Abstract
Following an outbreak of classical swine fever (CSF) in Japan, 2018, CSFV JPN/1/2018 was isolated from an infected pig sample. In this study, we carried out a comparative experimental infection in pigs using this strain and the highly virulent ALD strain and compared outcomes, including clinical manifestation, virus shedding patterns and antibody responses. Although pigs inoculated orally or intramuscularly with JPN/1/2018 developed hyperthermia and had decreased leucocyte numbers, they survived for the whole experimental period and showed less severe clinical signs than those infected with the ALD strain. We confirmed the presence of characteristic multifocal infarction of the margin of the spleen that arises following infection with JPN/1/2018, albeit that this finding was not observed in all infected pigs. Both viruses efficiently spread to contact pigs in a similar manner, suggesting in transmissibility between the two strains. Viral RNAs were detected in all clinical samples, especially whole blood samples, before the pigs developed hyperthermia until at least approximately 2 weeks after inoculation. Our findings will be valuable for the investigations into epidemic events occurring in Japan and for establishing diagnostic strategies and control measures against CSF.
Keywords: classical swine fever virus, experimental infection, Japan
Classical swine fever (CSF) is a highly contagious viral disease induced by the classical swine fever virus (CSFV) of the genus Pestivirus in the family Flaviviridae [7]. This disease is widely distributed around the world, including Asian countries. CSFV is classified into three genotypes (1, 2 and 3) and several subgenotypes (1.1–1.4, 2.1–2.3, and 3.1–3.4) [9, 14]. In East and Southeast Asia, several genotypes or subgenotypes of CSFV isolates including 1.1, 2.1–2.3 and 3.4 have been identified [1, 10]. The virulence of the CSFV ranges from highly virulent with almost 100% mortality to avirulent. Although, there is currently no clear evidence of a correlation between genotype and virulence, in general, the most virulent strains such as the ALD strain have genotype 1, while most strains of genotype 2 are moderately virulent. Severity also depends on the characteristics of the host animal including age, breed and health. Some reports have described difficulties obtaining clinical CSF diagnoses on infected farms because of low severities [17, 19]. In addition, a CSFV isolate newly classified into subgenotype 2.1d in China has been characterized as moderately virulent [11].
In Japan, the first outbreak of CSF was reported in 1888 and subsequent outbreaks occurred repeatedly for over one hundred years [2]. However, after the first use of GP live vaccine, which was developed from the attenuated CSFV strain GPE− [18], the number of CSF infections drastically decreased, with the last outbreak occurring in 1992. In 2007, Japan was given CSF-free status from World Organization for Animal Health. However, in September, 2018, a CSF outbreak occurred in Gifu Prefecture, Japan for the first time in 26 years. CSFV JPN/1/2018, which was isolated from an infected pig, was classified into the subgenotype 2.1 [13, 15]. This is the first isolate of CSFV in this lineage reported in Japan.
To evaluate the virulence of the isolate and the clinical manifestations of the associated infection, we conducted a comparative animal experiment using high virulent ALD strain.
MATERIALS AND METHODS
Ethics
The Animal Care and Use Committee of the National Institute of Animal Health (NIAH) approved all animal procedures prior to the initiation of this study (authorization number: 18-047). All experimental infections using live viruses were performed in a high-containment facility at the NIAH. The high-containment facility is compliant with a containment level for group 4 pathogens described in the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2018 [21].
Cells and viruses
CPK cells were used in this study. The cells were maintained using Dulbecco’s Modified Eagle Medium: Nutrient Mixture F12 (Thermo Fisher Scientific, Waltham, MA, U.S.A.) supplemented with 10% of pestivirus-free fetal calf serum. The CSFV JPN/1/2018 was isolated from a whole blood sample collected from the first reported case in the 2018 outbreak in Japan. It was initially isolated using CPK cells and propagated once by the same cells. The highly virulent ALD strain was also used as an inoculum for pigs. The ALD strain preserved in our institute was propagated once by CPK cells before use.
