Abstract
Background
HIV and bacterial sexually transmissible infection (STI) notifications among men who have sex with men (MSM) have increased in Australia and many other countries. The relationship between HIV infection and other STIs has been demonstrated previously. However, the relationship between the cumulative history of STIs and subsequent HIV infection remains largely unexplored and limits our understanding of the mechanisms underpinning the elevated HIV risk.
Methods
Data from HIV-negative MSM who attended high–HIV caseload primary care clinics in Melbourne, Australia, from 2007 to 2014 with 2 or more HIV and STI tests were included. Controlling for sexual behaviors self-reported at clinic visits, discrete time survival analyses using generalized linear modeling estimated the effect of an STI at the prior test event and the cumulative history of STIs (none, 1, 2, or more [repeated]) on risk of HIV infection.
Results
A total of 8941 MSM met the study criteria; 227 (2.5%) were diagnosed with HIV over the follow-up period. Adjusting for sexual behaviors, a cumulative history of repeated rectal gonorrhea infections (adjusted hazard ratio [aHR], 6.27; 95% confidence interval [CI], 2.68–14.50) and a single rectal gonorrhea infection (aHR, 2.09; 95% CI, 1.15–3.79) were associated with increased HIV infection risk.
Conclusions
Repeated and single rectal gonorrhea infections were independently associated with increased HIV infection risk. These findings suggest that MSM with any history of rectal gonorrhea, particularly repeat rectal gonorrhea, represent a group for whom preventive interventions for HIV should be emphasized.
Keywords: chlamydia, gonorrhoea, HIV, MSM, STI, syphilis
Over recent decades, HIV notifications among men who have sex with men (MSM) have increased in many high-income countries [1], and this has occurred in conjunction with increased notifications of bacterial sexually transmissible infections (STIs) in a number of countries including Australia [2–5]. Australia wide, in 2016, 75% of the HIV notifications were among MSM, and in the state of Victoria, the 312 HIV notifications made in 2016 represented an 18% increase since 2007. Additionally, there has been an increase in bacterial STI notifications with the 6328 gonorrhea and 1138 syphilis notifications made in 2016, representative of a ~6-fold increase in gonorrhea and almost a 3-fold increase in syphilis notifications compared with 2007 [6]. Australian guidelines recommend HIV and STI testing up to 4 times per year for HIV-negative MSM who report condomless anal sex and/or report >10 partners in 6 months [7]. Despite this recommendation, previous studies have reported quarterly HIV testing to be uncommon among MSM reporting these behaviors, with only 15% of MSM retested within 3 months [8].
HIV and bacterial STIs share common routes of sexual transmission, and risk is typically related to inconsistent condom use and a high number of sex partners [9], and the association between STIs and increased risk of HIV infection is well established among MSM [10–12]. However, there are few data describing the effect of repeated STIs on HIV infection risk. Findings from studies conducted in Baltimore [13] and Denmark [14] reported an increased risk of HIV infection among MSM following repeated compared with single syphilis infections, and another study in San Francisco reported that rectal chlamydia and/or gonorrhea reinfections among MSM increased risk of HIV infection approximately 8-fold compared with MSM with a single rectal infection [15]. Conversely, sentinel surveillance data from clinics in Melbourne showed an association between single syphilis infection in the previous 2 years and an HIV infection, but no association was found with repeat syphilis or chlamydia infections. This study, however, did not include data related to gonorrhea infection [16].
Some MSM experience multiple STIs, and there is evidence that this may be concentrated among MSM living with or at risk of HIV infection [17], yet no studies have examined the independent association between chlamydia, gonorrhea, or syphilis infection and HIV infection risk, including both single infections and repeated infections, while also controlling for sexual behaviors. Examining how HIV risk relates to single and repeated STIs may help inform our limited understanding of the biological [18] and potential causal [19] mechanisms underpinning the association between rectal bacterial STIs and HIV risk among MSM, which has been explored in more detail in relation to vaginal and penile inflammation among heterosexual females and males [20, 21].
Using patient-level linked data from a primary care sentinel surveillance system of high–HIV caseload clinics, we examined the relationship between patients’ cumulative history of STIs and having an STI at the prior test event and risk of subsequent HIV infection among MSM.
