Abstract
BACKGROUND
Hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) is a syndrome with a high short-term mortality rate, and it is crucial to identify those patients at a high mortality risk clinically.
AIM
To investigate the clinical value of soluble mannose receptor (sMR) in predicting the 90-day mortality of HBV-ACLF patients.
METHODS
A total of 43 patients were diagnosed with HBV-ACLF between October 2017 and October 2018 at the Second Hospital of Anhui Medical University, and all of them were enrolled in this retrospective study. Their serum sMR levels were determined using an enzyme-linked immunosorbent assay. Demographic and clinical data, including gender, age, albumin level, total bilirubin (TBIL) level, international normalized ratio, HBV-DNA level, HBV serological markers, procalcitonin level, interleukin-6 level, and model for end-stage liver disease (MELD) score were accessed at the time of diagnosis of HBV-ACLF. A multivariate logistic regression analysis was used to analyze the independent risk factors for mortality.
RESULTS
Serum sMR level was significantly increased in HBV-ACLF patients compared with chronic hepatitis B patients and healthy controls (P < 0.01). When compared with surviving patients, it was higher in those patients who succumbed to HBV-ACLF (P < 0.05). Serum sMR level was positively correlated with MELD score (rs = 0.533, P = 0.001), HBV-DNA level (rs = 0.497, P = 0.022), and TBIL level (rs = 0.894, P < 0.001). Serum sMR level (odds ratio = 1.007, 95% confidence interval: 1.004–1.012, P = 0.001) was an independent risk factor for the 90-day mortality in the HBV-ACLF cases. The patients with HBV-ACLF were stratified into two groups in accordance with their serum sMR levels at the baseline (low risk: < 99.84 pg/mL and high risk: ≥ 99.84 pg/mL). The 90-day mortality rates were 27.3% in the low-risk group and 87.5% in the high-risk group. Furthermore, sMR level apparently improved the performance of MELD score for predicting the prognosis of patients with HBV-ACLF.
CONCLUSION
Serum sMR level may be a predictor of the prognosis of HBV-ACLF patients.
Keywords: Acute-on-chronic liver failure, Mannose receptor, Model for end-stage liver disease, Prognosis, Risk factor
Core tip: This is a retrospective study to evaluate the value of soluble mannose receptor (sMR) level for predicting the 90-day mortality of patients with hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF). Serum sMR level was significantly higher in patients with HBV-ACLF, and it was an independent risk factor associated with the prognosis of HBV-ACLF. Furthermore, sMR level significantly improved the performance of model for end-stage liver disease score for predicting the prognosis of HBV-ACLF patients.
INTRODUCTION
Acute-on-chronic liver failure (ACLF) is a clinical syndrome of the acute decompensation of liver function based on chronic liver disease, and it is often accompanied by multiple organ failure[1]. The main cause of ACLF is the acute exacerbation of chronic hepatitis B (CHB), which accounts for more than 80% of all causes in China[2]. ACLF caused by CHB is called hepatitis B-related ACLF (HBV-ACLF).
The pathogenesis of HBV-ACLF is related to the activation of hepatic macrophages[3]. Macrophages can be rapidly activated under the stimulation of acute and chronic inflammatory reactions, and they are differentiated into Ml and M2 macrophages[4]. Mannose receptor (MR) is expressed mainly on the membrane surfaces of M2 macrophages and dendritic cells[5]. MR can recognize and bind specific carbohydrate molecules through the extracellular domain, which plays an important role in identifying pathogens, presenting antigens, and maintaining the homeostasis of the internal environment[6]. Its soluble form, soluble mannose receptor (sMR), is formed by the matrix metalloproteinase cleavage of MR on the cell membrane, and it is secreted into peripheral serum[7]. Serum sMR level is elevated in critical illnesses, including severe liver disease[8]. In addition, sMR level is associated with liver disease severity in patients with alcoholic hepatitis and chronic viral hepatitis[9-11].
Previous studies have shown that sMR levels gradually increase with ACLF severity, and they are closely related to mortality[12]. Nevertheless, the role of sMR in HBV-ACLF has not been elucidated. Therefore, this research was designed to assess the value of sMR level in predicting the 90-day mortality of HBV-ACLF patients.
