Abstract
Background and Purpose
Neurological involvement in Behçet's disease [neuro-Behçet's disease (NBD)] is uncommon, but it is worth investigating since it can cause substantial disability. However, difficulties exist in understanding the clinical features of NBD due to regional variations and the lack of studies utilizing well-established diagnostic criteria. We therefore analyzed the clinical features of patients with NBD based on the recent international consensus recommendation.
Methods
We retrospectively searched electronic databases for patients with Behçet's disease (BD) between 2000 and 2017, and reviewed their medical records. Based on the recent international consensus recommendation, patients with definite or probable NBD were included.
Results
Of 9,817 patients with the diagnosis code for BD, 1,682 (17.1%) visited the neurology clinic and 110 (1.1%) were classified as NBD. Ninety-eight patients exhibited parenchymal NBD and 12 exhibited nonparenchymal NBD. Their age at the onset of NBD was 37.6±10.6 years and the male-to-female ratio was 1.24:1. Brainstem syndrome (43.9%) was the most common condition in the 98 patients with parenchymal NBD, followed by multifocal (32.7%) and spinal cord (12.2%) syndromes. 72.4% exhibited acute NBD and 27.6% exhibited a progressive disease course. Frequent manifestations included pyramidal signs (52.0%), headache (45.9%), dysarthria (42.9%), and fever (31.6%). A frequent pattern in brain MRI was an upper brainstem lesion extending to the thalamus and basal ganglia.
Conclusions
Approximately 1% of the patients with suspected BD exhibited NBD. Neurologists must understand the clinical characteristics of NBD in order to perform the differential diagnosis and management of these patients.
Keywords: neuro-Behçet's disease, Behçet's disease, classification, treatment
INTRODUCTION
Behçet's disease (BD) is a multisystem inflammatory disease of uncertain etiology, with clinical features characterized by recurrent oral and genital ulcers, uveitis, and skin lesions.1 Neurological involvement in Behçet's disease [neuro-Behçet's disease (NBD)] has been reported to be less common than other systemic manifestations,2 but NBD can cause substantial disability. A previous study found that approximately 45% of patients with NBD deteriorated to a state requiring unilateral assistance for ambulation or worse within 10 years from the onset of neurological symptoms.3 In addition, approximately one-third of patients with NBD are known to convert to a progressive disease course.3,4
Several difficulties exist in understanding the clinical features of NBD. The prevalence of BD shows regional variations, with strikingly high prevalence rates in Middle Eastern and Far Eastern countries. In addition, the reported proportion of patients with neurological manifestations has varied markedly, from 1.3% to 59%.2,5 Further, the clinical manifestations NBD also show racial differences; for example, the incidence of intracranial hypertension is reportedly high in the Middle East,6 whereas seizure or optic neuritis appears frequently in Western countries.7 In addition, until recently there were no well-established diagnostic criteria for NBD, and so most previous studies have had to collect patients based on applying their own standards.
The present study analyzed the demographic, clinical, and imaging features of patients with NBD in South Korea based on recently suggested diagnostic criteria for NBD.8 We also analyzed the clinical manifestations and course of NBD.
METHODS
Subject enrollment
We retrospectively reviewed the medical records of patients with BD who visited the Department of Neurology of Severance Hospital, Seoul, South Korea. Patients with the diagnosis code for BD were identified between January 2000 and December 2017, and their medical records were reviewed from January 1985 to December 2017. The diagnosis of NBD was based on the recent international consensus recommendation.8 The patients were diagnosed as having “definite NBD” if they fulfilled the following criteria: 1) displayed a neurological syndrome recognized as being caused by BD, 2) exhibited characteristic abnormalities of NBD in neuroimaging or CSF analysis, and 3) satisfied the International Study Group (ISG) diagnostic criteria for BD. The patients were diagnosed as having “probable NBD” if they 1) displayed a neurological syndrome supported by neuroimaging or CSF analysis that did not satisfy the ISG criteria for BD or 2) displayed non-characteristic neurological symptoms while satisfying the ISG criteria for BD. The neurological syndromes recognized as being caused by BD included brainstem symptoms/signs, cerebral symptoms/signs, myelopathy, optic neuropathy, any combination of the aforementioned symptom/signs (multifocal), and nonparenchymal syndromes. The nonparenchymal syndromes included cerebral venous thrombosis, intracranial hypertension syndrome, and acute meningeal syndrome. Patients were included in the present study if they were classified as definite or probable NBD. The patients diagnosed with alternative causes for their neurological findings were excluded. The Institutional Review Board of Severance Hospital approved this study (approval no: 4-2018-0643) and the study was conducted in accordance with the Declaration of Helsinki.
Data collection and definition
Demographic data and clinical features of the patients were recorded by reviewing medical records. An attack was defined as an acute onset of neurological symptoms/signs that lasted for more than 24 hours. Cases with NBD were classified into nonparenchymal (cerebral venous thrombosis, intracranial hypertension syndrome, and acute meningeal syndrome) and parenchymal NBD. The clinical course of parenchymal NBD was classified into an acute course or a chronic progressive course. Patients were described as having acute NBD when they displayed acute focal neurological symptoms, which was usually accompanied by CSF pleocytosis. Depending on the number of acute attacks, acute NBD was further classified into a single episode (single acute attack) and the relapsing-remitting form (relapsing and remitting course with multiple attacks). Patients were classified into the chronic progressive type if they displayed the continuous worsening of neurological symptoms or signs over a period of months or years. Depending on the presence of preceding attack(s), chronic progressive NBD was classified into the primary progressive form (absence of preceding attacks) and the progressive form with relapse (one or more attacks during the progression course). MRI was performed during an acute attack or at the initial visit to the neurology department during the progressive disease course. CSF was analyzed in 64 patients.