METHODS
Data Collection
Data for this study were collected as part of the Victorian Primary Care Network for Sentinel Surveillance on Blood Borne Viruses and Sexually Transmissible Infections (VPCNSS), which has been described previously [22]. Briefly, VPCNSS links patients’ HIV and other STI testing data over time with demographic and behavioral data self-reported by patients at the time of testing. This study utilized VPCNSS data collected between 2007 and 2014 from a large sexual health center and 2 primary care clinics with a high-MSM patient base in inner-suburban Melbourne. Over this time, these clinics diagnosed approximately 50% of HIV notifications in the state of Victoria [23].
Ethics
Ethics approval for VPCNSS was obtained from 6 Human Research Ethics Committees. B.L.H. was added as a student researcher through The Alfred Hospital Ethics Committee.
Inclusion and Exclusion Criteria
To identify incident HIV infection, HIV-negative MSM were included in this study if they tested negative for HIV at their first test event and had at least 1 subsequent HIV test between 2007 and 2014. MSM status was determined as either a male with any history of self-reporting a male sex partner or males with any history of rectal STI testing [24]. Data from HIV-negative MSM reporting no male sexual partners in the previous 6 months, any history of injection drug use, and/or a current regular HIV-positive partner were excluded a priori on a test-by-test basis. HIV test events that did not include concurrent rectal chlamydia, rectal gonorrhea, and syphilis tests were also excluded on a test-by-test basis.
Outcome
The outcome was incident HIV infection, defined as a positive HIV diagnosis, measured as a positive enzyme-linked immunosorbent assay test and confirmed by Western blot during the study period, occurring among patients with at least 1 previous negative HIV test result.
Exposures
Patients’ STI diagnosis at their prior test and their cumulative history of STIs, including rectal chlamydia/gonorrhea and syphilis, were the primary exposures, and condom use and number of sexual partners were included to control for confounding from these sexual behaviors. All exposures were lagged to the prior test event(s) to temporally separate the exposure and response, accounting for the possibility of HIV infection preceding an STI.
Sexually Transmissible Infections at the Prior Test
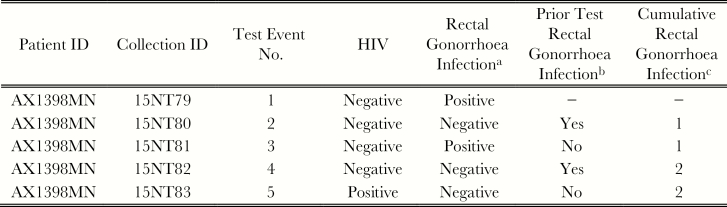
Rectal chlamydia, rectal gonorrhea, and syphilis infections were modeled as time-varying dichotomous measures of infection. Figure 1b shows an example testing history for hypothetical patient, defined as having a rectal gonorrhea infection at test events 2 and 4.
Figure 1.
Hypothetical HIV and sexually transmissible infection (STI) testing history. aResult of current test. bResult of STI test from prior test event. cCumulated history of positive STI diagnoses.
Cumulative Sexually Transmissible Infections
Cumulative STI histories were modeled as monotonic cumulative time-varying exposures permitting estimation of a cumulative effect of repeated infection on the risk of HIV infection. Patients were treated as having no, 1, or ≥2 (repeated) infections during the observation period. A positive rectal chlamydia or gonorrhea test within 45 or 60 days of a previous diagnosis, respectively, was treated as the same infection to account for tests of cure. Figure 1c shows an example testing history for a hypothetical patient, defined as having a cumulative history of 1 rectal gonorrhea infection at test events 2 and 3 and a cumulative history of 2 rectal gonorrhea infections at test events 4 and 5.
Sexual Behaviors
Self-reported condom use (any inconsistent condom use vs consistent condom use in the last 6 months) and number of sex partners in the last 6 months (1–5 partners vs 6+ partners) were treated as time-varying dichotomous exposures. The recall period was past 12 months at the sexual health center and past 6 months at primary care clinics. Number of sex partners was adjusted to the past 6 months by halving the number reported by sexual health center patients [16].