MATERIALS AND METHODS
Patients
Forty-three patients with HBV-ACLF and 43 patients with CHB from the Second Hospital of Anhui Medical University were enrolled in this study from October 2017 to October 2018. In addition, 20 healthy controls (HCs) were randomly selected during this time period. The diagnosis of HBV-ACLF was based on the consensus definition of the Asian Pacific Association for the Study of the Liver (2014 version)[13], as follows: Acute hepatic insult manifested as jaundice (serum bilirubin ≥ 5 mg/dL or 85 µmol/L) and coagulopathy [international normalized ratio (INR) > 1.5 or prothrombin activity < 40%], complicated within 4 wk by ascites and/or encephalopathy in a patient with CHB. According to the INR level, they were divided into three subgroups: Early stage (1.5 < INR ≤ 1.9), medium stage (1.9 < INR ≤ 2.6), and late stage (INR ≥ 2.6). Patients with liver failure caused by cirrhosis, other hepatitis virus infections or coinfections, alcoholic liver disease or nonalcoholic fatty liver, or hepatotoxic drugs were excluded from this research. CHB diagnosis was made according to the diagnostic criteria of the Chinese guidelines for the prevention and treatment of CHB (2015 version)[14]. Blood samples (5 mL) were collected at the time of HBV-ACLF or CHB diagnosis, and they were centrifuged at 1000 × g for 15 min and then stored at -80 °C. Demographic and clinical data, including gender, age, albumin (ALB) level, total bilirubin (TBIL) level, INR, HBV-DNA level, HBV serological markers, procalcitonin (PCT) level, and interleukin-6 (IL-6) level, were collected within the first day after HBV-ACLF diagnosis. We followed the patients with HBV-ACLF who met the inclusion criteria: The patients who survived within 90 d made up a survival group, and those who died within 90 d made up a non-survival group (including automatic discharge due to exacerbation).
According to the 1975 Helsinki Declaration, this study was approved by the Ethics Committee of the Second Hospital of Anhui Medical University. Written informed consent was obtained from all of the participants prior to their inclusion in this study.
Serological sMR detection
Serum concentrations of sMR were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (GTX, Inc., Los Angeles, CA, United States) in accordance with the instructions. Briefly, we added 50 µL of diluted (1:5) serum samples to the ELISA plates that were pre-coated with a captured sMR antibody, and the absorbance was measured at 450 nm by using an ELISA plate reader (ST-360; Shanghai Kehua Bio-engineering, Ltd., Shanghai, China). A duplicate assay was also performed. The concentration of the standard was set to the abscissa, and the absorbance value was set to the ordinate. A standard curve was drawn, and the absorbance value was substituted into the linear equation in order to calculate the sample concentration.
Scoring system
The model for end-stage liver disease (MELD) score was calculated using the formula as follows: MELD = 11.2 × ln (INR) + 9.6 × ln [creatinine (Cr, mg/dL)] + 3.8 × ln [TBIL (mg/dL)] + 6.4.
Statistical analysis
All of the data were analyzed using IBM SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY, United States). The normally distributed data are expressed as the mean ± standard deviation, and the non-normally distributed data are expressed as the median (interquartile range). The differences in the normally distributed data were analyzed using the Student’s t-test and analysis of variance. The comparisons of the non-normally distributed data were performed using the Mann-Whitney U test and the Kruskal-Wallis test. Spearman correlation analysis was used to evaluate the correlations between the sMR level and other clinical indicators. Multivariate logistic regression was employed to analyze the independent risk factors for mortality. The best sMR cutoff value for predicting the 90-day mortality was determined by adopting SPSS Modeler 18.0 software (IBM Corp., Armonk, NY, United States). The area under the receiver operating characteristics curve (AUROC) was used to compare the accuracy of the prediction. Survival analysis was performed using the Kaplan-Meier curve. A two-sided P-value < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Subject characteristics
The demographic and clinical characteristics of all of the subjects in the ACLF, CHB, and HC groups are listed in Table 1. There were no significant differences in the age or sex ratio between the three groups (P > 0.05). The patients with HBV-ACLF had elevated ALB, TBIL, Cr, INR, PCT, IL-6, and HBV-DNA levels when compared with the other two groups (P < 0.05). MELD score was significantly elevated in the HBV-ACLF patients when compared with CHB patients (P < 0.05). Out of the HBV-ACLF patients, there were 12 patients in the survival group and 31 in the non-survival group. The number of patients with HBV-ACLF in the early stage, medium stage, and late stage groups was 12, 10, and 21, respectively.