Experimental infections
Twelve 8-week-old crossbreed Landrace × Large White × Duroc pigs were used for this study. The pigs were confirmed not to have antibodies against pestivirus before the experimental infections. Half of them were directly inoculated with CSFVs and the remaining animals were cohabited with inoculated pigs (contact animals). Two pigs each were orally (Group 1, Nos. 1 and 2) or intramuscularly (Group 2, Nos. 5 and 6) administered 1 ml of 106.5 50% tissue culture infectious dose (TCID50) of JPN/1/2018 strain, and two pigs were injected intramuscularly with 1 ml of 106.5 TCID50 of the high virulent ALD strain (Group 3, Nos. 9 and 10). For oral administration, the strain was directly dropped onto tonsil. The day after inoculation, 2 more pigs were introduced to each group (Nos. 3 and 4 for Group 1, Nos. 7 and 8 for Group 2, and Nos. 11 and 12 for Group 3) and a total of 4 pigs were housed together in each room. During the experiment period, clinical samples (heparinized whole blood, sera, saliva, nasal swabs, and feces) were collected daily. Saliva, nasal swabs and feces were mixed with a 10 times volume of phosphate buffered saline and filtered by 0.45 µm centrifugal filter unit (Ultrafree-MC/CL, Merck Millipore, Darmstadt, Germany). For all animals, clinical signs were observed and total leucocyte numbers in the whole blood were counted daily using Turk’s solution and a disposable hemocytometer (C-Chip, NanoEn Tek Inc., Seoul, South Korea) for approximately 2 weeks.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Viral RNAs were extracted from the clinical samples using the High Pure Viral RNA kit (Roche Diagnostics, Basel, Switzerland) according to manufacturer’s instructions. Viral genes were amplified with SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Thermo Fisher Scientific) using primers 324 and 326 [20]. PCR products were separated by electrophoresis on a 1% agarose gels and visualized by staining with ethidium bromide and visualized using UV-light transillumination. The RT-PCR assay can detect the minimum 100.5 TCID50/ml of JPN/1/2018 strain according to our evaluation (data not shown).
Fluorescent antibody (FA) test
The tonsils of infected pigs were frozen in n-hexane cooled by liquid nitrogen. The frozen samples were cut into thin sections using a cryostat, reacted with FITC-labeled specific antibody for CSFV (Classical Swine Fever-FA, Kyoto Biken Lab. Inc., Kyoto, Japan) and observed under a fluorescence microscope (LSM 700, Carl Zeiss, Tokyo, Japan).
Enzyme-linked immunosorbent assay (ELISA)
Anti-CSFV antibodies in the serum samples collected from pigs were examined by a CSFV-specific ELISA kit (Classical Swine Fever ELISA kit II, JNC Corp., Tokyo, Japan) according to manufacturer’s instructions [16].
Scoring of CSF symptoms and pathological findings at autopsy
To evaluate the virulence of CSFV strains JPN/1/2018 and ALD, a clinical score (CS) was determined using the criteria of Mittelholzer et al. [12]. Virus strains were classified as highly virulent (peak CS>15), moderately virulent (CS 5 to 15), low virulent (CS<5) or avirulent (CS 0). While the original method is scoring all parameters daily, we estimated the score for the most severe symptoms in each pig throughout the experiment period. After clinical assessment, the pigs were euthanized then subjected to postmortem examination. A pathological score (PS) of each pig was determined using the method reported by Floegel-Niesmann et al. [3].
