Abstract
Context
Teriparatide and denosumab are effective treatments for osteoporosis and typically reserved as second-line options after patients have used bisphosphonates. However, limited head-to-head comparative effectiveness data exist between teriparatide and denosumab.
Objective
We compared changes in bone mineral density (BMD) between groups treated with teriparatide or denosumab after using bisphosphonates, focusing on the change in BMD while on either drug over 2 years.
Design
Observational cohort study using electronic medical records from two academic medical centers in the United States.
Participants
The study population included osteoporotic patients >45 years who received bisphosphonates >1 year before switching to teriparatide or denosumab.
Outcome Measures
Annualized BMD change from baseline at the lumbar spine, total hip, and femoral neck.
Results
Patients treated with teriparatide (n = 110) were compared with those treated with denosumab (n = 105); the mean (SD) age was 70 (10) years and median duration (interquartile range) of bisphosphonate use was 7.0 (5.6 to 9.7) years. Compared with denosumab users, teriparatide users had higher annualized BMD change at the spine by 1.3% (95% CI 0.02, 2.7%) but lower at the total hip by −2.2% (95% CI −2.9 to −1.5%) and the femoral neck by −1.1% (95% CI −2.1 to −0.1%). Those who switched to teriparatide had a transient loss of hip BMD for the first year, with no overall increase in the total hip BMD over 2 years.
Conclusions
Among patients who use long-term bisphosphonates, the decision of switching to teriparatide should be made with caution, especially for patients at high risk of hip fracture.
This is a head-to-head comparison study using 14 years of electronic medical records. We compared teriparatide with denosumab on BMD in patients switching from long-term bisphosphonate.
Therapeutic options for osteoporosis have increased over the past two decades (1). Bisphosphonates are the most widely used antiosteoporosis agents in clinical practice (2–4). The anabolic agent teriparatide (human parathyroid hormone 1-34) and the antiresorptive agent denosumab (monoclonal antibody to receptor activator of nuclear factor-κB ligand) are potent drugs, often reserved as second-line treatments for patients who lose bone mineral density (BMD) or fracture while on a bisphosphonate or who have severe disease (1).
In randomized controlled trials (RCTs) of bisphosphonate-naïve patients, the estimated fracture risk reduction using denosumab was 68% for vertebral fractures and up to 20% for nonvertebral fractures at 12 months compared with the placebo (5). In similar trials, teriparatide reduced vertebral fractures by 65% and nonvertebral fractures by 63% compared with the placebo over a median follow-up of 21 months (6). However, there is some evidence that prior antiresorptive therapy, in particular, bisphosphonates, may influence the effects of both teriparatide (7–12) and denosumab (13). Over 63% of teriparatide users (14) and 54% of denosumab (15) users in the United States had been prescribed a prior antiosteoporosis agent, mostly bisphosphonates. Thus, the therapeutic effect of teriparatide and denosumab in typical clinical practice may not be the same as reported in clinical trials.
There is only one head-to-head RCT comparing denosumab and teriparatide that included participants who switched from long-term bisphosphonates, but almost two-thirds of patients in this trial were bisphosphonate naïve (16). This head-to-head trial showed that denosumab and teriparatide improved BMD similarly at the lumbar spine, total hip, and femoral neck over 24 months. An indirect meta-analysis that included mostly bisphosphonate-naïve patients showed that teriparatide increased BMD 2.6% more than denosumab at the spine but 1.3% less than denosumab at the total hip over 24 months (17). These data conflict regarding the optimal medication if further treatment is needed after bisphosphonate use.
We used real-world data to compare the effectiveness of a switch to teriparatide vs denosumab on BMD in patients with prior long-term bisphosphonate use.
Methods
Study design
In a group of patients who had used bisphosphonates for >12 months, we compared changes in BMD between those switching to teriparatide or denosumab. The primary outcomes were the differences in annualized BMD change from baseline between two agents at the lumbar spine, total hip, and femoral neck for 2 years.
