Abstract
Intestinal smooth muscle hypertrophy and hyperplasia has been described in human and several mammal species. In birds, only one case of intestinal smooth muscle hyperplasia has been reported. This paper describes the anatomopathological and histological findings of three cases of intestinal smooth muscle hypertrophy/hyperplasia in two different avian species belonging to the family Gallinidae and Columbidae. Grossly, it involved all tracts of the small intestine. Histologically, hyperplasia involved the mucosal villi, muscularis mucosa and inner and outer layers of the tunica muscularis. Hypertrophy was apparently detected only in the inner circular muscle layer. Lack of submucosal plexuses was also observed in all three animals. The results confirm the remarkable histological difference between mammals and avian species and show as these pathological changes can occur in different species of birds.
Keywords: intestine, ornamental chicken, ringdove pigeon, smooth muscle hyperplasia
Intestinal smooth muscle hypertrophy has been reported in natural form in humans [30, 33] and in several mammal species including horses [5, 6, 19, 28] pigs [6, 22], sheeps [6], and cats [32]. Experimentally, it was observed in guinea pigs [9] and in rats as result of inflammation [2], anatomical surgical intestinal modification [24] and parasitic infestation with Trichinella spiralis larvae [34]. On the other hand, intestinal smooth muscle hyperplasia has been reported in natural form in goats [7] and experimentally in rat [2, 14]. In birds, a natural case of intestinal smooth muscle hyperplasia has been reported in an American rhea [25]. The objective of this paper is to describe the pathological findings of three cases of intestinal smooth muscle hypertrophy and hyperplasia in two different avian species belonging to the family Gallinidae and Columbidae.
One adult male ringdove pigeon (Columba palumbus) and two adult female ornamental chickens (Gallus gallus domesticus) coming from three different small breeders in central Italy were sent to the Diagnostics and Animal Welfare laboratories of Istituto Zooprofilattico of Umbria and Marche “Togo Rosati” for necropsy examination.
The clinical histories of the dead birds included lethargy, progressive weight loss, and finally death. Further significant anamnestic data were not reported.
A fully necropsy examination of all dead birds was performed and representative tissue samples were collected and fixed in 10% neutral buffered formalin for routine histological examination. Sections 5 µm thick were obtained and stained with hematoxylin and eosin (HE); Gomori’s trichrome staining and immunohistochemistry (IHC) on intestinal samples were also performed. Avidin biotin complex (ABC) method and monoclonal mouse anti-human smooth muscle actin (HSMA), clone 1A4 (1:400; Dako, Glostrup, Denmark) was used. 3-amino-9-ethylcarbazole (AEC Substrate-Chromogen, Abcam, Cambridge, U.K.) was used as a chromogen and Carazzi’s haematoxylin as a counterstain. Positive and negative control were used to confirm that the assay functioned properly.
Morphometric evaluation on mucosal smooth muscle, muscularis mucosae and smooth muscle layers of jejunum was performed. For this purpose, five selected random fields for three intestinal serial sections for each animal were evaluated. The measurements were recorded using a Nikon DS-Fi1 digital camera (Nikon Corp., Tokyo, Japan) connected to the microscope Leica DMR (Leica Microsystems, Milan, Italy), and NIS-Elements Br-2 as software. Minimum and maximum (range) measurement for every investigated histological parameter (number of smooth muscle cells for villus, muscularis mucosae and both muscle layers; thickness of muscularis mucosa and of both muscle layers; thickness of smooth muscle cell of villus, muscularis mucosae and of both muscle layers) were recorded for each animal. The thickness of villus smooth muscle cells layer was not measured because the mucosal cellular inflammation produced separation of muscular fibers (measurement bias).
Intestinal samples collected from healthy ringdove pigeon and chicken were used as control sample.