RESULTS
Clinical manifestations
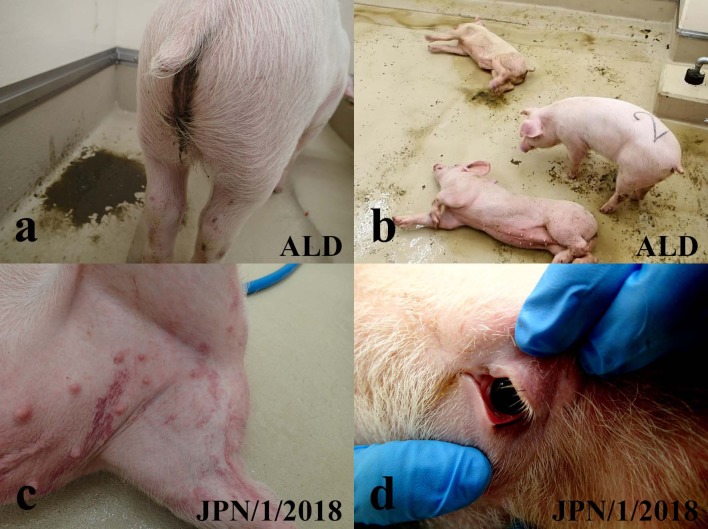
The inoculated pigs of Group 3 (inoculated with ALD) showed diarrhea, dysstasia, abolition of appetite and neurological symptoms (abnormal swimming behavior) at 5 days post infection (dpi) (Fig. 1). Those pigs were euthanized at 5 dpi because they were apparently moribund. Contact pigs housed with inoculated pigs in Group 3 also showed similar clinical symptoms, although these pigs survived for the whole experimental period (until 12 days post contact (dpc)). Therefore, estimated CSs for pigs in Group 3 were in the range 18–19, indicating high virulence (Table 1). In contrast, pigs in Group 2 (inoculated intramuscularly with JPN/1/2018) and Group 1 (inoculated orally with JPN/1/2018) showed less severe symptoms. Although they showed areas of reddened skin (Fig. 1c), and three of four pigs developed conjunctivitis (Fig. 1d) (Table 1), all pigs of Group 2 survived for the whole experiment period (14 dpi and 13 dpc). Pigs in Group 1 showed anorexia and stiffness/body tension, as well as areas of reddened skin and conjunctivitis, and survived for the whole experimental terms (15 dpi and 14 dpc). Estimated CSs for pigs in Groups 1 and 2 were in the range 1–5, suggesting low to moderate virulence.
Fig. 1.
Clinical findings of pigs of Groups 1–3. (a) Diarrhea in pig No. 10 at 3 dpi. (b) Severe neurological symptoms of abnormal swimming behavior in pigs Nos. 9 and 10 at 5 dpi. (c) Reddened skin in the hypogastrium in pig No. 6 at 15 dpi. (d) Congestion in the palpebral conjunctiva in pig No. 5 at 8 dpi.
Table 1. Clinical scores of experimental pigs.
| Group | Group 1 (JPN/1/2018 intraoral) |
Group 2 (JPN/1/2018 intramuscularly) |
Group 3 (ALD intramuscularly) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inoculated |
Contact |
Inoculated |
Contact |
Inoculated |
Contact |
|||||||
| Pig | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 | No. 12 |
| Liveliness | 1 (4 dpia)) | 1 (4 dpi) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (5 dpi) | 3 (5 dpi) | 3 (8 dpcb)) | 3 (8 dpc) |
| Body tension | 1 (5 dpi) | 1 (5 dpi) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (5 dpi) | 3 (5 dpi) | 3 (10 dpc) | 3 (10 dpc) |
| Body shape | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5 dpi) | 1 (5 dpi) | 0 | 1 (12 dpc) |
| Breathing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Walking (neurological signs) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (5 dpi) | 3 (5 dpi) | 3 (8 dpc) | 3 (8 dpc) |
| Skin | 1 (14 dpi) | 1 (14 dpi) | 1 (13 dpc) | 1 (13 dpc) | 1 (15 dpi) | 2 (15 dpi) | 0 | 1 (14 dpc) | 0 | 1 (5 dpi) | 2 (12 dpc) | 1 (12 dpc) |
| Eyes (conjunctivitis) | 1 (14 dpi) | 1 (9 dpi) | 2 (13 dpc) | 0 | 2 (8 dpi) | 1 (9 dpi) | 2 (14 dpc) | 0 | 2 (4 dpi) | 2 (5 dpi) | 2 (9 dpc) | 2 (9 dpc) |
| Appetite | 1 (7 dpi) | 1 (7 dpi) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (5 dpi) | 3 (5 dpi) | 3 (8 dpc) | 3 (8 dpc) |
| Defecation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (5 dpi) | 3 (3 dpi) | 3 (9 dpc) | 3 (12 dpc) |
| Total points | 5 | 5 | 3 | 1 | 3 | 3 | 2 | 1 | 18 | 19 | 19 | 19 |
a) Days post inoculation, b) days post contact.
