Abstract
BACKGROUND
Pancreatic cancer is a major cause of cancer-related death, with a 5-year overall survival rate being below 5%. The main causes of poor prognosis in pancreatic cancer include easy metastasis, high recurrence rate, and robust drug resistance. Gemcitabine is a first-line drug for patients with unresectable pancreatic cancer. However, due to drug resistance, the clinical effect is not satisfactory. ADAM28 is reported as a tumor promoter in some cancers, but its role in pancreatic cancer and gemcitabine chemoresistance in pancreatic cancer has not been elucidated.
AIM
To identify if ADAM28 can act as an important target to reverse the gemcitabine drug resistance in pancreatic cancer.
METHODS
RNA-sequence analysis was applied to explore the potential targets involved in the gemcitabine of pancreatic cancer. SW1990 pancreatic cancer cells were treated with an increased dose of gemcitabine, and the mRNA levels of ADAM28 were evaluated by RT-PCR. The protein and mRNA levels of ADAM28 were confirmed in the gemcitabine resistant and parallel SW1990 cells. The ADAM28 expression was also assessed in TCGA and GEO databases, and the results were confirmed in the collected tumor and adjacent normal tissues. The overall survival (OS) rate and relapse-free survival (RFS) rate of pancreatic cancer patients with high ADAM28 level and low ADAM28 level in TCGA were evaluated with Kaplan-Meier Plotter. Furthermore, the OS rate was calculated in pancreatic cancer patients with high tumor mutation burden (TMB) and low TMB. CCK-8 assay was used to examine the effect of ADAM28 on the viability of SW1990 cells. The ADAM28 and its co-expressed genes were analyzed in the cBioPortal for cancer genomics and subjected to GSEA pathway analysis. The correlations of ADAM28 with GSTP1, ABCC1, GSTM4, and BCL2 were analyzed based on TCGA data on pancreatic cancer.
RESULTS
RNA-sequence analysis identified that ADAM28 was overexpressed in gemcitabine-resistant cells, and gemcitabine treatment could induce the expression of ADAM28. The mRNA and protein levels of ADAM28 were elevated in gemcitabine-resistant SW1990 cells compared with parallel cells. Also, the expression of ADAM28 was upregulated in pancreatic tumor tissues against normal pancreatic tissues. Notably, ADAM28 was highly expressed in the classical type than in the basal tumor type. Furthermore, the high expression of ADAM28 was associated with low OS and RFS rates. Interestingly, the high levels of ADAM28 was associated with a significantly lower OS rate in the high TMB patients, but not in the low TMB patients. Moreover, overexpression of ADAM28 could reduce the cell viability inhibition by gemcitabine, and knockdown of ADAM28 could enhance the proliferation inhibition by gemcitabine. The GSEA analysis showed that ADAM28 was related to the regulation of drug metabolism, and ADAM28 was significantly positively correlated with GSTP1, ABCC1, GSTM4, and BCL2.
CONCLUSION
This study demonstrates that ADAM28 is overexpressed in pancreatic cancer, and closely involved in the regulation of gemcitabine resistance. Overexpression of ADAM28 is a novel prognostic biomarker in pancreatic cancer.
Keywords: ADAM28, Drug resistance, Overexpression, Poor prognosis, Drug metabolism, Gemcitabine
Core tip: This study demonstrated that ADAM28 was overexpressed in pancreatic cancer, and the ADAM28 level was elevated in gemcitabine-resistant pancreatic cancer cells. Furthermore, ADAM28 overexpression could predict a poor prognosis in pancreatic cancer. Also, overexpression of ADAM28 could attenuate the cell viability inhibition by gemcitabine, while knockdown of ADAM28 could enhance the cell viability inhibition by gemcitabine. Interestingly, ADAM28 was identified as a mediator which is closely involved in the regulation of drug resistance-related signaling pathways. ADAM28 was identified as a novel therapeutic target for pancreatic cancer, especially in case of resistance to gemcitabine.
INTRODUCTION
Pancreatic cancer is a common malignant tumor, and its symptoms are not obvious[1]. Even in the early stage, local tumor infiltration and distant metastasis are often observed in the clinical practice. Thus, most patients have no chance of radical surgery when they are diagnosed, and only about 15%-20% of patients with pancreatic carcinoma have the opportunity for surgery[2-4]. Chemotherapy is an important treatment method for pancreatic carcinoma. Gemcitabine is the first-line drug for chemotherapy in pancreatic carcinoma. Although the application of gemcitabine has a certain effect in terms of clinical benefit, it has limited control of progression of pancreatic carcinoma[5,6]. However, chemoresistance is a tough barrier in the treatment of pancreatic carcinoma, and the prognostic study has confirmed that the clinical effectiveness of gemcitabine is below 20%[7,8]. Therefore, an in-depth understanding of the molecular mechanism for the regulation of gemcitabine chemoresistance is important, and identification of novel drug targets is a viable strategy for drug resistance reversal.
ADAM28 is a membrane-located protein, which is implicated in a variety of biological process, including tooth development, muscle development, neurogenesis, catalytic activity, and cell-cell interactions[9-11]. The biological function study also reveals that ADAM28 is involved in hyperostosis cranialis interna and some cancers[12,13]. In breast and colorectal cancers, ADAM28 is highly expressed and promotes proliferation via regulation of insulin-like growth factor binding protein 3 (IGFBP-3)[14,15]. As a ligand for the integrin receptor, ADAM28 also mediates the metastasis of non-small cell lung carcinoma and the lymphocyte adhesion[16,17]. Also, ADAM28 was identified to bind P-selectin glycoprotein ligand-1, and the expression of ADAM28 enhanced the leukocyte adhesion to endothelial cells under inflammatory conditions[18]. In the aspect of diagnostic study, ADAM28 was overexpressed and characterized as a biomarker for bladder transitional cell carcinoma[19]. As a protein with catalytic activity, ADAM28 could cleave IGFBP-3 and von Willebrand factor[14,20]. Besides, ADAM28 could promote TNFα shedding and is involved in metabolic dysfunction[21]. The mechanism study also revealed that ADAM28 could activate the PI3K/AKT pathway, and ADAM28 was also reported as a key component in EGFR signaling[22,23].
In this study, we explored the role of ADAM28 in the development of drug resistance to gemcitabine. More importantly, we identified that ADAM28 was an important prognostic factor in pancreatic cancer. And the pathway analysis showed that ADAM28 was closely associated with the regulation of drug metabolism, especially the drug resistance-related genes. These findings give a better understanding of the role of ADAM28 in gemcitabine resistance of pancreatic cancer cells.