Statistical Analyses
Using patient test observation data, discrete time survival analysis using generalized linear modeling (GLM) with a binomial distribution and complementary log–log link function estimated the risk of HIV infection associated with STIs and sexual behavior. Discrete time modeling was used because the precise date of HIV infection was unknown and therefore interval-censored. Estimates from discrete time models were offset for the log of days between test events to account for patient variability in HIV exposure between test events. Discrete indicators for each test event were included in GLM analyses to permit variation in the baseline hazard across test events (ie, permitting the overall risk of HIV infection—independent of the effect of model covariates—to vary with time). STI and behavioral exposures were estimated as time-varying exposures and lagged to testing events before HIV test outcomes in GLM analyses (ie, risk of HIV infection was regressed against STIs, number of sexual partners, and condom use classified at the prior testing event).
Inference for differences in HIV infection risk between MSM included and not included in the analyses was estimated by a chi-square test of independence. Wald tests were used to provide inference for both the joint significance of polytomous indicator variables (ie, cumulative STI factors) and postestimation comparisons between these variables (eg, comparison of the difference in hazard ratios between 1 and ≥2 STIs). All analyses were completed with Stata/SE 13.1 (StataCorp, College Station, TX).
RESULTS
Between 2007 and 2014, 12 003 MSM who were HIV negative at their first test and had ≥2 HIV tests at 1 of the 3 clinics contributed 41 685 potentially eligible test events. There were 3550 test events, representing 688 individual MSM excluded based on a priori exclusions. A further 8723 test events among 1412 MSM were excluded due to no history of testing for STIs, with ~90% due to no concurrent rectal chlamydia and/or gonorrhea testing. Another 6597 tests among 962 MSM were excluded due to missing risk behavior data. During follow-up, there was no significant difference in the proportion of MSM diagnosed with new HIV infection among those included in (227, 2.54%) and excluded from (65, 2.12%) analyses (χ 2(1) = 1.66; P = .197).
A total of 22 815 test events, representing 8941 individual MSM, were included in further analyses. Demographic characteristics, self-reported sexual behaviors, and STIs diagnosed at each patient’s first test are shown in Table 1. The median age (interquartile range [IQR]) was 29 (24–38) years, and 61% were Australian born. Rectal gonorrhea was the most common STI diagnosed at the first test, followed by rectal chlamydia and syphilis. Approximately half reported inconsistent condom use, and one-third reported ≥6 sexual partners in the prior 6 months.
Table 1.
Demographics, STI Diagnoses, and Self-Reported Sexual Behaviors at First Test (n = 8941)
| Characteristic | No. | % |
|---|---|---|
| Demographics | ||
| Age group, y | ||
| 16–19 | 490 | 5.5 |
| 20–29 | 4083 | 45.7 |
| 30–39 | 2409 | 26.9 |
| 40–49 | 1256 | 14.0 |
| 50+ | 702 | 7.9 |
| Missing | 1 | |
| Country of birth | ||
| Australia | 5478 | 61.3 |
| Other | 2983 | 33.4 |
| Missing | 480 | 5.4 |
| Aboriginal or Torres Strait Islander | ||
| No | 8062 | 90.2 |
| Yes | 139 | 1.6 |
| Missing | 740 | 8.3 |
| STI diagnosis | ||
| Infectious syphilis | ||
| Negative | 8813 | 98.6 |
| Positive | 128 | 1.4 |
| Rectal chlamydia | ||
| Negative | 8659 | 96.8 |
| Positive | 282 | 3.2 |
| Rectal gonorrhea | ||
| Negative | 8391 | 93.8 |
| Positive | 550 | 6.2 |
| Sexual behaviors | ||
| No. of partners | ||
| 1 to 5 | 6352 | 71.0 |
| ≥6 | 2589 | 29.0 |
| Inconsistent condom use | ||
| No | 4451 | 49.8 |
| Yes | 4490 | 50.2 |
Abbreviation: STI, sexually transmissible infection.