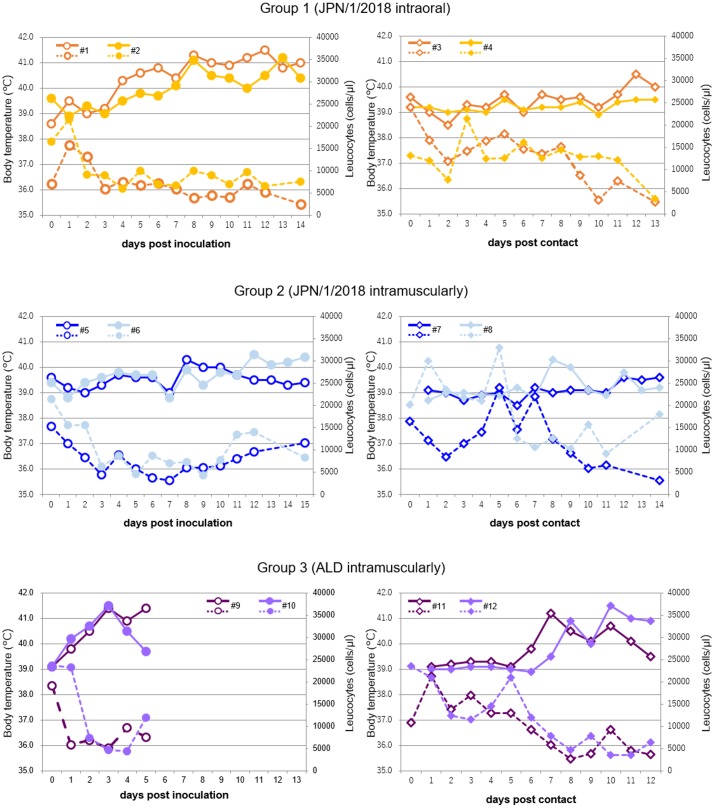
Body temperature and leucocyte number are shown in Fig. 2. The body temperature of inoculated pigs in Group 3 were over 40°C at 1–2 dpi. A decrease in leukocyte number (under 10,000 cells/µl) was simultaneously observed. Contact pigs that housed with the inoculated pigs showed high fever and decreased leukocyte number from 7–8 dpc and 6–7 dpc, respectively. In contrast, infected pigs in Groups 1 and 2 showed fever at 4–7 dpi and 8–12 dpi, respectively, while the decrease in leukocyte number was observed before the fever (2–3 dpi). One of two contact pigs housed with inoculated pigs in Groups 1 and 2 showed high fever at 8 and 12 dpc, respectively, and all contact pigs showed decreased leukocyte numbers until 13 or 14 dpc. In contrast, 2 contact pigs (Nos. 4 and 7) did not show fever over 40°C during the experimental period.
Fig. 2.
Body temperature and leucocyte number in pigs of Groups 1–3. Body temperature (solid line) and leucocyte counts (dotted line) from Groups 1–3 were shown. Pig Nos. 9 and 10 were euthanized at 5 dpi. (#) Pig number.
Detection of CSFV viral RNA (vRNA), antigen and antibody from clinical samples
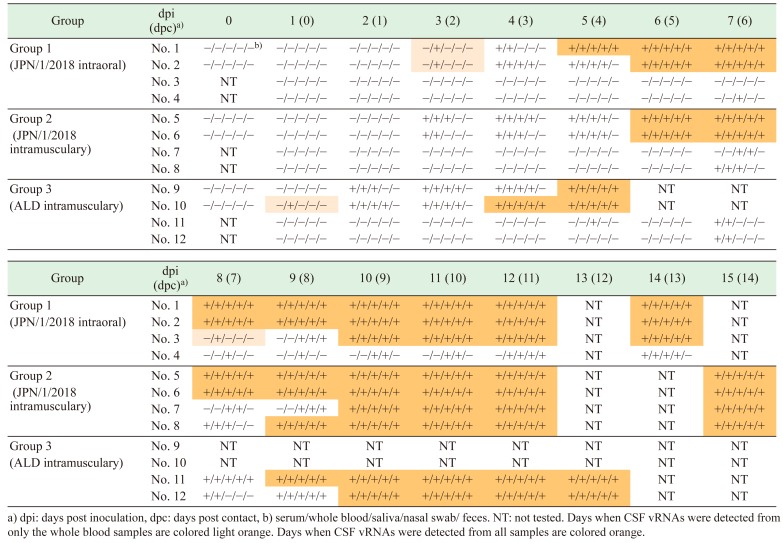
vRNAs were detected from whole blood, sera, saliva, nasal swabs, and feces of the all pigs, including contact animals (Table 2). vRNAs were detected at 1–2 dpi in samples from inoculated animals in Group 3, and at 3 dpi in samples from inoculated animals in Groups 1 and 2. In contrast, vRNAs were detected in the blood of contact pigs from 5, 4–8 and 4–7 days after virus shedding from inoculated pigs in Groups 3, 2 and 1, respectively (Table 2). Crypt epithelial cells in tonsils of all infected animals showed positive for CSFV antigen using the FA test (Fig. 3). Anti-CSF antibodies were only detected from 4 pigs (No. 2 on 14 dpi, No. 8 on 14 dpc, and Nos. 5 and 6 on 15 dpi) during the experimental period.
