Abstract
The toxicity caused by different organisms in septic shock is substantially complex and characterized by an intricate pathogenicity that involves several systems and pathways. Immune cells' pattern recognition receptors initiate the host response to pathogens after the recognition of pathogen-associated molecular patterns. In essence, the subsequent activation of downstream pathways may progress to infection resolution or to a dysregulated host response that represents the hallmark of organ injury in septic shock. Likewise, the management of organism toxicity in septic shock is complicated and comprises a multiplicity of suitable targets. In this review, the classic immune responses to pathogens are discussed as well as other factors that are relevant in the pathogenicity of septic shock, including sepsis-induced immune suppression, inflammasome activation, intestinal permeability, and the role of lipids and proprotein convertase subtilisin/kexin type 9. Current therapies aiming to eliminate the organisms causing septic shock, recent and ongoing trials in septic shock treatment, and potential new therapeutic strategies are also explored.
Keywords: Sepsis, Septic shock, Mediators, PCSK9, Therapies
Introduction
Any infection (bacterial, fungal, viral, parasitic) can potentially progress to sepsis and septic shock. Although different pathogens share several characteristics, there are also distinct pathogenicities and toxicities, i.e., that are specifically associated with each unique organism. This review will focus on the pathogenicity and toxicity associated with bacterial infections during sepsis as well as current management and potential new therapeutic targets. We discuss classic immune responses in sepsis, and add exciting recent information about the natural processes for endotoxin clearance during sepsis that could provide novel therapeutic strategies to complement management with antibiotics.
Organism Toxicity in Septic Shock
Pathogenicity
The innate immune system is an evolutionarily conserved system, acting as a first line of defense against exogenous and endogenous threats to the host, such as infections (Fig. 1). It includes diverse cells such as macrophages, dendritic cells, neutrophils, natural killer cells, and innate lymphoid cells. The destruction and clearance of invading pathogens and the resolution of other threats to the host require complex coordination of multiple innate immune pathways [1].
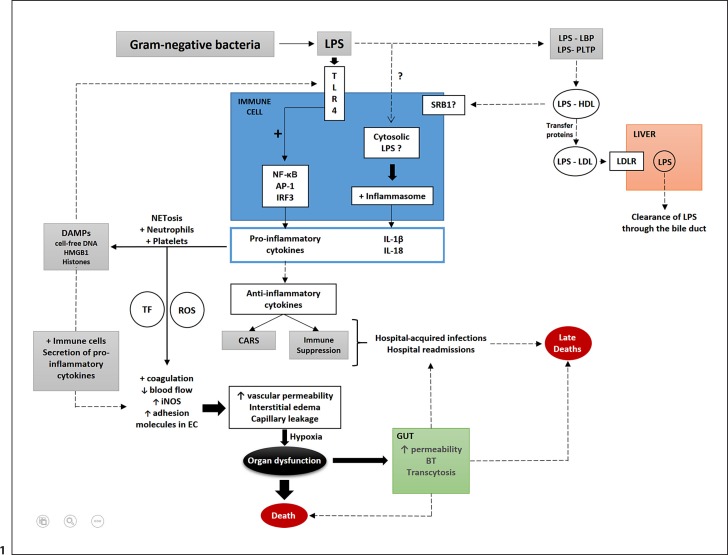
Fig. 1.
Schematic representation of important factors involved in the pathogenicity of septic shock caused by LPS from gram-negative bacteria. LPS from gram-negative bacteria are recognized by TLR4 on immune cells and activate the transcription of genes encoding the proinflammatory cytokines (IL-6, TNF, IFNα, and IL-8) released in the circulation. LPS may reach the cytosol of immune cells (likely mediated by an unknown endocytic pathway). LPS is then recognized by inflammatory caspases that activate the inflammasome, resulting in the release of mature IL-1β and IL-18. Proinflammatory cytokines activate neutrophils, platelets, and NET formation that generate DAMPs (histones, HMGB1, and cell-free DNA). DAMPs are recognized by TLR4 that further stimulates a proinflammatory response. Activated platelets and neutrophils lead to increases in TF and ROS that contribute to changes in endothelial cells (coagulation activation with clot formation, decreases in blood flow, and the upregulation of iNOS, ICAM, and VCAM-1 in endothelial cells. There is also upregulation of adhesion molecules in leukocytes (selectins and integrins). DAMPs also contribute to these effects on endothelial cells by the activation of immune cells, and further release of proinflammatory cytokines. Increased vascular permeability, interstitial edema, and capillary leakage cause tissue hypoxia that can progress to organ dysfunction and death. Intestinal dysfunction occurs mostly by the impairment of intestinal tight junctions and apoptosis, which lead to increases in intestinal permeability, bacterial translocation, and transcytosis. Anti-inflammatory cytokines that are also released into the circulation during sepsis may cause CARS and/or immune suppression. Both, in conjunction with the intestinal alterations in sepsis, contribute to increased rates of secondary and opportunistic hospital-acquired infections and hospital readmissions, factors associated with sepsis-associated late deaths. Alternatively, LPS can be sequestered within lipoprotein fractions in a process mediated by lipid transfer proteins such as LBP and PLTP. Initially, LPS is transferred to HDL and then from HDL to other lipoprotein fractions, such as LDL (again mediated by lipid transfer proteins such as LBP, PLTP, CETP, and possibly BPI). LPS within LDL is taken up by the LDLR and gets cleared from the body through the bile duct. Major pathways (black arrows); secondary pathways (dotted arrows); activation (+); increases (↑); decreases (↓). TLR, toll-like receptor; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; AP-1, activator protein 1; IRF3, interferon regulatory transcription factor 3; IL, interleukin; NET, neutrophil extracellular trap; DAMPs, damage-associated molecular patterns; HMGB1, high-mobility group box 1; TF, tissue factor; ROS, reactive oxygen species; EC, endothelial cell; iNOS, inducible nitric oxide synthase; CARS, compensatory anti-inflammatory response syndrome; BT, bacterial translocation; SRB1, scavenger receptor class B type 1; LBP, lipopolysaccharide binding protein; PLTP, phospholipid binding protein; CETP, cholesteryl-ester transfer protein; BPI, bactericidal/permeability increasing protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDLR, LDL receptor; ICAM, intercellular adhesion molecule; VCAM-1, vascular cell adhesion molecule.