Table 1.
Demographic and clinical characteristics of the study subjects
| Variable | HBV-ACLF (n = 43) | CHB (n = 43) | HCs (n = 20) |
| Age (yr) | 42.65 ± 13.93 | 40.24 ± 14.41 | 41.00 ± 11.20 |
| Male (%) | 36 (83.7%) | 35 (81.4%) | 16 (80%) |
| ALB (g/L) | 29.90 ± 4.45ab | 36.62 ± 4.60 | 45.07 ± 2.31 |
| TBIL (μmol/L) | 351.92 ± 190.27ab | 69.41 ± 95.43 | 8.00 ± 3.49 |
| Cr (μmol/L) | 95.56 ± 55.81ab | 63.82 ± 11.97 | 65.00 ± 11.61 |
| INR | 2.01 (1.13)ab | 1.13 (0.26) | 0.95 (0.10) |
| HBeAg (positive rate, %) | 14 (32.55%) | 21 (48.84%) | ND |
| HBV-DNA (log10 copies/mL) | 6.74 ± 7.35b | 4.88 ± 1.99 | ND |
| PCT (ng/mL) | 0.53 (0.43)ab | 0.07 (0.20) | 0.02 (0.02) |
| IL-6 (pg/mL) | 15.50 (24.00)ab | 7.50 (7.00) | 4.00 (4.00) |
| MELD | 33.48 ± 4.79b | 8.43 ± 7.42 | ND |
| sMR (pg/mL) | 114.94 ± 49.65ab | 80.75 ± 34.45 | 26.51 ± 8.62 |
The normally distributed data are expressed as the mean ± standard deviation. The non-normally distributed data are expressed as the median (interquartile range).
P < 0.05 vs HCs;
P < 0.05 vs CHB.
HBV: Hepatitis B virus; ACLF: Acute-on-chronic liver failure; CHB: Chronic hepatitis B; HCs: Healthy controls; ALB: Albumin; PTA: Prothrombin activity; TBIL: Total bilirubin; Cr: Creatinine; INR: International normalized ratio; HBeAg: Hepatitis B e antigen; PCT: Procalcitonin; IL-6: Interleukin-6; MELD: Model for end-stage liver disease; sMR: Soluble mannose receptor; ND: Not determined.
Serum sMR expression in HBV-ACLF patients
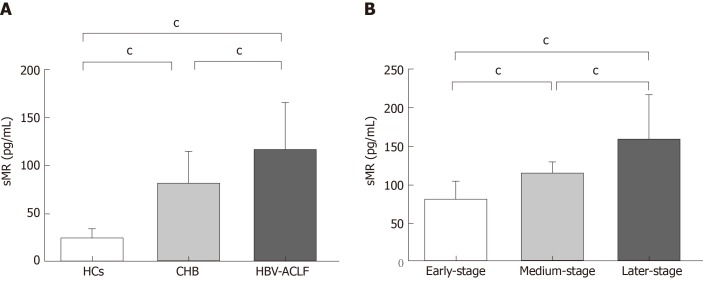
Serum sMR levels of HBV-ACLF patients, CHB patients, and HCs were 114.94 ± 49.65 pg/mL, 80.75 ± 34.45 pg/mL, and 26.51 ± 8.62 pg/mL, respectively. Serum sMR level of HBV-ACLF patients was significantly higher than those of CHB patients and HCs (P < 0.01; Figure 1A). Serum sMR levels in the early, medium, and late stage groups of HBV-ACLF patients increased gradually (79.42 ± 25.10 pg/mL, 113.56 ± 14.35 pg/mL, and 158.15 ± 59.13 pg/mL, respectively) (P < 0.01; Figure 1B).
Figure 1.
Serum soluble mannose receptor levels in different groups of subjects. A: Comparison of serum sMR levels among HBV-ACLF patients, CHB patients, and HCs; B: Comparison of sMR levels among different stages of HBV-ACLF patients. cP < 0.01. sMR: Soluble mannose receptor; HCs: Healthy controls; CHB: Chronic hepatitis B; HBV: Hepatitis B virus; ACLF: Acute-on-chronic liver failure.