Table 2. Detection of classical swine fever (CSF) vRNA from clinical samples by RT-PCR.
Fig. 3.
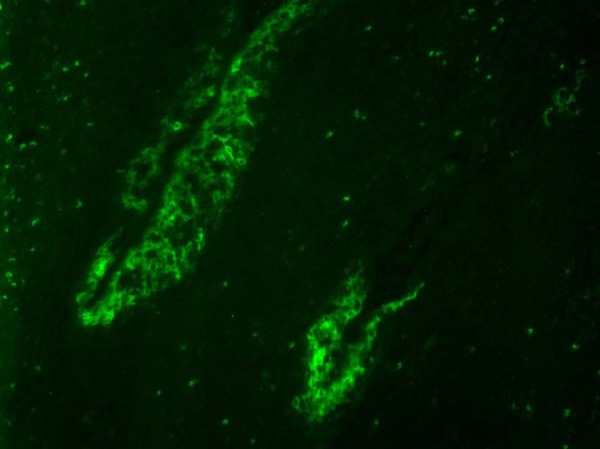
Fluorescent antibody assay. Pig No. 3. Specific antigen was is clearly detected in the crypt epithelial cells in the tonsil at 14 dpi.
Pathological findings
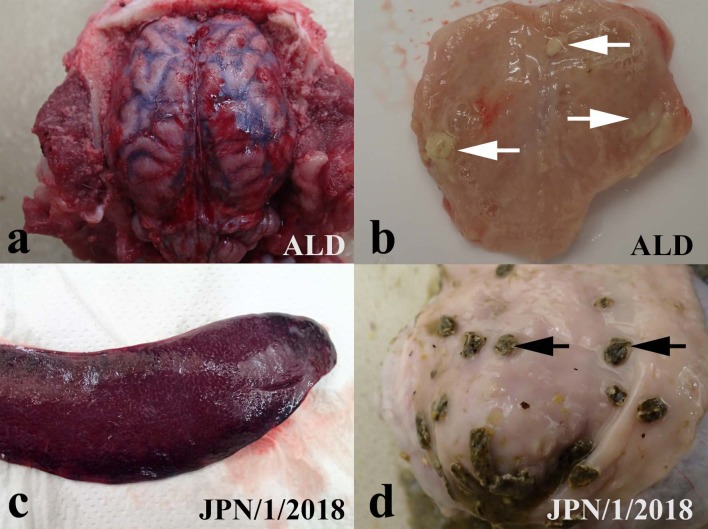
The PSs of the infected and contact pigs in Groups 1 and 2 (0–10) were lower than those in Group 3 (8–13) (Table 3). Among the pigs of Group 3, three of four pigs showed hyperemia in the brain, congestion and whitish clouding of the meninges or swollen of the meningeal blood vessels (Fig. 4a). In contrast, only one out of eight pigs in Groups 1 and 2 showed swelling of the meningeal blood vessels in the brain. Further, all four pigs in Group 3 showed multifocal necrosis and formation of small abscesses in the tonsil (Fig. 4b), while no significant lesions were observed in the tonsil of any pigs in Groups 1 and 2. Petechial hemorrhages in the kidney of the pigs in Group 3 appeared more severer than those of pigs in Groups 1 and 2. There was no significant differences in the pathological findings in the spleen, lymph nodes, intestine or skin between the pigs in Group 3 and those in Groups 1 and 2 (Table 3). Multifocal infarction of the margin of spleen and hemorrhagic lymph nodes were observed in four in eight pigs of Groups 1 and 2 (Fig. 4c) and three out of four pigs in Group 3. Button ulcers in the colon were found in two infected pigs in Groups 1 and 2 (Nos. 1 and 6) (Fig. 4d) and in one pig in Group 3 (No. 12). There was no pathological finding in the respiratory organs of any pig.