MATERIALS AND METHODS
Reagents
Leibovitz's L-15 cell culture medium (Cat. 11415114), fetal bovine serum (FBS), penicillin-streptomycin (Cat. 15140122), lipofectamine 3000, and TRIzol RNA purification kit (Cat. 12183555) were purchased from ThermoFisher Scientific. First-strand cDNA synthesis kit with genomic DNA digester, ECL reagents, and qPCR SYBR Green Mix were all purchased from Yeasen Biotech. ADAM28 polyclonal antibody (Cat. 22234-1) and β-actin antibody (Cat. 20536-1) were obtained from Proteintech Group. The primers of the indicated target genes for RT-PCR were produced by Invitrogen. Gemcitabine was from TCI, and other chemical reagents are analytical grade and were provided by Aladdin.
Cell culture
SW1990 pancreatic cancer cells, obtained from the American Type Culture Collection (ATCC), were cultured in L-15 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. And the culture condition was set at 37 °C in humidified air with 5% CO2. Gemcitabine resistant SW1990 cells were established as previously reported, and the resistance index was used to confirm the construction.
Gene Expression Omnibus (GEO) analysis
Differentially expressed genes (DEGs) were identified in the GEO database. The GSE35141 profile was included with GEO2R tool for DEGs identification in the gemcitabine-resistant cells and sensitive parallel cells[24]. The analysis is shown as a volcano plot, with a threshold of fold change (FC) ≥ 1.5 and a P-value < 0.01.
Reverse transcription-polymerase chain reaction (RT-PCR)
The cells were collected with a cell scraper and washed with cold PBS solution twice. Then, the cells were lysed with TRIzol RNA extraction reagent according to the manufacturer’s protocol. The purified RNA was used for the first-strand cDNA synthesis with a reverse transcription kit. SYBR Green qPCR was used to evaluate the mRNA levels of indicated genes. β-actin was used as a normalized gene, and the data were analyzed using the 2-ΔΔCT method.
Western blot assay
The indicated cells were washed with cold PBS twice, collected with a cell scraper, and treated with RIPA lysis buffer on ice for 30 min. Then, the whole solution was subjected to centrifugation at 12000 g/min for 15 min. The supernatant was collected, and protein loading was normalized with BCA assay. The total protein (20 μg) was then subjected to 10% SDS-PAGE, and transferred to PVDF membranes. With incubation of skimmed milk for 1 h at room temperature, the membranes were incubated with indicated primary antibody overnight. The detection was achieved using the enhanced chemiluminescence (ECL) system.
The Cancer Genome Atlas (TCGA) analysis
The expression data of pancreatic cancer were obtained from TCGA, and the ADAM28 levels were analyzed with transcripts per million (TPM) as log2 (TPM + 1) in Gene Expression Profiling Interactive Analysis (GPEIA) portal[25]. The clinical parameters and survival data were also acquired in the TCGA pancreatic cancer database, and the overall survival (OS) and relapse-free survival (RFS) rates were analyzed by the Kaplan-Meier Plotter. Also, the correlation analysis of indicated genes was evaluated in GPEIA portal.
Clinical tissue analysis
A total of 16 pancreatic ductal adenocarcinoma tissues and corresponding adjacent normal pancreatic tissues were collected in the surgery, and the tissues were stored at a –80 °C freezer. The tissues were subjected to homogenization, and then total RNA was extracted for RT-PCR. The study was approved by the Ethical Committee of the Third Affiliated Hospital of Sun Yat-Sen University.
Cloning and transfection
The full length of human ADAM28 transcript was cloned into the pcDNA3.1 (+) vector. The primers for the overexpression construct are: Forward primer, 5’- GGG GTA CCA TGT TGC AAG GTC TCC TGC CA-3’; reverse primer, 5’- CCG GAA TTC TCA TGC TTT TGG ATT TGA GTCC-3’. The knockdown construct was achieved with a pSuper knocking down system as previously reported[26]. The knockdown sequence was designed at the Sigma shRNA section, and the detailed sequence is: 5’-CCG GGC AAG AAC TAA TGG CTA AAT TCT CGA GAA TTT AGC CAT TAG TTC TTG CTT TTTG-3’. The constructs were confirmed by sequencing. Lipofectamine 3000 was used for transfection in SW1990 cells. Briefly, the plasmid and lipofectamine 3000 were diluted in Opti-MEM medium, respectively, and the two solutions were softly mixed at a ratio of 1:1, and stored at room temperature for 20 min. Then, the mixture was added into the cells.
Cell counting kit-8 (CCK-8) assay
The cells were plated in a 96-wells plate and treated for 24 h. Then, the CCK-8 reagent was added into cells for another 4 h culture. And the absorbance was examined with a microplate reader at 450 nm.
Gene set enrichment analysis (GSEA) for pathway analysis
ADAM28 and its co-expressed genes were identified by analyzing TCGA pancreatic cancer database. Then, the c2. Cp. kegg. V6.1. symbol. gmt and c5. all. v6. 1. Symbols. gmt gene datasets were downloaded from the GSEA and MsigDB portal. The expression data were analyzed using GSEA version 3.0 portal. The enrichment analysis was performed and showed from high enrichment score to low enrichment score. The gene clusters with a false discovery rate (FDR) < 0.25 and P-value < 0.05 were considered as significantly enriched genes[27].
Statistical analysis
The results were analyzed with GraphPad version 8. The Chi-squared test was used to analyze the counting data of RNA-sequence. Student’s t-test was used to analyze the difference between two groups. The log-rank test and Kaplan-Meier analysis were conducted to evaluate the survival rates. P < 0.05 was considered significant.