Statistical analysis
Data are expressed as mean±SD or median [interquartile range] values for continuous variables depending on whether or not they conformed to a normal distribution. Data are reported as number (percentage) values for categorical variables. Fisher's exact test or the chi-square test was used to compare categorical variables, and Mann-Whitney test was used to compare nonparametric data. A probability value of p<0.05 was considered statistically significant. Statistical analyses were performed using R software (version 3.4.0, R Foundation, Vienna, Austria).
RESULTS
Patient inclusion
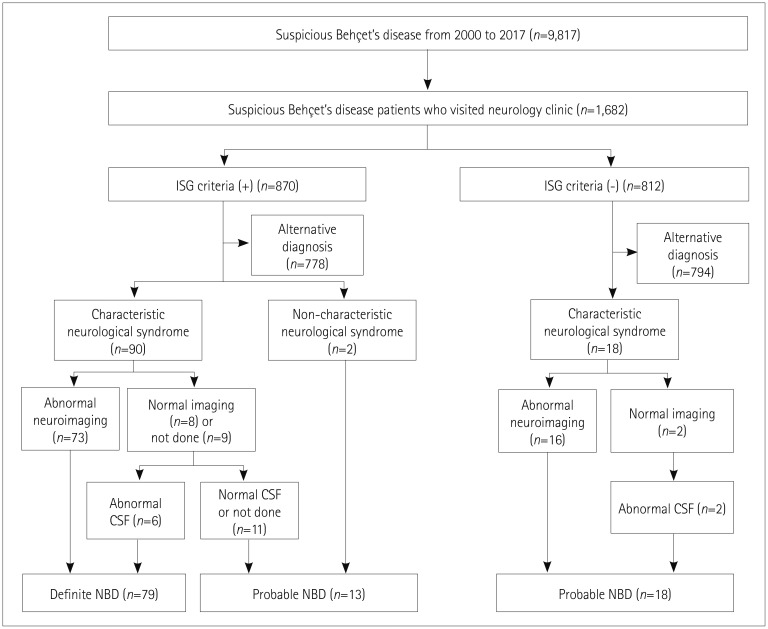
The patient inclusion flowchart is displayed in Fig. 1. There were 9,817 patients identified with the diagnosis code for BD during the study period, of which 1,682 (17.1%) visited the neurology clinic for various reasons. The most-frequent reasons for visiting the neurology department included the evaluation and management of headache (47.1%), other pain (12.5%), or dizziness (11.0%), or for managing previously diagnosed cerebrovascular disease (9.9%), peripheral neuropathy (8.7%), or movement disorders (6.6%). Among the 1,682 patients with suspected BD who visited the neurology clinic, 870 satisfied the ISG criteria for BD, of which 778 had alternative diagnoses that explained their symptoms better, including primary headache (48.3%), polyneuropathy associated with etiologies other than BD (12.3%), nonspecific dizziness (6.2%), and essential tremor or primary dystonia (3.8%). Of the remaining 92 patients satisfying the ISG criteria, 79 were finally classified as having definite NBD based on abnormal neuroimaging and/or CSF findings, and the other 13 were classified as having probable NBD. Of the 812 patients with suspected BD who did not satisfy the ISG criteria, 18 who had both the characteristic neurological syndrome and abnormal neuroimaging and/or CSF findings were classified as having probable NBD. Finally, 110 patients were included in this study. Their age at the onset of NBD was 37.6±10.6 years and the male-to-female ratio was 1.24:1.
Fig. 1. Flowchart of patient inclusion. CSF: cerebrospinal fluid, ISG: International Study Group, NBD: neuro-Behçet's disease.
Clinical features of patients with parenchymal NBD
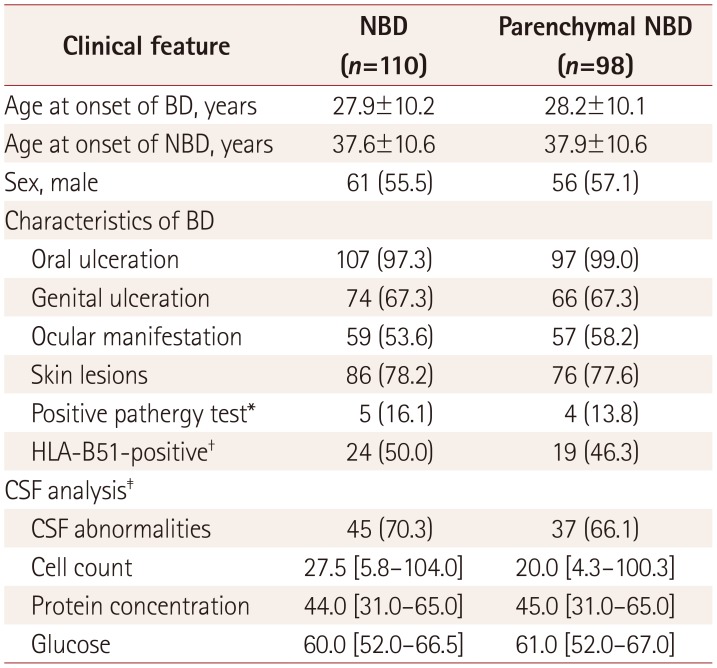
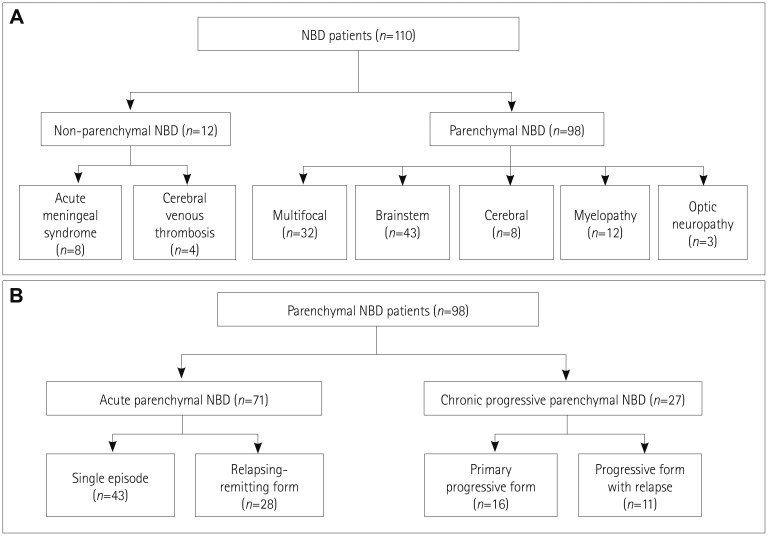
The clinical characteristics of the 98 patients with parenchymal NBD are presented in Table 1. Oral ulceration was observed in 97 (99.0%) patients, skin lesions in 76 (77.6%), genital ulceration in 66 (67.3%), and ocular manifestation in 57 (58.2%). The classification of patients with NBD according to the clinical syndrome and clinical course is demonstrated in Fig. 2. Thirty-two (32.7%) patients exhibited multifocal neurological syndrome, 43 (43.9%) exhibited brainstem syndrome, 12 (12.2%) exhibited spinal cord syndrome, 8 (8.2%) exhibited cerebral syndrome, and 3 (3.1%) exhibited optic neuropathy. In terms of the clinical course, 43 (43.9%) experienced a single episode, 28 (28.6%) exhibited the relapsingremitting form, 16 (16.3%) exhibited the primary progressive form, and 11 (11.2%) exhibited the progressive form with relapse. Sixty-two clinical relapses were observed in 39 patients, and the duration between the relapses was 2.5 [1–4] years. CSF was analyzed in 56 patients, and the results were abnormal in 37 (66.1%) of them.