In all three animals, intestinal, pulmonary and hepatic specimens were aseptically collected for bacteriological examinations. The samples were spread plated on MacConkey, mannitol salt agar and blood agar plates at 37°C for 3 days. Then, biochemical reactions were performed for identification of the suspicious colonies. Moreover, coprological examination of fresh stool specimens was also performed. Flotation with flotation solution-saturated sodium chloride (specific gravity 1.2 g/ml) was performed on 2 g of feces. Post mortem examination revealed severe dehydration and cachexia of all three animals. At necropsy, the ringdove pigeon showed pericarditis, air sacculitis, perihepatitis, and proventriculitis with abundant mucoid content in the lumen. On the other hand, the chickens showed air sacculitis. Moreover, in all three animals, duodenum, jejunum and ileum were thickened and had the turgid feel of a rubber tube. Catarrhal enteritis characterized by hyperaemic and oedematous mucosa was observed. On cross-section, the intestinal lumen appeared narrowed and occluded by numerous roundworms consistent with Ascaris spp. and Capillaria spp. in the pigeon, while only ascarids were observed in the two ornamental chickens. In the large intestine excessive intraluminal fluid and gas associated with moderate catarrhal typhlo-colitis were detected.
Microscopically, in the ringdove pigeon, sinuses of glands of the proventriculus contained desquamated epithelium and cellular debris in abundant mucoid content. Diffuse lymphocytic and plasmacytic infiltrate was observed in the lamina propria of the mucosa; small foci of similar inflammatory infiltrate were detected in glands expanding the glandular interstitium. Moreover, a variable degree of catarrhal typhlo-colitis was observed in all three animals. The cecal mucosa appeared eroded. The lamina propria showed from moderate to marked lymphocytic and plasmacytic infiltrate. Karyorrhectic debris, inflammatory cells and numerous bacilli were detected in the lumen. In all three animals a fibrinoheterophilic pneumonia was also observed.
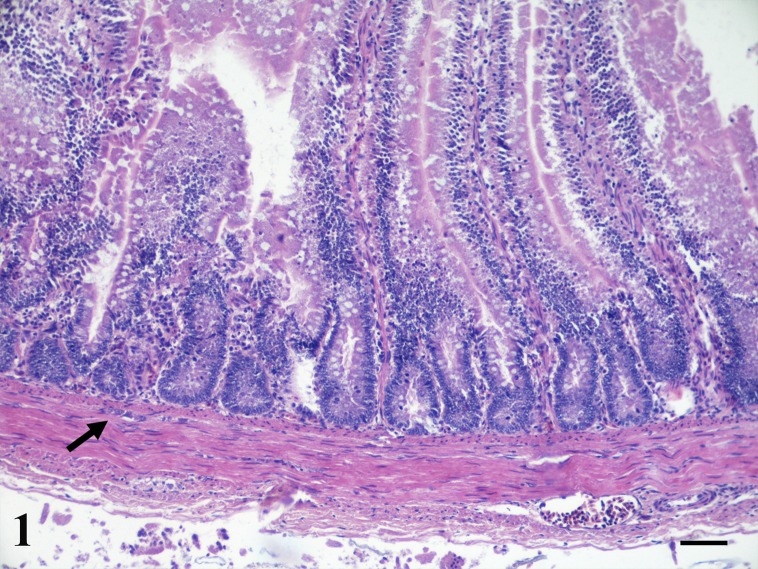
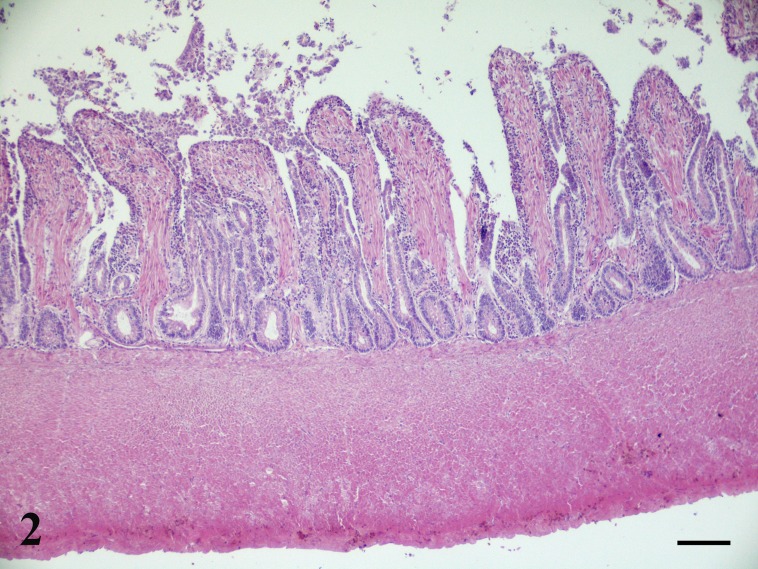