RESULTS
Identification of ADAM28 as a novel chemoresistance-related gene in pancreatic cancer cells
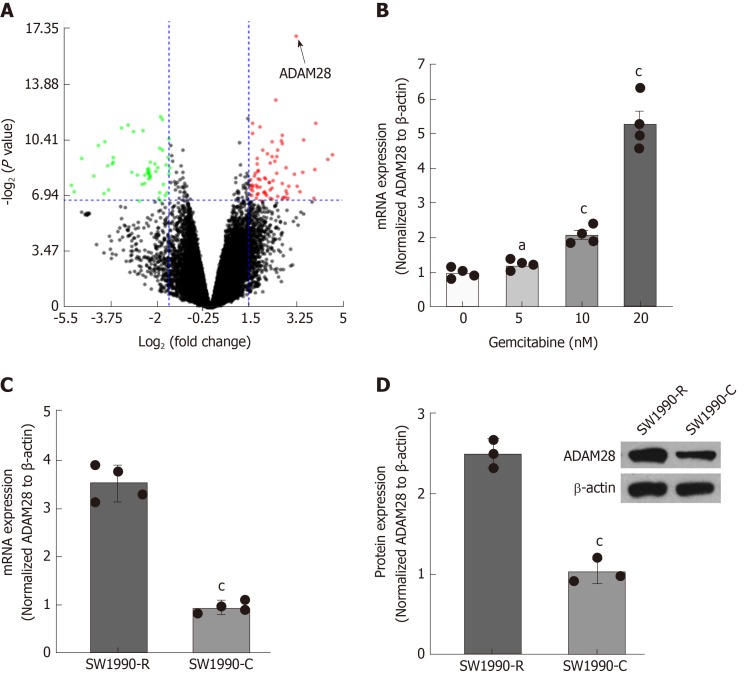
To explore a potential target to overcome gemcitabine resistance in pancreatic cancer therapy, GSE35141[28] was subjected to evaluation and the results showed that ADAM28 was a significantly upregulated gene in the gemcitabine-resistant cells compared with the parallel sensitive cells according to the fold change and statistical difference (Figure 1A), suggesting that ADAM28 might be involved in the regulation network of drug resistance, and it was therefore selected for the further characterization. To explore the potential role of ADAM28 in chemotherapy, human pancreatic cancer SW1990 cells were treated with different doses of gemcitabine, and the ADAM28 expression was examined by qRT-PCR. The data showed increased expression of ADAM28 in a dose-dependent manner with the treatment of gemcitabine for 24 h (Figure 1B). Furthermore, ADAM28 expression was determined in the gemcitabine-resistant SW1990 cells and parallel cells, as shown in Figure 1C and D, and the results revealed that ADAM28 was elevated in the gemcitabine-resistant cells at both the mRNA and protein levels, which is consistent with that shown in Figure 1A, suggesting that ADAM28 may be involved in the regulation of gemcitabine metabolism in chemoresistance.
Figure 1.
ADAM28 expression is elevated in gemcitabine-resistant pancreatic cancer cells. A: GSE35141 was used to evaluate DEGs, and the significant genes are showed by a volcano plot with a threshold of fold change ≥ 1.5 and P-value < 0.01. The figure was prepared with NetworkAnalyst 3.0[28]; B: The mRNA levels of ADAM28 detected by RT-PCR in SW1990 cells treated with different doses of gemcitabine as indicated for 24 h. aP < 0.05, cP < 0.001 vs without gemcitabine treatment; C: The mRNA levels of ADAM28 examined by RT-PCR in gemcitabine-resistant SW1990 cells (SW1990-R) and sensitive parallel SW1990 (SW1990-C) cells. cP < 0.001 vs SW1990 sensitive cells (SW1990-C); D: The protein expression of ADAM28 was examined in gemcitabine-resistant and sensitive parallel SW1990 cells by Western blot, and Quantity One software was applied to quantify the protein levels. cP < 0.001 vs with SW1990 sensitive parallel cells (SW1990-C). DEGs: Differentially expressed genes; FC: Fold change; RT-PCR: Reverse transcription-polymerase chain reaction; SW1990-C: SW1990 sensitive parallel cells; SW1990-R: Gemcitabine-resistant SW1990 cells.
ADAM28 is overexpressed in pancreatic cancer tissues
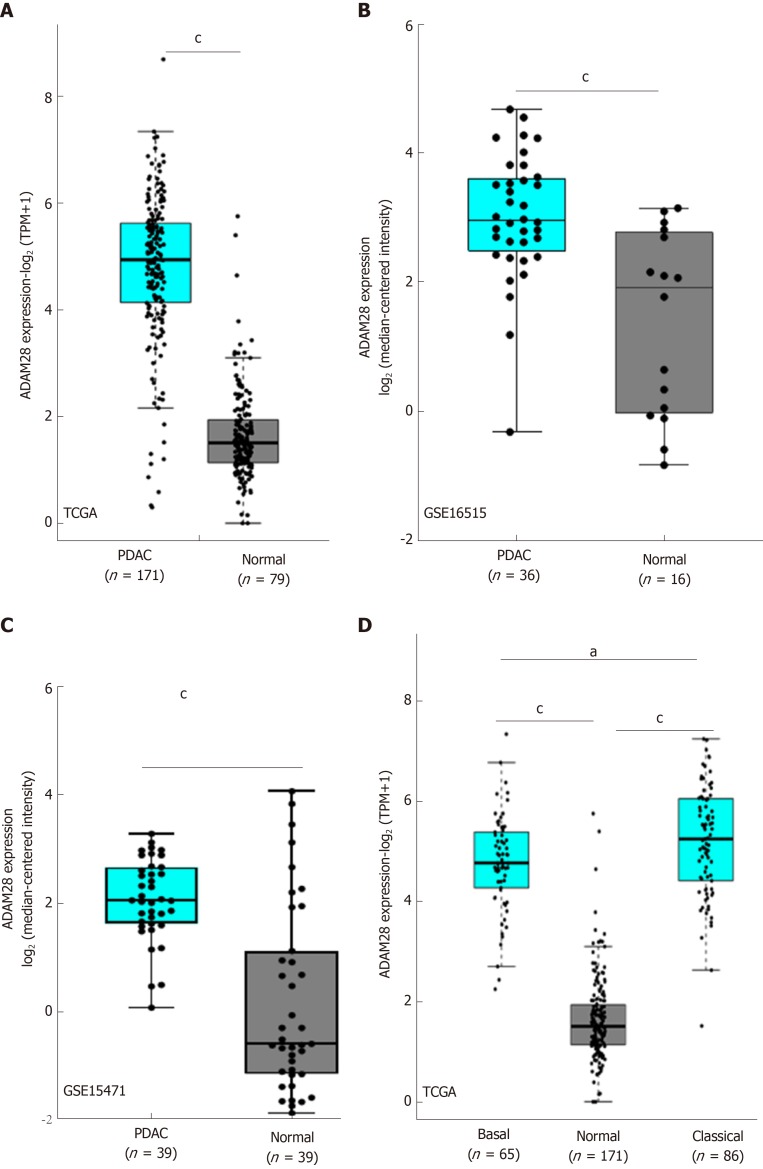
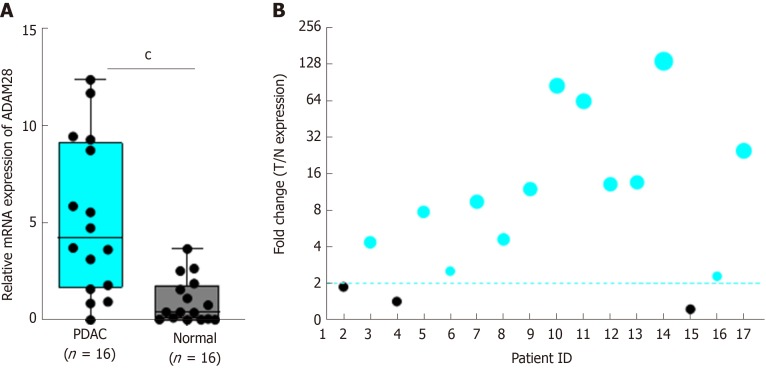
We next evaluated the expression of ADAM28 in the pancreatic tumor tissues and adjacent normal tissues based on GSE16515[29] and GSE15471[30]. The results showed that compared with adjacent normal pancreatic tissues, the expression level of ADAM28 was significantly higher in pancreatic cancer tissues (Figure 2A-C). As tumor type is an important factor in the target identification, especially for pancreatic cancer, whose tumor microenvironment is more complex than other tumors, we next determined that expression of ADAM28 in different types of pancreatic cancer. As shown in Figure 2D, ADAM28 was highly expressed both in basal and classical types of pancreatic tumors, and more interestingly, the expression level of ADAM28 was slightly increased in classical pancreatic tumors than in basal pancreatic tumors. To confirm the overexpression of ADAM28 in pancreatic cancer, a total of 16 patients with pancreatic tumor were subjected to examination of ADAM28 expression in the tumor and adjacent tissues. As shown in Figure 3A, the expression of ADAM28 was remarkably increased in pancreatic tumor tissues than in normal pancreatic tissues. The ratio between the expression of ADAM28 in the tumor and adjacent normal tissues is shown in Figure 3B. The 2-fold difference was used as a filter, which was considered that the high expression of ADAM28 in tumor than adjacent normal tissues. The results showed that 13 (about 80%) patients had overexpression of ADAM28 in the pancreatic tumor tissues, indicating the significance of ADAM28 in pancreatic cancer.