MSM received a median (IQR) of 4 (2–6) HIV tests, and the median time between HIV tests (IQR) was 6 (4–10) months. Two hundred twenty-seven (2.5%) were diagnosed with HIV over the observation period. More than 90% of the 8941 MSM were never diagnosed with rectal gonorrhea or syphilis, and >85% were never diagnosed with chlamydia (Table 2). Rectal chlamydia was the most common STI diagnosed among the 22 815 tests, with 1589 (7%) positive rectal chlamydia tests recorded among 1240 MSM; 965 (10.8%) MSM were diagnosed once, and 275 (3.1%) were diagnosed with repeat infections. There were 849 (3.7%) positive rectal gonorrhea tests recorded among 718 MSM, with 613 (6.9%) MSM diagnosed once and 105 (1.2%) diagnosed with repeat infections. There were 355 (1.6%) positive syphilis tests recorded among 332 MSM, with 312 (3.5%) MSM diagnosed once and 20 (0.2%) diagnosed with repeat infections. Of MSM classified as having repeat infections, the vast majority had only 2 rectal chlamydia (81%), rectal gonorrhea (83%), and syphilis (90%) infections. At the test event before a positive HIV diagnosis or end of study censoring for MSM who remained HIV negative, ~30% reported ≥6 sexual partners and 50% reported inconsistent condom use in the prior 6 months.
Table 2.
Cumulative STI Diagnoses Among 8941 MSM in Melbourne
| Cumulative STI | No. | % |
|---|---|---|
| Infectious syphilis | ||
| No infection | 8609 | 96.3 |
| 1 infection | 312 | 3.5 |
| ≥2 infections | 20 | 0.2 |
| Rectal chlamydia | ||
| No Infection | 7701 | 86.1 |
| 1 | 965 | 10.8 |
| ≥2 infections | 275 | 3.1 |
| Rectal gonorrhea | ||
| No infection | 8223 | 91.9 |
| 1 infection | 613 | 6.9 |
| ≥2 infections | 105 | 1.2 |
Abbreviations: MSM, men who have sex with men; STI, sexually transmissible infection.
There was no significant association between HIV diagnosis and age <30 years (hazard ratio [HR], 1.07; 95% confidence interval [CI], 0.76–1.51), Aboriginal or Torres Strait Islander status (HR, 1.10; 95% CI, 0.35–3.47), or non-Australian country of birth (HR, 0.95; 95% CI, 0.68–1.34) in unadjusted analyses, and these variables were not included in adjusted analyses.
Cumulative Sexually Transmitted Infections
Compared with MSM with no history of gonorrhea diagnosis, a history of single or repeated rectal gonorrhea diagnosis was jointly associated with risk of HIV infection (Wald χ 2(2) = 18.4; P < .001). Conversely, a history of single or repeated syphilis diagnosis (Wald χ 2(2) = 4; P = .138) was not jointly associated with an increased risk of HIV infection, nor was a history of single or repeated rectal chlamydia diagnosis (Wald χ 2(2) = 5.7; P = .058).
In adjusted analyses, as shown in Table 3, risk of HIV infection was higher for those with ≥2 previous rectal gonorrhea diagnoses (adjusted hazard ratio [aHR], 6.27; 95% CI, 2.68–14.50; Wald χ 2(1) = 18.1; P < .001) or 1 previous (aHR, 2.09; 95% CI, 1.15–3.79; Wald χ 2(1) = 5.82; P = .016) rectal gonorrhea diagnosis, compared with MSM with no rectal gonorrhea diagnosis. The 3-times-greater HIV risk for MSM with repeat rectal gonorrhea diagnoses compared with those with 1 diagnosis was statistically significant (Wald χ 2(1) = 7.56; P = .006).
Table 3.