Study population and data sources
Partners HeathCare electronic medical record (EMR) is used by several hospitals, including Brigham and Women’s Hospital and Massachusetts General Hospital. These hospitals provide care for ∼4.6 million patients in and around Boston, Massachusetts. We used the medical records of patients who took osteoporosis medications from January 2004 to December 2017.
Potentially eligible patients were over 45 years of age and had used at least 12 months of prior bisphosphonate, including alendronate, ibandronate, risedronate, pamidronate, or zoledronic acid. They were required to have subsequently used teriparatide or denosumab for >6 months and undergone at least two dual-energy X-ray absorptiometry (DXA) scans, as detailed below. From this group of potentially eligible patients, the following exclusion criteria were applied: a history of Paget’s disease, simultaneous use of denosumab, teriparatide and/or bisphosphonates, high-dosage denosumab (120 mg/month; prescribed for cancer patients), and a prior course of teriparatide. The Partners HealthCare Institutional Review Board approved all aspects of this study.
Exposure and outcome assessment
The exposure of interest was treatment with teriparatide or denosumab after at least 12 months of bisphosphonate use. First, we identified all patients who had a least one prescription of teriparatide or denosumab through an automated search of the EMR, and then drug-use details (duration and dosage) were verified by one author (H.L.) through chart review. For each patient, the dose, duration, and reason for discontinuation were documented based on the chart review. The date of the first dose of denosumab or teriparatide was defined as the index date. We classified drug brand or generic names into four categories: oral bisphosphonates (alendronate 10 mg once daily or 70 mg once weekly, ibandronate 150 mg once monthly, risedronate 35 mg once weekly, 75 mg on 2 consecutive days every month or 150 mg once monthly), intravenous bisphosphonate (zoledronic acid 5 mg once yearly or 2.5 mg every 6 months, ibandronate 3 mg every 3 months, pamidronate 60 mg every 6 months or 30 mg every 3 months), denosumab (60 mg subcutaneous every 6 months), and teriparatide (20 μg subcutaneous daily) for each patient.
We extracted BMD (grams per square centimeter) from routine DXA scans (QDR 4500/4500A; Hologic, Bedford, MA) of the posteroanterior lumbar spine, total hip, and femoral neck. The baseline DXA test window was defined as 2 years before through 3 months after the index date. Follow-up of teriparatide and denosumab use was truncated at 27 months to achieve similar drug-exposure durations for both treatment groups. Thus, the follow-up DXA test window was defined as 6 to 27 months after the index date so as to include all qualified DXA tests in this window. For patients with multiple DXA tests within the baseline or follow-up window, the DXA closest to the index date or last date of drug use was chosen. All interim DXA tests between baseline and last DXA were included for analysis (18).
Covariate assessment
Patient characteristics were collected from the EMR. Variables of interest included age, sex, race, body mass index (BMI), other medications related to BMD [hormone replacement therapy (HRT), raloxifene, glucocorticoids], and comorbidities included in the Charlson comorbidity index (19). Comorbidities were defined using corresponding International Classification of Diseases, 9th or 10th Revision, Clinical Modification codes before the index date. We also collected information on prior fragility fractures (20), defined as those occurring in the year before the index date. Prior bisphosphonate treatment (duration and washout period) was verified by chart review. Duration of prior bisphosphonate use was defined as the combined duration of all bisphosphonates (alendronate, ibandronate, risedronate, pamidronate, or zoledronic acid). The washout period was defined as the interval between bisphosphonate cessation and initiation of denosumab or teriparatide.