Microscopic examination of the jejunum of all three animals revealed similar lesions. Diffusely intestinal villi were lined by attenuated epithelial cells with multifocally slight increase of the intraepithelial lymphocytes. Sloughed necrotic epithelial cells with amounts of eosinophilic cellular and karyorrhectic debris were found in the lumen. The mucosal crypts were lined by columnar epithelium with increased numbers of mitotic figures. Moreover, the mucosa showed severe thickening caused by inflammation and smooth muscle hypertrophy and hyperplasia. In particular, expansion of the lamina propria by a mild to moderate inflammatory cellular infiltrate consisting mainly of plasma cells and scattered eosinophils and lymphocytes was detected. In the ringdove pigeon the inflammation was present especially in the deep layers of the lamina propria while in the chickens it appeared mostly in the superficial layer of the lamina propria. In all three birds, the villi resulted thickened by hyperplastic smooth muscle fibers than those the control animal (Figs. 1 and 2). From 15 to 30 and from 12 to 23 parallel thickened smooth muscle cells for villus were observed respectively in ringdove pigeon and ornamental chickens (in comparison with the 6–9 and 6–8 counted in the normal jejunum of control animals). The muscularis mucosae was also slightly thickened; lack of a discernible submucosa was observed (Fig. 2). In the ringdove pigeon the muscularis mucosae was composed of 7–8 smooth muscle cells (in comparison with the 5–6 parallel smooth muscle cells counted in the normal jejunum of the control animals); its thickness was 30–35 µm (in comparison with 20–27 µm of the control animals). In the ornamental chickens the muscularis mucosa was composed of 5–7 smooth muscle cells (in comparison with the 6–7 parallel smooth muscle cells counted in the normal jejunum of control animals); its thickness ranged from 24 to 33 µm (in comparison with 22–28 µm of the control animals). Submucosal plexuses were not highlighted in all three animals (Fig. 2).
Fig. 1.
Ornamental chicken, jejunum. Control chicken showing the normal histology of intestine. Submucosal (Meissner) plexus is present (arrow) (HE, Bar=100 µm).
Fig. 2.
Ornamental chicken, jejunum. Pathological chickens with marked hyperplasia of mucosal smooth muscle fibers, muscularis mucosa and inner intestinal smooth muscle layer. Lack both of a discernible submucosa and submucosal (Meissner) plexus. (HE, Bar=100 µm).