Risk of HIV Infection at Test Following Exposure Among MSM in Melbourne From Generalized Linear Modelinga; Hazard Ratio, Adjusted Hazard Ratio, and 95% Confidence Interval
| Exposure | HR | 95% CI | P Value | aHR | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Cumulative syphilis infectionb | ||||||
| No infection | 1 | − | 1 | − | ||
| 1 infection | 2.58 | 1.44–4.61 | .001 | 1.99 | 1.00–3.96 | .049 |
| ≥2 infectionsc | 2.87 | 0.38–21.9 | .309 | 0.93 | 0.11–7.66 | .947 |
| Syphilis infectionc | ||||||
| No | 1 | − | 1 | − | ||
| Yes | 2.88 | 1.07–7.77 | .037 | 1.13 | 0.35–3.67 | .839 |
| Cumulative rectal chlamydia infectionb | ||||||
| No infection | 1 | − | 1 | − | ||
| 1 infection | 2.96 | 1.99–4.41 | <.001 | 1.89 | 1.12–3.18 | .017 |
| ≥2 infectionsc | 3.55 | 1.79–7.04 | <.001 | 1.62 | 0.73–3.59 | .235 |
| Rectal chlamydia infectionc | ||||||
| No | 1 | − | 1 | − | ||
| Yes | 3.43 | 2.17–5.44 | <.001 | 1.43 | 0.76–2.69 | .264 |
| Cumulative rectal gonorrhea infectionb | ||||||
| No infection | 1 | − | 1 | − | ||
| 1 infection | 3.52 | 2.23–5.56 | <.001 | 2.09 | 1.15–3.79 | .016 |
| ≥2 infectionsc | 12.0 | 6.01–23.99 | <.001 | 6.27 | 2.68–14.5 | <.001 |
| Rectal gonorrhea infectionc | ||||||
| No | 1 | − | 1 | − | ||
| Yes | 5.25 | 3.17–8.69 | <.001 | 1.54 | 0.75–3.14 | .238 |
| Sexual partners | ||||||
| 1–5 | 1 | − | 1 | − | ||
| ≥6 | 1.71 | 1.24–2.35 | .001 | 1.57 | 1.13–2.16 | .007 |
| Inconsistent condom use | ||||||
| No | 1 | 1 | − | |||
| Yes | 2.17 | 1.56–3.01 | <.001 | 1.83 | 1.31–2.56 | <.001 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; MSM, men who have sex with men.
aGeneralized linear model with a binominal distribution and complementary log–log link function using patient test observation data. Models were offset for time between patients tests.
bOrdinal monotonic time-varying measure of infection whereby infections are cumulated for each patient’s STI test results across the study period.
cDichotomous time-varying measure of infection at the prior test event.
Sexually Transmitted Infection at Prior Test
A positive rectal chlamydia (aHR, 1.43; 95% CI, 0.76–2.69; Wald χ 2(1) = 1.23; P = .264), rectal gonorrhea (aHR, 1.54; 95% CI, 0.75–3.14; Wald χ 2(1) = 1.40; P = .238) or syphilis diagnosis (aHR, 1.13; 95% CI, 0.35–3.67; Wald χ 2(1) = 0.04; P = .839) at the prior test event was not associated with subsequent HIV infection risk in the adjusted analysis.
Sexual Behaviors
MSM who reported inconsistent condom use (aHR, 1.83; 95% CI, 1.31–2.56; Wald χ 2(1) = 12.5; P < .001) and ≥6 sexual partners (aHR, 1.57; 95% CI, 1.13–2.16; Wald χ 2(1) = 7.43; P = .007) in the previous 6 months at the preceding test event were at increased risk of HIV infection in the adjusted analysis.
DISCUSSION
To our knowledge, this is the first study to examine the independent effects of cumulative rectal chlamydia, rectal gonorrhea, and syphilis diagnoses on HIV infection risk among MSM. A cumulative history of repeated rectal gonorrhea diagnosis was strongly associated with increased risk of HIV infection. Although this result partly reflects previous findings showing elevated HIV risk among MSM with 2 rectal gonorrhea and/or chlamydia infections in the past 2 years [15], we show that the greatest association of HIV risk is with rectal gonorrhea and that it is independent of rectal chlamydia. Conversely, infection with syphilis or rectal chlamydia or gonorrhea at the prior test event was not associated with HIV risk when adjusted for self-reported condom use and number of sexual partners.