Table 1. Baseline characteristics of 110 patients with NBD and 98 patients with parenchymal NBD.
Data are n (%), mean±SD, or median [interquartile range] values.
*Pathergy test was conducted in 31 of all NBD patients and 29 of parenchymal NBD patients, †HLA-B51 test was conducted in 48 of all NBD patients and 41 of parenchymal NBD patients, ‡CSF analysis was performed in 64 of all NBD patients and 56 of parenchymal NBD patients.
BD: Behçet's disease, CSF: cerebrospinal fluid, NBD: neuro-Behçet's disease.
Fig. 2. Classification of patients with NBD. Classification of 110 patients with NBD based on the clinical syndrome (A) and classification of 98 patients with parenchymal NBD based on the clinical course (B). NBD: neuro-Behçet's disease.
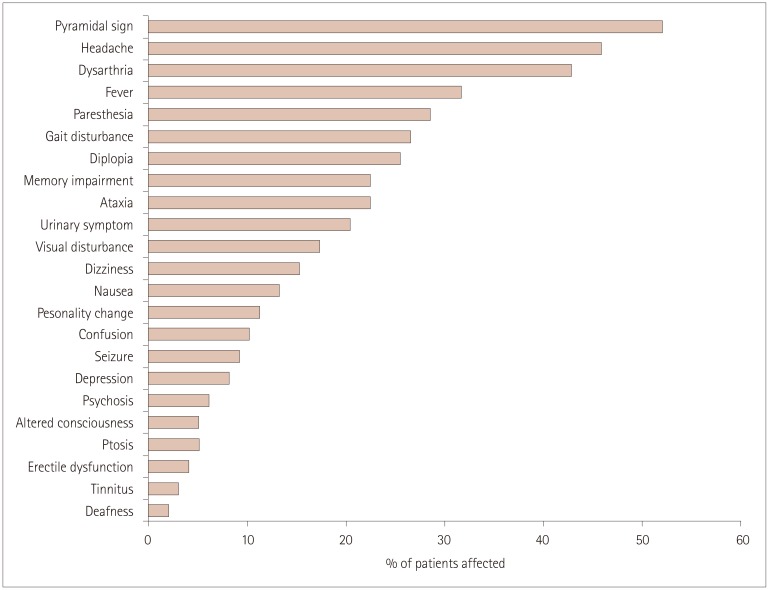
The detailed neurological manifestations of patients with parenchymal NBD are presented in Fig. 3. Overall, pyramidal signs or symptoms were the most common manifestation, observed in 51 (52.0%) patients. Headache and fever were observed in 45 (45.9%) and 31 (31.6%) patients, respectively. Symptoms or signs associated with brainstem involvement were also commonly observed in the present study, with dysarthria in 42 (42.9%) patients, diplopia in 25 (25.5%), and ataxia in 22 (22.4%). Memory impairment was the most common symptom associated with cerebral hemispheric involvement (22.4%), followed by personality change (11.2%), confusion (10.2%), and seizure (9.2%). While headache, paresthesia, and fever were relatively common in acute NBD, dysarthria, memory impairment, and gait disturbance were characteristic of chronic progressive NBD (Supplementary Fig. 1 in the online-only Data Supplement).
Fig. 3. Clinical manifestations in 98 patients with parenchymal neuro-Behçet's disease.
Clinical features of patients with nonparenchymal NBD
Twelve patients were classified as having nonparenchymal NBD, of which eight patients (four men and four women) exhibited acute meningeal syndrome. The ages at the onsets of BD and NBD were 29.5 [18.8–35.8] and 39 [24.5–49.3] years, respectively. Frequently presenting symptoms included headache (100%), fever (87.5%), and nausea (37.5%). All of the patients exhibited CSF pleocytosis. The CSF cell count was 96.0 [34.0–105.0]/mm3 and the CSF protein concentration was 39.8 [36.0–89.0] mg/dL. Seven patients were evaluated using brain MRI, which revealed leptomeningeal enhancement in two of them. Four patients exhibited venous sinus thrombosis. The thrombus was located in the transverse sinus in one patient, the transverse and sigmoid sinuses in one, the superior sagittal and transverse sinuses in one, and the superior sagittal, transverse, and sigmoid sinuses in one. The ages at the onsets of BD and NBD were 19.5 [15.8–21.8] and 33.5 [30.5–37.3] years, respectively. Frequently presenting symptoms included headache (100%), visual disturbance (50%), and nausea (25%).