The host response to a bacterial infection occurs initially by the recognition of pathogen-associated molecular patterns (PAMPs) by pathogen sensors known as pattern recognition receptors (PRRs) on immune cells. The leading families of PRRs are Toll-like receptors (TLRs), C-type lectin (CLEC)-like receptors, retinoic acid-inducible gene 1 (RIG-I)-like receptors, nucleotide-binding oligomerization domain (NOD)-like receptors, cytosolic DNA sensors, and inflammasomes. The interplay between these recognition molecule families ensures the efficient coordination of innate immune responses, through either synergistic or cooperative signaling [2]. Although pathogenicity of sepsis/septic shock involves specific (and complex) pathways, the inflammatory response to sepsis varies according to the specific organism, organism load, host genotype, underlying host conditions (especially immunodepression), and immunosuppressing drugs.
Toll-Like Receptors
TLRs are transmembrane receptors that specifically recognize PAMPs present in gram-positive (lipoteichoic acid, LTA), gram-negative (lipopolysaccharide, LPS) bacteria and viruses. They are located at the cellular membrane (TLR1, TLR2, TLR4, TLR5, TLR6) and in intracellular membranes (TLR3, TLR7, TLR8, TLR9), recognizing both PAMPs at the cell surface or in the cytosol, respectively [3]. Based on their primary sequences, TLRs can be further divided into several subfamilies, each one recognizing related PAMPs. Lipoproteins or lipopeptides in gram-positive bacteria are recognized by TLR2 complexed with TLR1 or TLR6, viral double-stranded RNAs by TLR3, LPS by the TLR4/MD2 complex, bacterial flagellins by TLR5, viral and bacterial single-stranded RNAs by TLR7 or TLR8, and CpG-rich undermethylated DNAs by TLR9. The activation of most TLRs by ligand binding results in dimerization, conformational changes and downstream signaling involving receptor/adaptor complexes that culminate with the translocation of nuclear factor (NF)-κB and/or interferon regulatory factor (IRF) 3 to the nucleus, which is followed by proinflammatory cytokine and type I interferon (IFN) transcription, respectively [3]. Importantly, the prompt production of proinflammatory mediators is accompanied by the production and release of anti-inflammatory mediators too [4]. Thus, the initial proinflammatory response is accompanied by increasing immunosuppression (see below).
NOD-Like Receptors
NOD-like receptors (NLRs) are special types of PRRs that sense cytosolic microbial and danger components. After the recognition of peptidoglycans on bacterial cell walls, NOD1 and NOD2 trigger inflammatory signaling, similar to the production of proinflammatory cytokines by TLRs, via the activation of NF-κB and activator protein 1 (AP-1). Inflammasome-associated NLRs such as NLRP1 and NLRP3 activate the inflammasome complex that is triggered by their recognition of PAMPs and DAMPs [5]. The inflammasome creates an unique inflammatory response, resulting in the maturation and release of IL-1β and IL-18.
RIG-I-Like Receptors
RIG-I-like receptors (RLRs) are a family of cytoplasmic RNA helicases that recognize viral RNA genomes, replication intermediates, and/or transcription products. RLRs include the RNA sensors RIG-I and melanoma differentiation-associated protein (MDA)-5, which, upon activation, induce transcription factors that control the transcription of genes encoding interferons and other cytokines. The cytosolic protein ATP-dependent helicase LGP2, which contains an RNA-binding domain, acts as a negative feedback regulator of both RIG-I and MDA-5 [6].
CLEC-Like Receptors
CLEC-like receptors (CLRs) encompass a large family of proteins characterized by the presence of at least one CLEC-like domain. They are involved mostly in fungal recognition and in the modulation of the innate immune mechanisms for pathogen clearance or antigen presentation to T lymphocytes. The principal human CLRs are the surface receptors dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN), Dectin-1, Dectin-2 and Mincle, and the soluble receptor mannose-binding lectin (MBL) [7].
The Host Response and the Development of Organ Dysfunction in Sepsis
Sepsis and septic shock result in both the widespread activation and the impairment of the innate immune system, characterized by disturbances in the fine balance between pro- and anti-inflammatory, and coagulation and anticoagulation responses [8]. Excessive inflammation is initiated by the production and release of proinflammatory cytokines mediated by PRR signaling (discussed earlier), followed by a crosstalk with the vascular endothelium and the coagulation systems [9].
Endothelial cells are constantly exposed to pathogens and endotoxins, so it is not surprising that endothelial injury is nearly universal in sepsis. Consequences of endothelial dysfunction and injury include blood flow changes (decreased flow and nonlaminar flow), vascular leakage, and coagulation activation. Cytokines and reactive oxygen species (ROS) released by activated immune cells such as neutrophils induce a global increase in the production of nitric oxide (NO) mediated by the expression of inducible NO synthase (iNOS) [10]. The heterogeneity of iNOS expression by endothelial cells contributes to a variety of alterations in blood flow (decreased in some vascular beds and increased in others) typical of sepsis. There is a concurrent presence of capillaries with increased flow, normal flow, intermittent flow, or even stopped flow [11].
Endothelial barrier disruption causes increased vascular permeability and leakage, the major phenotype of endothelial dysfunction [12]. The cytokine-induced upregulation of adhesion molecules on the endothelium (intracellular adhesion molecule [ICAM] and vascular cell adhesion molecule 1 [VCAM-1]) and neutrophils (selectins) promotes the adhesion and diapedesis of neutrophils and the consequent release of proteases and ROS [13]. These substances, in association with the effects of iNOS (the loss of inhibitory effects on platelet and neutrophil activation, mediated by endothelial NOS) contribute to denudation of glycocalyx, further exposure of adhesion molecules, and impairment in endothelial barrier function [12].
The intermittent or stopped blood flow induced by both excessive NO and coagulation activation and the interstitial edema and changes in organ architecture caused by vascular leakage lead to difficulties in the diffusion of metabolites and oxygen to parenchymal cells, causing hypoxia and hence organ dysfunction [14].
Immune Suppression of Sepsis
Septic shock can initiate an immunosuppressed state that can exceed the proinflammatory response, and this often starts about 3–4 days after the onset of septic shock [15]. This immunosuppressed state is characterized by the production of inhibitory cytokines such as interleukin (IL)-10 and IL-4, which limit the intensity of immune cell activation and negatively modulate inflammation. This immunosuppressed phase can progress to an upsurge in the release of anti-inflammatory cytokines that can progress to a state of quite profound immune suppression, the so-called compensatory anti-inflammatory response syndrome. This syndrome contributes to the increased risk of secondary and opportunistic infections, and late deaths associated with sepsis and septic shock [8].