Our findings showing a lack of association between HIV risk and an STI diagnosis at the prior test event contrast with previous analyses of data from the same sexual health center that contributed data to our analyses [10]. However, our analyses varied in 2 important ways. First, Cheung et al. discussed the importance of identifying risk before HIV infection to allow for intervention, particularly HIV PrEP, and we built on this by incorporating the cumulative history of STIs in addition to an STI at the prior test event. Second, we conducted a multivariable analysis, whereas Cheung et al. estimated univariable incidence rate ratios and calculated population attributable fractions (PAF) of HIV infection with inconsistent condom use during anal sex (PAF = 44.7%) and rectal STI diagnoses, including both chlamydia and gonorrhea (PAF = 23.9%), resulting in the highest and second highest PAFs, respectively. Although inconsistent condom use during anal intercourse remains an important risk factor for HIV infection, all MSM included in our analyses underwent rectal STI screening, and it is therefore assumed that they were engaging in receptive anal intercourse. Additionally, the behavioral data collected indicate that half were engaging in condomless anal intercourse. It has been suggested that condomless anal intercourse alters the rectal mucosa, increasing HIV risk [25]; however, our findings suggest that the act of condomless anal intercourse itself may not fully explain the increased risk of HIV infection and support a previous suggestion of rectal STIs potentially having a biological causative impact on HIV infection risk [18, 19].
There is a body of work indicating that STIs may enhance HIV vulnerability through genital inflammation [20] in the female reproductive tract and inner foreskin of the penis [21], but there is a paucity of data on the immune response to STIs in the rectum. The absence of an association of HIV risk with STIs at the prior test event in our study may be the result of successful treatment for STIs and subsequent reduction in rectal inflammation; given the high caseload nature of the clinics contributing data to this study, it is assumed that MSM were treated for their diagnosed STI, as per guidelines. It is possible that heightened HIV risk with repeated rectal gonorrhea may be associated with continuous or repeated influx of HIV target cells to the rectal mucosa, even following treatment, as has been reported to occur with herpes simplex virus [26]. Additionally, it is possible that the immunological response to gonorrhea infection following previous infection may increase HIV risk due to the recruitment of tissue-damaging neutrophils [27]. As suggested by Kelley et al., there is a clear need for longitudinal studies to understand the long-term effects of rectal inflammation on HIV risk among MSM [25]. Our findings suggest that this needs to include the role that STIs, including repeated STIs, have on this risk. In addition to potential immunological mechanisms, studies from England [28] and the Netherlands [29] incorporating molecular epidemiological methods have identified gonorrhea strains that existed in networks that included both HIV-negative and HIV-diagnosed MSM, and therefore it is also plausible that repeated gonorrhea infection is a marker of continuous sexual risk behavior among HIV-negative MSM in such networks.
It is well established that an STI diagnosis, including any one of syphilis, rectal chlamydia, or rectal gonorrhea, is indicative of suitability for HIV preexposure prophylaxis (PrEP) [30], and our data further support this. There is evidence that STIs may be increasing among some MSM using HIV PrEP [31] and further evidence of concentration of STIs among MSM with repeated infections [32]. Although difficult to quantify at this point, our data suggest that there may be important implications regarding HIV vulnerability for these MSM if they cease PrEP use or are not fully adherent to PrEP dosage recommendations.
Despite our study having strengths relative to previous studies, including analyzing the independent effects of cumulative diagnosis of STIs previously associated with increased risk of HIV and behavioral risk, there are limitations. Identifying an individual’s movement between clinic sites was not possible, and therefore the analyses do not include individuals’ tests and diagnoses that occurred between sentinel clinics or at clinics outside the sentinel network. Our data were also collected from high–HIV caseload clinics in inner-urban areas. These clinics diagnose a substantial percentage of jurisdictional HIV cases, and our results may not be generalizable to MSM attending general practices without a focus on sexual health or services in outer-suburban or regional areas. The lagging methodology utilized temporally separates the possibility of HIV infection preceding an STI; however, it is possible that an STI may have been diagnosed in the very early stages of HIV infection before seroconversion. Finally, we have assumed that censoring was noninformative, in that MSM who stopped testing did not have an increased risk of HIV infection due to increased risk behaviors. Due to the open nature of the surveillance system compared with a specifically recruited cohort, we did not analyze factors associated with loss to follow-up.
