Abstract
Background
Studies have identified that the telomerase reverse transcriptase (TERT) gene polymorphism rs10069690 (C>T) is associated with cancer risk, but the results remain inconclusive.
Methods
To provide a more precise estimation of the relationship, we performed a meta‐analysis of 45 published studies including 329,035 cases and 730,940 controls. We conducted a search in PubMed, Google Scholar and Web of Science to select studies on the association between rs10069690 and cancer risk. Stratification by ethnicity, cancer type, cancers’ classification, source of control, sample size, and genotype method was used to explore the source of heterogeneity. The pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were evaluated using random effects models. Sensitivity, publication bias, false‐positive report probability (FPRP) and statistical power were also assessed.
Results
The result demonstrated that rs10069690 was significantly associated with an increased risk of cancer overall (OR = 1.09, 95% CI: 1.06–1.12, p < .001) under the allele model. Stratification analysis revealed an increased cancer risk in subgroups of breast cancer, ovarian cancer, lung cancer, thyroid cancer, and renal cell carcinoma (RCC). However, a significantly decreased association was observed in pancreatic cancer in the European population (OR = 0.93,95% CI: 0.87–0.99, p = .031). In the subgroup analysis based on cancer type, no significant association was found in prostate cancer, leukemia, colorectal cancer and glioma.
Conclusions
This meta‐analysis suggested that the TERT rs10069690 polymorphism may be a risk factor for cancer, especially breast cancer, ovarian cancer, lung cancer, thyroid cancer, and RCC. Further functional studies are warranted to reveal the role of the polymorphism in carcinogenesis.
Keywords: cancer, meta‐analysis, polymorphism, TERT
1. INTRODUCTION
Cancer is one of the leading causes of human death worldwide and constitutes an enormous burden on the society in both economically developed and developing countries (Are et al., 2013). Based on GLOBOCAN estimates, about 18.1 million newly diagnosed cancer patients and 9.6 cancer million deaths occurred in 2018 worldwide (Bray et al., 2018). The mechanism of occurrence and development of cancer remains unclear. People generally agree that a complex interaction between genetic and environmental factors may contribute to cancer development. Recently, genome‐wide association studies (GWAS) have demonstrated that single nucleotide polymorphisms (SNPs) in Chromosome 5p15.33, which is a crucial genomic region for telomere biology and contains two well‐known genes: telomerase reverse transcriptase (TERT) and cleft lip and palate transmembrane 1‐like (CLPTM1L), are significantly associated with cancer risk (Bojesen et al., 2013; Haiman et al., 2011; Rafnar et al., 2009; Wolpin et al., 2014).
Telomeres consisting of TTAGGG repeats are specialized structures at the end of eukaryotic chromosomes that protect chromosomes from degradation, end‐to‐end fusion, and atypical recombination; thus, telomeres are crucial in maintaining chromosome integrity and genomic stability (Blackburn, 2005). Telomere length is maintained by telomerase, a ribonucleoprotein enzyme that adds the telomeric repeat sequence directly to the single‐strand 3’ overhang to maintain telomere ends that have been incrementally shortened by each cell division (Collins & Mitchell, 2002). The expression of telomerase is extremely low in most normal human somatic cells, but is present in over 90% of human malignancies. As the catalytic subunit of telomerase, TERT is the most important determinant in the regulation of telomerase expression (Zhang et al., 2000).
TERT, located on the short (p) arm of chromosome 5 at position 15.33 (5p15.33), encodes a catalytic subunit of telomerase and exerts a pivotal role in the maintenance of telomere DNA length and carcinogenesis. Mutations in the coding regions of TERT can affect telomerase activity and telomere length, and generate severe clinical phenotypes, including a substantive increase in cancer frequency (Baird, 2010). Previous studies have demonstrated that rs10069690 (C>T) polymorphism in the TERT is associated with susceptibility to multiple types of cancer, such as breast cancer (Bojesen et al., 2013; Haiman et al., 2011; Huo et al., 2016; Michailidou et al., 2015, 2017), ovarian cancer (Bojesen et al., 2013; Earp et al., 2016; Kuchenbaecker et al., 2015; Lee et al., 2016; Phelan et al., 2017), lung cancer (Landi et al., 2009; Ye et al., 2017), and thyroid cancer (Gong et al., 2016; Gudmundsson et al., 2017). However, studies have yet to reach a consensus.
Meanwhile, a single study might have been underpowered to detect the overall effects. A quantitative synthesis of the accumulated data from different studies is important to provide evidence on the association of rs10069690 polymorphism with cancer risk. Therefore, in this study, we performed a comprehensive meta‐analysis including the latest and relevant articles to explore the association between the TERT rs10069690 polymorphism and cancer risk.
2. METHODS
2.1. Search strategy
According to the Meta‐analysis of Observational Studies in Epidemiology guidelines, we performed a systematic literature search on PubMed, Google Scholar, Embase, Web of Science, China national knowledge infrastructure (CNKI) and Wan fang electronic databases and sample size limitations covering all publications regarding the association between TERT polymorphisms and cancer susceptibility up to the end of May 2019. The search terms were as follows: “TERT”, “telomerase reverse transcriptase”, “5p15”, “polymorphism”, ‘“SNP”’, “variant’’, “cancer”, “tumor” “carcinoma” and ‘“malignancy”’. The search was limited to English language papers and human studies. In addition, references of articles and reviews were also searched to find other eligible studies. When an article reported results on different subpopulations, we treated each subpopulation as a separate comparison.
2.2. Inclusion and exclusion criteria
In this meta‐analysis, the following inclusion criteria were used for selecting the studies: (a) population‐ or hospital‐based case–control studies published in English as original articles; (b) investigating TERT rs10069690 polymorphism and cancer susceptibility; (c) studies provided the odds ratios (OR) estimates and their 95% confidence intervals (CIs) in allele model. The exclusion criteria were: (a) not involving TERT and rs10069690 polymorphism research; (b) case reports, reviews, repeated literature, nonhuman studies; (c) no available data presented.
2.3. Data extraction
Two investigators independently extracted the data from all eligible publications, according to the inclusion and exclusion criteria listed above. Discrepancies were resolved by discussion and consensus. We extracted the following information from each study when available: the first author's last name, year of publication, cancer type, patient ethnicity, number of cases and controls, genotyping method, the odds ratios (ORs) estimates and their 95% confidence intervals (CIs) in allele model. Quality scores of studies ranged from 0 (lowest) to 15 (highest). Studies with scores ≤9 were categorized into low quality, while those with scores >9 were considered as high quality (Fu et al., 2017).
2.4. Statistical analysis
We used the ORs with 95% CIs to assess the strength of association between the TERT rs10069690 polymorphism and cancers risk. The OR and the 95% CI in each comparison were assessed in the allele model. Stratified analyses were performed by cancer type (if one cancer type contained less than two individual studies, it was combined into the “other cancers” group), ethnicity, sample size, and genotyping method under the allele model. Heterogeneity was checked using the Chi‐square‐based Q statistic test. If the result of heterogeneity test was p > .05, then the pooled ORs were calculated using the fixed‐effects model with the Mantel–Haenszel method. If heterogeneity was present (p < .05), the random effects model (the DerSimonian and Laird method) was selected. The literature publication bias was estimated using the Funnel plot and Egger's linear regression test (p < .05 was considered a significant publication bias). The false‐positive report probability (FPRP) was calculated to evaluate the significant findings. We set 0.2 as an FPRP threshold and assigned a prior probability of 0.1 to detect an odds ratio (OR) of 0.67/1.50 (protective/risk effects) for an association with cancer risk under investigation. Only the significant result with an FPRP value less than 0.2 was considered a noteworthy finding (He et al., 2013). All statistical analyses were conducted using the Stata software (version 11.0; Stata Corporation), using two‐sided p values.