Headache in patients with BD
Headache was present in 793 (47.1%) of the 1,682 patients with suspected BD who visited the neurology clinic: in 58 (52.7%) of the 110 patients with NBD and in 735 (46.8%) of the remaining 1,572 patients with BD (non-NBD). Further, 89.4% of the patients with BD who visited the neurology department had alternative diagnoses other than NBD that better explained their symptoms, and this was primary headache in 48.6% of cases. Overall, only 7.3% of the headaches in patients with suspected BD were attributed to NBD. We further compared basic demographic and concomitant neurological features between the patients with and without NBD who had headache. The male-to-female ratio indicated a marked female dominance (1:4.6) in patients without NBD having headache, whereas there was no significant sex difference (1:1.1) in patients with NBD having headache (p<0.001). Patients with NBD having headache were significantly younger (36.7±11.1 years) than those without NBD having headache (44.4±11.7 years, p<0.001). Moreover, 86.2% of the patients with NBD exhibited other neurological signs, whereas these signs were observed in only 7.5% of the patients without NBD having headache (p<0.001).
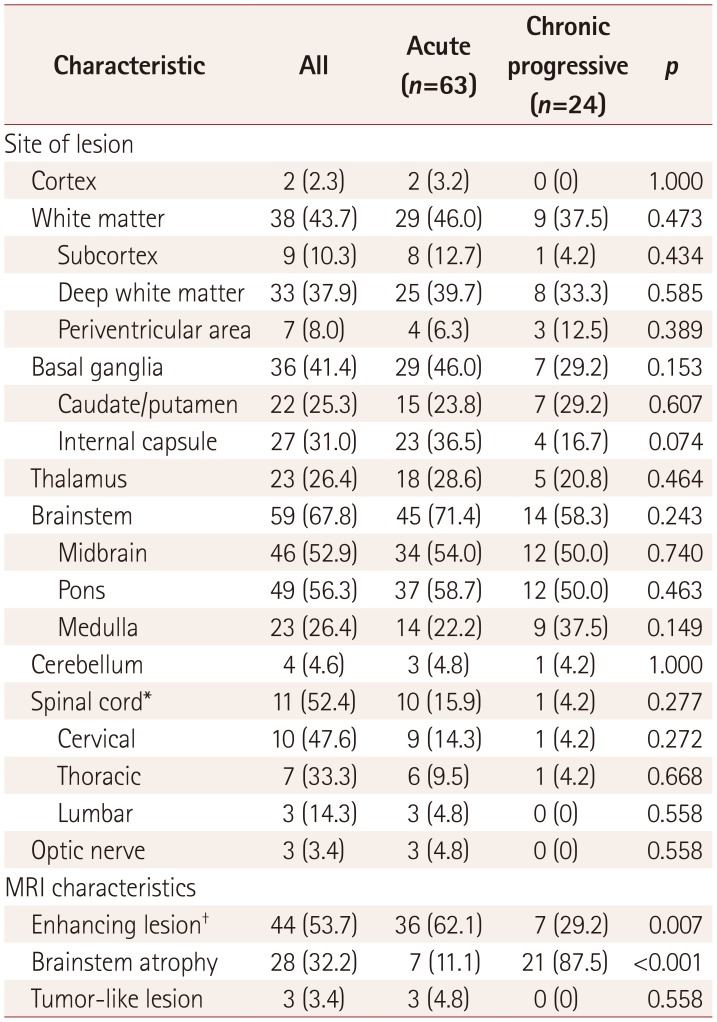
MRI findings of patients with parenchymal NBD
Brian MRI was conducted in 87 patients with parenchymal NBD. The characteristics of the MRI findings are summarized in Table 2. The most frequent site of involvement was the brainstem (67.8%), followed by the white matter (43.7%), basal ganglia (41.4%) and thalamus (26.4%). Most of the white-matter lesions were located in the deep white matter. Only a few patients had lesions in the subcortex or periventricular area. Cortical lesions were observed only in two (2.3%) patients. The location of the brain lesions did not differ significantly between patients with acute NBD and chronic progressive NBD. Contrast enhancement occurred significantly more frequently in patients with acute NBD (p=0.007) than in those with chronic progressive NBD, whereas the latter were strongly associated with brainstem atrophy (p<0.001). Lesions were observed in 11 (52.4%) of the 21 patients who underwent spinal cord MRI. Lesions were most frequently located in the cervical area, followed by the thoracic and lumbar segments. The length of the spinal cord lesion was one or two vertebral bodies in two patients, three to five vertebral bodies in four patients, and more than five vertebral bodies in five patients.
Table 2. Brain and spinal cord MRI findings in 87 patients with parenchymal NBD.
Data are n (%) values.
*Spinal cord MRI was conducted in 21 patients (14 with acute NBD and 7 with chronic progressive NBD), †Contrast-enhanced MRI was conducted in 82 patients (58 with acute NBD and 24 with chronic progressive NBD).
NBD: neuro-Behçet's disease.