CONCLUSIONS
There is a limited understanding of the long-term impact of repeated bacterial STIs on HIV infection risk among MSM. Using data from high–HIV caseload clinics, we found that a cumulated history of rectal gonorrhea infection increased risk of subsequent HIV infection among MSM, and this relationship was independent of other STI diagnoses and sexual behaviors. These findings suggest that MSM with a history of rectal gonorrhea, and particularly repeated rectal gonorrhea infection, represent a high-risk group for HIV infection and further support STIs as a marker of MSM who should be offered HIV PrEP. Additionally, a history of STI diagnoses, particularly repeated rectal gonorrhea, may warrant specific clinical counseling among MSM who self-report suboptimal PrEP adherence or who are considering ceasing PrEP use. Future studies combining longitudinal epidemiological and immunological data may be useful to examine the independent effects of rectal inflammation as a result of STIs and sexual behaviors on HIV infection. Similarly, incorporating molecular epidemiological data into routine surveillance may help better identify how the relationship between STI and HIV infection vulnerability is related to sexual networks.
Acknowledgments
We would like to acknowledge and thank patients and clinicians at each site who contribute data to the Burnet Institute sentinel surveillance systems, as well as past and present members of the Burnet Institute Surveillance Group for their work in establishing and maintaining the surveillance systems.
Author contributions. B.L.H., P.A.A., C.E.H., and M.S. conceived and designed the study; P.A.A., C.E.H., and M.S. provided supervision to B.L.H.; C.K.F., E.P.F.C., N.R., B.K.T., and D.L.* acquired and contributed data; M.H. designed the surveillance system and provided clinical expertise; G.T. provided mucosal immunology expertise; B.L.H. conceived and performed the analyses under the supervision of P.A.A. and drafted the manuscript; all authors critically revised the manuscript and approved the final version for submission. *Dr. David Leslie passed away in July 2018. He made vital contributions to the VPCNSS and particularly to the understanding of the overlapping epidemiology of syphilis and HIV among MSM in Victoria.
Financial support. Funding for VPCNSS was received from the Department of Health and Human Services, Victorian Government. Burnet Institute receives ongoing infrastructure support from the Victorian Government. C.K.F., E.P.F.C., M.S., G.T., and M.H. have received and/or currently receive personal fellowship funding from the Australian National Health and Medical Research Council.
Potential conflicts of interest. M.H. and M.S. have received unrelated investigator-initiated funding from Gilead Sciences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sullivan PS, Hamouda O, Delpech V, et al. Annecy MSM Epidemiology Study Group Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996-2005. Ann Epidemiol 2009; 19:423–31. [DOI] [PubMed] [Google Scholar]
- 2. Savage EJ, Hughes G, Ison C, Lowndes CM. Syphilis and gonorrhoea in men who have sex with men: a European overview. Euro Surveill 2009; 14(47). [DOI] [PubMed] [Google Scholar]
- 3. Aral SO, Fenton KA, Holmes KK. Sexually transmitted diseases in the USA: temporal trends. Sex Transm Infect 2007; 83:257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Read P, Fairley CK, Chow EP. Increasing trends of syphilis among men who have sex with men in high income countries. Sex Health 2015; 12:155–63. [DOI] [PubMed] [Google Scholar]
- 5. Chapin-Bardales J, Schmidt AJ, Guy RJ, et al. Trends in human immunodeficiency virus diagnoses among men who have sex with men in North America, Western Europe, and Australia, 2000-2014. Ann Epidemiol 2018; 28:874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirby Institute. HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia: Annual Surveillance Report 2017. Sydney, Australia: Kirby Institute; 2017. [Google Scholar]
- 7. STIs in Gay Men Action Group. Australian STI & HIV testing guidelines 2014 for asymptomatic MSM. 2014. Available at: https://stipu.nsw.gov.au/stigma/stihiv-testing-guidelines-for-msm. Viewed 8 June 2019. [Google Scholar]
- 8. Wilkinson AL, El-Hayek C, Spelman T, et al. A ‘test and treat’ prevention strategy in Australia requires innovative HIV testing models: a cohort study of repeat testing among ‘high-risk’ men who have sex with men. Sex Transm Infect 2016; 92:464–6. [DOI] [PubMed] [Google Scholar]
- 9. Mayer KH. Sexually transmitted diseases in men who have sex with men. Clin Infect Dis 2011; 53(Suppl 3):S79–83. [DOI] [PubMed] [Google Scholar]