3. RESULTS
3.1. Characteristics of studies
The detailed process of study selection is summarized in the flow diagram (Figure 1). According to the inclusion criteria, a total of 45 eligible studies involving 329,035 cases and 730,940 controls were included in this meta‐analysis. The characteristics of selected studies are summarized in Table1. The 45 studies included nine on breast cancer (Garcia‐Closas et al., 2013; Haiman et al., 2011; Huo et al., 2016; Michailidou et al., 2015, 2017; Palmer et al., 2013; Purrington et al., 2014) and, six on ovarian cancer (Bojesen et al., 2013; Earp et al., 2016; Kuchenbaecker et al., 2015; Lee et al., 2016; Phelan et al., 2017; Terry et al., 2012); five on lung cancer (Gao et al., 2014; Landi et al., 2009; Wang et al., 2016; Ye et al., 2017; Zhao et al., 2013). two each on glioma (Melin et al., 2017; Zhao et al., 2012), thyroid cancer (Gong et al., 2016; Gudmundsson et al., 2017), pancreatic cancer (Campa, Rizzato, et al., 2015; Petersen et al., 2010), prostate cancer (Panagiotou et al., 2015; Schumacher et al., 2011), colorectal cancer (Li et al., 2017; Pellatt, Wolff, Herrick, Lundgreen, & Slattery, 2013), RCC (Martino et al., 2016; Wu, Yan, et al., 2017), and leukemia (Sheng et al., 2013; Speedy et al., 2014); and one each on endometrial cancer (Prescott, McGrath, Lee, Buring, & De Vivo, 2010), bladder cancer (Rothman et al., 2010), testicular germ cell tumor (TGCTs) (Schumacher et al., 2013), melanoma (Llorca‐Cardenosa et al., 2014), multiple myeloma (Campa, Martino, et al., 2015), gastrointestinal stromal tumors (GISTs) (Zhang et al., 2015), non‐Hodgkin's lymphoma (NHL), diffuse Large B‐cell lymphoma (DLBCL), small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL) (Shadrina et al., 2015), nasopharyngeal carcinoma (NPC) (Zhang et al., 2016), gastric cancer (Duan et al., 2016), esophageal cancer (Wu, Yan, et al., 2017), gastric cardia adenocarcinoma (GCA) (Zhang et al., 2019), hepatocellular carcinoma (HCC) (Zhang et al., 2017). One study focused on Caucasians (Prescott et al., 2010); two studies focused on Africans (Huo et al., 2016; Long et al., 2013); three studies on African‐Americans (Bojesen et al., 2013; Haiman et al., 2011; Palmer et al., 2013), 16 studies on Asians (Bojesen et al., 2013; Duan et al., 2016; Gao et al., 2014; Gong et al., 2016; Li et al., 2017; Sheng et al., 2013; Wang et al., 2016; Wu, Yan, et al., 2017; Wu, Zhu, et al., 2017; Zhang et al., 2017; Ye et al., 2017; Zhang et al., 2019; Zhang et al., 2015; Zhang et al., 2016; Zhao et al., 2012; Zhao et al., 2013); twenty studies on European (Campa, Martino, et al., 2015; Campa, Rizzato, et al., 2015; Earp et al., 2016; Garcia‐Closas et al., 2013; Gudmundsson et al., 2017; Haiman et al., 2011; Kuchenbaecker et al., 2015; Landi et al., 2011; Llorca‐Cardenosa et al., 2014; Martino et al., 2016; Melin et al., 2017; Michailidou et al., 2015, 2017; Mosrati et al., 2015; Panagiotou et al., 2015; Petersen et al., 2010; Prescott et al., 2010; Rothman et al., 2010; Schumacher et al., 2013; Shadrina et al., 2015), and six studies on multiple populations (Lee et al., 2016; Pellatt et al., 2013; Purrington et al., 2014; Schumacher et al., 2011; Speedy et al., 2014; Terry et al., 2012). The studies used genotyping methods such as Illumina, TaqMan, MassArray, Agarose gel electrophoresis, KASP technology, and polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP).
Figure 1.
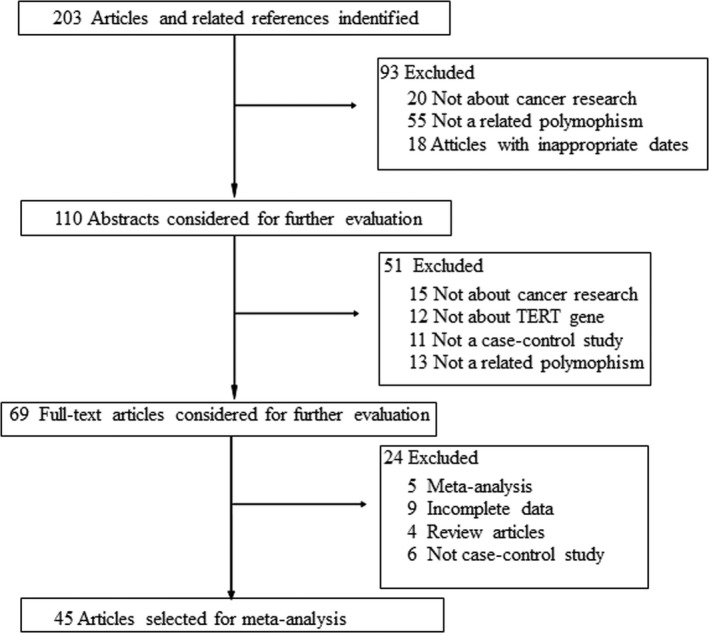
Study inclusion and exclusion procedures
Table 1.
Study characteristics of the association between the rs10069690 polymorphism and cancer risk in this meta‐analysis
| Study (y) | Cancer type | Ethnicity | Method | Source of control | Case | Control | OR (95% CI) | Score |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. (2019) | GCA | Asian | MassArray | HB | 1,024 | 1,118 | 1.42 (1.22–1.66) | 10 |
| Gudmundsson et al. (2017) | Thyroid cancer | European | Illumina | Multiple | 3,001 | 287,550 | 1.20 (1.12–1.29) | 12 |
| Michailidou et al. (2017) | Breast cancer | European | Illumina | Multiple | 61,282 | 45,494 | 1.06 (1.04–1.08) | 13 |
| Zhang et al. (2017) | HCC | Asian | MassArray | HB | 473 | 564 | 0.75 (0.59–0.96) | 8 |
| Wu, Yan, et al. (2017) | Esophageal cancer | Asian | MassArray | HB | 386 | 495 | 1.70 (1.33–2.18) | 6 |
| Ye et al. (2017) | Lung cancer | Asian | MassArray | PB | 554 | 603 | 1.41 (1.14–1.76) | 8 |
| Wu, Zhu, et al. (2017) | RCC | Asian | MassArray | PB | 293 | 459 | 1.39 (1.07–1.81) | 6 |
| Melin et al. (2017) | Glioma | European | Illumina | Multiple | 1591 | 804 | 1.40 (1.20–1.63) | 10 |
| Phelan et al. (2017) | Ovarian cancer | European | Illumina | Multiple | 16,924 | 68,502 | 1.08 (1.05–1.11) | 14 |
| Kuchenbaecker et al. (2015) | Ovarian cancer | European | Illumina | Multiple | 30,845 | 9,627 | 1.14 (1.10–1.19) | 12 |
| Li et al. (2017) | Colorectal cancer | Asian | MassArray | PB | 247 | 300 | 1.30 (0.94–1.80) | 5 |
| Lee et al. (2016) | Ovarian cancer | Multiple | Illumina | PB | 1,414 | 4,051 | 1.14 (1.03–1.26) | 13 |
| Earp et al. (2016) | Ovarian cancer | European | Affymetrix | Multiple | 3,573 | 5,640 | 1.14 (1.06–1.22) | 12 |
| Martino et al. (2016) | RCC | European | Agarose gel electrophoresis | PB | 243 | 420 | 1.20 (0.93–1.55) | 5 |
