Abstract
To evaluate the preventive effects of combined vaccination for Pasteurella multocida, Mannheimia haemolytica and Histophilus somni on respiratory diseases in Japanese Black calves, 295 calves at one farm were alternately assigned to two groups; 147 calves received the vaccine at 4 and 8 weeks of age (vaccination group), and the other 148 calves did not receive vaccine (control group). The incidences of respiratory diseases were 25.9 and 70.9% in the vaccination and control groups, respectively, and the odds ratio for comparison between the two groups was 0.143 (95% confidence interval: 0.086–0.238). Administration of the multiple vaccine to Japanese black calves might be one of effective factor for prevention of respiratory diseases.
Keywords: bacteria, bovine respiratory disease, Japanese Black calves, vaccine
Pasteurella multocida, Mannheimia haemolytica, and Histophilus somni are common inhabitants of the upper respiratory tract of healthy cattle [2]. These bacteria have been most commonly isolated in bovine respiratory diseases [7, 20]. The Japanese Black is a breed of beef cattle, which originated and is mainly distributed in Japan [10]. Young calves have an immature immune system [3, 11], demonstrated by a low antibody response [6, 12]. Furthermore, Japanese Black calves have fewer peripheral blood T and B cells compared to Holstein calves, making them more susceptible to infections [14].
Antimicrobial agents are generally used to treat bacterial infections. However, in recent years, the use of antimicrobial agents for livestock animals has been limited [18]. To prevent respiratory infections in cattle by bacteria such as P. multocida, M. haemolytica, and H. somni, vaccination, rather than antibiotics, has importance. In this study, we evaluated the preventive effects of combined vaccination for P. multocida, M. haemolytica, and H. somni on respiratory diseases in young Japanese Black calves in one field farm.
In this study, we examined 295 Japanese Black calves, kept at one farm in Kagoshima Prefecture, Japan, born from December 2016 to November 2018, and we alternately categorized them under two groups: vaccination group, with 147 calves receiving 2 ml of inactivated vaccine (Cattle Vact 3 [P. multocida, BP165 strain; M. haemolytica, HL strain; H. somni, M-1 Br strain], Kyoto Biken Laboratories, Kyoto, Japan) intramuscularly at 4 and 8 weeks of age following manufacturer’s instruction, and control group, with 148 calves receiving no vaccines. The calves were fed to meet the nutritional requirement, based on the Japanese Feeding Standard for Beef Cattle [1], and managed similarly. The experimental procedures were performed following the Guide for the Care and Use of Laboratory Animals of the Joint Faculty of Veterinary Medicine, Kagoshima University. The calves were allowed to remain with their dams to consume colostrum freely for 4 days after birth. Subsequently, they were separated from their dams and housed in individual calf pens (in nose-to-nose contact with their peers) until about 10 weeks of age. Finally, they were moved to the group pens.
We randomly selected 18 calves each from the vaccination and control groups to determine the antibody titers against P. multocida, M. haemolytica, and H. somni. Blood was derived from the jugular vein of the calves selected for antibody determination and collected in vacutainer tubes at 1 (7 days), 4 (28–34 days), 8 (56–62 days), 12 (84–90 days), 16 (112–118 days), and 20 (140–146 days) weeks after birth. Serum was separated by centrifugation and stored at −30°C until the analysis. There were no seasonal variations in blood sampling for antibody titers.