Table 3. Pathological scores of experimental pigs.
| Group | Group 1 (JPN/1/2018 intraoral) |
Group 2 (JPN/1/2018 intramuscularly) |
Group 3 (ALD intramuscularly) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Administrated |
Contact |
Inoculated |
Contact |
Inoculated |
Contact |
|||||||
| Pig | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 | No. 12 |
| Skin | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 2 | 1 |
| Subcutis and serosae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tonsil | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 3 | 3 |
| Spleen | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 1 |
| Kidney | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Lymph nodes | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 0 |
| Intestine from ileum to rectum | 3 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 3 |
| Brain | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 3 |
| Respiratory system | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total points | 10 | 6 | 6 | 1 | 1 | 5 | 0 | 4 | 8 | 13 | 11 | 11 |
Fig. 4.
Pathological findings in pigs of Groups 1–3. (a) Congestion and whitish clouding of the meninges in pig No. 10 at 5 dpi. (b) Formation of multiple abscesses (white arrows) in the tonsil in pigs No. 9 at 5 dpi. (c) Multifocal hemorrhagic infarction of the margin of the spleen in pig No. 2 at 14 dpi. (d) Button ulcers (black arrows) in the colon in pig No. 1 at 14 dpi.
DISCUSSION
In this study, vRNAs of JPN/1/2018 and ALD strains were detected from clinical samples of all infected pigs (Table 2), and anti-CSFV antibodies were detected in some pigs on day 14–15 (data not shown). We confirmed that JPN/1/2018 infection of pigs via both oral and intramuscular routes and transmitted horizontally to contact pigs in a similar manner to the virulent ALD strain. The clinical symptoms of JPN/1/2018 infected pigs were only pyrexia, hypodynamia, anorexia and conjunctivitis only. These were milder than the symptoms observed in ALD infected pigs, and no pigs infected with JPN/1/2018 died or were euthanized within the 2-week experimental period. Two of five pigs infected experimentally with a subgenotype 2.1 CSFV isolated in Mongolia did not show any clinical signs during an experimental period of 2 weeks [3]. In addition, all pigs were alive during the experimental period. Authors of the experiment judged that pathogenicity of the subgenotype 2.1 CSFV is moderately virulent by comparing the pathogenicity with those of highly virulent and vaccine strains. Clinical signs in this study were similar with those in their study; therefore, pathogenicity of JPN/1/2018 strain is also moderately virulent.
To assess the pathogenicity, we scored the clinical and pathological symptoms [4, 12]. ALD infected pigs showed high PCs due to necrosis in the tonsil tissue and vascular swelling in the brain. Estimation of CSs in this study was simpler than the original method. Scores of the JPN/1/2018 infected pigs were markedly lower than those of the ALD infected animals. These results suggest that the JPN/1/2018 strain is less virulent than ALD. Meanwhile, there was no significant difference in the period of transmission from infected to contact pigs between the ALD and JPN/1/2018 strains (Table 2), suggesting similar transmissibility of both virus strains. Analysis of the pathogenicity of a subgenotype 2.1 CSFV isolated in China showed that transmissibility of the subgenotype 2.1 CSFV was expected to be significantly lower than that of a high virulent strain because virus excretion in pigs inoculated with the subgenotype 2.1 CSFV was significantly delayed than that in pigs inoculated with the high virulent strain [11]. Results in the previous and our studies cannot compare directly because virus strains, infectious doses and ages of pigs were different in both the studies; however, it is interesting that transmissibility of JPN/1/2018 and ALD strain is similar in this study although pathogenicity of JPN/1/2018 strain was apparently milder than that of ALD strain. In contrast, although we confirmed the presence of characteristic multifocal infarction of the margin of the spleen after oral and intramuscular inoculation with JPN/1/2018, these lesions were not observed in all infected pigs and it was difficult to diagnose CSF only by antemortem inspection because multifocal infarction of spleen are not always confirmed in all CSF-affected pigs [7]. Therefore, in addition to the antemortem inspection, the FA test needs to be performed using tonsil samples collected from suspected pigs that are affected CSF. In contrast, there were several cases that apparent clinical signs were not confirmed in recent CSF cases in Japan [personal communication). Taken together, these findings suggest that in addition to clinical and antemortem inspections including the FA test, molecular and serological laboratory tests should be performed to accurately determine the presence of infection with JPN/1/2018.