Serum sMR levels help to differentiate between survivors and non-survivors
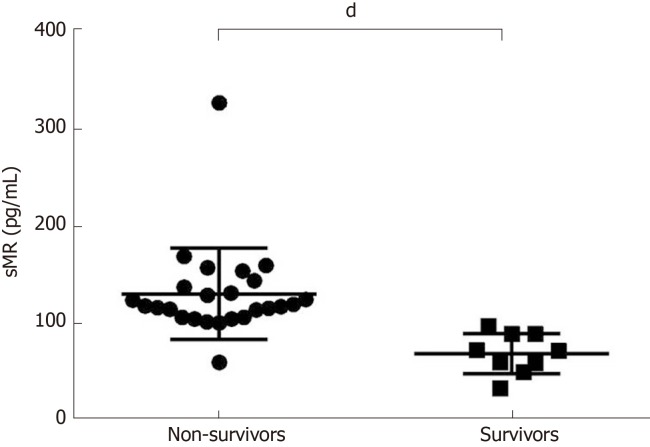
Serum sMR level of non-survivors was significantly increased (131.20 ± 9.40 pg/mL), and it was much higher than that of the survival group (69.89 ± 6.92 pg/mL) (P < 0.05; Figure 2).
Figure 2.
Comparison of soluble mannose receptor levels between non-survivors and survivors. Serum sMR level in the non-survivor group was significantly higher than that of the survivor group. dP < 0.05. sMR: Soluble mannose receptor.
Correlations between sMR level and liver injury indicators
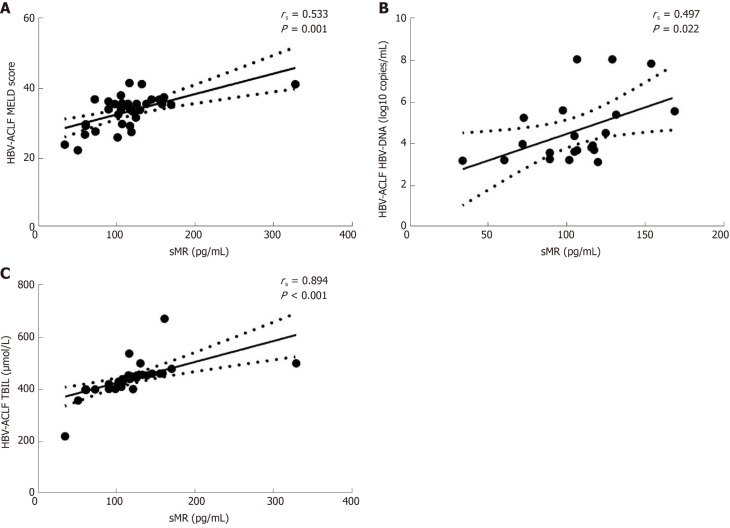
To further explore the clinical value of sMR level in developing HBV-ACLF, we analyzed the correlations between serum sMR level and liver injury parameters, including MELD score, HBV-DNA level, and TBIL level. Serum sMR level was positively correlated with MELD score (rs = 0.533, P = 0.001), HBV-DNA level (rs = 0.497, P = 0.022), and TBIL level (rs = 0.894, P < 0.001) (Figure 3A-C).
Figure 3.
Correlations between serum soluble mannose receptor level and liver injury parameters. rs: Spearman correlation coefficient; sMR: Soluble mannose receptor; HBV: Hepatitis B virus; ACLF: Acute-on-chronic liver failure; MELD: Model for end-stage liver disease; TBIL: Total bilirubin.
Serum sMR level is an independent risk factor for mortality in HBV-ACLF patients
To determine the potential of serum sMR level to predict the prognosis of HBV-ACLF, univariate and multivariate logistic regression analyses were employed to test the following parameters (see Table 2). MELD score was calculated using the TBIL, Cr, and INR, so these three values were not used in the logistic regression. The multivariate analysis indicated that MELD score [odds ratio (OR) = 1.132, 95% confidence interval (CI): 1.046-1.396, P = 0.001] and sMR level (OR = 1.007, 95%CI: 1.004-1.012, P = 0.001) were independent risk factors for the 90-day mortality (Table 2).
Table 2.