Sepsis-induced immune suppression is mediated by lymphocyte exhaustion, increased rates of B and T cell apoptosis (mediated by programmed death-1/programmed death ligand-1 [PD1/PDL1] signaling), [16] and immunoparalysis (or endotoxin tolerance) [17]. The latter is characterized by decreased expression of human leukocyte antigen type 2 (HLA-DR) on monocytes and a reduction in the production of cytokines by macrophages and monocytes upon inflammatory stimulus [17]. Other late features of septic shock are the expansion of regulatory T cells [18] and myeloid suppressor cells [17], Th2 cell polarization, and reprogramming of macrophages to an M2 phenotype, all of which contribute to decreasing inflammation and impaired phagocytosis [17].
Other Factors Involved in the Pathogenicity of Sepsis and Septic Shock
Lipoproteins and Proprotein Convertase Subtilisin/Kexin Type 9
LPS in gram-negative bacteria and LTA in gram-positive bacteria are key lipid moieties of bacterial cell walls that stimulate the immune system. These pathogen lipids (PLs) can enter injury pathways or enter clearance pathways. PLs are recognized by TLRs as PAMPs, and promote a proinflammatory response, or can be sequestered within any lipoprotein particles present, e.g., high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and chylomicrons [19, 20]. When released into the circulation, PLs are initially bound to transfer proteins, notably LPS-binding protein (LBP) and phospholipid-transfer protein (PLTP), and then incorporated into HDL particles [21]. Next, in a process also mediated by transfer proteins (including LBP, PLTP, and cholesteryl ester transfer protein [CETP]), PLs are transferred to ApoB-containing lipoproteins (LDL, VLDL, and chylomicrons). PLs within lipoproteins are then cleared from the circulation by the liver (Fig. 2). Hepatic clearance of LPS involves primarily the LDL receptor (LDLR) and possibly other receptors such as scavenger receptor class B type 1 (SRB1). After uptake by hepatocytes, PLs, mostly within LDL particles, are then excreted into the bile [22]. Recent evidence suggests that PL clearance can be modulated and that proprotein convertase subtilisin/kexin type 9 (PCSK9) is one of the key players in this process. PCSK9 targets the LDLR on hepatocytes for lysosomal degradation within cells, preventing LDLR recycling, and thereby decreasing LDLR concentrations and also the clearance of LPS. PCSK9 is thus a “bad guy.” In contrast, reduced PCSK9 activity increases LDLR concentration on the surface of hepatocytes and also the clearance of PLs transported in LDL by the liver [22]. Accordingly (discussed below in detail), PCSK9 inhibition may be a novel strategy to increase PL clearance and thus complement the established efficacy of early antibiotics in sepsis.
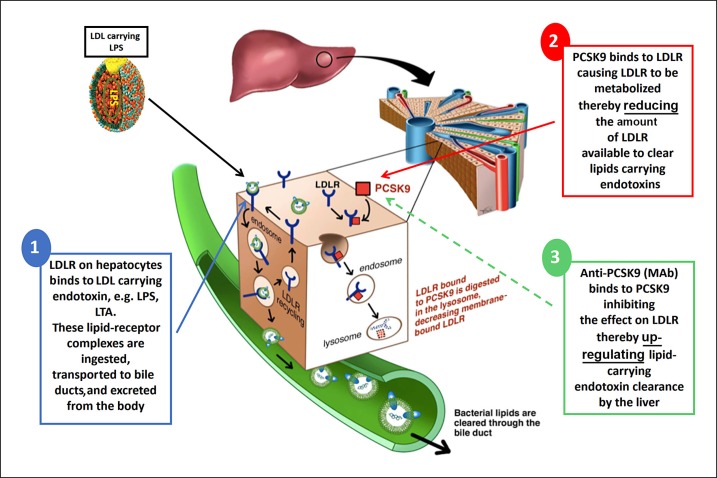
Fig. 2.
LPS clearance in the human host. (1) LPS from gram-negative bacteria carried within LDL particles binds to the LDLR on the surface of liver cells. The LPS-LDLR complex is then internalized, and while LDLR is recycled to the cell surface, LPS is cleared from the circulation through the bile duct. (2) When present, PCSK9 binds to LDLR on the cell surface and targets its lysosomal degradation. Therefore PCSK9, by preventing LDLR recycling, reduces the amount of LDLR available to clear LPS carried within LDL particles. (3) Anti-PCSK9 therapy (MAb) binds to PCSK9 and inhibits PCSK9-induced degradation of LDLR, upregulating the hepatic clearance of LPS carried within LDL. Adapted from [22]. LPS, lipopolysaccharide; LDL, low-density lipoprotein; LDLR, LDL receptor; PCSK9, proprotein convertase subtilisin/kexin type 9; Ab, monoclonal antibody.
Inflammasomes
PAMPs and DAMPs in sepsis activate NF-κB and thus promote the transcription of NLR3, the best characterized inflammasome. Cytosolic LPS is detected by inflammatory caspases (caspases 4 and 5 in humans and caspase-11 in mice), mediated by the specific binding of LPS (the lipid A portion) and the caspase activation and recruitment domain of caspases. Oligomerization and activation of caspases (4, 5, or 11) activate inflammasomes, causing the release of mature IL-1β and IL-18 (by cleaving the inactive precursors pro-IL-1β and pro-IL-18 in the cytosol). Moreover, excessive concentrations of LPS can cause hyperactivation of the caspase 4, 5, and 11 pathways, the induction of uncontrolled inflammation, and septic shock [23].
Intestinal Permeability, Bacterial Translocation, and Intestinal Epithelial Apoptosis
Gut integrity is compromised in critical illness such as sepsis, with increases in intestinal cell apoptosis and permeability. The major functions of the intestinal epithelium (nutrient absorption and barrier) are mediated by cell-cell intramembrane protein interactions within tight junctions [24]. Reduced perfusion and impaired oxygen delivery to the gastrointestinal tract in sepsis increases intestinal permeability and bacterial translocation (the passage of viable bacteria or their products from the intestinal lumen to extraintestinal sites) [24]. Apoptosis of intestinal cells by the host immune response in sepsis contributes to impaired gut integrity and increased intestinal permeability. In addition, transcellular transport of bacteria and endotoxin can occur through lipid rafts (membrane microdomains activated by receptor molecules such as CD14 and TLR4), permitting the passage of bacteria and endotoxins without disrupting the tight junctions. Pathogens can therefore migrate across the intestinal cell cytoplasm and exit from the opposite surface [25]. Sepsis-induced increased intestinal cell apoptosis, permeability, and bacterial transcytosis contribute to the spread of bacteria and endotoxins into the systemic circulation, increasing the risk of organ dysfunction.
Management of Organism and Organism Toxicity in Septic Shock
Antimicrobial Therapy
Early antibiotics increase survival from septic shock, and other aspects of septic shock management have improved, in particular better and more appropriate supportive therapy (e.g., ventilatory support, renal replacement therapy, nutrition, and limited transfusion and sedation). However, many clinical trials of drugs and devices that aimed to modulate the host immune response to sepsis have failed [26, 27].