Figure 2.
ADAM28 is overexpressed in pancreatic cancer tissues. A: The TCGA database was used to evaluate the transcript of ADAM28 in pancreatic tumor tissues and adjacent normal pancreatic tissues. cP < 0.001 vs normal group; B: GSE16515 was included to analyze the ADAM28 gene levels in 36 pancreatic cancer tissues and 16 adjacent normal tissues. cP < 0.001 vs normal group; C: GSE15471 was included to analyze the ADAM28 gene levels in 36 pancreatic cancer tissues and 16 adjacent normal tissues. cP < 0.001 vs normal group; D: Difference of ADAM28 expression between basal and classical types of pancreatic cancer. cP < 0.001 vs normal group; aP < 0.05 vs basal group. TCGA: The Cancer Genome Atlas; PDAC: Pancreatic ductal adenocarcinoma.
Figure 3.
Expression of ADAM28 in collected clinical pancreatic cancer tissues. A: A total of 16 pancreatic cancer tissues and the corresponding adjacent normal tissues were analyzed. cP < 0.001 vs normal group; B: The respective ratio between tumor and adjacent normal tissues (T/N) showed as a bubble chart. T: Tumor tissue; N: Normal tissue; PDAC: Pancreatic ductal adenocarcinoma.
High ADAM28 expression predicts a poor prognosis in pancreatic cancer
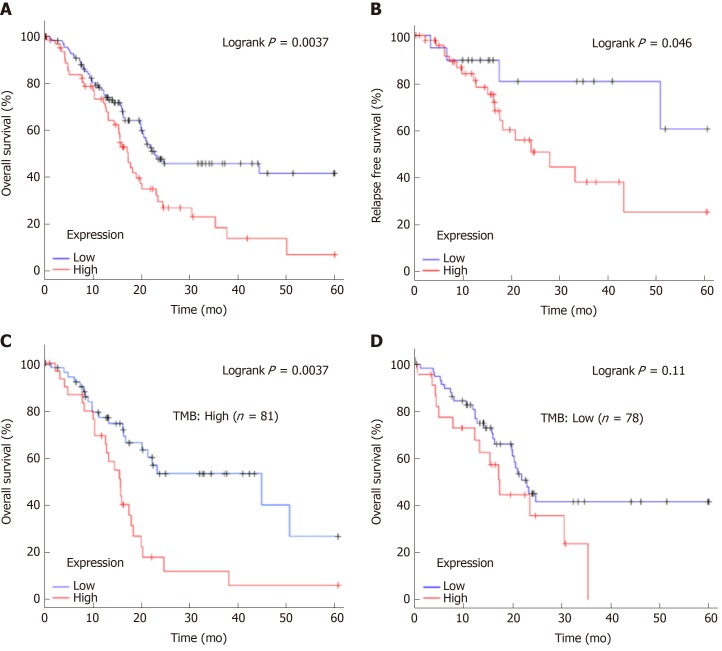
To explore the clinical significance of ADAM28 in pancreatic cancer, Kaplan-Meier analysis was performed. The results showed that patients with higher expression of ADAM28 had shorter OS and RFS (Figure 4A and B). The median OS of the high expression cohort was 17.23 mo, which was less than that of the low expression cohort (22.8 mo). The median RFS of the low ADAM28 expression cohort was 50.37 mo, which was obviously longer than that of the high expression cohort (16.2 mo). These results revealed the prognostic value of ADAM28 in pancreatic cancer patients. As recently reported, tumor mutation burden (TMB) with some specific genes could predict the prognosis outcome in some cancers[31]. Hence, we evaluated the role of TMB of ADAM28 in the prediction of outcome in pancreatic cancer patients. The data showed that in patients with high TMB, the OS of the high ADAM28 expression cohort showed was poorer than that of the low ADAM28 expression cohort (15.53 mo vs 44.4 mo) (Figure 4C and D). Conversely, in patients with low TMB, compared with the high ADAM28 expression cohort (17.23 mo), the median OS of the low expression cohort slightly increased (22.8 mo). These data suggested that high ADAM28 mutation burden in pancreatic cancer is a marker for poor prognosis.
Figure 4.
Overall survival and relapse-free survival evaluated between pancreatic cancer patients with high and low expression of ADAM28. A: OS of pancreatic cancer patients from TCGA; B: RFS of pancreatic cancer patients from TCGA; C: OS of pancreatic cancer patients with high TMB between the high and low ADAM28 expression cohorts; D: OS of pancreatic cancer patients with low TMB between the high and low ADAM28 expression cohorts. OS: Overall survival; RFS: Relapse-free survival; TMB: Tumor mutation burden; TCGA: The Cancer Genome Atlas.