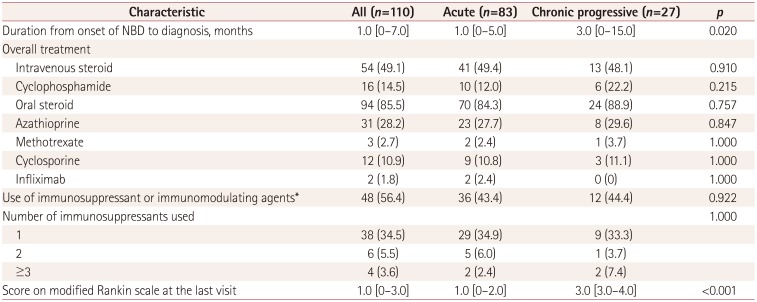
Treatment approach in patients with NBD
Table 3 lists the treatment approaches and prognoses in the 110 patients with NBD. The duration from the onset of NBD to the diagnosis was significantly shorter in patients with acute NBD [1.0 (0–5.0) months] than in those with chronic progressive NBD [3.0 (0–15.0) months, p=0.02]. There was no overall difference in treatment between acute NBD and chronic progressive NBD. Corticosteroids were most frequently selected as the treatment option: they were used for the initial treatment of NBD in 89 (80.9%) patients and were used overall in 96 (87.3%) patients. Immunosuppressive or immunomodulating agents were used in 48 (56.4%) patients. Azathioprine was the most common agent, followed by cyclophosphamide and cyclosporine. Seven of the 12 patients who received treatment with cyclosporine had been taking the medication prior to the NBD onset and continued the treatment for the management of the disease. Cyclosporine was primarily used for managing NBD in the remaining five patients. At the end of the follow-up, the score on the modified Rankin Scale was significantly higher in chronic progressive NBD [3.0 (3.0–4.0)] than in acute NBD [1.0 (0–3.0), p<0.001].
Table 3. Comparison of treatment approaches between acute NBD and chronic progressive NBD.
Data are n (%) or median [interquartile range] values.
*Includes azathioprine, methotrexate, cyclosporine, cyclophosphamide, and infliximab.
NBD: neuro-Behçet's disease.
DISCUSSION
The current study identified the characteristics of patients with NBD from a large study population observed over a period of 17 years. Patient inclusion criteria were determined based on the diagnostic criteria suggested by the recent international consensus recommendation.8 Although 17% of the patients with suspected BD visited the neurology department, the prevalence of NBD was 1.1% among the patients with suspected BD. Nearly 90% of the patients with suspected BD who visited the neurology department had alternative diagnoses, including primary headache. These findings demonstrate the importance of neurologists understanding the basic characteristics of NBD in order to properly diagnose and manage patients with BD exhibiting neurological symptoms.
The results of the present study are consistent with previous reports in terms of demographic and clinical features. The mean age at the onset of NBD was 37.6 years in the present study, which is within the range of 25.0–39.8 years reported previously.4,5,9,10,11,12 High male-to-female ratios for patients with NBD have been observed in most of the previous studies, with an overall male-to-female ratio of 2.8:1.1 However, lower male-to-female ratios have been reported for South Korea, ranging from 0.8:1 to 1.8:1,13,14 which may result from the low male-to-female ratio for BD in South Korea.15 Thus, the sex ratio of 1.2:1 in the present study may reflect both the overall male predominance in NBD and the racial characteristics of South Korean patients. The frequency of major clinical symptoms of BD and the proportion of patients testing positive for HLA–B51 are also consistent with previous reports.10,11,12,16 The percentage of positive pathergy test results has varied widely, from 8% to 70%, being high mostly in Mediterranean countries.1,17 In contrast, the proportion of positive pathergy test results has been reported to be low in both South Korea and Western countries.12,13,18 A pathergy reaction was observed in 13.8% of the patients in the present study, which is slightly higher than the rate of 6.7% observed previously in South Korea.13
The prevalence of NBD was 1.1% among all of the patients with suspected BD, 6.5% among the patients with suspected BD who visited the neurology clinic, and 12.6% among the patients with BD who fulfilled the ISG criteria and visited the neurology clinic. The prevalence of NBD among the patients with BD was lower than that found in previous prospective studies, which ranged from 5.3% to 14.3%.19,20,21 This discrepancy may have resulted from differences in the applied inclusion criteria. In contrast to the previous studies only including patients who fulfilled the diagnostic criteria for BD,19,20,21 all patients with suspected BD were included in the present study regardless of whether or not they satisfied the ISG criteria. Since the prevalence of NBD was higher among the patients with BD satisfying the ISG criteria (10.6%) than among those who did not fulfill the criteria (2.2%), the overall prevalence of NBD would have been higher if only the patients fulfilling the ISG criteria were included. However, we also included the patients who did not satisfy the ISG criteria since these patients can be classified as having probable NBD based on the recent international consensus recommendation.8 In addition, the reported prevalence of NBD among patients with BD has varied widely, from 1.3% to 59% depending on the study population, definition of NBD, and study design.2,5 Although the estimated prevalence of NBD has ranged between 5% and 30%,17 several studies—including another study from South Korea—have found low prevalence rates of NBD ranging from 1.3% to 3.5%.2,13,22
Headache has been reported to be the most common neurological symptom of BD.23,24,25 Consistently, headache was observed in nearly half of the present patients with NBD and those with BD without neurological involvement. Although headache can present as a sign of NBD, it also frequently occurs independently in patients with BD. Previous studies of the occurrence of headache in patients with BD found that tension-type headache and migraine are common,23,24,25 as they are in the general population. Both the present and previous studies found that only 10% of the headache in patients with BD is due to NBD.26,27 Most of the headaches in the patients with BD are benign, showing no abnormal findings in brain MRI.28 Thus, isolated headache in patients with BD is generally not attributed to neurological involvement in BD.3,4,11,12 Headache in NBD differs from benign headache, in that nearly 80% of patients with NBD demonstrate other neurological syndromes.3 In addition, we further demonstrated the differences in the demographic features between the patients with and without NBD having headache: the male-to-female ratio was significantly higher and the mean age was lower in patients with NBD than in those without NBD. These observations support the assumption that the headaches experienced by patients with and without NBD are different disease entities.