| Zhang et al. (2016) | NPC | Asian | MassArray | PB | 855 | 1,036 | 1.16 (0.96–1.41) | 10 |
| Wang et al. (2016) | Lung cancer | Asian | MassArray | HB | 228 | 301 | 1.34 (0.98–1.83) | 6 |
| Duan et al. (2016) | Gastric cancer | Asian | MassArray | HB | 302 | 300 | 1.56 (1.15–2.11) | 6 |
| Huo et al. (2016) | Breast cancer | African | Illumina | PB | 6,657 | 7,713 | 1.13 (1.07–1.19) | 13 |
| Gong et al. (2016) | Thyroid cancer | Asian | PCR‐RFLP | HB | 452 | 452 | 1.38 (1.10–1.72) | 7 |
| Zhang et al. (2015) | GISTs | Asian | TaqMan | HB | 300 | 300 | 1.40 (1.04–1.88) | 6 |
| Michailidou et al. (2015) | Breast cancer | European | TaqMan | Multiple | 62,533 | 60,976 | 1.06 (1.04–1.09) | 14 |
| Campa, Rizzato, et al. (2015) | Multiple myeloma | European | TaqMan | PB | 2,267 | 2,796 | 0.88 (0.79–0.97) | 11 |
| Shadrina et al. (2015) | NHL | European | TaqMan | PB | 344 | 893 | 1.01 (0.83–1.24) | 8 |
| Shadrina et al. (2015) | DLBCL | European | TaqMan | PB | 139 | 893 | 0.85 (0.63–1.16) | 7 |
| Shadrina et al. (2015) | SLL/CLL | European | TaqMan | PB | 77 | 893 | 1.21 (0.84–1.73) | 5 |
| Campa, Rizzato, et al. (2015) | Pancreatic cancer | European | Illumina | Multiple | 1901 | 4,106 | 0.95 (0.87–1.05) | 12 |
| Panagiotou et al. (2015) | Prostate cancer | European | Illumina | Multiple | 23,631 | 24,534 | 1.15 (1.12–1.19) | 13 |
| Speedy et al. (2014) | Leukemia | Multiple | Illumina | Multiple | 2,883 | 8,350 | 1.03 (0.96–1.10) | 11 |
| Llorca‐Cardenosa et al. (2014) | Melanoma | European | KASPtechnology | HB | 648 | 381 | 1.02 (0.83–1.23) | 8 |
| Gao et al. (2014) | Lung cancer | Asian | MassArray | HB | 309 | 310 | 1.28 (0.96–1.71) | 6 |
| Long et al. (2013) | Breast cancer | African | Illumina | Multiple | 1,112 | 930 | 0.86 (0.75–0.97) | 11 |
| Palmer et al. (2013) | Breast cancer | African‐American | MassArray | Multiple | 1,199 | 1948 | 1.05 (0.94–1.17) | 12 |
| Garcia‐Closas et al. (2013) | Breast cancer | European | Illumina | Multiple | 4,193 | 35,194 | 1.15 (1.11–1.20) | 14 |
| Purrington et al. (2013) | Breast cancer | Multiple | Illumina | Multiple | 3,677 | 4,708 | 1.24 (1.14–1.34) | 11 |
| Bojesen et al. (2013) | Breast cancer | European | Illumina | Multiple | 46,451 | 42,599 | 1.06 (1.04–1.08) | 14 |
| Bojesen et al. (2013) | Breast cancer | Asian | Illumina | Multiple | 6,269 | 6,624 | 1.04 (0.98–1.10) | 13 |
| Bojesen et al. (2013) | Breast cancer | African‐American | Illumina | Multiple | 1,116 | 932 | 1.19 (1.05–1.35) | 10 |
| Bojesen et al. (2013) | Ovarian cancer | European | Illumina | Multiple | 986 | 23,491 | 1.33 (1.20–1.47) | 12 |
| Bojesen et al. (2013) | Ovarian cancer | European | Illumina | Multiple | 8,371 | 23,491 | 1.15 (1.11–1.20) | 13 |
| Zhao et al. (2013) | Lung cancer | Asian | SNPscanTM | PB | 784 | 782 | 1.14 (0.98–1.32) | 9 |
| Pellatt et al. (2013) | Colorectal cancer | Multiple | Illumina | PB | 2,309 | 2,915 | 1.06 (0.97–1.15) | 12 |
| Schumacher et al. (2013) | TGCTs | European | TaqMan | Multiple | 940 | 1559 | 0.66 (0.53–0.82) | 10 |
| Terry et al. (2012) | Ovarian cancer | Multiple | TaqMan | Multiple | 2,112 | 2,456 | 1.11 (1.00–1.23) | 11 |
| Sheng et al. (2013) | leukemia | Asian | TaqMan | HB | 570 | 673 | 1.27 (1.04–1.56) | 10 |
| Haiman et al. (2011) | Breast cancer | African‐American | Illumina | Multiple | 1,002 | 2,743 | 0.76 (0.68–0.84) | 12 |
| Haiman et al. (2011) | Breast cancer | European | Illumina | Multiple | 5,007 | 17,965 | 1.19 (1.13–1.25) | 14 |
| Zhao et al. (2011) | Glioma | Asian | MassArray | HB | 983 | 1,024 | 0.99 (0.84–1.18) | 12 |
| Schumacher et al. (2011) | Prostate cancer | Multiple | Illumina | Multiple | 2,782 | 4,458 | 0.80 (0.73–0.89) | 11 |
| Petersen et al. (2010) | Pancreatic cancer | European | Illumina | Multiple | 3,851 | 3,934 | 0.91 (0.83–1.00) | 10 |
| Rothman et al. (2010) | Bladder cancer | European | Illumina | Multiple | 3,532 | 5,120 | 0.90 (0.84–0.96) | 12 |
| Prescott et al. (2010) | Endometrial cancer | Caucasian | TaqMan | PB | 674 | 1685 | 1.08 (0.92–1.26) | 10 |
| Landi et al. (2009) | Lung cancer | European | Illumina | Multiple | 5,739 | 5,848 | 1.02 (0.95–1.10) | 13 |
Abbreviations: 95% CI: 95% confidence interval; DLBCL, diffuse Large B‐cell lymphoma; GCA, gastric cardia adenocarcinoma; GISTs, gastrointestinal stromal tumors; HB, hospital based; HCC, hepatocellular carcinoma; NHL, non‐Hodgkin's lymphomas; NPC, nasopharyngeal carcinoma; OR, odds ratio; PB, population based; PCR‐RFLP, polymerase chain reaction‐restriction fragment length polymorphism; RCC, renal cell carcinoma; SLL/CLL, small lymphocytic lymphoma/chronic lymphocytic leukemia; TGCTs, testicular germ cell tumors.
3.2. Association between rs10069690 polymorphism and cancer risk
Based on the data from all 45 studies, we found a significant increased cancer risk for the TERT rs10069690 under a per‐allele risk analysis (OR = 1.09, 95% CI: 1.06–1.12, p < .001), with a statistical power of 100%. The results from a random effect model showed significant heterogeneity (p‐heterogeneity < .001, I 2 = 86.3%) (Figure 2 and Table 2).
Figure 2.
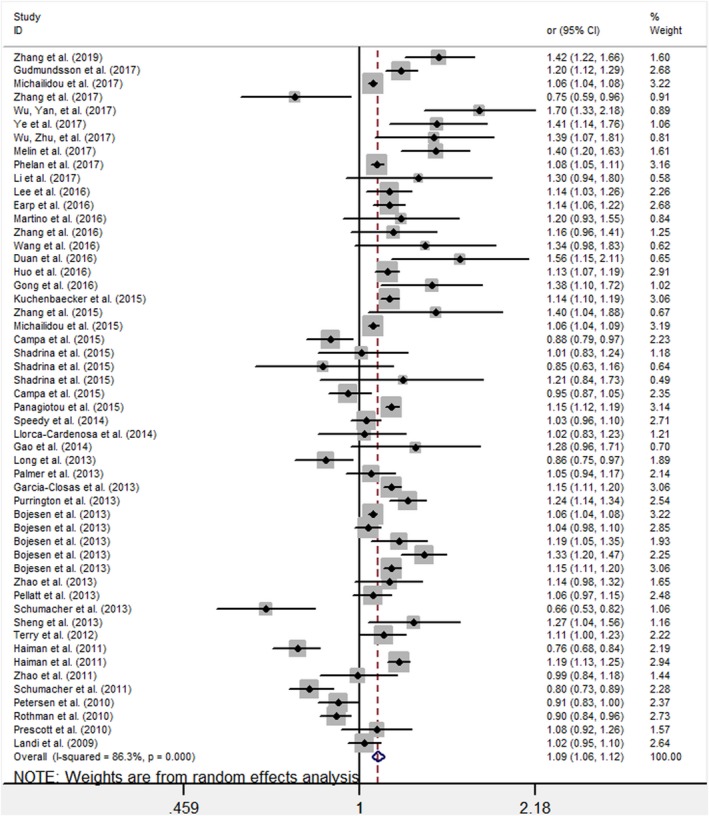
Forest plot of the ORs for the overall cancer risk associated with the TERT variant rs10069690 polymorphism
Table 2.