Risk factors for 90-d mortality in patients with hepatitis B virus-related acute-on-chronic liver failure
| Baseline variable | OR | Univariate 95%CI | P-value | OR | Multivariate 95%CI | P-value |
| Age (yr) | 0.850 | 0.701–1.051 | 0.642 | |||
| Gender | 0.519 | 0.096–2.741 | 0.422 | |||
| ALB (g/L) | 0.722 | 0.567–1.052 | 0.102 | |||
| PCT (ng/mL) | 1.003 | 0.999–1.006 | 0.152 | |||
| IL-6 (pg/mL) | 1.022 | 1.005–1.038 | 0.187 | |||
| MELD | 1.422 | 1.038–2.693 | 0.001 | 1.132 | 1.046-1.396 | 0.001 |
| sMR (pg/mL) | 1.062 | 1.005–1.038 | 0.001 | 1.007 | 1.004-1.012 | 0.001 |
HBV: Hepatitis B virus; ACLF: Acute-on-chronic liver failure. OR: Odds ratio; CI: Confidence interval; ALB: Albumin; PCT: Procalcitonin; IL-6: Interleukin-6; MELD: Model for end-stage liver disease; sMR: Soluble mannose receptor.
Serum sMR level apparently improves performance of MELD score for predicting prognosis of HBV-ACLF patients
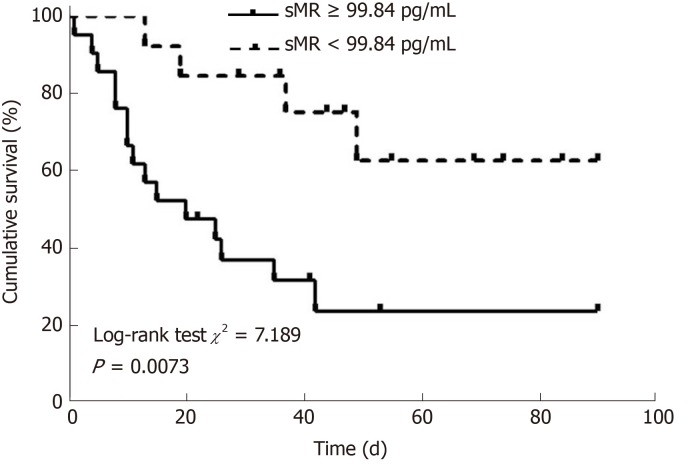
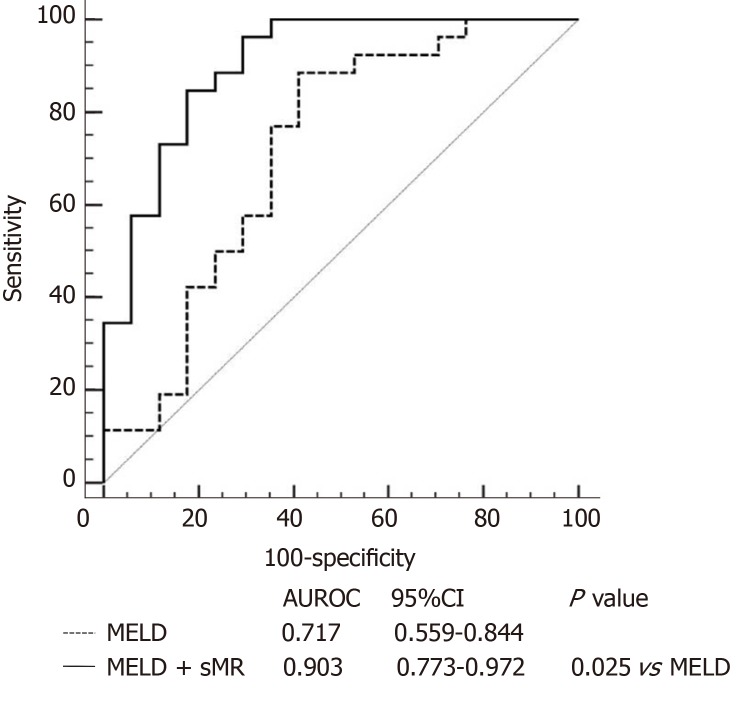
Patients with HBV-ACLF were stratified into two groups in accordance with the cutoff value of serum sMR level. The best cutoff value of sMR level was identified using SPSS Modeler 18.0 software (low risk: < 99.84 pg/mL and high risk: ≥ 99.84 pg/mL). The 90-day mortality rates were 27.3% (3/11) for the low-risk group and 87.5% (28/32) for the high-risk group k, and patients with sMR levels ≥ 99.84 pg/mL had poor prognoses (Figure 4). When the optimal cut-off value was 99.84 pg/mL, the sensitivity and specificity of sMR level for predicting the 90-day mortality were 70.6% and 83.3%, respectively. In addition, compared with the single MELD score, the new prognostic model combining MELD with sMR showed a significant advantage in terms of prognostic accuracy (AUROC = 0.903 and 0.717, respectively, P = 0.025) (Figure 5).