The clinical and radiographic features of NBD generally show a characteristic pattern despite some regional differences being reported. Overall, more than 80% of patients with NBD present with the parenchymal form, and approximately 10% of these patients exhibit spinal cord involvement.1 The prevalence rates of cerebral venous thrombosis and optic neuropathy show regional differences. The reported prevalence of cerebral venous thrombosis in Far Eastern and European countries has ranged from 0% to 9.1%,10,13,29,30,31 whereas it has been higher in the Middle East, ranging up to 42%.5 Optic neuropathy has been considered rare in patients with BD, with a prevalence of less than 5% among those with NBD,4,30,31 but this has been observed to be 9% in Caucasian patients.7 Regarding parenchymal NBD, the acute parenchymal form is more common than the chronic progressive form, with an approximate ratio of 2.2:1.9,32,33,34 This is similar to the ratio of 2.6:1 observed in the present study. Headache and pyramidal signs are the most commonly reported clinical signs, followed by sensory abnormality and ataxia.10,16 Although seizure usually occurs in fewer than 5% of cases,35 its prevalence has been reported to be as high as 27% in Caucasian patients.7 Radiographic findings of NBD patients characteristically show upper brainstem involvement, frequently extending to the basal ganglia and thalamus.14 Although the white matter is frequently involved, white-matter lesions show no predilection for the periventricular area.30 Brain atrophy was observed in 10–30% of the patients in previous studies,9,36,37 and was present in 32.2% of the patients in the current study.
The recent international consensus recommendation suggests that intravenous methylprednisolone should be administered in an acute attack or relapse.8 It is generally recommended that steroids should be used for the initial attack, with other oral immunosuppressants added if the response is inadequate.1 Among oral immunosuppressants, the recent international consensus recommendation suggests azathioprine as a first-line disease-modifying therapy.8 It is recommended to use cyclosporine with caution due to its potential association with the development of CNS complications.38 However, none of the patients included in the present study discontinued cyclosporine treatment following the diagnosis of NBD, suggesting that the potential neurotoxicity of cyclosporine is not widely known among physicians. Although no difference in therapeutic agents was found between acute NBD and chronic progressive NBD, those patients with chronic progressive NBD displayed both a progression of the neurological symptoms and a worse score on the modified Rankin Scale compared to subjects with acute NBD at the last visit. This finding suggests that the currently recommended therapeutic guideline is ineffective in the treatment of the chronic progressive form of NBD. The findings of some recent studies suggest that methotrexate or infliximab is effective against the progressive form of NBD.39,40 The duration from the onset of NBD to a diagnosis was significantly longer for the chronic progressive form. It has been suggested that the early initiation of treatment is associated with better outcomes.5 However, the initial diagnosis is frequently delayed in the progressive form of NBD due to its insidious onset, and it is unclear whether early treatment of these patients influences the prognosis.
Several limitations existed in the present study. First, information regarding the presence of neurological symptoms in the remaining 8,135 patients with suspected BD who did not visit the neurology department could not be obtained, and it is possible that some of these patients exhibited NBD. Second, the serum and CSF levels of various disease markers were not evaluated in the present study. Recent studies have demonstrated that interleukin-6, tumor necrosis factor alpha, and matrix metallopeptidase-9 levels are associated with disease activity in patients with NBD,32,41,42 and measuring these markers may have provided useful information for the diagnosis of NBD. Third, although the acute meningeal syndrome was classified as nonparenchymal NBD following the recent international consensus recommendation, microscopic inflammatory lesions may be present in the brain parenchyma. In addition, the acute meningeal syndrome could not be fully discriminated from simple headache in NBD in the present investigation, considering that CSF was not analyzed in each patient. However, given that it is not practically feasible to perform a CSF analysis for all NBD patients with headaches, we classified headaches as primary headaches despite CSF analysis not being conducted.
In conclusion, the present study has revealed the clinical features of a large population of patients with NBD in South Korea. Approximately 1% of the patients with suspected BD exhibited NBD, and most of the patients who visited the neurology department had alternative diagnoses that provided a better explanation of their symptoms. These findings indicate the importance of neurologists understanding the clinical and radiographic characteristics of NBD in order to perform the differential diagnosis and management of these patients.
Footnotes
- Conceptualization: Dongsik Bang, Ha Young Shin, Seung Woo Kim, Tae-Gyun Kim.
- Data curation: Seung Woo Kim, Tae-Gyun Kim, Jongwook Oh, Do-Young Kim.
- Formal analysis: Ha Young Shin, Seung Woo Kim.
- Investigation: Seung Woo Kim, Tae-Gyun Kim, Jongwook Oh, Do-Young Kim, Young-Chul Choi, Seung Min Kim, Ha Young Shin, Dongsik Bang.
- Methodology: Seung Woo Kim, Tae-Gyun Kim, Jongwook Oh, Do-Young Kim, Young-Chul Choi, Seung Min Kim, Ha Young Shin, Dongsik Bang.
- Supervision: Do-Young Kim, Young-Chul Choi, Seung Min Kim, Ha Young Shin, Dongsik Bang.
- Validation: Seung Woo Kim, Tae-Gyun Kim, Jongwook Oh, Do-Young Kim, Young-Chul Choi, Seung Min Kim, Ha Young Shin, Dongsik Bang.
- Visualization: Seung Woo Kim.
- Writing—original draft: Seung Woo Kim, Tae-Gyun Kim, Ha Young Shin, Dongsik Bang.
- Writing—review & editing: Seung Woo Kim, Tae-Gyun Kim, Jongwook Oh, Do-Young Kim, Young-Chul Choi, Seung Min Kim, Ha Young Shin, Dongsik Bang.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2019.15.4.429.
Clinical manifestations in (A) 71 patients with acute parenchymal NBD and (B) 27 patients with chronic progressive parenchymal NBD. NBD: neuro-Behçet's disease.