Stratified analyses of the rs10069690 polymorphism and cancer risk
| Category | No. | Cases/Controls | OR (95% CI) | P | I 2 (%) |
P‐ heterogeneity |
P‐ egger |
Power (%) | Prior probability |
Statistical power |
P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.1 | 0.01 | 0.001 | |||||||||||
| Total | 52 | 329035/730940 | 1.09 (1.06–1.12) | <.001 | 86.3 | <.001 | .592 | 100.00 | 1.48E‐09 | 4.44E‐09 | 4.89E‐08 | 4.93E‐07 | 1.000 | 4.94E‐10 |
| Ethnicity | ||||||||||||||
| European | 24 | 288069/672710 | 1.08 (1.04–1.11) | <.001 | 88.3 | <.001 | .866 | 55.02 | 1.10E‐07 | 3.31E‐07 | 3.65E‐06 | 3.68E‐05 | 1.000 | 3.68E‐08 |
| Asian | 16 | 14029/15341 | 1.24 (1.13–1.37) | <.001 | 74.8 | <.001 | .018 | 17.86 | 7.05E‐05 | 2.11E‐04 | 0.002 | 0.023 | 1.000 | 2.35E‐05 |
| Multiple | 6 | 15177/26938 | 1.06 (0.94–1.18) | .351 | 89.8 | <.001 | .77 | 14.49 | 0.463 | 0.721 | 0.966 | 0.997 | 1.000 | .287 |
| African | 2 | 7769/8643 | 0.99 (0.76–1.30) | .955 | 93.2 | <.001 | / | 4.800 | 0.739 | 0.895 | 0.989 | 0.999 | 0.998 | .942 |
| African‐American | 3 | 3317/5623 | 0.98 (0.75–1.28) | .889 | 93.9 | <.001 | .394 | 6.26 | 0.726 | 0.888 | 0.989 | 0.999 | 0.998 | .882 |
| Caucasian | 1 | 674/1685 | 1.08 (0.92–1.26) | .337 | 0.0 | / | / | 1.57 | 0.496 | 0.747 | 0.970 | 0.997 | 1.000 | .328 |
| Cancer type | ||||||||||||||
| Breast cancer | 12 | 200498/227826 | 1.07 (1.03–1.11) | <.001 | 89.5 | <.001 | .941 | 32.08 | 0.001 | 0.003 | 0.029 | 0.232 | 1.000 | 3.02E‐04 |
| Ovarian cancer | 7 | 64225/137258 | 1.14 (1.10–1.19) | <.001 | 70.8 | .002 | .140 | 18.70 | 6.58E‐09 | 1.97E‐08 | 2.17E‐07 | 2.19E‐06 | 1.000 | 2.19E‐09 |
| Lung cancer | 5 | 7614/7844 | 1.19 (1.03–1.36) | .015 | 66.0 | .019 | .022 | 6.68 | 0.031 | 0.088 | 0.514 | 0.914 | 1.000 | .011 |
| Thyroid cancer | 2 | 3453/288002 | 1.23 (1.11–1.38) | <.001 | 26.8 | .243 | / | 3.70 | 0.001 | 0.004 | 0.040 | 0.296 | 1.000 | 4.22E‐04 |
| RCC | 2 | 536/879 | 1.29 (1.07–1.55) | .007 | 0.0 | .432 | / | 1.65 | 0.020 | 0.059 | 0.407 | 0.874 | 0.946 | .007 |
| Prostate cancer | 2 | 26413/28992 | 0.96 (0.67–1.37) | .832 | 97.9 | <.001 | / | 5.42 | 0.716 | 0.883 | 0.988 | 0.999 | 0.978 | .822 |
| Pancreatic cancer | 2 | 5752/8040 | 0.93 (0.87–0.99) | .031 | 0.0 | .524 | / | 4.72 | 0.064 | 0.171 | 0.694 | 0.958 | 1.000 | .023 |
| Leukemia | 2 | 3453/9023 | 1.12 (0.92–1.37) | .275 | 72.9 | .055 | / | 3.88 | 0.448 | 0.709 | 0.964 | 0.996 | 0.998 | .270 |
| Colorectal cancer | 2 | 2556/3215 | 1.10 (0.94–1.29) | .225 | 29.5 | .234 | / | 3.06 | 0.420 | 0.684 | 0.960 | 0.996 | 1.000 | .241 |
| Glioma | 2 | 2574/1828 | 1.18 (0.84–1.66) | .340 | 88.7 | .003 | / | 3.05 | 0.528 | 0.771 | 0.974 | 0.997 | 0.916 | .342 |
| Other | 14 | 11961/18033 | 1.06 (0.94–1.20) | .338 | 85.6 | <.001 | .122 | 17.06 | 0.517 | 0.763 | 0.973 | 0.997 | 1.000 | .357 |
| Cancer classification | ||||||||||||||
| Gynecological cancer | 19 | 264395/364026 | 1.11 (1.09–1.14) | <.001 | 82.0 | .002 | .050 | 50.15 | 5.13E‐14 | 1.54E‐13 | 1.69E‐12 | 1.71E‐11 | 1.000 | 1.71E‐14 |
| Gastrointestinal cancer | 8 | 10320/13468 | 1.21 (1.05–1.41) | .010 | 87.2 | .035 | .021 | 11.58 | 0.042 | 0.116 | 0.592 | 0.936 | 0.997 | .015 |
| Hematological tumor | 7 | 7282/17241 | 0.97 (0.85–1.11) | .663 | 82.9 | .023 | .814 | 10.61 | 0.664 | 0.856 | 0.985 | 0.998 | 1.000 | .658 |
| Urinary tumor | 5 | 30481/34991 | 1.04 (0.87–1.25) | .660 | 95.3 | .036 | .548 | 9.81 | 0.670 | 0.859 | 0.985 | 0.999 | 1.000 | .676 |
| Head and neck cancer | 3 | 4308/289038 | 1.21 (1.14–1.29) | <.001 | 0.0 | <.001 | .667 | 4.95 | 1.61E‐08 | 4.82E‐08 | 5.30E‐07 | 5.35E‐06 | 1.000 | 5.35E‐09 |
| Other | 10 | 12249/12176 | 1.07 (0.93–1.22) | .340 | 82.6 | .035 | .843 | 12.90 | 0.484 | 0.737 | 0.969 | 0.997 | 1.000 | .312 |
| Source of control | ||||||||||||||
| Multiple | 27 | 304912/699583 | 1.06 (1.03–1.09) | <.001 | 90.8 | <.001 | .496 | 69.18 | 1.28E‐04 | 3.84E‐04 | 0.004 | 0.041 | 1.000 | 4.27E‐05 |
| Population based | 14 | 16857/25439 | 1.11 (1.04–1.18) | .003 | 61.9 | <.001 | .604 | 19.95 | 0.002 | 0.007 | 0.075 | 0.451 | 1.000 | .001 |
| Hospital based | 11 | 5675/5918 | 1.24 (1.08–1.43) | .002 | 75.1 | .001 | .579 | 10.87 | 0.009 | 0.027 | 0.236 | 0.757 | 0.996 | .003 |
| Sample size | ||||||||||||||
| Large | 34 | 320240/720885 | 1.07 (1.04–1.10) | <.001 | 89.6 | <.001 | .551 | 83.67 | 4.86E‐06 | 1.46E‐05 | 1.60E‐04 | 0.002 | 1.000 | 1.62E‐06 |
| Small | 18 | 7204/10055 | 1.21 (1.11–1.33) | <.001 | 60.3 | .001 | .378 | 16.33 | 2.33E‐04 | 0.001 | 0.008 | 0.072 | 1.000 | 7.78E‐05 |
| Method | ||||||||||||||
| Illumina | 25 | 244935/641683 | 1.07 (1.04–1.11) | <.001 | 90.9 | <.001 | .687 | 65.54 | 0.001 | 0.003 | 0.029 | 0.232 | 1.000 | 3.02E‐04 |
| MassArray | 12 | 6853/8458 | 1.24 (1.10–1.40) | .001 | 75.2 | <.001 | .21 | 12.64 | 0.002 | 0.005 | 0.048 | 0.339 | 0.999 | .001 |
| TaqMan | 10 | 69956/73124 | 1.02 (0.93–1.12) | .639 | 77.6 | <.001 | .618 | 14.41 | 0.670 | 0.859 | 0.985 | 0.999 | 1.000 | .678 |
| Other | 5 | 5700/7675 | 1.15 (1.08–1.22) | <.001 | 3.7 | .386 | .667 | 7.420 | 1.07E‐05 | 3.20E‐05 | 3.52E‐04 | 0.004 | 1.000 | 3.55E‐06 |
p < .05 indicates statistical significance.
Abbreviations: 95% CI, 95% confidence interval; OR, odds ratio.
Stratification analysis identified increased cancer risk in subgroups of ethnicity in European (OR = 1.08, 95% CI: 1.04–1.11, p‐heterogeneity < .001, I 2 = 88.3%), Asian (OR = 1.24, 95% CI: 1.13–1.37, p‐heterogeneity = <.001, I 2 = 88.3%), multiple (OR = 1.06, 95% CI: 0.94–1.18, p‐heterogeneity = .351, I 2 = 89.8%), African (OR = 0.99, 95% CI: 0.76–1.30, p‐heterogeneity = .955, I 2 = 93.2%), African‐American (OR = 0.98, 95% CI: 0.75–1.28, p‐heterogeneity = .889, I 2 = 93.9%) and (OR = 1.08, 95% CI: 0.92–1.26, p‐heterogeneity = .337, I 2 = 0.0%) (Table 2). Subgroup analysis based on cancer type indicated that the TERT rs10069690 polymorphism was associated with an increased risk of breast cancer (OR = 1.07, 95% CI: 1.03–1.11, p‐heterogeneity < .001, I 2 = 89.5%), ovarian cancer (OR = 1.14, 95% CI: 1.10–1.19, p‐heterogeneity = .002, I 2 = 70.8%), lung cancer (OR = 1.19, 95% CI: 1.03–1.36, p‐heterogeneity = .019, I 2 = 66%), thyroid cancer (OR = 1.23, 95% CI: 1.11–1.38, p‐heterogeneity = .243, I 2 = 26.8%), and RCC (OR = 1.29, 95% CI: 1.07–1.55, p‐heterogeneity < .001, I 2 = 0.0%). No significant increase in risk was found in prostate cancer, leukemia, colorectal cancer, glioma and other cancers. However, a significantly decreased association was observed in pancreatic cancer (OR = 0.93, 95% CI: 0.87–0.99, p‐heterogeneity = .524, I 2 = 0.0%), as shown in Table 2.
