;315:F364–f373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171 [DOI] [PubMed] [Google Scholar]
- 28.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector ct: Comparison with electron-beam ct. Radiology. 2007;243:405–412 [DOI] [PubMed] [Google Scholar]
- 30.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. American journal of physiology. Renal physiology. 2001;281:F630–638 [DOI] [PubMed] [Google Scholar]
- 31.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1706–1708 [DOI] [PubMed] [Google Scholar]
- 32.Kapetanovic R, Fairbairn L, Beraldi D, Sester DP, Archibald AL, Tuggle CK, Hume DA. Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. The Journal of Immunology. 2012;188:3382–3394 [DOI] [PubMed] [Google Scholar]
- 33.Schroder K, Irvine KM, Taylor MS, et al. Conservation and divergence in toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chade AR, Tullos N, Stewart NJ, Surles B. Endothelin-a receptor antagonism after renal angioplasty enhances renal recovery in renovascular disease. Journal of the American Society of Nephrology : JASN. 2015;26:1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tullos NA, Stewart NJ, Davidovich R, Chade AR. Chronic blockade of endothelin a and b receptors using macitentan in experimental renovascular disease. Nephrol Dial Transplant. 2015;30:584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapter 1: Definition and classification of ckd. Kidney International Supplements. 2013;3:19–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrelli S, Leonardis D, Minutolo R, Chiodini P, De Nicola L, Esposito C, Mallamaci F, Zoccali C, Conte G. Epidemiology of ckd regression in patients under nephrology care. PLoS One. 2015;10:e0140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moresco RN, Bochi GV, Stein CS, De Carvalho JAM, Cembranel BM, Bollick YS. Urinary kidney injury molecule-1 in renal disease. Clinica chimica acta; international journal of clinical chemistry. 2018;487:15–21 [DOI] [PubMed] [Google Scholar]
- 39.Rysz J, Gluba-Brzózka A, Franczyk B, Jabłonowski Z, Ciałkowska-Rysz A. Novel biomarkers in the diagnosis of chronic kidney disease and the prediction of its outcome. Int J Mol Sci. 2017;18:1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung CM, Chrysochou C, Shurrab AE, Buckley DL, Cowie A, Kalra PA. Effects of renal volume and single-kidney glomerular filtration rate on renal functional outcome in atherosclerotic renal artery stenosis. Nephrol Dial Transplant. 2010;25:1133–1140 [DOI] [PubMed] [Google Scholar]
- 41.Warncke J, David S, Kumpers P, Opherk JP, Haller H, Fliser D. A hibernating kidney - ischemic preconditioning in a renal transplant recipient with a proximal stenosis of the iliac artery. Clin Nephrol. 2008;70:168–171 [DOI] [PubMed] [Google Scholar]
- 42.Eichmann A, Simons M. Vegf signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24:188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mansour SG, Zhang WR, Moledina DG, Coca SG, Jia Y, Thiessen-Philbrook H, McArthur E, Inoue K, Koyner JL, Shlipak MG, Wilson FP, Garg AX, Ishibe S, Parikh CR. The association of angiogenesis markers with acute kidney injury and mortality after cardiac surgery. Am J Kidney Dis. 2019;74:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Nino MD, Poveda J, Sanz AB, Mezzano S, Carrasco S, Fernandez-Fernandez B, Burkly LC, Nair V, Kretzler M, Hodgin JB, Ruiz-Ortega M, Selgas R, Egido J, Ortiz A. Fn14 in podocytes and proteinuric kidney disease. Biochimica et biophysica acta. 2013;1832:2232–2243 [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Chen ZJ. Regulation of nf-kappab by ubiquitination. Current opinion in immunology. 2013;25:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chade AR, Best PJ, Rodriguez-Porcel M, Herrmann J, Zhu X, Sawamura T, Napoli C, Lerman A, Lerman LO. Endothelin-1 receptor blockade prevents renal injury in experimental hypercholesterolemia. Kidney Int. 2003;64:962–969 [DOI] [PubMed] [Google Scholar]