Figure 4.
Kaplan-Meier survival curves of soluble mannose receptor levels for predicting 90-day mortality. sMR: Soluble mannose receptor.
Figure 5.
Areas under the receiver operating characteristics curves of model for end-stage liver disease score alone and model for end-stage liver disease score plus serum soluble mannose receptor. AUROC: Area under the receiver operating characteristics curve; CI: Confidence interval; MELD: Model for end-stage liver disease; sMR: Soluble mannose receptor.
DISCUSSION
Our study demonstrated that serum sMR levels in patients with HBV-ACLF were increased significantly, and they helped to distinguish the survivors from non-survivors. Serum sMR level was positively correlated with MELD score and HBV-DNA and TBIL levels. In addition, the results of this study showed that serum sMR level was an independent risk factor for the prognosis of HBV-ACLF.
As described previously, HBV-ACLF refers to liver failure caused by the acute aggravation of liver injury in CHB patients[1,2]. At present, the pathogenesis of the disease has not been fully clarified, but most researchers believe that immune damage plays a key role in HBV-ACLF. Immunocytes, such as macrophages, are activated in the pathogenesis of HBV-ACLF; they release a large number of inflammatory mediators and cytokines which participate in the inflammatory response of the liver. The expression level of the macrophages is closely related to the development and prognosis of the disease[3,15]. As a macrophage activation marker, MR mediates a series of immune responses that recognize, phagocytose, and clear pathogens[6]. The serum containing sMR can be stored at - 80 °C for 20 mo, and it can be detected by ELISA[8].
This study found that compared with HCs and CHB patients, the expression level of sMR in serum of HBV-ACLF patients was significantly increased. Moreover, sCD163, a marker of macrophage activation, is also significantly elevated in patients with HBV-ACLF[3]. Therefore, we speculate that sMR and sCD163 may play important roles in the immune process of acute liver inflammation. Laursen et al[11] found that the macrophage activation markers sCD163 and sMR were associated with activity and fibrosis in the liver biopsies of patients with CHB. At the same time, we found that the sMR level of CHB patients was significantly higher than that of HCs, suggesting that sMR may also play an important role in the pathogenesis of CHB. We also analyzed the sMR levels in the early, middle, and late stages of HBV-ACLF. The results showed that the sMR expression level increased gradually with the increase in the severity of the disease, indicating that sMR level is closely related to disease severity in HBV-ACLF patients.
MELD score has been used widely to evaluate the prognosis of HBV-ACLF. Through a correlation analysis, we found that sMR level was positively correlated with MELD score, HBV-DNA level, and TBIL level. When combined with the previous experimental results, we concluded that sMR level was closely related to liver inflammatory injury and disease severity in patients with HBV-ACLF. Previous studies have indicated that sMR may be a potential biomarker for liver diseases and infectious diseases[8]. Moreover, Japanese scholars have found that serum sCD206 was increased in pulmonary tuberculosis (PTB), and that it was associated with the prognosis; therefore, it could be a potential biomarker for PTB[16]. Relster et al showed that the macrophage activation marker sMR holds potential as a new diagnostic and early prognostic biomarker for patients with acute infectious diseases, with or without sepsis[17]. In order to verify whether sMR level could be a predictor of HBV-ACLF prognosis, a multivariate logistic regression analysis was performed, and the results showed that sMR level was an independent risk factor for mortality in HBV-ACLF patients. In addition, it apparently improved the performance of MELD score for predicting HBV-ACLF prognosis.