Subgroup analysis based on cancer classification indicated that the TERT rs10069690 polymorphism was associated with an increased risk of gynecological cancer (OR = 1.11, 95% CI: 1.09–1.14, p‐heterogeneity < .001, I 2 = 82.0%), gastrointestinal cancer (OR = 1.21, 95% CI: 1.05–1.41, p‐heterogeneity = .035, I 2 = 87.2%) and head and neck cancer (OR = 1.21, 95% CI: 1.14–1.29, p‐heterogeneity < .001, I 2 = 0.0%). No significant increase in risk was found in hematological tumor, urinary tumor and other cancer (Table 2).A stratified analysis by source of controls indicated a significantly increased cancer risk in population based, hospital based, and multiple with ORs of 1.11 (95% CI: 1.04–1.18), 1.24 (95% CI: 1.08–1.43), and 1.06 (95% CI: 1.03–1.09), respectively. Moreover, a stratified analysis performed on the sample size revealed that the significant increased risk of cancer was also observed in large and small groups with ORs of 1.07 (95% CI: 1.04–1.10), 1.21 (95% CI: 1.11–1.33), respectively, as shown in Table 2. The stratified analysis based on Method of genotype indicated that the TERT rs10069690 polymorphism was associated with an increased risk of cancer in the Illumina (OR = 1.07, 95% CI: 1.04–1.11, p‐heterogeneity < .001, I2 = 90.9%) and MassArray groups (OR = 1.24, 95% CI: 1.10–1.40, p‐heterogeneity < .001, I 2 = 75.2%) (Table 2).
3.3. FPRP and statistical power
The FPRP values for significant findings at different prior probability levels are shown in Table 2. For a prior probability of 0.1, assuming that the statistical power was 1.00, the FPRP values were 4.44E‐09 for an association of rs10069690 allele with an increased risk of cancer. Positive associations with the rs10069690 observed in the subgroups of ethnicity (European and Asian), cancer type (breast cancer, ovarian cancer, lung cancer, thyroid cancer, RCC, and pancreatic cancer), cancer classification (gynecological cancer, gastrointestinal cancer, and head and neck cancer), source of control (PB and HB), sample size (large and small), and genotype method (Illumina and MassArray) were significant (Table 2).
3.4. Sensitivity analyses and publication bias
Sensitivity analyses were performed to conclude whether modification of the inclusion criteria of the meta‐analysis affected the final results. The results showed that the significance of the OR was not affected by any single study (Figure 3). We used Begg's funnel plot and Egger's test to assess publication bias of the literatures. As shown in Figure 4, the shapes of the funnel plots seemed symmetrical and did not indicate any evidence of publication bias (p = .653). Egger's test results also did not show any evidence of publication bias (p = .592), indicating our results to be statistically robust.
Figure 3.
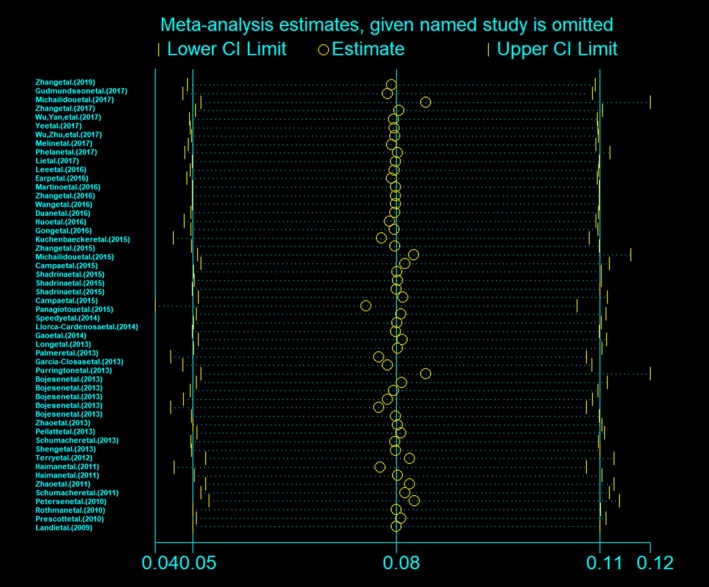
Sensitivity analyses of the overall ORs. The results were calculated by omitting each eligible study. Meta‐analysis random effects estimates were used
Figure 4.
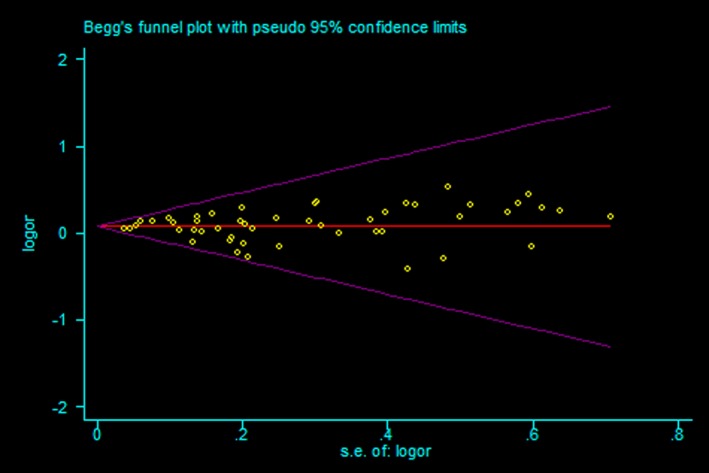
Begg's funnel plot for publication bias
4. DISCUSSION
A single nucleotide polymorphism (SNP) rs10069690 located in intron 4 of TERT, has been hypothesized to be associated with the risk of cancers development by many researchers, however, the results are conflicting and heterogeneous. Here, we performed a meta‐analysis included 45 case–control studies, including 329,035 cancer cases and 730,940 controls to explore the association between the TERT rs10069690 polymorphism and cancer risk. The result demonstrated that the TERT rs10069690 polymorphism was found to be associated with a significantly increased cancer risk overall. The association mainly existed in the European and Asian population, especially for breast cancer, ovarian cancer, lung cancer, thyroid cancer and RCC; but a significantly decreased association was observed in pancreatic cancer. In the subgroup analyses by cancer type, no significant association was found in prostate cancer, leukemia, colorectal cancer and glioma. The significant association between rs10069690 and cancer risk was also found in the stratification by cancer classification, source of controls, sample size, and genotype method.
TERT is mapped to chromosome 5p15.33 and consists of 16 exons and 15 introns spanning about 35 kb (Wick, Zubov, & Hagen, 1999). It encodes the catalytic protein subunit of telomerase and adds nucleotide repeats to chromosome ends in cooperation with a telomere RNA component (Cheung & Deng, 2008). A high level of TERT expression is involved in many tumors and it possibly contributes to unlimited cell division and carcinogenesis. The expression of the functional TERT protein is a prerequisite for acquisition of telomerase activity (Artandi & DePinho, 2000). Activation of telomerase has been implicated in human cell immortalization and cancer cell pathogenesis and telomerase expression is a key factor in cancer cell biology, enabling malignant cells to proliferate indefinitely (Greider, 1998). The biology of TERT makes it a compelling candidate gene for factors that influence cancer risk and TERT has been recognized as one of the most common tumor markers. A growing number of epidemiological studies have provided evidence that TERT polymorphisms contribute to cancer development (Jin et al., 2013; Li et al., 2012; Rafnar et al., 2009).
It has been reported that rs10069690 was associated with an increased risk of breast cancer (Bojesen et al., 2013; Haiman et al., 2011; Huo et al., 2016; Michailidou et al., 2015, 2017), ovarian cancer (Bojesen et al., 2013; Earp et al., 2016; Kuchenbaecker et al., 2015; Lee et al., 2016; Phelan et al., 2017), thyroid cancer (Gudmundsson et al., 2017), prostate cancer (Panagiotou et al., 2015), and glioma (Kinnersley et al., 2015; Melin et al., 2017; Ostrom et al., 2018; Rajaraman et al., 2012), through GWASs, but other studies have shown that the T allele was associated with a remarkably decreased risk of prostate cancer (Schumacher et al., 2011; Thomas et al., 2008), bladder cancer (Rothman et al., 2010), and testicular germ cell tumor (Schumacher et al., 2013). Additionally, a recent study composed of 386 patients and 495 controls suggested that the rs10069690 T allele was associated with increased risk of lung cancer (Ye et al., 2017), while other studies did not find any significant association between rs10069690 and risk of lung cancer (Gao et al., 2014; Landi et al., 2011; Wang et al., 2016). Other studies reported that the rs10069690 T allele was also not associated with risk of nasopharyngeal carcinoma (Zhang et al., 2016), melanoma (Llorca‐Cardenosa et al., 2014), colorectal cancer (Li et al., 2017), non‐Hodgkin's lymphoma (Prescott et al., 2010), and endometrial cancer (Burghaus et al., 2017; Prescott et al., 2010). As above, the results remain controversial and ambiguous.