Kumar et al. [28] were the first to describe the striking association of early antimicrobial administration (within the first hour of documented hypotension) with increased survival. In agreement with the Canadian study, Seymour et al. [29] showed, in a large retrospective study on 50,000 septic patients in the USA, that for every hour antimicrobial administration is delayed, the likelihood of in-hospital death increases by 4%. Delays of > 6 h were associated with hospital mortality rates of > 25%.
Antibiotics decrease the bacterial load in septic shock [30], so their early administration must be efficacious. Early antibiotics are commonly selected based empirically on: (1) infection characteristics such as the source of infection, the local organism resistance pattern, and community-acquired versus nosocomial infection, and (2) host factors such as age, concomitant chronic diseases, and immune status. Accordingly, empirical broad-spectrum antibiotics that cover all likely pathogens are recommended in the Surviving Sepsis Guidelines [31].
Among patients with septic shock caused by gram-negative bacteria, the incidence of extended-spectrum β-lactamase-producing bacteria has increased. Both β- lactam/β-lactamase inhibitors and carbapenems are currently used to treat these patients, but neither one was found to be superior in randomized clinical trials. In a meta-analysis, the efficacy of β-lactam/β-lactamase inhibitors and carbapenems appears equivalent in sepsis [32].
Other issues to consider are how to administer antimicrobials, the duration of treatment, and the use of procalcitonin-guided antibiotics. Continuous infusion and intermittent bolus dosing of β-lactams were compared in septic patients not receiving renal replacement therapy [33]. Patients on continuous-infusion antibiotics had higher clinical cure rates within 14 days after the cessation of antibiotics (primary outcome), greater pharmacokinetic/pharmacodynamic target attainment, and more ventilator-free days than the intermittent bolus group, but no difference in 30-day survival. In another trial, discontinuation of antibiotics based on a procalcitonin-guided protocol decreased the duration of antibiotic adminstration (7.5 vs. 9.3 days) and mortality at 28 days and 1 year, respectively, compared to usual care in critically ill patients with infection [34]. However, the patients on the procalcitonin-guided protocol had greater rates of re-infection than those who had their antibiotics discontinued according to usual care. Earlier discontinuation of antimicrobials might attenuate the disruption of the gut microbiome caused by the use of broad-spectrum antibiotics [35].
Antimicrobial Peptides
Antimicrobial peptides (AMPs) are a natural part of the host innate immune system [36], and have antimicrobial, immunostimulatory, and anti-inflammatory properties [37]. AMP levels are increased in septic shock [38]. AMPs include cathelicidin (LL-37) [39], bactericidal/permeability-increasing protein (BPI) [40], and lactoferrin [41]. An infectious stimulus triggers the release of AMPs from activated neutrophils, causing a proinflammatory response. However, anti-endotoxin activity directly associated with AMPs may lead to an anti-inflammatory response, mediated by a decrease in the activation of immune cells [42]. Some AMPs have shown positive correlations with procalcitonin (e.g., HDB-2) [43] or white blood cell count (e.g., lactoferrin) [44], while BPI has been associated with severe organ dysfunction in sepsis [45].
AMPs have been studied in sepsis and septic shock [46, 47]. Talactoferrin-α, a recombinant human form of lactoferrin, was associated with a trend towards a decreased 28-day mortality rate, especially in patients with APACHE II (Acute Physiology and Chronic Health Evaluation II) scores of > 25 in a phase II trial [46]. However, it was negative in a subsequent phase II/III trial that was prematurely terminated (NCT 01273779). Recently, MD54 (an MD2-derivative peptide that encompasses a sequence from human MD2 that is involved with hydrophobic and electrostatic interactions with LPS) was investigated for its effects on LPS binding and neutralization, and also on anti-inflammation, and showed that MD54 interacts with LPS, promotes its aggregation and inhibits the LPS-induced nuclear translocation of NF-κB in a human macrophage cell line. MD54 was also associated with inhibition of IL-6 and TNF-α production and improved survival in a murine LPS injection model [47].
Anti-Cytokine Trials in Sepsis
Numerous trials of anti-cytokines have been done and all were negative. One interpretation that aligns with the efficacy of early antibiotics is that inhibiting the natural defense against the infecting organism is not effective. We suggest that these results, i.e., the efficacy of early antibiotics and the failure of host response inhibition to fight infection, emphasize the need for more research into agents that inhibit the organism and organism endotoxins.
Recently, a recombinant IL-1 receptor antagonist (anakinra) was associated with improved 28-day survival rates in a reanalysis of its original phase III trial in a subgroup of patients with severe sepsis associated with macrophage activation syndrome. The 28-day survival rate in patients who received anakinra was 65.4% versus 35.3% receiving placebo [48].
Anti-Endotoxin Therapies
Therapies aiming to neutralize or remove endotoxins evaluated over the last 20 years include agents that block LPS binding to TRL4 (eritoran) [49] and monoclonal antibodies against endotoxins (monoclonal antibody E5) [50]. Studies analyzing these drugs did not demonstrate a survival benefit (28-day mortality rate for eritoran and 14-day mortality rate for E5 when compared to placebo) in patients with severe sepsis.
The removal of endotoxins and/or cytokines using blood purification techniques is an attractive option in sepsis and septic shock. Polymixin B hemoperfusion was associated with improvements in hemodynamics and organ dysfunction in patients with intra-abdominal sepsis in a nonblinded trial [51]. However, the EUPHRATES (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized Controlled Trial of Adults Treated for Endotoxemia and Septic Shock) study, which included patients with all sources of septic shock was essentially negative; there was no difference in 28-day survival between patients receiving polymyxin B hemoperfusion and those under usual care [52].
There are at least 2 ongoing trials analyzing other blood purification techniques in sepsis. The first is evaluating the effects on the inflammatory response of cytokine absorption (CytoSorb®) in patients with septic shock (NCT 02288975), and the second (in the preclinical phase) is investigating the use of magnetic nanobeads coated with an engineered human MBL, opsonin, for the removal of pathogens and toxins [53].
Modulating the Endotoxin Clearance Cascade
The human host has developed a natural system for endotoxin removal that we call the “endotoxin clearance cascade.” This endotoxin clearance cascade can be modulated to increase natural endotoxin removal in sepsis.