- 47.Chade AR, Herrmann J, Zhu X, Krier JD, Lerman A, Lerman LO. Effects of proteasome inhibition on the kidney in experimental hypercholesterolemia. Journal of the American Society of Nephrology : JASN. 2005;16:1005–1012 [DOI] [PubMed] [Google Scholar]
- 48.Zhu XY, Chade AR, Krier JD, Daghini E, Lavi R, Guglielmotti A, Lerman A, Lerman LO. The chemokine monocyte chemoattractant protein-1 contributes to renal dysfunction in swine renovascular hypertension. Journal of hypertension. 2009;27:2063–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. The Journal of Clinical Investigation. 2012;122:787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connelly L, Barham W, Onishko HM, Chen L, Sherrill TP, Zabuawala T, Ostrowski MC, Blackwell TS, Yull FE. Nf-kappab activation within macrophages leads to an anti-tumor phenotype in a mammary tumor lung metastasis model. Breast Cancer Research : BCR. 2011;13:R83–R83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, Susanti HE, Kobayashi KS, Flavell RA, Anders H-J. Macrophage phenotype controls long-term aki outcomes—kidney regeneration versus atrophy. Journal of the American Society of Nephrology : JASN. 2014;25:292–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikezumi Y, Suzuki T, Hayafuji S, Okubo S, Nikolic-Paterson DJ, Kawachi H, Shimizu F, Uchiyama M. The sialoadhesin (cd169) expressing a macrophage subset in human proliferative glomerulonephritis. Nephrol Dial Transplant. 2005;20:2704–2713 [DOI] [PubMed] [Google Scholar]
- 53.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant. 2006;21:1231–1239 [DOI] [PubMed] [Google Scholar]
- 54.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory m2, but not pro-inflammatory m1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118 [DOI] [PubMed] [Google Scholar]
- 55.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized m2 mononuclear phagocytes. Trends in immunology. 2002;23:549–555 [DOI] [PubMed] [Google Scholar]
- 56.Huang H, Langenkamp E, Georganaki M, Loskog A, Fuchs PF, Dieterich LC, Kreuger J, Dimberg A. Vegf suppresses t-lymphocyte infiltration in the tumor microenvironment through inhibition of nf-kappab-induced endothelial activation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:227–238 [DOI] [PubMed] [Google Scholar]
- 57.Kim M-G, Kim SC, Ko YS, Lee HY, Jo S-K, Cho W. The role of m2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLOS ONE. 2015;10:e0143961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saito H, Tanaka T, Tanaka S, Higashijima Y, Yamaguchi J, Sugahara M, Ito M, Uchida L, Hasegawa S, Wakashima T, Fukui K, Nangaku M. Persistent expression of neutrophil gelatinase-associated lipocalin and m2 macrophage markers and chronic fibrosis after acute kidney injury. Physiological reports. 2018;6:e13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marshall SM. Natural history and clinical characteristics of ckd in type 1 and type 2 diabetes mellitus. Advances in chronic kidney disease. 2014;21:267–272 [DOI] [PubMed] [Google Scholar]
- 60.Futrakul N, Futrakul P. Renal microvascular disease predicts renal function in diabetes. Ren Fail. 2012;34:126–129 [DOI] [PubMed] [Google Scholar]
- 61.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iliescu R, Chade AR. Progressive renal vascular proliferation and injury in obese zucker rats. Microcirculation. 2010;17:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Advances in chronic kidney disease. 2010;17:225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuot DS, Zhu Y, Velasquez A, Espinoza J, Mendez CD, Banerjee T, Hsu C-y, Powe NR. Variation in patients’ awareness of ckd according to how they are asked. Clinical Journal of the American Society of Nephrology. 2016;11:1566–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.