The heterogeneity among studies in this meta‐analysis was significantly reduced in stratified analyses by the cancer type subgroups. These results suggested that the the role of polymorphism is potentially influenced by the tumor origins, and that stratified analysis is reasonable. Therefore, we can infer that rs10069690 had cancer‐specific contributions and may play different roles in the etiology of different tumor sites. More recently, a meta‐analysis study showed that rs10069690 polymorphism was associated with an increased breast cancer risk (Li, Dong, Feng, Zhang, & Cao, 2016). An agnostic subset‐based meta‐analysis (association analysis based on subsets) across six distinct cancers in 34,248 cases and 45,036 controls identified that rs10069690 T allele was positively associated with glioma, while being negatively associated with testicular, prostate, bladder and pancreatic cancer (Wang et al., 2014). The association between TERT rs10069690 polymorphism and longer telomere length has been recently reported (Pellatt, Wolff, Lundgreen, Cawthon, & Slattery, 2012). However, the exact biological function of rs10069690 has not been clarified until now. TERT rs10069690 polymorphism may contribute directly to disease predisposition by modifying the function of TERT, or it is in linkage disequilibrium (LD) with other disease‐causing mutations.
There are some limitations that should be addressed in interpreting the results of this meta‐analysis. First, due to insufficient genotype frequencies, we were unable to calculate the pooled ORs in other genetic models except allele model. Second, the origins of heterogeneity may include many factors, such as the ethnicity, cancer type, source of control, genotyping method and sample size. Finally, gene–gene and gene–environment interactions may have influenced our results, as cancer is mainly caused by genetic and environmental factors. In addition, the lack of detailed information, such as age and sex of the subjects, in some studies limited a more accurate OR would be corrected for age, sex and other factors that are associated with cancer risk.
5. CONCLUSIONS
The results of this meta‐analysis have shown that the TERT rs10069690 polymorphism is associated with an increased cancer risk overall. These results suggested that the TERT rs10069690 polymorphism may be a potential biomarker of cancer susceptibility. Overall, these results would help in understanding the role of this variant rs10069690 in cancer development and can aid in identifying new molecular targets focusing on cancer. However, the effect on cancer risk may be modified by ethnicity, cancer type, source of controls, sample size and genotype method. Considering the limitations of the present meta‐analysis, future studies with standardized unbiased methods, larger sample studies and well‐matched controls are required to validate the current findings and functional studies are warranted to reveal the role of the polymorphism rs10069690 in carcinogenesis.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
The study was supported by the Hainan Provincial Key Research and Development Program Project Fund (No. ZDYF2017087). We are grateful to all of the patients and other individuals who made this work possible.
He G, Song T, Zhang Y, et al. TERT rs10069690 polymorphism and cancers risk: A meta‐analysis. Mol Genet Genomic Med. 2019;7:e903 10.1002/mgg3.903
REFERENCES
- Are, C. , Rajaram, S. , Are, M. , Raj, H. , Anderson, B. O. , Chaluvarya Swamy, R. , … Cazap, E. L. (2013). A review of global cancer burden: Trends, challenges, strategies, and a role for surgeons. Journal of Surgical Oncology, 107(2), 221–226. 10.1002/jso.23248 [DOI] [PubMed] [Google Scholar]
- Artandi, S. E. , & DePinho, R. A. (2000). A critical role for telomeres in suppressing and facilitating carcinogenesis. Current Opinion in Genetics and Development, 10(1), 39–46. 10.1016/S0959-437X(99)00047-7 [DOI] [PubMed] [Google Scholar]
- Baird, D. M. (2010). Variation at the TERT locus and predisposition for cancer. Expert Reviews in Molecular Medicine, 12, e16 10.1017/S146239941000147X [DOI] [PubMed] [Google Scholar]
- Blackburn, E. H. (2005). Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Letters, 579(4), 859–862. 10.1016/j.febslet.2004.11.036 [DOI] [PubMed] [Google Scholar]
- Bojesen, S. E. , Pooley, K. A. , Johnatty, S. E. , Beesley, J. , Michailidou, K. , Tyrer, J. P. , … Dunning, A. M. (2013). Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nature Genetics, 45(4), 371–384. 10.1038/ng.2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, F. , Ferlay, J. , Soerjomataram, I. , Siegel, R. L. , Torre, L. A. , & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Burghaus, S. , Fasching, P. A. , Häberle, L. , Rübner, M. , Büchner, K. , Blum, S. , … Renner, S. P. (2017). Genetic risk factors for ovarian cancer and their role for endometriosis risk. Gynecologic Oncology, 145(1), 142 10.1016/j.ygyno.2017.02.022 [DOI] [PubMed] [Google Scholar]
- Campa, D. , Martino, A. , Varkonyi, J. , Lesueur, F. , Jamroziak, K. , Landi, S. , … Canzian, F. (2015). Risk of multiple myeloma is associated with polymorphisms within telomerase genes and telomere length. International Journal of Cancer, 136(5), E351–E358. 10.1002/ijc.29101 [DOI] [PubMed] [Google Scholar]
- Campa, D. , Rizzato, C. , Stolzenberg‐Solomon, R. , Pacetti, P. , Vodicka, P. , Cleary, S. P. , … Canzian, F. (2015). TERT gene harbors multiple variants associated with pancreatic cancer susceptibility. International Journal of Cancer, 137(9), 2175–2183. 10.1002/ijc.29590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, A. L. , & Deng, W. (2008). Telomere dysfunction, genome instability and cancer. Frontiers in Bioscience, 13, 2075–2090. 10.2741/2825 [DOI] [PubMed] [Google Scholar]
- Collins, K. , & Mitchell, J. R. (2002). Telomerase in the human organism. Oncogene, 21(4), 564–579. 10.1038/sj.onc.1205083 [DOI] [PubMed] [Google Scholar]
- Duan, X. , Cao, W. , Wang, L. , Liu, S. , Liu, Z. , Zhang, B. , … Jin, T. (2016). Genetic variants in TERT are associated with risk of gastric cancer in a Chinese Han population. Oncotarget, 7(50), 82727–82732. 10.18632/oncotarget.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp, M. , Winham, S. J. , Larson, N. , Permuth, J. B. , Sicotte, H. , Chien, J. , … Goode, E. L. (2016). A targeted genetic association study of epithelial ovarian cancer susceptibility. Oncotarget, 7(7), 7381–7389. 10.18632/oncotarget.7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, W. , Zhuo, Z. J. , Chen, Y. C. , Zhu, J. , Zhao, Z. , Jia, W. , … Liu, G. C. (2017). NFKB1 ‐94insertion/deletion ATTG polymorphism and cancer risk: Evidence from 50 case‐control studies. Oncotarget, 8(6), 9806–9822. 10.18632/oncotarget.14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, L. , Thakur, A. , Liang, Y. , Zhang, S. , Wang, T. , Chen, T. , … Chen, M. (2014). Polymorphisms in the TERT gene are associated with lung cancer risk in the Chinese Han population. European Journal of Cancer Prevention, 23(6), 497–501. 10.1097/CEJ.0000000000000086 [DOI] [PubMed] [Google Scholar]
- Garcia‐Closas, M. , Couch, F. J. , Lindstrom, S. , Michailidou, K. , Schmidt, M. K. , Brook, M. N. , … Kraft, P. (2013). Genome‐wide association studies identify four ER negative‐specific breast cancer risk loci. Nature Genetics, 45(4), 392–398. 10.1038/ng.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, L. , Xu, Y. , Hu, Y. Q. , Ding, Q. J. , Yi, C. H. , Huang, W. , & Zhou, M. (2016). hTERT gene polymorphism correlates with the risk and the prognosis of thyroid cancer. Cancer Biomark, 17(2), 195–204. 10.3233/CBM-160631 [DOI] [PubMed] [Google Scholar]