Endotoxins are bound to HDL, LDL, and VLDL in the circulation via the actions of cholesterol transport proteins. Scavenger receptor class B type 1-mediated hepatic elimination and detoxification clears the LPS that is sequestered in HDL. Hepatic uptake of pathogen lipids (e.g., LPS and LTA) bound to LDL occurs by hepatocyte clearance through the LDLR, and ultimately to bile excretion [54]. PCSK9 degrades the LDLR, thereby decreasing LDLR-mediated LPS removal [55]. So PCSK9 inhibition could be an effective strategy to increase endotoxin removal, by increasing hepatocyte LDLR expression and the subsequent hepatocyte clearance of LPS and other toxic bacterial lipids carried within LDL. In a murine model of sepsis, the treatment with a PCSK9 inhibitor 6 h after cecal ligation and puncture improved survival compared with placebo. Furthermore, in humans, PCSK9 loss of function genotype was associated with increased 28-day survival (compared with the wild-type genotype) in 2 cohorts of patients with septic shock [56]. In an animal model of sepsis, PCSK9 knockout mice subjected to cecal ligation and puncture had lower bacterial concentrations in the blood, lungs, and peritoneal fluid than wild-type controls; PCSK9-overexpressing mice had higher bacterial concentrations in the blood, lungs, and peritoneal fluid [57]. Thus, the inhibition of PCSK9 has several potentially beneficial effects in sepsis and septic shock: (1) it increases hepatic clearance of pathogen endotoxins by increasing LDLR expression, and (2) it decreases the bacterial load in the peritoneum and, remarkably, also at remote sites (blood and lung).
We suggest that modulation of the whole endotoxin clearance cascade to increase natural endotoxin removal in sepsis is a promising and exciting novel therapeutic strategy for managing sepsis and septic shock. This strategy would provide a broad-spectrum adjunct to all antibiotics in severe infection.
Anti-PCSK9 antibodies did not decrease mortality in a high-dose LPS model [58]. Further studies are needed in more appropriate live-organism and infection models of sepsis and septic shock.
Fecal Microbiota Transplantation
Sepsis changes the fecal, oral, lung, and skin microbiomes, i.e., so-called dysbiosis [59]. Overuse of antibiotics in the intensive care unit (ICU) is likely the most important factor associated with dysbiosis, and it contributes to the increased incidence of Clostridium difficile infection (CDI) and nosocomial infections [59]. Both CDI and nosocomial infections can progress to sepsis or be a consequence of sepsis treatment. The reestablishment of a healthy microbiome and the normal dominance of healthy bacteria, can be achieved by a fecal microbiota transplant (FMT), in which feces from a healthy donor are instilled into the patient's gastrointestinal tract. FMT reduces colitis in patients with CDI and might be another strategy to treat dysbiosis by restoring a healthy microbiome. A more in-depth understanding of the microbiome changes caused by sepsis is needed to define how to treat this condition [60].
Drugs Targeting Intestinal Epithelial Cell Barriers
The maintenance of gut barrier function by healthy intestinal epithelial cells is crucial for the host's defense and response to infection. A dysfunctional intestinal barrier causes microbiome alterations (dysbiosis) [60] that contribute to a sustained and systemic immune dysfunction in sepsis and septic shock [61].
Drugs associated with intestinal barrier protection, antiapoptotic properties, and/or protection against increases in intestinal permeability, such as IL-11 [62] and insulin-like growth factor (IGF) [63], have been analyzed in preclinical studies. Reduced levels of IGF were previously demonstrated in patients with sepsis [64]. Additionally, a decline in IGF1 level was inversely correlated with the degree of bacterial translocation across the gastrointestinal epithelium in patients with gram-positive sepsis [63].
Immunomodulatory Drugs
Sepsis-associated excessive inflammation and immune suppression are both potential therapeutic targets that have been evaluated in preclinical and clinical trials. Sepsis-associated immune suppression is characterized by lymphocyte exhaustion, increased apoptosis [16], and reduced expression of HLA-DR in monocytes [17]. Preclinical studies have shown associations between the therapeutic use of IL-7 and the induction of T and B cell growth and inhibition of lymphocyte apoptosis [65]. Another strategy that has been analyzed is the use of monoclonal antibodies against PD1 and its ligand PDL1. In vitro studies in lymphocytes from septic patients incubated with both antibodies demonstrated improvements in cell function and reductions in apoptosis [66]. A phase Ib/IIa study analyzing the use of PDL1 antibody in patients with sepsis has just been terminated due to “changes in business objectives,” and there are no results available so far (NCT 02576457). In a multicenter, double-blind, placebo-controlled randomized trial, the use of granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with sepsis-associated immune suppression was able to normalize HLA-DR expression in monocytes and restore monocyte cytokine production. GM-CSF use was also associated with nonsignificant decreases in the duration of mechanical ventilation, ICU and hospital length of stay, and improvements in APACHE II scores when compared to placebo [67]. In addition, mesenchymal stem cells have been associated with anti-inflammatory, immunomodulatory, antiapoptotic, and antimicrobial properties [68]. A phase I clinical trial in patients with infections who were admitted to the ICU is ongoing (NCT 02421484).
A Novel Strategy to Complement Antibiotics in Sepsis
Over 100 randomized controlled trials have tested the hypothesis that modulating the host response to infection improves survival; none of them resulted in new treatments. In contrast, early antibiotics have repeatedly been shown to decrease sepsis mortality. Sepsis is a risky field for drug development because it is extremely complex, being characterized by the heterogeneity of infecting organisms and of human hosts.
We selected 2 novel strategies in an effort to discover novel sepsis therapies. First, we focused on the early infectious phase of sepsis to develop adjuncts to antibiotics. Second, we inverted the usual drug discovery sequence (Fig. 3). The majority of previous drug trials used a classic research approach; researchers identified a mechanism implicated in sepsis pathophysiology in animal models and then proceeded to human clinical trials. Unfortunately, this strategy did not take into account the marked genetic heterogeneity of the human host response, and we believe this is why so many previous drug trials of sepsis have failed.