Serum antibodies against P. multocida were detected on enzyme-linked immune sorbent assay (ELISA), which was performed as described in a previous study [16]. Capsular antigens of P. multocida serotype A3 (strain BP165) were dispensed in the wells of a microtiter plate. Serum samples at 2-fold serial dilution (starting from 1/100) were added in the wells and incubated. After washing, peroxidase-conjugated anti-bovine IgG was added and incubated. After washing, o-Phenylenediamine was added in a citrate–phosphate buffer and incubated at 30°C for 30 min. After stopping the reaction, the optical density was 492 nm, using a reference of 630 nm. The highest dilution, which showed an optical density higher than 0.4, was used as an antibody titer. Serum antibodies against M. haemolytica were detected on ELISA, which was performed as described in a previous study [15]. M. haemolytica serotype 1 (HL2 strain) was dispensed in the wells of a microtiter plate. Serum samples at 2-fold serial dilution (starting from 1/100) were incubated in the wells. After washing, peroxidase-conjugated anti-bovine IgG was added and incubated. After washing, o-Phenylenediamine was added in a citrate–phosphate buffer and incubated at 30°C for 30 min. After stopping the reaction, the optical density was 492 nm, using a reference of 630 nm. The highest dilution, which showed an optical density higher than 0.4, was used as an antibody titer. For P. multocida and M. haemolytica, when the antibody titer was detected with less than 100-fold dilution, it was calculated as a 50-fold dilution. Serum antibodies against H. somni were detected on ELISA, which was performed as described in a previous study [5, 17]. H. somni (M-1Br) was dispensed in the wells of a microtiter plate. Serum samples at 400-fold dilution were added in the wells and incubated. After washing, peroxidase-conjugated anti-bovine IgG was added and incubated. After washing, o-Phenylenediamine was added in a citrate–phosphate buffer and incubated at 30°C for 30 min. After stopping the reaction, the optical density was 492 nm, using a reference of 630 nm.
The 295 calves enrolled in this study were monitored clinically from birth to 20 weeks of age. For the calves with rectal temperature ≥39.7°C and symptoms of nasal discharge, coughing, depression, and anorexia, incidence of a respiratory disease was considered for the treatment by the veterinarian, as previously reported [8].
The data of antibody titers against P. multocida and M. haemolytica were expressed as geometric mean ± standard error (SE) and for H. somni, as mean ± SE. The odds ratio and 95% confidence interval (CI) of comparison between the vaccination and control groups were determined. Comparisons between the two groups at the same age were performed for antibody titers and respiratory disease incidence using the Student’s t-test and chi-squared test, respectively. P values less than 0.05 were considered statistically significant. The analyses were performed using SPSS software version 24 (IBM, Tokyo, Japan).