Loforban et al. previously described a difference in infectivity between intramuscular and intranasal infections of CSFV [8]. Pigs intramuscularly inoculated with CSFV showed typical lesions while those that were intranasally inoculated did not. In contrast, the pathological findings in JPN/1/2018 orally- and intramuscularly-inoculated pigs in the present study were comparable. The reason of this discrepancy between the previous and our present study is unclear. However, the fact that we conducted oral administration by directly dropping CSFV onto the tonsil may be a contributing factor. Previous studies have reported that epithelial cells in the tonsillar crypt and mononuclear cells that accumulate to tonsils are sensitive to CSFVs [5, 6]. Therefore, direct administration of the CSFV onto the tonsils presumably enhances primary virus propagation at the site of infection and affects aggravations in orally infected pigs.
In conclusion, the results of this study show that the pigs infected with the JPN/1/2018 strain shed viruses into clinical samples, especially whole blood samples, before the appearance of high fever, until at least approximately 2 weeks after infection. The results are expected to be of significant value in establishing appropriate countermeasures and diagnostic strategies for the JPN/1/2018 strain.
Acknowledgments
The author would like to thank Mr. Takayoshi Shiratori (Yamagata Prefectural Shonai Animal Hygiene Service Center, Yamagata), Mr. Shunjiro Koizumi (Saitama Prefectural Central District Animal Hygiene Service Center, Saitama) and Mr. Kosuke Ohashi (Osaka Prefectural Animal Hygiene Service Center) for technical assistance. We also thank Mr. Yuuki Takahashi, Mr. Masayuki Kanda, Mr. Kenichi Ishii, Mr. Tatsuo Nakamura, Mr. Masami Wada and Mr. Shigeo Mizumura for their care of the animals.
REFERENCES
- 1.Beer M., Goller K. V., Staubach C., Blome S.2015. Genetic variability and distribution of Classical swine fever virus. Anim. Health Res. Rev. 16: 33–39. doi: 10.1017/S1466252315000109 [DOI] [PubMed] [Google Scholar]
- 2.Edwards S., Fukusho A., Lefèvre P. C., Lipowski A., Pejsak Z., Roehe P., Westergaard J.2000. Classical swine fever: the global situation. Vet. Microbiol. 73: 103–119. doi: 10.1016/S0378-1135(00)00138-3 [DOI] [PubMed] [Google Scholar]
- 3.Enkhbold B., Shatar M., Wakamori S., Tamura T., Hiono T., Matsuno K., Okamatsu M., Umemura T., Damdinjav B., Sakoda Y.2017. Genetic and virulence characterization of classical swine fever viruses isolated in Mongolia from 2007 to 2015. Virus Genes 53: 418–425. doi: 10.1007/s11262-017-1442-2 [DOI] [PubMed] [Google Scholar]
- 4.Floegel-Niesmann G., Bunzenthal C., Fischer S., Moennig V.2003. Virulence of recent and former classical swine fever virus isolates evaluated by their clinical and pathological signs. J. Vet. Med. B Infect. Dis. Vet. Public Health 50: 214–220. doi: 10.1046/j.1439-0450.2003.00663.x [DOI] [PubMed] [Google Scholar]
- 5.Gisler A. C., Nardi N. B., Nonnig R. B., Oliveira L. G., Roehe P. M.1999. Classical swine fever virus in plasma and peripheral blood mononuclear cells of acutely infected swine. Zentralbl. Veterinarmed. B. 46: 585–593. [DOI] [PubMed] [Google Scholar]
- 6.Horter D. C., Yoon K. J., Zimmerman J. J.2003. A review of porcine tonsils in immunity and disease. Anim. Health Res. Rev. 4: 143–155. doi: 10.1079/AHRR200358 [DOI] [PubMed] [Google Scholar]