- Greider, C. W. (1998). Telomerase activity, cell proliferation, and cancer. Proceedings of the National Academy of Sciences of the United States of America, 95(1), 90–92. 10.1073/pnas.95.1.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson, J. , Thorleifsson, G. , Sigurdsson, J. K. , Stefansdottir, L. , Jonasson, J. G. , Gudjonsson, S. A. , … Stefansson, K. (2017). A genome‐wide association study yields five novel thyroid cancer risk loci. Nature Communications, 8, 14517 10.1038/ncomms14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman, C. A. , Chen, G. K. , Vachon, C. M. , Canzian, F. , Dunning, A. , Millikan, R. C. , … Couch, F. J. (2011). A common variant at the TERT‐CLPTM1L locus is associated with estrogen receptor‐negative breast cancer. Nature Genetics, 43(12), 1210–1214. 10.1038/ng.985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J. , Wang, M. Y. , Qiu, L. X. , Zhu, M. L. , Shi, T. Y. , Zhou, X. Y. , … Wei, Q. Y. (2013). Genetic variations of mTORC1 genes and risk of gastric cancer in an Eastern Chinese population. Molecular Carcinogenesis, 52(Suppl 1), E70–E779. 10.1002/mc.22013 [DOI] [PubMed] [Google Scholar]
- Huo, D. , Feng, Y. E. , Haddad, S. , Zheng, Y. , Yao, S. , Han, Y.‐J. , … Haiman, C. A. (2016). Genome‐wide association studies in women of African ancestry identified 3q26.21 as a novel susceptibility locus for oestrogen receptor negative breast cancer. Human Molecular Genetics, 25(21), 4835–4846. 10.1093/hmg/ddw305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, T.‐B. , Zhang, J.‐Y. , Li, G. , Du, S.‐L. , Geng, T.‐T. , Gao, J. , … Li, S.‐Q. (2013). RTEL1 and TERT polymorphisms are associated with astrocytoma risk in the Chinese Han population. Tumor Biology, 34(6), 3659–3666. 10.1007/s13277-013-0947-0 [DOI] [PubMed] [Google Scholar]
- Kinnersley, B. , Labussière, M. , Holroyd, A. , Di Stefano, A.‐L. , Broderick, P. , Vijayakrishnan, J. , … Houlston, R. S. (2015). Genome‐wide association study identifies multiple susceptibility loci for glioma. Nature Communications, 6, 8559 10.1038/ncomms9559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchenbaecker, K. B. , Ramus, S. J. , Tyrer, J. , Lee, A. , Shen, H. C. , Beesley, J. , … Chenevix‐Trench, G. (2015). Identification of six new susceptibility loci for invasive epithelial ovarian cancer. Nature Genetics, 47(2), 164–171. 10.1038/ng.3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi, M. T. , Chatterjee, N. , Yu, K. , Goldin, L. R. , Goldstein, A. M. , Rotunno, M. , … Caporaso, N. E. (2009). A genome‐wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. American Journal of Human Genetics, 85(5), 679–691. 10.1016/j.ajhg.2009.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi, M. T. , Chatterjee, N. , Yu, K. , Goldin, L. R. , Goldstein, A. M. , Rotunno, M. , … Caporaso, N. E. (2011). A genome‐wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. American Journal of Human Genetics, 88(6), 861 10.1016/j.ajhg.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, A. W. , Bomkamp, A. , Bandera, E. V. , Jensen, A. , Ramus, S. J. , Goodman, M. T. , … Chang‐Claude, J. (2016). A splicing variant of TERT identified by GWAS interacts with menopausal estrogen therapy in risk of ovarian cancer. International Journal of Cancer, 139(12), 2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Zhao, Z. , Zhou, J. , Liu, Y. , Wang, H. , & Zhao, X. (2017). Relationship between the TERT, TNIP1 and OBFC1 genetic polymorphisms and susceptibility to colorectal cancer in Chinese Han population. Oncotarget, 8(34), 56932–56941. 10.18632/oncotarget.18378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Jin, T.‐B. , Wei, X.‐B. , He, S.‐M. , Liang, H.‐J. , Yang, H.‐X. , … Gao, G.‐D. (2012). Selected polymorphisms of GSTP1 and TERT were associated with glioma risk in Han Chinese. Cancer Epidemiology, 36(6), 525 10.1016/j.canep.2012.06.008 [DOI] [PubMed] [Google Scholar]
- Li, Z. Y. , Dong, Y. L. , Feng, Y. , Zhang, Z. , & Cao, X. Z. (2016). Polymorphisms in the telomerase reverse transcriptase promoter are associated with risk of breast cancer: A meta‐analysis. Journal of Cancer Research and Therapeutics, 12(2), 1040 10.4103/0973-1482.164701 [DOI] [PubMed] [Google Scholar]
- Llorca‐Cardeñosa, M. J. , Peña‐Chilet, M. , Mayor, M. , Gomez‐Fernandez, C. , Casado, B. , Martin‐Gonzalez, M. , … Ribas, G. (2014). Long telomere length and a TERT‐CLPTM1 locus polymorphism association with melanoma risk. European Journal of Cancer, 50(18), 3168–3177. 10.1016/j.ejca.2014.09.017 [DOI] [PubMed] [Google Scholar]
- Long, J. , Zhang, B. , Signorello, L. B. , Cai, Q. , Deming‐Halverson, S. , Shrubsole, M. J. , … Zheng, W. (2013). Evaluating genome‐wide association study‐identified breast cancer risk variants in African‐American women. PLoS ONE, 8(4), e58350 10.1371/journal.pone.0058350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, M. , Taus, C. , Lucca, I. , Hofbauer, S. L. , Haitel, A. , Shariat, S. F. , & Klatte, T. (2016). Association of human telomerase reverse transcriptase gene polymorphisms, serum levels, and telomere length with renal cell carcinoma risk and pathology. Molecular Carcinogenesis, 55(10), 1458–1466. 10.1002/mc.22388 [DOI] [PubMed] [Google Scholar]
- Melin, B. S. , Barnholtz‐Sloan, J. S. , Wrensch, M. R. , Johansen, C. , Il'yasova, D. , Kinnersley, B. , … Bondy, M. L. (2017). Genome‐wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non‐glioblastoma tumors. Nature Genetics, 49(5), 789–794. 10.1038/ng.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidou, K. , Beesley, J. , Lindstrom, S. , Canisius, S. , Dennis, J. , Lush, M. J. , … Easton, D. F. (2015). Genome‐wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nature Genetics, 47(4), 373–380. 10.1038/ng.3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidou, K. , Lindström, S. , Dennis, J. , Beesley, J. , Hui, S. , Kar, S. , … Easton, D. F. (2017). Association analysis identifies 65 new breast cancer risk loci. Nature, 551(7678), 92–94. 10.1038/nature24284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosrati, M. A. , Willander, K. , Falk, I. J. , Hermanson, M. , Höglund, M. , Stockelberg, D. , … Söderkvist, P. (2015). Association between TERT promoter polymorphisms and acute myeloid leukemia risk and prognosis. Oncotarget, 6(28), 25109–25120. 10.18632/oncotarget.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom, Q. T. , Kinnersley, B. , Wrensch, M. R. , Eckel‐Passow, J. E. , Armstrong, G. , Rice, T. , … Barnholtz‐Sloan, J. S. (2018). Sex‐specific glioma genome‐wide association study identifies new risk locus at 3p21.31 in females, and finds sex‐differences in risk at 8q24.21. Scientific Reports, 8(1), 7352 10.1038/s41598-018-24580-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, J. R. , Ruiz‐Narvaez, E. A. , Rotimi, C. N. , Cupples, L. A. , Cozier, Y. C. , Adams‐Campbell, L. L. , & Rosenberg, L. (2013). Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiology, Biomarkers and Prevention, 22(1), 127–134. 10.1158/1055-9965.EPI-12-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotou, O. A. , Travis, R. C. , Campa, D. , Berndt, S. I. , Lindstrom, S. , Kraft, P. , … Tsilidis, K. K. (2015). A genome‐wide pleiotropy scan for prostate cancer risk. European Urology, 67(4), 649–657. 10.1016/j.eururo.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellatt, A. J. , Wolff, R. K. , Herrick, J. , Lundgreen, A. , & Slattery, M. L. (2013). TERT's role in colorectal carcinogenesis. Molecular Carcinogenesis, 52(7), 507–513. 10.1002/mc.21885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellatt, A. J. , Wolff, R. K. , Lundgreen, A. , Cawthon, R. , & Slattery, M. L. (2012). Genetic and lifestyle influence on telomere length and subsequent risk of colon cancer in a case control study. International Journal of Molecular Epidemiology and Genetics, 3(3), 184–194. [PMC free article] [PubMed] [Google Scholar]