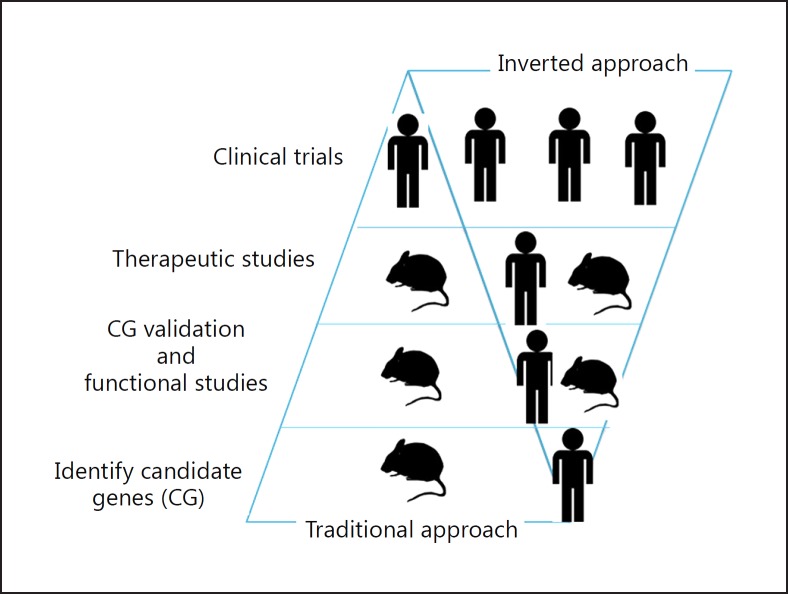
Fig. 3.
We invert the usual drug discovery sequence. We start with human 'omics for drug candidate discovery (instead of animal models), confirm mechanisms in human-cell sepsis models and clinically relevant murine sepsis models, and then make go/no-go decisions for potential validated targets for clinical trials.
Instead, we inverted (as shown by our PCSK9 discovery) the candidate target discovery sequence (Fig. 3). We started with human 'omics for drug candidate discovery (instead of animal models), confirmed the mechanisms in human-cell sepsis models and clinically relevant murine sepsis models, and then made go/no-go decisions for potential validated targets for clinical trials. We intentionally focused on the acute early infection stage of sepsis, not the later host inflammatory and coagulation response stage, because early administration (within 1 h) of antibiotics has been shown to be the most effective treatment for sepsis. However, because antibiotics do not remove bacterial endotoxins, we hope to discover novel validated targets that act as broad-spectrum adjuncts to all antibiotics in severe infections by enhancing the body's natural mechanisms for removing endotoxins.
Personalized Medicine for Sepsis
Sepsis is a complex syndrome, associated with enormous heterogeneity of both the causative pathogens and the human hosts. Importantly, responses to sepsis are highly variable, both among different patients and within the same patient over time [69]. All patients with sepsis and septic shock have a distinct immune status, comorbidities, pathogens, sources of infection, and genetic predisposition. Such heterogeneity explains, in part, why many clinical trials of sepsis have failed.
Personalized medicine for sepsis could mitigate its heterogeneity and increase the chances of attaining positive trials by using tailored therapies (i.e., use specific therapies to treat specific groups or endotypes of patients). Early organism detection in blood within a few hours by new real-time multiplex polymerase chain reaction tests [70], with its rapid and accurate identification of organisms can facilitate earlier specific antibiotic treatment. This strategy could replace the recommended initiation of empirical broad-spectrum antibiotics for all patients with sepsis.
Similarly, the use of PCSK9 inhibitors in patients with septic shock who carry a PCSK9 gain of function (GOF) genotype, allowing the use of specific therapies based on personal genotype information [56], is another example of personalized medicine. We took a candidate gene approach, studying the role of PCSK9 genetic variants during sepsis, because PCSK9 inhibitors were actually developed as a novel class of drugs for lowering cholesterol in atherosclerosis patients. PCSK9 impedes LPS clearance from the blood by decreasing LDLR density on hepatocytes (Fig. 2). We genotyped candidate single-nucleotide polymorphisms in 2 patient cohorts and discovered that septic patients with PCSK9 loss-of-function (LOF) genotypes have significantly increased survival and decreased inflammatory response [71]. Patients carrying a PCSK9 LOF allele had lower plasma cytokine concentrations than patients carrying a GOF allele.
We then showed that healthy humans with PCSK9 LOF, given a low-dose LPS infusion, have significantly reduced inflammatory responses, suggesting a mechanism of the survival benefit of the PCSK9 LOF. This benefit endures; PCSK9 LOF patients had significantly lower rates of readmission or death after sepsis [71]. In a clinically relevant murine model of sepsis, we showed that: (1) PCSK9 knockout mice and (2) mice treated 6 h after the induction of peritonitis with a PCSK9-blocking antibody had a higher survival rate and lower inflammatory responses [57]. The therapies discussed in this section are summarized in Table 1.
Table 1.
Therapies for sepsis and septic shock: aims and major findings
| Therapy aim | Drug/ therapy | Major findings in studies |
|---|---|---|
| Pathogen “killing” (bactericidal drugs) | Antibiotics Antimicrobial peptides (in association with antibiotics) |
Observation studies: early antibiotics increased survival in human septic shock [28, 29] Talactoferrin-α: trend to decreased 28-day mortality in phase 2 trial [46]; negative in phase 3 trial |
| Endotoxin removal | Polymyxin B hemoperfusion Cytosorb® Nanobeads |
Hemodynamics and organ dysfunction improvements in phase 2 trial; negative phase 3 trial in human septic shock [51, 52] Ongoing studies in human septic shock [NCT 02288975] Ongoing preclinical study [53] |
| Neutralization of pathogens or cytokines | Antimicrobial peptide MD54 Anakinra Eritoran (blocks LPS binding to TLR4) and monoclonal antibody against endotoxin (E5) |
Interacts with LPS and promotes LPS aggregation [47] Decreased 28-day mortality in human septic shock and macrophage activation syndrome [48] Negative phase 3 trial in human septic shock [49, 50] |
| Attenuation of excessive inflammatory response induced by sepsis | Antimicrobial peptide MD54 | Inhibition of proinflammatory cytokines transcription in in vitro studies; decreased IL-6 and TNF-α production in animal studies; increased survival in murine CLP model [47] |
| GM-CSF | Normalization of monocyte HLA-DR expression; restoration of monocyte cytokine production; nonsignificant decreases in time of mechanical ventilation, ICU, and hospital length of stay; improvements in APACHE-II in human septic shock [67] | |
| Attenuation of immune suppression induced by sepsis | IL-7 Monoclonal antibody against PD1 and PDL1 |
Induction of T and B cell growth; inhibition of lymphocyte apoptosis in preclinical studies [65] Improvements in lymphocyte function and reduction of apoptosis in vitro [66] |
| Intestinal barrier protection | IL-11, insulin-like growth factor | Protection against increased intestinal permeability and anti-apoptotic properties in preclinical studies [62, 63] |
| Microbiome reestablishment |
Fecal microbiota transplant | Attenuation of colitis in patients with Clostridium difficile infection [59] |
| Pathogen clearance | Monoclonal antibody against PCSK9 | Greater survival in murine CLP model [56] |
APACHE-II, Acute Physiology and Chronic Health Evaluation II; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; CLP, cecal ligation and puncture; GM-CSF, granulocyte macrophage colony-stimulating factor; HLA-DR, human leukocyte antigen class 2; ICU, intensive care unit; IL, interleukin; PD1, programmed death-1; PDL1, programmed death ligand-1; PCSK9, proprotein convertase subtylisin/kexin type 9.