Mild hypertrophy of cells of intestinal smooth muscle layers and marked hyperplasia of the intestinal smooth muscle layers were detected compared to controls (Figs. 1 and 2). In the pigeon, the inner circular muscle layer was composed of 115–130 smooth muscle cells (in comparison with the 32–40 counted in the healthy jejunum). Its thickness ranged from 600 to 630 µm (in comparison with 160–190 µm detected in the control animal). The outer longitudinal muscle layer was composed of 7–9 parallel smooth muscle cells; the control animal showed the same range of value. Its thickness ranged from 30 to 35 µm (in comparison with 25–27 µm detected in the control animal). In the ornamental chickens, the inner circular muscle layer was composed of 35–80 smooth muscle cells (in comparison with the 28–35 counted in the healthy jejunum). Its thickness ranged from 170 to 400 µm (in comparison with 100–163 µm detected in the control). From 10 to 12 parallel smooth muscle cells for the outer longitudinal muscle layer were detected (in comparison with the 6–10 counted in the normal jejunum of the control animal). On the other hand, the outer longitudinal muscle layer thickness ranged from 42 to 54 µm (in comparison with 27–40 of the control). In both pathological and healthy animals, the diameter of individual myofiber was relatively uniform (3–4.5 µm for smooth muscle of mucosa, muscularis mucosae and for the outer longitudinal muscle layer); only a slight greater variability was observed for the inner circular muscle layer (5–7 µm); the highest values were found in the pathological subjects. All the morphometric results are summed in Table 1.
Table 1. Morphometric evaluation on mucosal smooth muscle, muscularis mucosae and smooth muscle layers of jejunum.
| Sex | Age | Body weight (g) |
Range of No. of smooth muscle cells for villus | Range of No. of cells for the muscolaris mucosa | Range of muscularis mucosa thickness | Range of No. of cells for the inner circular muscle layer | Range of inner circular muscle layer thickness | Range of No. of cells for the outer longitudinal muscle layer | Range of outer longitudinal muscle layer thickness | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control animals | |||||||||||
| Columba palumbus | Male | Adult | 610 | 6–9 (6.6) | 5–6 (5.27) | 20–27 (21.87) | 32–40 (34.73) | 160–190 (178.20) | 5–7 (5.53) | 25–27 (25.47) | |
| Gallus gallus domesticus | Female | Adult | 2,010 | 6–8 (6.47) | 6–7 (6.07) | 22–28 (23.87) | 28–35 (29.53) | 100–163 (127.80) | 6–10 (6.87) | 24–30 (26.20) | |
| Pathological animals | |||||||||||
| Columba palumbus | Male | Adult | 550 | 15–30 (25.73) | 7–8 (7.6) | 30–35 (32.53) | 115–130 (125.33) | 600–630 (621.20) | 7–9 (8.33) | 30–35 (33.20) | |
| Gallus gallus domesticus 1 | Female | Adult | 1,725 | 12–20 (17.07) | 6–7 (6.73) | 24–30 (27.53) | 35–70 (54.20) | 170–390 (316.33) | 10–12 (11.20) | 25–27 (26.40) | |
| Gallus gallus domesticus 2 | Female | Adult | 1,690 | 15–23 (20.07) | 5–6 (5.67) | 27–33 (30.13) | 65–84 (76.87) | 365–420 (402.06) | 10–12 (11.47) | 26–29 (28.07) | |
Date are presented as minimum–maximum and means in parentheses.
In all three birds, the smooth muscles fibers appeared spindle-shaped and characterized by eosinophilic cytoplasm with variable staining intensity and nuclei with normal shape and size. Between the inner and outer muscle layers isolated myenteric plexuses (one or two for each slide) with normal morphology and size were observed.
Gomori’s trichrome staining stained mucosal smooth muscle, muscularis mucosa and both muscle layers in red. Multifocal mild fibrosis of lamina propria was also revealed.The IHC highlighted numerous smooth muscle immunoreactive cells in the lamina propria. E. coli was isolated in intestine and in lung of all investigated animals.