References
- 1.Al-Araji A, Kidd DP. Neuro-Behçet's disease: epidemiology, clinical characteristics, and management. Lancet Neurol. 2009;8:192–204. doi: 10.1016/S1474-4422(09)70015-8. [DOI] [PubMed] [Google Scholar]
- 2.Tursen U, Gurler A, Boyvat A. Evaluation of clinical findings according to sex in 2313 Turkish patients with Behçet's disease. Int J Dermatol. 2003;42:346–351. doi: 10.1046/j.1365-4362.2003.01741.x. [DOI] [PubMed] [Google Scholar]
- 3.Siva A, Kantarci OH, Saip S, Altintas A, Hamuryudan V, Islak C, et al. Behçet's disease: diagnostic and prognostic aspects of neurological involvement. J Neurol. 2001;248:95–103. doi: 10.1007/s004150170242. [DOI] [PubMed] [Google Scholar]
- 4.Akman-Demir G, Serdaroglu P, Tasçi B. Clinical patterns of neurological involvement in Behçet's disease: evaluation of 200 patients. The Neuro-Behçet Study Group. Brain. 1999;122:2171–2182. doi: 10.1093/brain/122.11.2171. [DOI] [PubMed] [Google Scholar]
- 5.Farah S, Al-Shubaili A, Montaser A, Hussein JM, Malaviya AN, Mukhtar M, et al. Behçet's syndrome: a report of 41 patients with emphasis on neurological manifestations. J Neurol Neurosurg Psychiatry. 1998;64:382–384. doi: 10.1136/jnnp.64.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda K, Abe M, Iwasaki Y, Kinoshita M. Neuro-Behçet's disease in Japan. Neurology. 1996;47:614–615. doi: 10.1212/wnl.47.2.614. [DOI] [PubMed] [Google Scholar]
- 7.Joseph FG, Scolding NJ. Neuro-Behçet's disease in Caucasians: a study of 22 patients. Eur J Neurol. 2007;14:174–180. doi: 10.1111/j.1468-1331.2006.01613.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalra S, Silman A, Akman-Demir G, Bohlega S, Borhani-Haghighi A, Constantinescu CS, et al. Diagnosis and management of neuro-Behçet's disease: international consensus recommendations. J Neurol. 2014;261:1662–1676. doi: 10.1007/s00415-013-7209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirohata S, Kikuchi H, Sawada T, Nagafuchi H, Kuwana M, Takeno M, et al. Clinical characteristics of Neuro-Behcet's disease in Japan: a multicenter retrospective analysis. Mod Rheumatol. 2012;22:405–413. doi: 10.1007/s10165-011-0533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ideguchi H, Suda A, Takeno M, Kirino Y, Ihata A, Ueda A, et al. Neurological manifestations of Behçet's disease in Japan: a study of 54 patients. J Neurol. 2010;257:1012–1020. doi: 10.1007/s00415-010-5454-2. [DOI] [PubMed] [Google Scholar]
- 11.Houman MH, Bellakhal S, Ben Salem T, Hamzaoui A, Braham A, Lamloum M, et al. Characteristics of neurological manifestations of Behçet's disease: a retrospective monocentric study in Tunisia. Clin Neurol Neurosurg. 2013;115:2015–2018. doi: 10.1016/j.clineuro.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Talarico R, D'Ascanio A, Figus M, Stagnaro C, Ferrari C, Elefante E, et al. Behçet's disease: features of neurological involvement in a dedicated centre in Italy. Clin Exp Rheumatol. 2012;30:S69–S72. [PubMed] [Google Scholar]
- 13.Yoon DL, Kim YJ, Koo BS, Kim YG, Lee CK, Yoo B. Neuro-Behçet's disease in South Korea: clinical characteristics and treatment response. Int J Rheum Dis. 2014;17:453–458. doi: 10.1111/1756-185X.12265. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Yoon PH, Park SJ, Kim DI. MRI findings in neuro-Behçet's disease. Clin Radiol. 2001;56:485–494. doi: 10.1053/crad.2000.0675. [DOI] [PubMed] [Google Scholar]
- 15.Davatchi F, Shahram F, Chams-Davatchi C, Sadeghi Abdollahi B, Shams H, Nadji A, et al. Behcet's disease: is there a gender influence on clinical manifestations. Int J Rheum Dis. 2012;15:306–314. doi: 10.1111/j.1756-185X.2011.01696.x. [DOI] [PubMed] [Google Scholar]
- 16.Noel N, Bernard R, Wechsler B, Resche-Rigon M, Depaz R, Le Thi Huong Boutin D, et al. Long-term outcome of neuro-Behçet's disease. Arthritis Rheumatol. 2014;66:1306–1314. doi: 10.1002/art.38351. [DOI] [PubMed] [Google Scholar]
- 17.Borhani Haghighi A, Pourmand R, Nikseresht AR. Neuro-Behçet disease: a review. Neurologist. 2005;11:80–89. doi: 10.1097/01.nrl.0000156343.16797.c4. [DOI] [PubMed] [Google Scholar]
- 18.Hatemi I, Hatemi G, Celik AF, Melikoglu M, Arzuhal N, Mat C, et al. Frequency of pathergy phenomenon and other features of Behçet's syndrome among patients with inflammatory bowel disease. Clin Exp Rheumatol. 2008;26:S91–S95. [PubMed] [Google Scholar]
- 19.Al-Araji A, Sharquie K, Al-Rawi Z. Prevalence and patterns of neurological involvement in Behcet's disease: a prospective study from Iraq. J Neurol Neurosurg Psychiatry. 2003;74:608–613. doi: 10.1136/jnnp.74.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashjazadeh N, Borhani Haghighi A, Samangooie S, Moosavi H. Neuro-Behcet's disease: a masquerader of multiple sclerosis. A prospective study of neurologic manifestations of Behcet's disease in 96 Iranian patients. Exp Mol Pathol. 2003;74:17–22. doi: 10.1016/s0014-4800(03)80004-7. [DOI] [PubMed] [Google Scholar]
- 21.Serdaroğlu P, Yazici H, Ozdemir C, Yurdakul S, Bahar S, Aktin E. Neurologic involvement in Behçet's syndrome. A prospective study. Arch Neurol. 1989;46:265–269. doi: 10.1001/archneur.1989.00520390031011. [DOI] [PubMed] [Google Scholar]