Our study did have some limitations. First of all, serum sMR level was obviously correlated with HBV-ACLF prognosis, but the mechanisms underlying the role of sMR were not investigated. Second, the dynamic changes in serum sMR should be determined during the progression of HBV-ACLF. Finally, multicenter studies with large sample sizes are also required to confirm the current findings.
In conclusion, serum sMR level is significantly elevated in HBV-ACLF patients, and it is apparently associated with indicators of liver injury and disease severity. In addition, sMR level is an independent risk factor for the prognosis of HBV-ACLF. The present findings collectively suggest that sMR level may be a predictor of the prognosis in patients with HBV-ACLF.
ARTICLE HIGHLIGHTS
Research background
Since hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) is a syndrome with a high short-term mortality rate, and clinically identifying those patients at a high mortality risk is of great significance.
Research motivation
The aim of this study was to investigate the clinical value of soluble mannose receptor (sMR) in patients with HBV-ACLF.
Research objectives
To investigate the clinical value of sMR for predicting 90-d mortality of patients with HBV-ACLF.
Research methods
A total of 43 patients with HBV-ACLF were enrolled in this retrospective study, and their serum sMR levels were determined using an enzyme-linked immunosorbent assay. A multivariate logistic regression analysis was used to analyze the independent risk factors for mortality.
Research results
When compared with chronic hepatitis B patients and healthy controls, serum sMR level was significantly higher in HBV-ACLF patients. Serum sMR helped to distinguish the survivors from non-survivors, and it was positively correlated with model for end-stage liver disease (MELD) score, HBV-DNA level, and total bilirubin level. In addition, serum sMR level was an independent risk factor and significantly improved the performance of MELD score in predicting the prognosis of HBV-ACLF patients.
Research conclusions
Serum sMR level is significantly elevated in patients with HBV-ACLF, and it is significantly associated with indicators of liver injury and disease severity. Additionally, sMR level is negatively correlated with patients outcome. Finally, sMR level may be a predictor of the prognosis of patients with HBV-ACLF.
Research perspectives
Future studies are needed to investigate the mechanisms underlying the role of sMR. And it is necessary to determine the dynamic changes in serum sMR during the progression of HBV-ACLF. Multicenter trials with large sample sizes are also required to confirm the current findings.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Second Hospital of Anhui Medical University.
Informed consent statement: All study participants provided written informed consent prior to study enrolment.
Conflict-of-interest statement: There are no potential conflicts of interest relevant to this article to be reported.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Peer-review started: July 16, 2019
First decision: August 2, 2019
Article in press: September 11, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed M, Farshadpour F, Surani S, Sandhu SD S-Editor: Wang J L-Editor: Wang TQ E-Editor: Ma YJ
Contributor Information
Tai-Ping Li, Department of Clinical Laboratory, The Second Hospital of Anhui Medical University, Hefei 230601, Anhui Province, China.
Shi-He Guan, Department of Clinical Laboratory, The Second Hospital of Anhui Medical University, Hefei 230601, Anhui Province, China. shiheguan@126.com.
Qin Wang, Department of Clinical Laboratory, The Second Hospital of Anhui Medical University, Hefei 230601, Anhui Province, China.
Li-Wen Chen, Department of Clinical Laboratory, The Second Hospital of Anhui Medical University, Hefei 230601, Anhui Province, China.
Kai Yang, Department of Clinical Laboratory, The Second Hospital of Anhui Medical University, Hefei 230601, Anhui Province, China.
Hao Zhang, Department of Clinical Laboratory, The Second Hospital of Anhui Medical University, Hefei 230601, Anhui Province, China.