Conclusions
Great advances in the understanding of organism infection and organism toxicity in sepsis include therapeutic approaches related to the genetics of the infecting organism and the host. However, early antibiotic administration and appropriate supportive care are still the only therapies associated with improved outcomes in sepsis. Moreover, to date, no biomarker can completely determine the diagnosis and/or prognosis of sepsis. Further research and trials investigating better antimicrobial strategies and personalized medicine are needed in septic patients.
Disclosure Statement
J.A.R. reports patents owned by the University of British Columbia (UBC) that are related to PCSK9 inhibitor(s) and sepsis and to the use of vasopressin in septic shock; he is an inventor on these patents. He is a founder, Director, and shareholder in Cyon Therapeutics Inc. (developing a sepsis therapy), has share options in Leading Biosciences Inc., and is a shareholder in Molecular You Corp. He received consulting fees from: Cubist Pharmaceuticals (now owned by Merck; formerly Trius Pharmaceuticals; developing antibiotics), Leading Biosciences (developing a sepsis therapeutic), Ferring Pharmaceuticals (manufactures vasopressin and is developing selepressin), Grifols (sells albumin), La Jolla Pharmaceuticals (developing angiotensin II; Dr. Russell chairs the DSMB of a trial of angiotensin II), CytoVale Inc. (developing a sepsis diagnostic), and Asahi Kesai Pharmaceuticals of America (AKPA) (developing recombinant thrombomodulin). K.R.W. and J.H.B. report patents owned by the University of British Columbia (UBC) that are related to PCSK9 inhibitor(s) and sepsis. K.R.G. and T.S. have no conflicts of interest to declare.
Acknowledgements
J.A.R. received an investigator-initiated grant from Grifols that is provided to and administered by UBC. K.R.G. is sponsored by CNPq-Brazil.
References
- 1.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 2.Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 4.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- 5.Jin HS, Park JK, Jo EK. Toll-like receptors and NOD-like receptors in innate immune defense during pathogenic infection. J Bacteriol Virol. 2014;44:215–225. [Google Scholar]
- 6.Vazquez C, Horner SM. MAVS Coordination of antiviral innate immunity. J Virol. 2015;89:6974–6977. doi: 10.1128/JVI.01918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015;32:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao YM, Luan YY, Zhang QH, Sheng ZY. Pathophysiological aspects of sepsis: an overview. Methods Mol Biol. 2015;1237:5–15. doi: 10.1007/978-1-4939-1776-1_2. [DOI] [PubMed] [Google Scholar]
- 9.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 10.Cunha FQ, Assreuy J, Moss DW, Rees D, Leal LM, Moncada S, et al. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-α and IL-1β. Immunology. 1994;81:211–215. [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman D, Bateman RM, Ellis CG. Effect of decreased O2 supply on skeletal muscle oxygenation and O2 consumption during sepsis: role of heterogeneous capillary spacing and blood flow. Am J Physiol Heart Circ Physiol. 2006;290:H2277–H2285. doi: 10.1152/ajpheart.00547.2005. [DOI] [PubMed] [Google Scholar]
- 12.Opal SM, van der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med. 2015;277:277–293. doi: 10.1111/joim.12331. [DOI] [PubMed] [Google Scholar]
- 13.Russell JA, Rush B, Boyd J. Pathophysiology of septic shock. Crit Care Clin. 2018;34:43–61. doi: 10.1016/j.ccc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10:37–47. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- 15.Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 16.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 18.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, et al. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J Immunol. 2006;177:6540–6547. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- 19.Levels JH, Abraham PR, van Barreveld EP, Meijers JC, van Deventer SJ. Distribution and kinetics of lipoprotein-bound lipoteichoic acid. Infect Immun. 2003;71:3280–3284. doi: 10.1128/IAI.71.6.3280-3284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levels JH, Abraham PR, van den Ende A, van Deventer SJ. Distribution and kinetics of lipoprotein-bound endotoxin. Infect Immun. 2001;69:2821–2828. doi: 10.1128/IAI.69.5.2821-2828.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levels JH, Marquart JA, Abraham PR, van den Ende AE, Molhuizen HO, van Deventer SJ, et al. Lipopolysaccharide is transferred from high-density to low-density lipoproteins by lipopolysaccharide-binding protein and phospholipid transfer protein. Infect Immun. 2005;73:2321–2326. doi: 10.1128/IAI.73.4.2321-2326.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walley KR, Francis GA, Opal SM, Stein EA, Russell JA, Boyd JH. The central role of proprotein convertase subtilisin/kexin type 9 in septic pathogen lipid transport and clearance. Am J Respir Crit Care Med. 2015;192:1275–1286. doi: 10.1164/rccm.201505-0876CI. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Zhao Y, Shao F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr Opin Immunol. 2015;32:78–83. doi: 10.1016/j.coi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability - a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle EC, Finlay BB. Leaky guts and lipid rafts. Trends Microbiol. 2005;13:560–563. doi: 10.1016/j.tim.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 26.McAuley DF, Laffey JG, O'Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 27.Opal SM, Dellinger RP, Vincent JL, Masur H, Angus DC. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C? Crit Care Med. 2014;42:1714–1721. doi: 10.1097/CCM.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 29.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376:2235–44. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak R, Charpentier E, Braun JS, Tuomanen E. Signal transduction by a death signal peptide: uncovering the mechanism of bacterial killing by penicillin. Mol Cell. 2000;5:49–57. doi: 10.1016/s1097-2765(00)80402-5. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 32.Shiber S, Yahav D, Avni T, Leibovici L, Paul M. β-Lactam/β-lactamase inhibitors versus carbapenems for the treatment of sepsis: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2015;70:41–47. doi: 10.1093/jac/dku351. [DOI] [PubMed] [Google Scholar]
- 33.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, Rai V, Wong KK, Hasan MS, et al. β-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent β-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016;42:1535–1545. doi: 10.1007/s00134-015-4188-0. [DOI] [PubMed] [Google Scholar]