Role of ADAM28 in resistance of SW1990 pancreatic cancer cells to gemcitabine
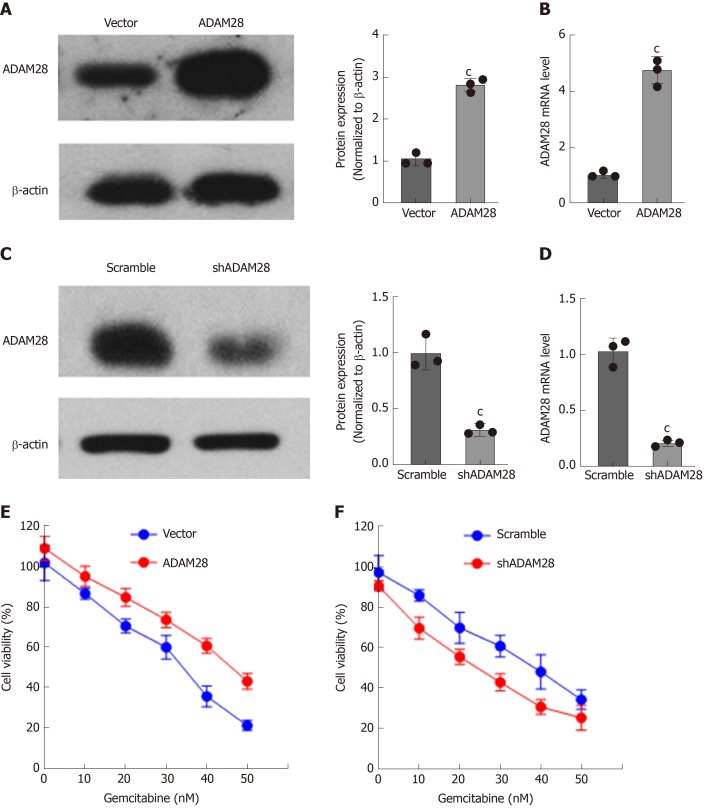
Treatment with gemcitabine could enhance the ADAM28 mRNA expression, and compared with parallel sensitive cells, ADAM28 expression was upregulated at both the mRNA and protein levels. These results indicated that ADAM28 is a novel chemoresistance target in pancreatic cancer. Then, to study the potential role of ADAM28 in the chemoresistance to pancreatic cancer therapy, we constructed SW1990 cell lines with stable overexpression or knock-down of ADAM28, and as shown in Figure 5A-D, the efficiency of overexpression or knock-down was remarkable at both the protein and mRNA levels. We then examined the role of ADAM28 in cell viability inhibition by gemcitabine in SW1990 cells. As shown in Figure 5E and 5F, overexpression of ADAM28 was able to significantly impair the cell viability inhibition by gemcitabine, and this result is consistent with the overexpression of ADAM28 in the resistant cells. Thus, ADAM28 may contribute to the occurrence of gemcitabine resistance in pancreatic cancer cells. We then hypothesized that inhibition of ADAM28 might restore the gemcitabine sensitivity in pancreatic cancer cells. Subsequently, we tested the response to gemcitabine in ADAM28 knock-down cells and scramble control cells, and the data showed that ADAM28 inhibition partially enhanced sensitivity to gemcitabine in SW1990 cells.
Figure 5.
Effect of ADAM28 on cell viability inhibition by gemcitabine. A: Western blot analysis of overexpression of ADAM28 in SW1990 cells. The right panel is the semi-quantitative result for ADAM28 protein expression. cP < 0.001 vs vector group; B: The mRNA expression of ADAM28 in ADAM28 overexpressing and vector control cells. cP < 0.001 vs vector group; C: Western blot analysis of ADAM28 protein in SW1990 cells treated with shRNA against ADAM28 and scramble control. The right panel is the semi-quantitative result for ADAM28 protein. cP < 0.001 vs scramble group; D: The mRNA expression of ADAM28 in ADAM28 knock-down and scramble control cells. cP < 0.001 vs scramble group; E: Relative cell viability evaluated according to absorbance value in ADAM28 overexpressing and vector control SW1990 cells treated with gemcitabine. cP < 0.001 vs vector group; F: Cell viability of ADAM28 knock-down and scramble SW1990 cells treated with different doses of gemcitabine for 24h. cP < 0.001 vs scramble group. shADAM28: shRNA against ADAM28; Scramble: Knockdown control.
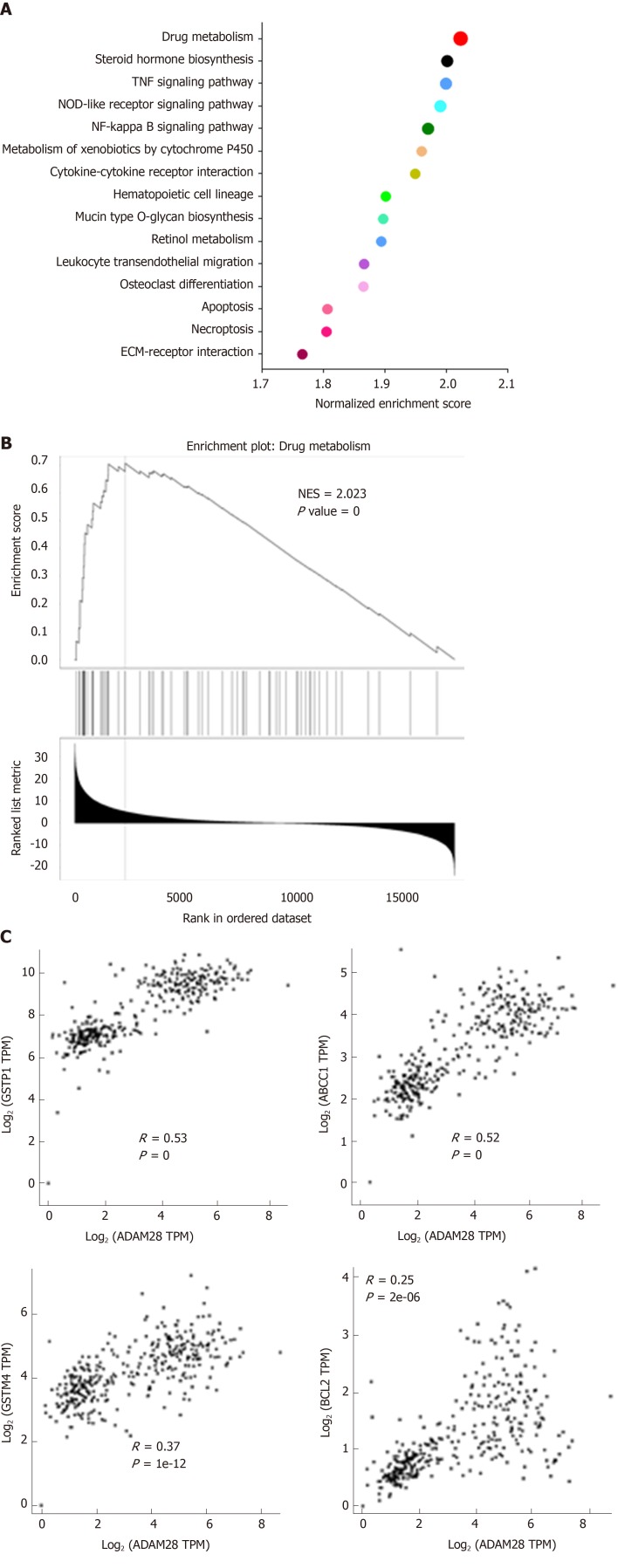
ADAM28 is closely associated with drug resistance-related signaling pathways
To study the molecular mechanism of ADAM28 in the gemcitabine resistance of pancreatic cancer cells, the enrichment analysis of ADAM28 and its co-expression network in pancreatic cancer was performed. GSEA analysis showed that ADAM28 was closely correlated with drug metabolism, steroid hormone biosynthesis, TNF signaling pathway, NOD-like receptor signaling pathway, NF kappa B signaling pathway and so on (Figure 6A). And the most significantly different pathway was drug metabolism, and the normalized enrichment score of drug metabolism was up to 2.023 (Figure 6B). This result was certified by the involvement of ADAM28 in gemcitabine resistance in pancreatic cancer. Then, to examine whether ADAM28 functions as a mediator in drug resistance and its relationship with other drug resistance-related molecules, the correlations of ADAM28 with GSTP1, ABCC1, GSTM4, and BCL2, which were reported as drug resistance-related genes in the regulation of drug delivery and apoptotic pathways[32], were determined. The correlation coefficients of ADAM28 with GSTP1, ABCC1, GSTM4, and BCL2 were 0.53, 0.52, 0.37, and 0.25, respectively (Figure 6C), suggesting that ADAM28 contributes to gemcitabine resistance possibly by regulation of these molecules.
Figure 6.