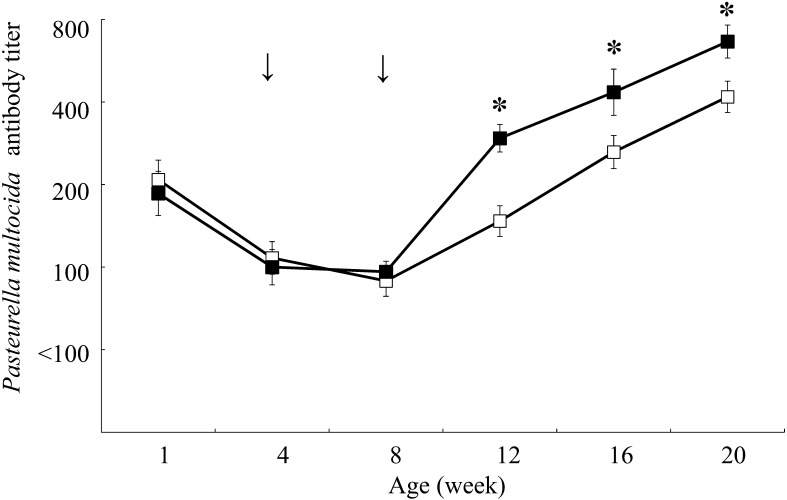
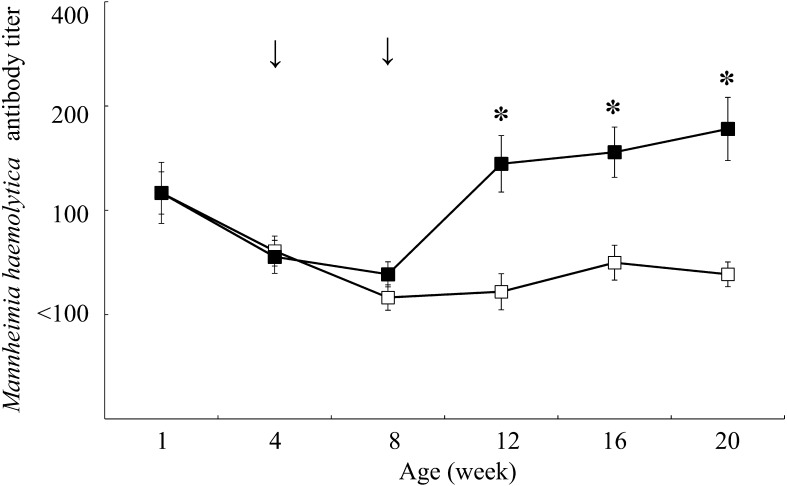
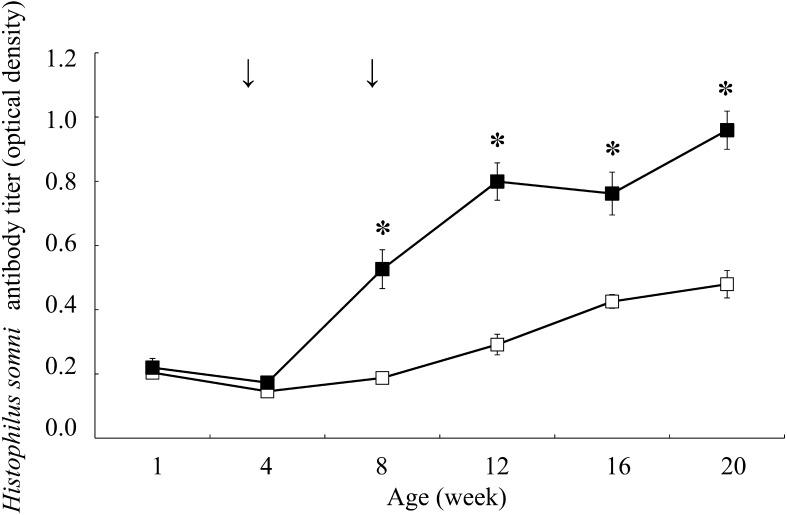
Figures 1, 2, 3 showed the changes in antibody titers against P. multocida, M. haemolytica, and H. somni, respectively. In the vaccination group, the antibody titers against P. multocida and M. haemolytica were significantly higher from 12 weeks of age (P<0.05), and against H. somni were significantly higher from 8 weeks of age compared to the control group at the same age (P<0.05). The incidence of respiratory disease was in 38 out of 147 calves (25.9%) in the vaccination group and in 105 out of 148 calves (70.9%) in the control group (Table 1), which showed a significantly lower rate in the vaccination group compared to the control group (P<0.01). The odds ratio for comparison between the vaccination and control groups was 0.143 (95% CI: 0.086–0.238).
Fig. 1.
Changes in antibody titers against Pasteurella multocida in the vaccination (n=18, dark square) and control (n=18, empty square) groups. Antibody titers are expressed as geometric mean ± standard error. Arrows indicate inoculation with combined vaccine containing inactivated Pasteurella multocida, Mannheimia haemolytica, and Histophilus somni. The asterisk indicates a significant difference between groups at the same age (*P<0.05).
Fig. 2.
Changes in antibody titers against Mannheimia haemolytica in the vaccination (n=18, dark square) and control (n=18, empty square) groups. Antibody titers are expressed as geometric mean ± standard error. Arrows indicate inoculation with combined vaccine containing inactivated Pasteurella multocida, Mannheimia haemolytica, and Histophilus somni. The asterisk indicates a significant difference between groups at the same age (*P<0.05).
Fig. 3.
Changes in antibody titers against Histophilus somni in the vaccination (n=18, dark square) and control (n=18, empty square) groups. Antibody titers (optical density) are expressed as mean ± standard error. Arrows indicate inoculation with combined vaccine containing inactivated Pasteurella multocida, Mannheimia haemolytica, and Histophilus somni. The asterisk indicates a significant difference between groups at the same age (*P<0.05).
Table 1. Incidence of respiratory diseases in calves.
| Group | No. of incidence | No. of no incidence | Incidence rate (%) |
|---|---|---|---|
| Vaccination group (n=147) | 38 | 109 | 25.9 a) |
| Control group (n=148) | 105 | 43 | 70.9 |
The superscript symbol indicates a significant difference between groups (a) P<0.01).