Coprological examination of fresh stool specimens revealed coccidian oocyst, Capillaria spp and Ascaris spp. eggs in the ringdove pigeon, while only coccidian oocyst and Ascaris spp. eggs in the chickens. It is well known that smooth muscle tissue has got remarkable plasticity responding with adaptive changes when stimulated. Hypertrophy and hyperplasia generally occur when there is an imbalance between functional capacity and demand and it represents the response to an effective persistent stimulus [31]. Humoral factors such as pro-inflammatory factor, or increased mechanical load are considered causes responsible of smooth muscle hypertrophy and hyperplasia [1, 3, 4, 8,9,10,11,12,13, 15,16,17,18, 20, 23, 24, 26, 27, 29, 30, 33, 35]. Unfortunately, there are no studies to confirm whether any or all of these mechanisms can occur in avian species. In this study, grossly, hypertrophy and hyperplasia involved all tracts of small intestine. In the only previous report on avian species, the lower small intestine was affected but no specific information about the extension of the intestinal smooth muscle hyperplastic changes was reported [25]. In our study, microscopically, an increase in the number (hyperplasia) of cells of mucosal smooth muscle, muscularis mucosae and smooth muscle layers of jejunum were detected in all three animals while slight hyperthophy was found only in the inner smooth muscle layers in both species. In particular, these changes appeared more severe in the mucosa and in the intestinal smooth muscle inner layer as previously recorded in Rhea americana [25]. These results confirm the remarkable histological difference between mammals and avian species. Moreover, our results showed greater hyperplastic changes in the intestinal smooth muscle of ringdove pigeon compared with ornamental chicken. Although, no specific molecular and mechanical pathological process can be suggested for the avian species, in the present study all three animals were affected by inflammation of the intestine and increase in intraluminal pressure caused by large amounts of intestinal parasites. Moreover, lack of submucosal plexuses was observed in all three animals. This singular finding associated with marked thickening of mucosal smooth muscle and muscularis mucosae detected only in avian species till now, could suggest a possible pathological link. Indeed, as known, the Meissner’s plexuses sense the lumen environment and control the smooth muscle of the muscularis mucosae as well as the epithelial cell functions and secretion and regulate gastrointestinal blood flow [21].
Based on macroscopic, microscopic, histological and immunohistochemical findings, the diagnosis of intestinal smooth muscle hypertrophy and hyperplasia in the small intestine was confirmed in the ringdove pigeon and in the two ornamental chickens. Other two cases not well documented in ringdove pigeon and in chicken were histologically observed (data not published) during the last few years from our laboratory. Therefore, a possible underestimation of this phenomenon in avian species may be suspected.
In conclusion, to the authors’ knowledge, this is the first report of intestinal smooth muscle hypertrophy/hyperplasia in ringdove pigeon and ornamental chickens. Further studies are necessary to clarify the mechanisms responsible of smooth muscle hypertrophy and hyperplasia in avian species.
REFERENCES