ADAM28 is closely related to drug resistance-related signaling pathways. A: Enrichment analysis of ADAM28 and its co-expression network in pancreatic cancer; B: GSEA analysis showed that ADAM28 was closely associated with drug metabolism; C: Correlations of ADAM28 with GSTP1, ABCC1, GSTM4, and BCL2 evaluated by the Spearman method. GSEA: Gene set enrichment analysis. ADAM28: AMAM metallopeptidase domain 28; NES: Normalized enrichment score; GSTP1: Glutathione S-transferase pi 1; ABCC1: ATP binding cassette subfamily C member 1; GSTM4: Glutathione S-transferase mu 4; TPM: Transcripts per million.
DISCUSSION
Pancreatic cancer is one of the most serious malignant tumors. Among digestive system tumors, the incidence and mortality of pancreatic cancer are second only to colorectal cancer according to the cancer statistics in 2019. Clinical studies have demonstrated that resistance to chemotherapy is the most important factor for restricting the therapy of pancreatic cancer and contributes to its low survival rate and poor prognosis. Thus, identification of novel targets for overcoming drug resistance is a practical strategy for the treatment of pancreatic cancer.
In this study, we identified a novel gene in gemcitabine-resistant pancreatic cancer cells. Compared with the sensitive cells, ADAM28 was overexpressed in gemcitabine-resistant pancreatic cancer cells, and treatment with a low dose of gemcitabine could induce the expression of ADAM28. Thus, ADAM28 may be involved in the occurrence of resistance to gemcitabine therapy in pancreatic cancer. Furthermore, to evaluate the role of ADAM28 in pancreatic cancer, we examined the expression of ADAM28 in pancreatic cancer and adjacent normal tissues, and we found that ADAM28 was highly expressed in tumor tissues, suggesting the significance of ADAM28 in pancreatic cancer. Previous studies also revealed the overexpression of ADAM28 in prostate cancer and breast cancer[14,33]. Thus, ADAM28 may be a drug target in a variety of tumors. More importantly, the OS and RFS analysis revealed that high ADAM28 expression in pancreatic tumor tissues predicted a poor prognosis. Similarly, ADAM28 expression was correlated with lymph node metastasis[33,34]. And more surprisingly, the prognostic prediction by high ADAM28 expression was only applied to patients with high TMD. Also, we confirmed that overexpression of ADAM28 in SW1990 cells could decrease the effect of growth inhibition by gemcitabine. Considering the overexpression of ADAM28 in pancreatic tumors, we concluded that ADAM28 may contribute to the occurrence of gemcitabine resistance in pancreatic cancer cells. And the knockdown of ADAM28 could enhance the sensitivity of gemcitabine to pancreatic cancer cells. Also, the enrichment analysis of ADAM28 in pancreatic tumors revealed that ADAM28 was closely associated with drug metabolism, steroid hormone biosynthesis[35,36], TNF signaling pathway[37], NOD-like receptor signaling pathway[38], and NF kappa B signaling pathway[39], all of which were reported as key mediators for the regulation of the multidrug resistance-related signaling pathways. In addition, ADAM28 was positively correlated with some key multidrug resistant-related genes, such as ABCC1, GSTP1, GSTM4, and BCL2. Taken together, this discovery gave us an idea that inhibition of ADAM28 may be a potential strategy for rescuing drug resistance in the therapy of pancreatic cancer.
In conclusion, we show for the first time that ADAM28 is a novel chemoresistance-related gene, and its overexpression in pancreatic tumors may be a window for overcoming drug resistance and provide guidance for prognostic evaluation.
ARTICLE HIGHLIGHTS
Research background
Pancreatic cancer, called the king of cancer, contributes to high mortality rates. Drug resistance is a major concern to the treatment of pancreatic cancer, and the mechanism of the occurrence of drug resistance in pancreatic cancer is complex and still not clear. ADAM28 was previously reported as an oncogene in some cancers, but its role in pancreatic cancer is not clear, especially in the development of chemoresistance to gemcitabine.
Research motivation
To fully understand the role of gemcitabine in the development of chemoresistance to gemcitabine, and to discover a novel the therapeutic target for pancreatic cancer.
Research objectives
To explore the expression and significance of ADAM28 in pancreatic cancer, especially in the regulation of chemoresistance to gemcitabine.