References
- 1.Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–553. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.You S, Rong Y, Zhu B, Zhang A, Zang H, Liu H, Li D, Wan Z, Xin S. Changing etiology of liver failure in 3,916 patients from northern China: a 10-year survey. Hepatol Int. 2013;7:714–720. doi: 10.1007/s12072-013-9424-5. [DOI] [PubMed] [Google Scholar]
- 3.Ye H, Wang LY, Zhao J, Wang K. Increased CD163 expression is associated with acute-on-chronic hepatitis B liver failure. World J Gastroenterol. 2013;19:2818–2825. doi: 10.3748/wjg.v19.i18.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 5.Rey-Giraud F, Hafner M, Ries CH. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. PLoS One. 2012;7:e42656. doi: 10.1371/journal.pone.0042656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 7.Jordens R, Thompson A, Amons R, Koning F. Human dendritic cells shed a functional, soluble form of the mannose receptor. Int Immunol. 1999;11:1775–1780. doi: 10.1093/intimm/11.11.1775. [DOI] [PubMed] [Google Scholar]
- 8.Rødgaard-Hansen S, Rafique A, Christensen PA, Maniecki MB, Sandahl TD, Nexø E, Møller HJ. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin Chem Lab Med. 2014;52:453–461. doi: 10.1515/cclm-2013-0451. [DOI] [PubMed] [Google Scholar]
- 9.Sandahl TD, Støy SH, Laursen TL, Rødgaard-Hansen S, Møller HJ, Møller S, Vilstrup H, Grønbæk H. The soluble mannose receptor (sMR) is elevated in alcoholic liver disease and associated with disease severity, portal hypertension, and mortality in cirrhosis patients. PLoS One. 2017;12:e0189345. doi: 10.1371/journal.pone.0189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laursen TL, Rødgaard-Hansen S, Møller HJ, Mortensen C, Karlsen S, Nielsen DT, Frevert S, Clemmesen JO, Møller S, Jensen JS, Bendtsen F, Grønbaek H. The soluble mannose receptor is released from the liver in cirrhotic patients, but is not associated with bacterial translocation. Liver Int. 2017;37:569–575. doi: 10.1111/liv.13262. [DOI] [PubMed] [Google Scholar]
- 11.Laursen TL, Wong GL, Kazankov K, Sandahl T, Møller HJ, Hamilton-Dutoit S, George J, Chan HL, Grønbaek H. Soluble CD163 and mannose receptor associate with chronic hepatitis B activity and fibrosis and decline with treatment. J Gastroenterol Hepatol. 2018;33:484–491. doi: 10.1111/jgh.13849. [DOI] [PubMed] [Google Scholar]
- 12.Grønbæk H, Rødgaard-Hansen S, Aagaard NK, Arroyo V, Moestrup SK, Garcia E, Solà E, Domenicali M, Piano S, Vilstrup H, Møller HJ CANONIC study investigators of the EASL-CLIF Consortium. Macrophage activation markers predict mortality in patients with liver cirrhosis without or with acute-on-chronic liver failure (ACLF) J Hepatol. 2016;64:813–822. doi: 10.1016/j.jhep.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, Hamid S, Kim DJ, Komolmit P, Lata S, Lee GH, Lesmana LA, Mahtab M, Maiwall R, Moreau R, Ning Q, Pamecha V, Payawal DA, Rastogi A, Rahman S, Rela M, Saraya A, Samuel D, Saraswat V, Shah S, Shiha G, Sharma BC, Sharma MK, Sharma K, Butt AS, Tan SS, Vashishtha C, Wani ZA, Yuen MF, Yokosuka O APASL ACLF Working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 14.Tang CM, Yau TO, Yu J. Management of chronic hepatitis B infection: current treatment guidelines, challenges, and new developments. World J Gastroenterol. 2014;20:6262–6278. doi: 10.3748/wjg.v20.i20.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han M, Yan W, Guo W, Xi D, Zhou Y, Li W, Gao S, Liu M, Levy G, Luo X, Ning Q. Hepatitis B virus-induced hFGL2 transcription is dependent on c-Ets-2 and MAPK signal pathway. J Biol Chem. 2008;283:32715–32729. doi: 10.1074/jbc.M806769200. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, Shirai M, Asada K, Yasui H, Karayama M, Hozumi H, Furuhashi K, Enomoto N, Fujisawa T, Nakamura Y, Inui N, Shirai T, Hayakawa H, Suda T. Macrophage mannose receptor, CD206, predict prognosis in patients with pulmonary tuberculosis. Sci Rep. 2018;8:13129. doi: 10.1038/s41598-018-31565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marie Relster M, Gaini S, Møller HJ, Johansen IS, Pedersen C. The macrophage activation marker sMR as a diagnostic and prognostic marker in patients with acute infectious disease with or without sepsis. Scand J Clin Lab Invest. 2018;78:180–186. doi: 10.1080/00365513.2018.1431841. [DOI] [PubMed] [Google Scholar]