Most respiratory diseases of calves are caused by bacterial, mycoplasma, and viral infections [4]. P. multocida, M. haemolytica, and H. somni are the predominant bacteria that cause bovine respiratory diseases [7, 20]. When each of these bacterial infections is combined with bovine respiratory syncytial virus or bovine herpesvirus-1, the respiratory disease becomes more severe [9, 21]. Therefore, effective prevention against P. multocida, M. haemolytica, and H. somni are important for calves. Vaccination is an important prevention technique for respiratory diseases. However, vaccination does not produce sufficient antibodies when calves possess a maternal antibody titer [3, 13, 19]. In Japanese Black calves, Otomaru et al. [15,16,17] reported that the antibody titers against P. multocida, M. haemolytica and H. somni were decreased at around 4, 8, and 4 weeks of age, respectively, because of transmission of maternal antibody titers against them. Therefore, vaccination against P. multocida, M. haemolytica, and H. somni should be performed at around 4, 8, and 4 weeks of age, respectively. However, there have been no reports of Japanese Black calves vaccinated against P. multocida, M. haemolytica and H. somni at the aforementioned ages. In the present study, the calves were vaccinated at 4 and 8 weeks of age, and the antibody response and respiratory disease incidence were assessed. The antibody titers against P. multocida, M. haemolytica, and H. somni in the vaccination group were significantly higher than those in the control group after vaccination. In particular, antibody titers against H. somni in the vaccination group remained significantly higher than those in the control group at 8 weeks of age before the second administration of the vaccine. These results indicated that the calf produced reliable antibody production against the combined vaccine. The incidence of respiratory disease was also significantly lower in the vaccination group compared to the control group, showing a low odds ratio.
Although the study was conducted at a single farm with a limited number of calves, administration of combined inactivated vaccine against P. multocida, M. haemolytica, and H. somni to Japanese black calves might be one of effective prevention for respiratory diseases. Lager field trials are needed to monitor the duration of immunity with vaccination and test the effect of protection against naturally occurring respiratory diseases in the field.
Acknowledgments
We would like to acknowledge Dr. Shuichi Kubota and Dr. Takahiro Kaneshige of the Kyoto Biken Laboratories for their help with this study. This study was supported by grants from a project of the National Agricultural Research Organization, Bio-oriented Technology Research Advancement Institution, Japan.
REFERENCES
- 1.Agriculture, Factory and Fisheries Research Council Secretariat. 2008. Japanese Feeding Standard for Beef Cattle, Japan Livestock Industry Association, Tokyo (in Japanese). [Google Scholar]
- 2.Allen J. W., Viel L., Bateman K. G., Rosendal S., Shewen P. E., Physick-Sheard P.1991. The microbial flora of the respiratory tract in feedlot calves: associations between nasopharyngeal and bronchoalveolar lavage cultures. Can. J. Vet. Res. 55: 341–346. [PMC free article] [PubMed] [Google Scholar]
- 3.Ayoub I. A., Yang T. J.1996. Age-dependent changes in peripheral blood lymphocyte subpopulations in cattle: a longitudinal study. Dev. Comp. Immunol. 20: 353–363. doi: 10.1016/S0145-305X(96)00024-9 [DOI] [PubMed] [Google Scholar]
- 4.Bryson D. G.1985. Calf pneumonia. Vet. Clin. North Am. Food Anim. Pract. 1: 237–257. doi: 10.1016/S0749-0720(15)31326-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canto J., Biberstein E. L., Schulte T. A., Behymer D.1983. Cross-reactivity of Haemophilus somnus antibody in agglutination and complement fixation tests and in the enzyme-linked immunosorbent assay. J. Clin. Microbiol. 17: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da Roden L., Smith B. P., Spier S. J., Dilling G. W.1992. Effect of calf age and Salmonella bacterin type on ability to produce immunoglobulins directed against Salmonella whole cells or lipopolysaccharide. Am. J. Vet. Res. 53: 1895–1899. [PubMed] [Google Scholar]