Statistical analyses
Baseline characteristics were compared between the two groups using descriptive statistics. There were imbalances between the two groups in baseline characteristics; thus, we used matching weights—an extension of inverse probability of the treatment weighting method—to improve balance across the two treatment groups (21, 22). We first fit a propensity score logistic model in which the treatment group (teriparatide or denosumab) was the dependent variable, and all potential confounders (age, sex, race, BMI, prior oral bisphosphonate duration, prior intravenous bisphosphonate duration, prior bisphosphonate washout period, baseline BMD, prior fragility fracture, glucocorticoids history, HRT history, raloxifene history, hyperthyroidism, any malignancy, renal disease, rheumatoid arthritis, osteoarthritis, esophageal disease, diabetes, anemia, hypertension, hyperlipidemia, congestive heart disease, peripheral cardiovascular disease, stroke, chronic obstructive pulmonary disease, hemiplegia or paraplegia, and Charlson comorbidity index) were independent variables. The predicted probability from this model represents each patient’s probability of receiving teriparatide. These probabilities were used to assign each patient a weight, such that the weighted teriparatide group and weighted denosumab group were balanced in their baseline characteristics (23), similar to a 1:1 propensity score-matched cohort. In contrast to propensity score matching, the weighting method retains all patients, thereby maximizing the use of all available data (21, 22).
We then used weighted generalized estimating equations to compare BMD change in the treatment groups. Change in BMD from baseline to follow-up was modeled as a linear term to provide annualized change estimates. The models included interaction terms between the treatment group and the time variable; their coefficients are interpreted as the difference in annualized change between the two treatments. To aid interpretability and comparability with prior clinical trials (5, 6, 24–26), we calculated the percentage change in BMD from baseline. To explore nonlinear BMD changes, we performed the same analysis with time categorized into baseline (−24 to 3 months), 12 months (9 to 15 months), and 24 months (21 to 27 months). As consolidation with antiresorptive agents are typically recommended after 2 years of teriparatide, we also estimated the BMD response through the consolidation stage, thus assessing denosumab over 4 years and teriparatide for 2 years plus 2 further years of consolidation.
We performed a series of sensitivity analyses to test the robustness of the primary analysis. First, we conducted a 1:1 propensity score-matched analysis using greedy matching within a caliper of 0.1 SDs of the propensity score to provide estimates for a subgroup matched for baseline characteristics. Second, as percentage change from baseline might be vulnerable to extreme values, we repeated the same analysis with actual BMD (grams per square centimeter) and converted resulting differences to percentage change from the mean baseline BMD (27). Third, we repeated the above analyses, excluding patients who had baseline DXA >12 months before the index date to improve the accuracy of baseline BMD. Fourth, we excluded patients with very low BMD (the lowest 10%) to improve comparability further between the two groups. Fifth, patients who did not complete 2 years of treatment were excluded. Sixth, patients in the teriparatide group with the index date before 6 June 2010 were excluded, as denosumab was not on the market before this date. Last, we excluded rare cases of patients who had >10 years of prior bisphosphonate use. All analyses were performed using R-3.4.3 (The Comprehensive R Archive Network; https://cran.r-project.org).
Results
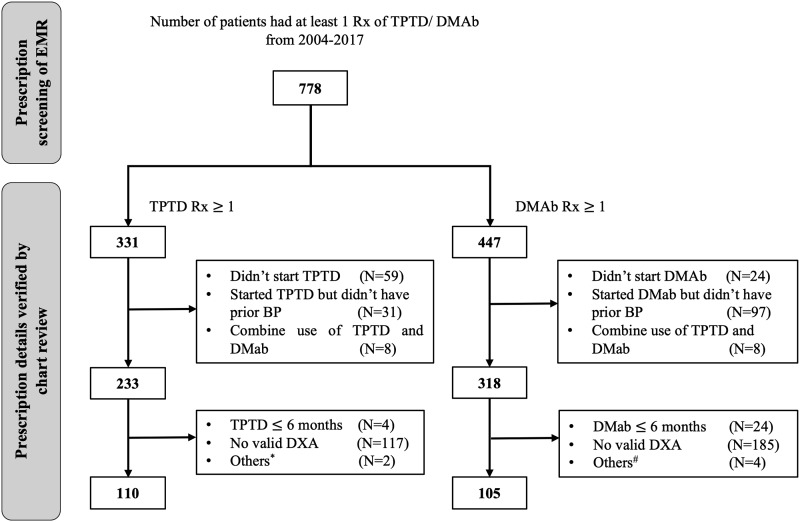
Among 778 patients with at least one prescription of denosumab or teriparatide, 215 patients were eligible for the current analysis (Fig. 1). Patients were 94% women with a mean (SD) age of 70 (10) years. The median duration (interquartile range) of prior bisphosphonate use was 7.0 (5.6 to 9.7) years.