Research methods
Bioinformatic analysis was performed to explore novel targets for the chemoresistance to gemcitabine in pancreatic cancer. RT-PCR and Western blot were used to study the expression of ADAM28. GEO and TCGA analyses were conducted to analyze the expression of ADAM28 in pancreatic cancer. Kaplan-Meier Plotter was used to show the OS and RFS rates of pancreatic cancer patients. Cell viability was performed by CCK-8 assay. GSEA was used to explore the ADAM28 and its co-expression network.
Research results
We first identified that ADAM28 was a novel gene that was involved in the regulation of chemoresistance to gemcitabine. We further analyzed the expression of ADAM28 and its significance in pancreatic cancer, indicating that ADAM28 could be a good biomarker to predict the prognosis. However, the detailed regulation mechanism of ADAM28 in pancreatic cancer needs further evaluation.
Research conclusions
ADAM28 is overexpressed in pancreatic cancer, and its overexpression contributes to gemcitabine chemoresistance. ADAM28 is an important mediator that participates in the regulation of chemoresistance-related signaling pathway. High expression of ADAM28 in pancreatic cancer can be used as a biomarker to predict the poor prognosis. ADAM28 might be a potential therapeutic target for overcoming chemoresistance to gemcitabine.
Research perspectives
Based on the bioinformatic analysis and in vitro experiments, ADAM28 was identified as a novel therapeutic target for pancreatic cancer, especially in the occurrence of drug resistance. In the future studies, an in-depth exploration of the ADAM28-mediated drug resistance signaling pathway will be very meaningful.
Footnotes
Institutional review board statement: This study was approved by the Scientific Committee of the Third Affiliated Hospital of Sun Yat-Sen University.
Institutional animal care and use committee statement: This study was approved by the Ethical Committee of the Third Affiliated Hospital of Sun Yat-Sen University.
Conflict-of-interest statement: The authors declare no conflict of interest.
Data sharing statement: The bioinformatic data could be downloaded from the public databases, and no additional data are available.
ARRIVE guidelines statement: This study was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: July 30, 2019
First decision: August 17, 2019
Article in press: September 9, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aykan NF, Mikulic D, González González R S-Editor: Wang J L-Editor: Wang TQ E-Editor: Ma YJ
Contributor Information
Li Wei, Department of Medical Oncology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, Guangdong Province, China.
Jing-Yun Wen, Department of Medical Oncology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, Guangdong Province, China.
Jie Chen, Department of Medical Oncology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, Guangdong Province, China.
Xiao-Kun Ma, Department of Medical Oncology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, Guangdong Province, China.
Dong-Hao Wu, Department of Medical Oncology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, Guangdong Province, China.
Zhan-Hong Chen, Department of Medical Oncology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, Guangdong Province, China.
Jiang-Long Huang, Department of Gastrointestinal Surgery, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, Guangdong Province, China. drhjl@aliyun.com.
References
- 1.Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3:e99263. doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin JF, Poruk KE, Wolfgang CL. Pancreatic cancer surgery: past, present, and future. Chin J Cancer Res. 2015;27:332–348. doi: 10.3978/j.issn.1000-9604.2015.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fogel EL, Shahda S, Sandrasegaran K, DeWitt J, Easler JJ, Agarwal DM, Eagleson M, Zyromski NJ, House MG, Ellsworth S, El Hajj I, O’Neil BH, Nakeeb A, Sherman S. A Multidisciplinary Approach to Pancreas Cancer in 2016: A Review. Am J Gastroenterol. 2017;112:537–554. doi: 10.1038/ajg.2016.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S, Batra SK. Pancreatic cancer metastasis: are we being pre-EMTed. Curr Pharm Des. 2015;21:1249–1255. doi: 10.2174/1381612821666141211115234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajatdoost L, Sedaghat K, Walker EJ, Thomas J, Kosari S. Chemotherapy in Pancreatic Cancer: A Systematic Review. Medicina (Kaunas) 2018;54:48. doi: 10.3390/medicina54030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martín AM, Hidalgo M, Alvarez R, Arrazubi V, Martínez-Galán J, Salgado M, Macarulla T, Carrato A. From First Line to Sequential Treatment in the Management of Metastatic Pancreatic Cancer. J Cancer. 2018;9:1978–1988. doi: 10.7150/jca.23716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amrutkar M, Gladhaug IP. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel) 2017;9:157. doi: 10.3390/cancers9110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamska A, Domenichini A, Falasca M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int J Mol Sci. 2017;18:1338. doi: 10.3390/ijms18071338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard L, Zheng Y, Horrocks M, Maciewicz RA, Blobel C. Catalytic activity of ADAM28. FEBS Lett. 2001;498:82–86. doi: 10.1016/s0014-5793(01)02506-6. [DOI] [PubMed] [Google Scholar]
- 10.Howard L, Maciewicz RA, Blobel CP. Cloning and characterization of ADAM28: evidence for autocatalytic pro-domain removal and for cell surface localization of mature ADAM28. Biochem J. 2000;348 Pt 1:21–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Z, Wen LY, Jin M, Deng ZH, Jin Y. ADAM28 participates in the regulation of tooth development. Arch Oral Biol. 2006;51:996–1005. doi: 10.1016/j.archoralbio.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Borra VM, Waterval JJ, Stokroos RJ, Manni JJ, Van Hul W. Localization of the Gene for Hyperostosis Cranialis Interna to Chromosome 8p21 with Analysis of Three Candidate Genes. Calcif Tissue Int. 2013;93:93–100. doi: 10.1007/s00223-013-9732-8. [DOI] [PubMed] [Google Scholar]
- 13.Satsuki M, Yasunori O. ADAM28 as a Target for Human Cancers. Curr Pharm Des. 2009;15:2349–2358. doi: 10.2174/138161209788682424. [DOI] [PubMed] [Google Scholar]