- 7.Gagea M. I., Bateman K. G., van Dreumel T., McEwen B. J., Carman S., Archambault M., Shanahan R. A., Caswell J. L.2006. Diseases and pathogens associated with mortality in Ontario beef feedlots. J. Vet. Diagn. Invest. 18: 18–28. doi: 10.1177/104063870601800104 [DOI] [PubMed] [Google Scholar]
- 8.Galyean M. L., Gunter S. A., Malcolm-Callis K. J.1995. Effects of arrival medication with tilmicosin phosphate on health and performance of newly received beef cattle. J. Anim. Sci. 73: 1219–1226. doi: 10.2527/1995.7351219x [DOI] [PubMed] [Google Scholar]
- 9.Gershwin L. J.2007. Bovine respiratory syncytial virus infection: immunopathogenic mechanisms. Anim. Health Res. Rev. 8: 207–213. doi: 10.1017/S1466252307001405 [DOI] [PubMed] [Google Scholar]
- 10.Gotoh T., Albrecht E., Teuscher F., Kawabata K., Sakashita K., Iwamoto H., Wegner J.2009. Differences in muscle and fat accretion in Japanese Black and European cattle. Meat Sci. 82: 300–308. doi: 10.1016/j.meatsci.2009.01.026 [DOI] [PubMed] [Google Scholar]
- 11.Kampen A. H., Olsen I., Tollersrud T., Storset A. K., Lund A.2006. Lymphocyte subpopulations and neutrophil function in calves during the first 6 months of life. Vet. Immunol. Immunopathol. 113: 53–63. doi: 10.1016/j.vetimm.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 12.King N. B., Frank N. A.1961. Effect of age on resistance and retention of titer in cattle vaccinated with strain 19 Brucella abortus vaccine. J. Am. Vet. Med. Assoc. 139: 100–103. [PubMed] [Google Scholar]
- 13.Menanteau-Horta A. M., Ames T. R., Johnson D. W., Meiske J. C.1985. Effect of maternal antibody upon vaccination with infectious bovine rhinotracheitis and bovine virus diarrhea vaccines. Can. J. Comp. Med. 49: 10–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Ohtsuka H., Ono M., Saruyama Y., Mukai M., Kohiruimaki M., Kawamura S.2011. Comparison of the peripheral blood leukocyte population between Japanese Black and Holstein calves. Anim. Sci. J. 82: 93–98. doi: 10.1111/j.1740-0929.2010.00833.x [DOI] [PubMed] [Google Scholar]
- 15.Otomaru K., Kubota S., Tokimori M.2013. Maternally and naturally acquired antibody to Mannheimia haemolytica in Japanese Black calves. J. Vet. Med. Sci. 75: 1675–1677. doi: 10.1292/jvms.13-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otomaru K., Kubota S., Tokimori M.2015. Maternally and naturally acquired antibodies to Pasteurella multocida in Japanese Black calves. Pak. Vet. J. 35: 108–110. [Google Scholar]
- 17.Otomaru K., Kubota S., Kaneshige T.2018. Maternally and naturally acquired antibodies to Histophilus somni in Japanese Black calves. J. Jpn. Vet. Med. Assoc. 71: 298–301 (in Japanese). [Google Scholar]
- 18.Schrijver R., Stijntjes M., Rodríguez-Baño J., Tacconelli E., Babu Rajendran N., Voss A.2018. Review of antimicrobial resistance surveillance programmes in livestock and meat in EU with focus on humans. Clin. Microbiol. Infect. 24: 577–590. doi: 10.1016/j.cmi.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 19.Vangeel I., Antonis A. F., Fluess M., Riegler L., Peters A. R., Harmeyer S. S.2007. Efficacy of a modified live intranasal bovine respiratory syncytial virus vaccine in 3-week-old calves experimentally challenged with BRSV. Vet. J. 174: 627–635. doi: 10.1016/j.tvjl.2006.10.013 [DOI] [PubMed] [Google Scholar]
- 20.Virtala A. M., Mechor G. D., Gröhn Y. T., Erb H. N., Dubovi E. J.1996. Epidemiologic and pathologic characteristics of respiratory tract disease in dairy heifers during the first three months of life. J. Am. Vet. Med. Assoc. 208: 2035–2042. [PubMed] [Google Scholar]
- 21.Yates W. D., Jericho K. W., Doige C. E.1983. Effect of viral dose on experimental pneumonia caused by aerosol exposure of calves to bovine herpesvirus 1 and Pasteurella haemolytica. Can. J. Comp. Med. 47: 57–63. [PMC free article] [PubMed] [Google Scholar]