- 22.Borhani-Haghighi A, Samangooie S, Ashjazadeh N, Nikseresht A, Shariat A, Yousefipour G, et al. Neurological manifestations of Behçet's disease. Saudi Med J. 2006;27:1542–1546. [PubMed] [Google Scholar]
- 23.Moghaddassi M, Togha M, Shahram F, Hanif H, Dadkhah S, Jahromi SR, et al. Headache in Behcet's disease: types and characteristics. Springerplus. 2016;5:1077. doi: 10.1186/s40064-016-2721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fountain EM, Dhurandhar A. Neuro-Behçet's disease: an unusual cause of headache. J Gen Intern Med. 2014;29:956–960. doi: 10.1007/s11606-014-2781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd D. The prevalence of headache in Behçet's syndrome. Rheumatology (Oxford) 2006;45:621–623. doi: 10.1093/rheumatology/kei255. [DOI] [PubMed] [Google Scholar]
- 26.Saip S, Siva A, Altintas A, Kiyat A, Seyahi E, Hamuryudan V, et al. Headache in Behçet's syndrome. Headache. 2005;45:911–919. doi: 10.1111/j.1526-4610.2005.05160.x. [DOI] [PubMed] [Google Scholar]
- 27.Borhani Haghighi A, Aflaki E, Ketabchi L. The prevalence and characteristics of different types of headache in patients with Behçet's disease, a case-control study. Headache. 2008;48:424–429. doi: 10.1111/j.1526-4610.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- 28.Gökçay F, Celebisoy N, Gökçay A, Aksu K, Keser G. Neurological symptoms and signs in Behçet disease: a Western Turkey experience. Neurologist. 2011;17:147–150. doi: 10.1097/NRL.0b013e3182173379. [DOI] [PubMed] [Google Scholar]
- 29.Lee HS, Kim DY, Shin HY, Choi YC, Kim SM. Spinal cord involvement in Behçet's disease. Mult Scler. 2016;22:960–963. doi: 10.1177/1352458515613642. [DOI] [PubMed] [Google Scholar]
- 30.Kidd D, Steuer A, Denman AM, Rudge P. Neurological complications in Behçet's syndrome. Brain. 1999;122:2183–2194. doi: 10.1093/brain/122.11.2183. [DOI] [PubMed] [Google Scholar]
- 31.Lo Monaco A, La Corte R, Caniatti L, Borrelli M, Trotta F. Neurological involvement in North Italian patients with Behçet disease. Rheumatol Int. 2006;26:1113–1119. doi: 10.1007/s00296-006-0149-9. [DOI] [PubMed] [Google Scholar]
- 32.Akman-Demir G, Tüzün E, Içöz S, Yeşilot N, Yentür SP, Kürtüncü M, et al. Interleukin-6 in neuro-Behçet's disease: association with disease subsets and long-term outcome. Cytokine. 2008;44:373–376. doi: 10.1016/j.cyto.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Coban O, Bahar S, Akman-Demir G, Taşci B, Yurdakul S, Yazici H, et al. Masked assessment of MRI findings: is it possible to differentiate neuro-Behçet's disease from other central nervous system diseases? [corrected] Neuroradiology. 1999;41:255–260. doi: 10.1007/s002340050742. [DOI] [PubMed] [Google Scholar]
- 34.Sumita Y, Murakawa Y, Sugiura T, Wada Y, Nagai A, Yamaguchi S. Elevated BAFF levels in the cerebrospinal fluid of patients with neuro-Behçet's disease: BAFF is correlated with progressive dementia and psychosis. Scand J Immunol. 2012;75:633–640. doi: 10.1111/j.1365-3083.2012.02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aykutlu E, Baykan B, Serdaroglu P, Gökyigit A, Akman-Demir G. Epileptic seizures in Behçet disease. Epilepsia. 2002;43:832–835. doi: 10.1046/j.1528-1157.2002.01102.x. [DOI] [PubMed] [Google Scholar]
- 36.Farahangiz S, Sarhadi S, Safari A, Borhani-Haghighi A. Magnetic resonance imaging findings and outcome of neuro-Behçet's disease: the predictive factors. Int J Rheum Dis. 2012;15:e142–e149. doi: 10.1111/1756-185X.12013. [DOI] [PubMed] [Google Scholar]
- 37.Borhani Haghighi A, Sarhadi S, Farahangiz S. MRI findings of neuro-Behcet's disease. Clin Rheumatol. 2011;30:765–770. doi: 10.1007/s10067-010-1650-9. [DOI] [PubMed] [Google Scholar]
- 38.Kotake S, Higashi K, Yoshikawa K, Sasamoto Y, Okamoto T, Matsuda H. Central nervous system symptoms in patients with Behçet disease receiving cyclosporine therapy. Ophthalmology. 1999;106:586–589. doi: 10.1016/S0161-6420(99)90120-3. [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi H, Aramaki K, Hirohata S. Effect of infliximab in progressive neuro-Behçet's syndrome. J Neurol Sci. 2008;272:99–105. doi: 10.1016/j.jns.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Hirohata S, Kikuchi H, Sawada T, Nagafuchi H, Kuwana M, Takeno M, et al. Retrospective analysis of long-term outcome of chronic progressive neurological manifestations in Behcet's disease. J Neurol Sci. 2015;349:143–148. doi: 10.1016/j.jns.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Santos Lacomba M, Marcos Martín C, Gallardo Galera JM, Gómez Vidal MA, Collantes Estévez E, Ramírez Chamond R, et al. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001;33:251–255. doi: 10.1159/000055677. [DOI] [PubMed] [Google Scholar]
- 42.Aldinucci A, Bonechi E, Biagioli T, Repice AM, D'Elios MM, Emmi L, et al. CSF/serum matrix metallopeptidase-9 ratio discriminates neuro Behçet from multiple sclerosis. Ann Clin Transl Neurol. 2018;5:493–498. doi: 10.1002/acn3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical manifestations in (A) 71 patients with acute parenchymal NBD and (B) 27 patients with chronic progressive parenchymal NBD. NBD: neuro-Behçet's disease.