- 14.Mitsui Y, Mochizuki S, Kodama T, Shimoda M, Ohtsuka T, Shiomi T, Chijiiwa M, Ikeda T, Kitajima M, Okada Y. ADAM28 Is Overexpressed in Human Breast Carcinomas: Implications for Carcinoma Cell Proliferation through Cleavage of Insulin-like Growth Factor Binding Protein-3. Cancer Res. 2006;66:9913–9920. doi: 10.1158/0008-5472.CAN-06-0377. [DOI] [PubMed] [Google Scholar]
- 15.Nowakowska-Zajdel E, Mazurek U, Wierzgoń J, Kokot T, Fatyga E, Ziółko E, Klakla K, Błażelonis A, Waniczek D, Głogowski Ł, Kozowicz A, Niedworok E, Muc-Wierzgoń M. Expression of ADAM28 and IGFBP-3 Genes in Patients with Colorectal Cancer—A Preliminary Report. Int J Immunopathol Pharmacol. 2013;26:223–228. doi: 10.1177/039463201302600122. [DOI] [PubMed] [Google Scholar]
- 16.Bridges LC, Sheppard D, Bowditch RD. ADAM disintegrin-like domain recognition by the lymphocyte integrins alpha4beta1 and alpha4beta7. Biochem J. 2005;387:101–108. doi: 10.1042/BJ20041444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtsuka T, Shiomi T, Shimoda M, Kodama T, Amour A, Murphy G, Ohuchi E, Kobayashi K, Okada Y. ADAM28 is overexpressed in human non-small cell lung carcinomas and correlates with cell proliferation and lymph node metastasis. International Journal of Cancer. 2006;118(2):263–273. doi: 10.1002/ijc.21324. [DOI] [PubMed] [Google Scholar]
- 18.Shimoda M, Hashimoto G, Mochizuki S, Ikeda E, Nagai N, Ishida S, Okada Y. Binding of ADAM28 to P-selectin glycoprotein ligand-1 enhances P-selectin-mediated leukocyte adhesion to endothelial cells. J Biol Chem. 2007;282:25864–25874. doi: 10.1074/jbc.M702414200. [DOI] [PubMed] [Google Scholar]
- 19.Yang MH, Chu PY, Chen SCJ, Chung TW, Chen WC, Tan LB, Kan WC, Wang HY, Su SB, Tyan YC. Characterization of ADAM28 as a biomarker of bladder transitional cell carcinomas by urinary proteome analysis. Biochem Biophys Res Commun. 2011;411(4):714–720. doi: 10.1016/j.bbrc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki S, Soejima K, Shimoda M, Abe H, Sasaki A, Okano HJ, Okano H, Okada Y. Effect of ADAM28 on carcinoma cell metastasis by cleavage of von Willebrand factor. J Natl Cancer Inst. 2012;104:906–922. doi: 10.1093/jnci/djs232. [DOI] [PubMed] [Google Scholar]
- 21.Jowett JBM, Okada Y, Leedman PJ, Curran JE, Johnson MP, Moses EK, Goring HHH, Mochizuki S, Blangero J, Stone L, Allen H, Mitchell C, Matthews VB. ADAM28 is elevated in humans with the metabolic syndrome and is a novel sheddase of human tumour necrosis factor-α. Immunol Cell Biol. 2012;90:966–973. doi: 10.1038/icb.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XH, Wang CC, Jiang Q, Yang SM, Jiang H, Lu J, Wang QM, Feng FE, Zhu XL, Zhao T, Huang XJ. ADAM28 overexpression regulated via the PI3K/Akt pathway is associated with relapse in de novo adult B-cell acute lymphoblastic leukemia. Leuk Res. 2015;39:1229–1238. doi: 10.1016/j.leukres.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Saiki Y, Yoshino Y, Fujimura H, Manabe T, Kudo Y, Shimada M, Mano N, Nakano T, Lee Y, Shimizu S, Oba S, Fujiwara S, Shimizu H, Chen N, Nezhad Z, Jin G, Fukushige S, Sunamura M, Ishida M, Motoi F, Egawa S, Unno M, Horii A. DCK is frequently inactivated in acquired gemcitabine-resistant human cancer cells. Biochem Biophys Res Commun. 2012;421:98–104. doi: 10.1016/j.bbrc.2012.03.122. [DOI] [PubMed] [Google Scholar]
- 25.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strillacci A, Griffoni C, Spisni E, Manara MC, Tomasi V. RNA interference as a key to knockdown overexpressed cyclooxygenase-2 gene in tumour cells. Br J Cancer. 2006;94:1300–1310. doi: 10.1038/sj.bjc.6603094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou G, Soufan O, Ewald J, Hancock REW, Basu N, Xia J. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019;47:W234–W241. doi: 10.1093/nar/gkz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016–2027. [PubMed] [Google Scholar]
- 31.Penault-Llorca F, Radosevic-Robin N. Tumor mutational burden in non-small cell lung cancer-the pathologist's point of view. Transl Lung Cancer Res. 2018;7:716–721. doi: 10.21037/tlcr.2018.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jjg M, Lozano E, Briz O, Al-Abdulla R, Serrano MA, Rir M. Molecular Bases of Chemoresistance in Cholangiocarcinoma. Curr Drug Targets. 2015;18:889–900. doi: 10.2174/1389450116666150223121508. [DOI] [PubMed] [Google Scholar]
- 33.Rudnicka C, Mochizuki S, Okada Y, Mclaughlin C, Leedman PJ, Stuart L, Epis M, Hoyne G, Boulos S, Johnson L. Overexpression and knock-down studies highlight that a disintegrin and metalloproteinase 28 controls proliferation and migration in human prostate cancer. Medicine. 2016;95:e5085. doi: 10.1097/MD.0000000000005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zucker S, Cao J. New Wrinkle Between Cancer and Blood Coagulation: Metastasis and Cleavage of von Willebrand Factor by ADAM28. J Natl Cancer Inst. 2012;104:887. doi: 10.1093/jnci/djs251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Kalken CK, Broxterman HJ, Pinedo HM, Feller N, Dekker H, Lankelma J, Giaccone G. Cortisol is transported by the multidrug resistance gene product P-glycoprotein. Br J Cancer. 1993;67:284–289. doi: 10.1038/bjc.1993.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo G, Gong K, Ali S, Ali N, Shallwani S, Hatanpaa KJ, Pan E, Mickey B, Burma S, Wang DH, Kesari S, Sarkaria JN, Zhao D, Habib AA. A TNF-JNK-Axl-ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma. Nat Neurosci. 2017;20:1074–1084. doi: 10.1038/nn.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han J, Jun Y, Kim SH, Hoang HH, Jung Y, Kim S, Kim J, Austin RH, Lee S, Park S. Rapid emergence and mechanisms of resistance by U87 glioblastoma cells to doxorubicin in an in vitro tumor microfluidic ecology. Proc Natl Acad Sci USA. 2016;113:14283–14288. doi: 10.1073/pnas.1614898113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung H, Kim JS, Kim WK, Oh KJ, Kim JM, Lee HJ, Han BS, Kim DS, Seo YS, Lee SC, Park SG, Bae KH. Intracellular annexin A2 regulates NF-κB signaling by binding to the p50 subunit: implications for gemcitabine resistance in pancreatic cancer. Cell Death Dis. 2015;6:e1606. doi: 10.1038/cddis.2014.558. [DOI] [PMC free article] [PubMed] [Google Scholar]