- 34.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16:819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 35.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehrer RI, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol. 1999;11:23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 37.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 38.Steinstraesser L, Kraneburg UM, Hirsch T, Kesting M, Steinau HU, Jacobsen F, et al. Host defense peptides as effector molecules of the innate immune response: a sledgehammer for drug resistance? Int J Mol Sci. 2009;10:3951–3970. doi: 10.3390/ijms10093951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciornei CD, Sigurdardottir T, Schmidtchen A, Bodelsson M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob Agents Chemother. 2005;49:2845–2850. doi: 10.1128/AAC.49.7.2845-2850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J Leukoc Biol. 2004;76:909–925. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 41.Masson PL, Heremans JF. Metal-combining properties of human lactoferrin (red milk protein) 1. The involvement of bicarbonate in the reaction. Eur J Biochem. 1968;6:579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 42.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 43.Book M, Chen Q, Lehmann LE, Klaschik S, Weber S, Schewe JC, et al. Inducibility of the endogenous antibiotic peptide beta-defensin 2 is impaired in patients with severe sepsis. Crit Care. 2007;11:R19. doi: 10.1186/cc5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas NJ, Carcillo JA, Doughty LA, Sasser H, Heine RP. Plasma concentrations of defensins and lactoferrin in children with severe sepsis. Pediatr Infect Dis J. 2002;21:34–38. doi: 10.1097/00006454-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Rintala E, Peuravuori H, Pulkki K, Voipio-Pulkki LM, Nevalainen T. Bactericidal/permeability-increasing protein (BPI) in sepsis correlates with the severity of sepsis and the outcome. Intensive Care Med. 2000;26:1248–1251. doi: 10.1007/s001340000616. [DOI] [PubMed] [Google Scholar]
- 46.Guntupalli K, Dean N, Morris PE, Bandi V, Margolis B, Rivers E, et al. A phase 2 randomized, double-blind, placebo-controlled study of the safety and efficacy of talactoferrin in patients with severe sepsis. Crit Care Med. 2013;41:706–716. doi: 10.1097/CCM.0b013e3182741551. [DOI] [PubMed] [Google Scholar]
- 47.Tandon A, Harioudh MK, Ishrat N, Tripathi AK, Srivastava S, Ghosh JK. An MD2-derived peptide promotes LPS aggregation, facilitates its internalization in THP-1 cells, and inhibits LPS-induced pro-inflammatory responses. Cell Mol Life Sci. 2018 doi: 10.1007/s00018-017-2735-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309:1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 50.Angus DC, Birmingham MC, Balk RA, Scannon PJ, Collins D, Kruse JA, et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA. 2000;283:1723–1730. doi: 10.1001/jama.283.13.1723. [DOI] [PubMed] [Google Scholar]
- 51.Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 52.Klein DJ, Foster D, Schorr CA, Kazempour K, Walker PM, Dellinger RP. The EUPHRATES trial (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized Controlled Trial of Adults Treated for Endotoxemia and Septic Shock): study protocol for a randomized controlled trial. Trials. 2014;15:218. doi: 10.1186/1745-6215-15-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang JH, Super M, Yung CW, Cooper RM, Domansky K, Graveline AR, et al. An extracorporeal blood-cleansing device for sepsis therapy. Nat Med. 2014;20:1211–1216. doi: 10.1038/nm.3640. [DOI] [PubMed] [Google Scholar]
- 54.Topchiy E, Cirstea M, Kong HJ, Boyd JH, Wang Y, Russell JA, et al. Lipopolysaccharide Is cleared from the circulation by hepatocytes via the low density lipoprotein receptor. PLoS One. 2016;11:e0155030. doi: 10.1371/journal.pone.0155030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 56.Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF, et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008782. 258ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dwivedi DJ, Grin PM, Khan M, Prat A, Zhou J, Fox-Robichaud AE, et al. Differential expression of PCSK9 modulates infection, inflammation, and coagulation in a murine model of sepsis. Shock. 2016;46:672–680. doi: 10.1097/SHK.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 58.Berger JM, Loza Valdes A, Gromada J, Anderson N, Horton JD. Inhibition of PCSK9 does not improve lipopolysaccharide-induced mortality in mice. Journal of lipid research. 2017;58:1661–1669. doi: 10.1194/jlr.M076844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wischmeyer PE, McDonald D, Knight R. Role of the microbiome, probiotics, and ‘dysbiosis therapy’ in critical illness. Curr Opin Crit Care. 2016;22:347–353. doi: 10.1097/MCC.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han S, Shannahan S, Pellish R. Fecal microbiota transplant: treatment options for Clostridium difficile infection in the intensive care unit. J Intensive Care Med. 2016;31:577–586. doi: 10.1177/0885066615594344. [DOI] [PubMed] [Google Scholar]
- 61.Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg. 2010;2:61–69. doi: 10.4240/wjgs.v2.i3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Opal SM, Keith JC, Jhung J, Palardy JE, Parejo N, Marchese E, et al. Orally administered recombinant human interleukin-11 is protective in experimental neutropenic sepsis. J Infect Dis. 2003;187:70–76. doi: 10.1086/345864. [DOI] [PubMed] [Google Scholar]
- 63.Hunninghake GW, Doerschug KC, Nymon AB, Schmidt GA, Meyerholz DK, Ashare A. Insulin-like growth factor-1 levels contribute to the development of bacterial translocation in sepsis. Am J Respir Crit Care Med. 2010;182:517–525. doi: 10.1164/rccm.200911-1757OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ashare A, Nymon AB, Doerschug KC, Morrison JM, Monick MM, Hunninghake GW. Insulin-like growth factor-1 improves survival in sepsis via enhanced hepatic bacterial clearance. Am J Respir Crit Care Med. 2008;178:149–157. doi: 10.1164/rccm.200709-1400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venet F, Foray AP, Villars-Mechin A, Malcus C, Poitevin-Later F, Lepape A, et al. IL-7 restores lymphocyte functions in septic patients. J Immunol. 2012;189:5073–5081. doi: 10.4049/jimmunol.1202062. [DOI] [PubMed] [Google Scholar]
- 66.Chang K, Svabek C, Vazquez-Guillamet C, Sato B, Rasche D, Wilson S, et al. Targeting the programmed cell death 1:programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care. 2014;18:R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 68.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2:1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- 69.Pinheiro da Silva F, Cesar Machado MC. Personalized medicine for sepsis. Am J Med Sci. 2015;350:409–413. doi: 10.1097/MAJ.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 70.Schaub N, Boldanova T, Noveanu M, Arenja N, Hermann H, Twerenbold R, et al. Incremental value of multiplex real-time PCR for the early diagnosis of sepsis in the emergen cy department. Swiss Med Wkly. 2014;144:w13911. doi: 10.4414/smw.2014.13911. [DOI] [PubMed] [Google Scholar]
- 71.Genga KR, Lo C, Cirstea M, et al. PCSK9 loss-of-function genotype is associated with better short and long-term outcomes in sepsis. Crit Care. 2017;21((suppl 1: 58)) [Google Scholar]