- 10. Cheung KT, Fairley CK, Read TR, et al. HIV incidence and predictors of incident HIV among men who have sex with men attending a sexual health clinic in Melbourne, Australia. PLoS One 2016; 11:e0156160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pathela P, Braunstein SL, Blank S, et al. The high risk of an HIV diagnosis following a diagnosis of syphilis: a population-level analysis of New York City men. Clin Infect Dis 2015; 61:281–7. [DOI] [PubMed] [Google Scholar]
- 12. Katz DA, Dombrowski JC, Bell TR, et al. HIV incidence among men who have sex with men after diagnosis with sexually transmitted infections. Sex Transm Dis 2016; 43:249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooley LA, Pearl ML, Flynn C, et al. Low viral suppression and high HIV diagnosis rate among men who have sex with men with syphilis—Baltimore, Maryland. Sex Transm Dis 2015; 42:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salado-Rasmussen K, Katzenstein TL, Gerstoft J, et al. Risk of HIV or second syphilis infection in Danish men with newly acquired syphilis in the period 2000-2010. Sex Transm Infect 2013; 89:372–6. [DOI] [PubMed] [Google Scholar]
- 15. Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 2010; 53:537–43. [DOI] [PubMed] [Google Scholar]
- 16. Guy RJ, Spelman T, Stoove M, et al. Risk factors for HIV seroconversion in men who have sex with men in Victoria, Australia: results from a sentinel surveillance system. Sex Health 2011; 8:319–29. [DOI] [PubMed] [Google Scholar]
- 17. Hsu KK, Molotnikov LE, Roosevelt KA, et al. Characteristics of cases with repeated sexually transmitted infections, Massachusetts, 2014-2016. Clin Infect Dis 2018; 67:99–104. [DOI] [PubMed] [Google Scholar]
- 18. Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaughan AS, Kelley CF, Luisi N, et al. An application of propensity score weighting to quantify the causal effect of rectal sexually transmitted infections on incident HIV among men who have sex with men. BMC Med Res Methodol 2015; 15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masson L, Mlisana K, Little F, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 2014; 90:580–7. [DOI] [PubMed] [Google Scholar]
- 21. Esra RT, Olivier AJ, Passmore JA, et al. Does HIV exploit the inflammatory milieu of the male genital tract for successful infection? Front Immunol 2016; 7:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goller JL, Guy RJ, Gold J, et al. Establishing a linked sentinel surveillance system for blood-borne viruses and sexually transmissible infections: methods, system attributes and early findings. Sex Health 2010; 7:425–33. [DOI] [PubMed] [Google Scholar]
- 23. Feigin A, El-Hayek C, Hellard M, et al. Increases in newly acquired HIV infections in Victoria, Australia: epidemiological evidence of successful prevention? Sex Health 2013; 10:166–70. [DOI] [PubMed] [Google Scholar]
- 24. Ampt FH, El Hayek C, Agius PA, et al. Anorectal swabs as a marker of male-to-male sexual exposure in STI surveillance systems. Epidemiol Infect 2017; 145:2530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelley CF, Kraft CS, de Man TJ, et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention. Mucosal Immunol 2017; 10:996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu J, Hladik F, Woodward A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med 2009; 15:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Feinen B, Russell MW. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol 2011; 2:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peters J, Cresswell F, Amor L, et al. Whole genome sequencing of Neisseria gonorrhoeae reveals transmission clusters involving patients of mixed HIV serostatus. Sex Transm Infect 2018; 94:138–43. [DOI] [PubMed] [Google Scholar]
- 29. Heymans R, A Matser A, Bruisten SM, et al. Distinct Neisseria gonorrhoeae transmission networks among men who have sex with men in Amsterdam, the Netherlands. J Infect Dis 2012; 206:596–605. [DOI] [PubMed] [Google Scholar]
- 30. Cornelisse VJ, Fairley CK, Stoove M, et al. PrEPX Study Team Evaluation of preexposure (PrEP) eligibility criteria, using sexually transmissible infections as markers of human immunodeficiency virus (HIV) risk at enrollment in PrEPX, a large Australian HIV PrEP trial. Clin Infect Dis 2018; 67:1847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis 2018; 67:676–86. [DOI] [PubMed] [Google Scholar]
- 32. Traeger MW, Cornelisse VJ, Asselin J, et al. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA 2019; 321:1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]