- 1.Alexander M. R., Murgai M., Moehle C. W., Owens G. K.2012. Interleukin-1β modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiol. Genomics 44: 417–429. doi: 10.1152/physiolgenomics.00160.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blennerhassett M. G., Vignjevic P., Vermillion D. L., Collins S. M.1992. Inflammation causes hyperplasia and hypertrophy in smooth muscle of rat small intestine. Am. J. Physiol. 262: G1041–G1046. [DOI] [PubMed] [Google Scholar]
- 3.Bowers R. L., Eeckhout C., Weisbrodt N. W.1986. Actomyosin, collagen, and cell hypertrophy in intestinal muscle after jejunoileal bypass. Am. J. Physiol. 250: G70–G75. [DOI] [PubMed] [Google Scholar]
- 4.Castro G. A., Badial-Aceves F., Smith J. W., Dudrick S. J., Weisbrodt N. W.1976. Altered small bowel propulsion associated with parasitism. Gastroenterology 71: 620–625. [PubMed] [Google Scholar]
- 5.Chaffin M. K., Fuenteabla I. C., Schumacher J., Welch R. D., Edwards J. F.1992. Idiopathic muscular hypertrophy of the equine small intestine: 11 cases (1980–1991). Equine Vet. J. 24: 372–378. doi: 10.1111/j.2042-3306.1992.tb02858.x [DOI] [PubMed] [Google Scholar]
- 6.Cordes D. O., Dewes H. F.1971. Diverticulosis and muscular hypertrophy of the small intestine of horses, pigs and sheep. N. Z. Vet. J. 19: 108–111. doi: 10.1080/00480169.1971.33943 [DOI] [PubMed] [Google Scholar]
- 7.de Lara F. C. n-M, Hervæs J., Bautista M. J, Pørez J., mez-Villamandos J. C. G., Mulas J. M, Carrasco L.1996. Intestinal smooth muscle hyperplasia in a goat. J. Vet. Diagn. Invest. 8: 390–392. [DOI] [PubMed] [Google Scholar]
- 8.de Mattos C. E., Kempson R. L., Erdos T., Csapo A.1967. Stretch-induced myometrial hypertrophy. Fertil. Steril. 18: 545–556. doi: 10.1016/S0015-0282(16)36373-7 [DOI] [PubMed] [Google Scholar]
- 9.Gabella G.1975. Hypertrophy of intestinal smooth muscle. Cell Tissue Res. 163: 199–214. doi: 10.1007/BF00221727 [DOI] [PubMed] [Google Scholar]
- 10.Gabella G.1979. Hypertrophic smooth muscle. I. Size and shape of cells, occurrence of mitoses. Cell Tissue Res. 201: 63–78. doi: 10.1007/BF00238048 [DOI] [PubMed] [Google Scholar]
- 11.Gabella G.1979. Hypertrophic smooth muscle. II. Sarcoplasmic reticulum, caveolae and mitochondria. Cell Tissue Res. 201: 79–92. doi: 10.1007/BF00238049 [DOI] [PubMed] [Google Scholar]
- 12.Gabella G.1979. Hypertrophic smooth muscle. III. Increase in number and size of gap junctions. Cell Tissue Res. 201: 263–276. doi: 10.1007/BF00235062 [DOI] [PubMed] [Google Scholar]
- 13.Gabella G.1979. Hypertrophic smooth muscle. IV. Myofilaments, intermediate filaments and some mechanical properties. Cell Tissue Res. 201: 277–288. doi: 10.1007/BF00235063 [DOI] [PubMed] [Google Scholar]
- 14.Gabella G.1990. Hypertrophy of visceral smooth muscle. Anat. Embryol. (Berl.) 182: 409–424. doi: 10.1007/BF00178906 [DOI] [PubMed] [Google Scholar]
- 15.Johansson B.1984. Different types of smooth muscle hypertrophy. Hypertension 6: III64–III68. doi: 10.1161/01.HYP.6.6_Pt_2.III64 [DOI] [PubMed] [Google Scholar]
- 16.Kitagaki M., Isoda K., Kamada H., Kobayashi T., Tsunoda S., Tsutsumi Y., Niida T., Kujiraoka T., Ishigami N., Ishihara M., Matsubara O., Ohsuzu F., Kikuchi M.2012. Novel TNF-α receptor 1 antagonist treatment attenuates arterial inflammation and intimal hyperplasia in mice. J. Atheroscler. Thromb. 19: 36–46. doi: 10.5551/jat.9746 [DOI] [PubMed] [Google Scholar]
- 17.Kuemmerle J. F.2012. Insulin-like growth factors in the gastrointestinal tract and liver. Endocrinol. Metab. Clin. North Am. 41: 409–423, vii. doi: 10.1016/j.ecl.2012.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby P., Warner S. J., Friedman G. B.1988. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J. Clin. Invest. 81: 487–498. doi: 10.1172/JCI113346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsay W. A., Confer A. W., Ochoa R.1981. Ileal smooth muscle hypertrophy and rupture in a horse. Equine Vet. J. 13: 66–67. doi: 10.1111/j.2042-3306.1981.tb03458.x [DOI] [PubMed] [Google Scholar]