- 7.Kirkland P. D., Le Potier M. F., Vannier P., Finlaison D.2012. Pestiviruses. pp. 538–553. In: Diseases of swine, 10th ed. (Zimmerman, J. J., Karriker, L. A., Ramirez, A., Schwartz, K. J. and Stevenson, G. W. eds.), John Wiley & Sons, West Sussex. [Google Scholar]
- 8.Leforban Y., Cariolet R.1992. Characterization and pathogenicity for pigs of a hog cholera virus strain isolated from wild boars. Ann. Rech. Vet. 23: 93–100. [PubMed] [Google Scholar]
- 9.Lowings P., Ibata G., Needham J., Paton D.1996. Classical swine fever virus diversity and evolution. J. Gen. Virol. 77: 1311–1321. doi: 10.1099/0022-1317-77-6-1311 [DOI] [PubMed] [Google Scholar]
- 10.Luo Y., Li S., Sun Y., Qiu H. J.2014. Classical swine fever in China: a minireview. Vet. Microbiol. 172: 1–6. doi: 10.1016/j.vetmic.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 11.Luo Y., Ji S., Liu Y., Lei J. L., Xia S. L., Wang Y., Du M. L., Shao L., Meng X. Y., Zhou M., Sun Y., Qiu H. J.2017. Isolation and characterization of a moderately virulent classical swine fever virus emerging in China. Transbound. Emerg. Dis. 64: 1848–1857. doi: 10.1111/tbed.12581 [DOI] [PubMed] [Google Scholar]
- 12.Mittelholzer C., Moser C., Tratschin J. D., Hofmann M. A.2000. Analysis of classical swine fever virus replication kinetics allows differentiation of highly virulent from avirulent strains. Vet. Microbiol. 74: 293–308. doi: 10.1016/S0378-1135(00)00195-4 [DOI] [PubMed] [Google Scholar]
- 13.Nishi T., Kameyama K. I., Kato T., Fukai K.2019. Genome sequence of a classical swine fever virus of subgenotype 2.1 isolated from a pig in Japan in 2018. Microbiol Resour Announc 8: e01362–e18. doi: 10.1128/MRA.01362-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paton D. J., McGoldrick A., Greiser-Wilke I., Parchariyanon S., Song J. Y., Liou P. P., Stadejek T., Lowings J. P., Björklund H., Belák S.2000. Genetic typing of classical swine fever virus. Vet. Microbiol. 73: 137–157. doi: 10.1016/S0378-1135(00)00141-3 [DOI] [PubMed] [Google Scholar]
- 15.Postel A., Nishi T., Kameyama K. I., Meyer D., Suckstorff O., Fukai K., Becher P.2019. Reemergence of classical swine fever, Japan, 2018. Emerg. Infect. Dis. 25: 1228–1231. doi: 10.3201/eid2506.181578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakoda Y., Wakamoto H., Tamura T., Nomura T., Naito M., Aoki H., Morita H., Kida H., Fukusho A.2012. Development and evaluation of indirect enzyme-linked immunosorbent assay for a screening test to detect antibodies against classical swine fever virus. Jpn. J. Vet. Res. 60: 85–94. [PubMed] [Google Scholar]
- 17.Sandvik T., Drew T., Paton D.2000. CSF virus in East Anglia: where from? Vet. Rec. 147: 251. [PubMed] [Google Scholar]
- 18.Shimizu Y., Furuuchi S., Kumagai T., Sasahara J.1970. A mutant of hog cholera virus inducing interference in swine testicle cell cultures. Am. J. Vet. Res. 31: 1787–1794. [PubMed] [Google Scholar]
- 19.Terpstra C., de Smit A. J.2000. The 1997/1998 epizootic of swine fever in the Netherlands: control strategies under a non-vaccination regimen. Vet. Microbiol. 77: 3–15. doi: 10.1016/S0378-1135(00)00252-2 [DOI] [PubMed] [Google Scholar]
- 20.Vilcek S., Herring A. J., Herring J. A., Nettleton P. F., Lowings J. P., Paton D. J.1994. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. 136: 309–323. doi: 10.1007/BF01321060 [DOI] [PubMed] [Google Scholar]
- 21.World Organization for Animal Health. 2018. Chapter 1.1.4. Biosafety and biosecurity: Standard for managing biological risk in the veterinary laboratory and animal facilities, http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/1.01.04_BIOSAFETY_BIOSECURITY.pdf [accessed on February 27, 2019].