- Petersen, G. M. , Amundadottir, L. , Fuchs, C. S. , Kraft, P. , Stolzenberg‐Solomon, R. Z. , Jacobs, K. B. , … Chanock, S. J. (2010). A genome‐wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nature Genetics, 42(3), 224–228. 10.1038/ng.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan, C. M. , Kuchenbaecker, K. B. , Tyrer, J. P. , Kar, S. P. , Lawrenson, K. , Winham, S. J. , … Pharoah, P. D. P. (2017). Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nature Genetics, 49(5), 680–691. 10.1038/ng.3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott, J. , McGrath, M. , Lee, I. M. , Buring, J. E. , & De Vivo, I. (2010). Telomere length and genetic analyses in population‐based studies of endometrial cancer risk. Cancer, 116(18), 4275–4282. 10.1002/cncr.25328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purrington, K. S. , Slager, S. , Eccles, D. , Yannoukakos, D. , Fasching, P. A. , Miron, P. , … Couch, F. J. (2014). Genome‐wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple‐negative breast cancer. Carcinogenesis, 35(5), 1012–1019. 10.1093/carcin/bgt404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafnar, T. , Sulem, P. , Stacey, S. N. , Geller, F. , Gudmundsson, J. , Sigurdsson, A. , … Stefansson, K. (2009). Sequence variants at the TERT‐CLPTM1L locus associate with many cancer types. Nature Genetics, 41(2), 221–227. 10.1038/ng.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman, P. , Melin, B. S. , Wang, Z. , McKean‐Cowdin, R. , Michaud, D. S. , Wang, S. S. , … Chanock, S. J. (2012). Genome‐wide association study of glioma and meta‐analysis. Human Genetics, 131(12), 1877–1888. 10.1007/s00439-012-1212-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, N. , Garcia‐Closas, M. , Chatterjee, N. , Malats, N. , Wu, X. , Figueroa, J. D. , … Chanock, S. J. (2010). A multi‐stage genome‐wide association study of bladder cancer identifies multiple susceptibility loci. Nature Genetics, 42(11), 978–984. 10.1038/ng.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, F. R. , Berndt, S. I. , Siddiq, A. , Jacobs, K. B. , Wang, Z. , Lindstrom, S. , … Kraft, P. (2011). Genome‐wide association study identifies new prostate cancer susceptibility loci. Human Molecular Genetics, 20(19), 3867–3875. 10.1093/hmg/ddr295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, F. R. , Wang, Z. , Skotheim, R. I. , Koster, R. , Chung, C. C. , Hildebrandt, M. A. T. , … McGlynn, K. A. (2013). Testicular germ cell tumor susceptibility associated with the UCK2 locus on chromosome 1q23. Human Molecular Genetics, 22(13), 2748–2753. 10.1093/hmg/ddt109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadrina, A. S. , Berezina, O. V. , Ovchinnikov, V. S. , Voropaeva, E. N. , Pospelova, T. I. , & Filipenko, M. L. (2015). TERT polymorphisms rs2853669, rs2736100, rs7726159 and rs10069690 and the risk of non‐Hodgkin’s lymphoma in ethnical Russians. Telomere & Telomerase, 2(2). 10.14800/tt.736 [DOI] [Google Scholar]
- Sheng, X. , Tong, N. A. , Tao, G. , Luo, D. , Wang, M. , Fang, Y. , … Wu, D. (2013). TERT polymorphisms modify the risk of acute lymphoblastic leukemia in Chinese children. Carcinogenesis, 34(1), 228–235. 10.1093/carcin/bgs325 [DOI] [PubMed] [Google Scholar]
- Speedy, H. E. , Di Bernardo, M. C. , Sava, G. P. , Dyer, M. J. S. , Holroyd, A. , Wang, Y. , … Houlston, R. S. (2014). A genome‐wide association study identifies multiple susceptibility loci for chronic lymphocytic leukemia. Nature Genetics, 46(1), 56–60. 10.1038/ng.2843 [DOI] [PubMed] [Google Scholar]
- Terry, K. L. , Tworoger, S. S. , Vitonis, A. F. , Wong, J. , Titus‐Ernstoff, L. , De Vivo, I. , & Cramer, D. W. (2012). Telomere length and genetic variation in telomere maintenance genes in relation to ovarian cancer risk. Cancer Epidemiology, Biomarkers and Prevention, 21(3), 504–512. 10.1158/1055-9965.EPI-11-0867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G. , Jacobs, K. B. , Yeager, M. , Kraft, P. , Wacholder, S. , Orr, N. , … Chanock, S. J. (2008). Multiple loci identified in a genome‐wide association study of prostate cancer. Nature Genetics, 40(3), 310–315. 10.1038/ng.91 [DOI] [PubMed] [Google Scholar]
- Wang, H. , Yang, H. , Feng, T. , Geng, T. , Chen, C. , & Jin, T. (2016). Effects of TERT gene polymorphism and environmental factor interactions on lung cancer risk in the Xi’an Han population. International Journal of Clinical and Experimental Medicine, 9(2), 4200–4210. [Google Scholar]
- Wang, Z. , Zhu, B. , Zhang, M. , Parikh, H. , Jia, J. , Chung, C. C. , … Amundadottir, L. T. (2014). Imputation and subset‐based association analysis across different cancer types identifies multiple independent risk loci in the TERT‐CLPTM1L region on chromosome 5p15.33. Human Molecular Genetics, 23(24), 6616–6633. 10.1093/hmg/ddu363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick, M. , Zubov, D. , & Hagen, G. (1999). Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT). Gene, 232(1), 97–106. 10.1016/S0378-1119(99)00108-0 [DOI] [PubMed] [Google Scholar]
- Wolpin, B. M. , Rizzato, C. , Kraft, P. , Kooperberg, C. , Petersen, G. M. , Wang, Z. , … Amundadottir, L. T. (2014). Genome‐wide association study identifies multiple susceptibility loci for pancreatic cancer. Nature Genetics, 46(9), 994–1000. 10.1038/ng.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D. , Zhu, G. , Zeng, J. , Song, W. , Wang, K. , Wang, X. , … He, D. (2017). Genetic variations in TERC and TERT genes are associated with renal cell carcinoma risk in a Chinese Han population. Oncotarget, 8(44), 76832–76842. 10.18632/oncotarget.20163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Yan, M. , Li, J. , Li, J. , Chen, Z. , Chen, P. , … Chen, C. (2017). Genetic polymorphisms in TERT are associated with increased risk of esophageal cancer. Oncotarget, 8(6), 10523–10530. 10.18632/oncotarget.14451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, G. , Tan, N. , Meng, C. , Li, J. , Jing, L. , Yan, M. , … Chen, F. (2017). Genetic variations in TERC and TERT genes are associated with lung cancer risk in a Chinese Han population. Oncotarget, 8(66), 110145–110152. 10.18632/oncotarget.22329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, A. , Zheng, C. , Lindvall, C. , Hou, M. , Ekedahl, J. , Lewensohn, R. , … Xu, D. (2000). Frequent amplification of the telomerase reverse transcriptase gene in human tumors. Cancer Research, 60(22), 6230–6235. [PubMed] [Google Scholar]
- Zhang, N. , Zheng, Y. , Liu, J. , Lei, T. , Xu, Y. , & Yang, M. (2019). Genetic variations associated with telomere length confer risk of gastric cardia adenocarcinoma. Gastric Cancer, 10.1007/s10120-019-00954-8 [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Zhao, J. , Xu, J. , Liu, F. , Xu, Y. , Bu, X. , … Song, C. (2015). Genetic variations in the TERT and CLPTM1L gene region and gastrointestinal stromal tumors risk. Oncotarget, 6(31), 31360–31367. 10.18632/oncotarget.5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, S. , Shi, Y. , Cao, Y. , He, H. , Zhou, S. , & Zhang, S. (2017). Associations of TERT polymorphisms with hepatocellular carcinoma risk in a Han Chinese population. International Journal of Clinical and Experimental Pathology, 10(7), 7776–7783. [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Zhang, X. , Zhang, H. , Zhai, Y. , Wang, Z. , Li, P. , … Zhou, G. (2016). Common variations in TERT‐CLPTM1L locus are reproducibly associated with the risk of nasopharyngeal carcinoma in Chinese populations. Oncotarget, 7(1), 759–770. 10.18632/oncotarget.6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Chen, G. , Zhao, Y. , Song, X. , Chen, H. , Mao, Y. , & Lu, D. (2012). Fine‐mapping of a region of chromosome 5p15.33 (TERT‐CLPTM1L) suggests a novel locus in TERT and a CLPTM1L haplotype are associated with glioma susceptibility in a Chinese population. International Journal of Cancer, 131(7), 1569–1576. 10.1002/ijc.27417 [DOI] [PubMed] [Google Scholar]
- Zhao, Z. , Li, C. , Yang, L. , Zhang, X. , Zhao, X. , Song, X. , … Lu, D. (2013). Significant association of 5p15.33 (TERT‐CLPTM1L genes) with lung cancer in Chinese Han population. Experimental Lung Research, 39(2), 91–98. 10.3109/01902148.2012.762436 [DOI] [PubMed] [Google Scholar]