- 20.Malmqvist U., Arner A., Uvelius B.1991. Cytoskeletal and contractile proteins in detrusor smooth muscle from bladders with outlet obstruction—a comparative study in rat and man. Scand. J. Urol. Nephrol. 25: 261–267. doi: 10.3109/00365599109024556 [DOI] [PubMed] [Google Scholar]
- 21.Nezami B. G., Srinivasan S.2010. Enteric nervous system in the small intestine: pathophysiology and clinical implications. Curr. Gastroenterol. Rep. 12: 358–365. doi: 10.1007/s11894-010-0129-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen S. W.1955. Muscular hypertrophy of the ileum in relation to terminal ileitis in pigs; a preliminary report. J. Am. Vet. Med. Assoc. 127: 437–441. [PubMed] [Google Scholar]
- 23.Peterson C. M., Goss R. J., Atryzek V.1974. Hypertrophy of the rat urinary bladder following reduction of its functional volume. J. Exp. Zool. 187: 121–126. doi: 10.1002/jez.1401870113 [DOI] [PubMed] [Google Scholar]
- 24.Philipson B. M., Leth R., Kock N. G.1985. Hypertrophy of ileal smooth muscle after construction of ileal reservoir in the rat. Virchows Arch. A Pathol. Anat. Histopathol. 406: 417–424. doi: 10.1007/BF00710233 [DOI] [PubMed] [Google Scholar]
- 25.Prantner M. M.1995. Intestinal smooth muscle hyperplasia in a rhea (Rhea americana). Avian Dis. 39: 197–200. doi: 10.2307/1592004 [DOI] [PubMed] [Google Scholar]
- 26.Raines E. W., Dower S. K., Ross R.1989. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science 243: 393–396. doi: 10.1126/science.2783498 [DOI] [PubMed] [Google Scholar]
- 27.Reynolds S. R. M., Kaminester S.1937. The rate of uterine growth resulting from chronic distension. Anat. Rec. 69: 281–286. doi: 10.1002/ar.1090690303 [DOI] [Google Scholar]
- 28.Rooney J. R., Jeffcott L. B.1968. Muscular hypertrophy of the ileum in a horse. Vet. Rec. 83: 217–219. doi: 10.1136/vr.83.9.217 [DOI] [PubMed] [Google Scholar]
- 29.Ross R., Raines E. W., Bowen-Pope D. F.1986. The biology of platelet-derived growth factor. Cell 46: 155–169. doi: 10.1016/0092-8674(86)90733-6 [DOI] [PubMed] [Google Scholar]
- 30.Seidel C. L.1987. Hypertrophy of vascular smooth muscle. pp. 18–44. In: Hypertrophic Response in Smooth Muscle (Seidel, C. L. and Weisbrodt, N. W. eds.), CRC Press, Boca Raton. [Google Scholar]
- 31.Siegman M. J., Butler T. M., Mooers S. U., Trinkle-Mulcahy L., Narayan S., Stirewalt W. S., Starcher B. C.1997. Hypertrophy of colonic smooth muscle: structural remodeling, chemical composition, and force output. Am. J. Physiol. 272: G1560–G1570. [DOI] [PubMed] [Google Scholar]
- 32.Silva J. F., Bruno H. A., Paiva B. H. A., Natália M., Ocarino N. M., Marília M., Melo M. M., Rogéria Serakides R.2015. Primary muscle hypertrophy of the colon in a cat: a rare presentation. Braz. J. Vet. Pathol. 8: 107–111. [Google Scholar]
- 33.Stelling C. B., Straus F. H.1977. Roentgenographic findings in work hypertrophy of the muscularis propria of the terminal ileum. Am. J. Dig. Dis. 22: 1117–1121. doi: 10.1007/BF01072869 [DOI] [PubMed] [Google Scholar]
- 34.Tanović A., Fernández E., Jiménez M.2006. Alterations in intestinal contractility during inflammation are caused by both smooth muscle damage and specific receptor-mediated mechanisms. Croat. Med. J. 47: 318–326. [PMC free article] [PubMed] [Google Scholar]
- 35.Wolinsky H.1970. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ. Res. 26: 507–522. doi: 10.1161/01.RES.26.4.507 [DOI] [PubMed] [Google Scholar]