Figure 1.
Flowchart showing the cohort selection process. BP, bisphosphonate; DMAb, denosumab; Rx, prescription; TPTD, teriparatide.* Used DMAb before. # Used TPTD before, uncertain DMAb use, or DMAb prescribed monthly for cancer patients.
The baseline characteristics of the two exposure groups are shown in Table 1. Most baseline characteristics were quite similar between the two groups. The teriparatide group had lower BMD at all three anatomic sites (the lumbar spine, total hip, and femoral neck), shorter duration of prior bisphosphonate, and lower prevalence of prior fractures than the denosumab group. After the application of propensity score-based weighting, baseline characteristics were well balanced across both exposure groups (28). Potential confounders, such as age, BMI, hyperthyroidism, esophageal disease, prior fragility fracture, any malignancy, hemiplegia/paraplegia, rheumatoid arthritis, osteoarthritis, baseline BMD (the lumbar spine, total hip, and femoral neck), and prior bisphosphonate treatment duration, were all balanced between the two exposure groups (Table 1). In the 1:1 propensity score-matched subset, the above-mentioned potential confounders were also adequately balanced (29).
Table 1.
Baseline Characteristics of Study Cohorts Before and After Weighting
| Variables | Unweighted Cohort | Weighted Cohort | ||||||
| DMAb | TPTD | SMD | DMAb | TPTD | SMD | |||
| n | 105 | 110 | ||||||
| Age, mean | 70.2 | 70.3 | 0.014 | 67.9 | 66.8 | 0.018 | ||
| Men, % | 7.6 | 4.5 | 0.129 | 5.4 | 5.8 | 0.015 | ||
| Race, white, % | 93.3 | 91.8 | 0.058 | 90.2 | 90.3 | 0.003 | ||
| BMI, mean | 24.1 | 22.8 | 0.287 | 23.2 | 23.9 | 0.049 | ||
| Smoking history, % | 23.8 | 10.9 | 0.346 | 12.2 | 12.0 | 0.010 | ||
| Obesity, % | 18.1 | 8.2 | 0.297 | 9.6 | 11.9 | 0.082 | ||
| Hyperthyroidism, % | 12.4 | 12.7 | 0.010 | 13.1 | 13.8 | 0.020 | ||
| Esophagus disease, % | 54.3 | 41.8 | 0.252 | 42.3 | 42.2 | 0.002 | ||
| Any malignancy, % | 36.2 | 11.8 | 0.595 | 16.8 | 17.0 | 0.005 | ||
| Renal disease, % | 29.5 | 9.1 | 0.536 | 10.6 | 11.4 | 0.024 | ||
| Diabetes, % | 24.8 | 14.5 | 0.259 | 17.0 | 16.6 | 0.012 | ||
| Hypertension, % | 64.8 | 57.3 | 0.154 | 57.6 | 57.0 | 0.012 | ||
| Hyperlipidemia, % | 78.1 | 69.1 | 0.205 | 70.8 | 72.4 | 0.034 | ||
| Cerebrovascular disease, % | 18.1 | 19.1 | 0.026 | 11.3 | 9.7 | 0.053 | ||
| Chronic pulmonary disease, % | 36.2 | 37.3 | 0.022 | 37.7 | 38.0 | 0.006 | ||
| Anemia, % | 48.6 | 37.3 | 0.230 | 43.9 | 45.3 | 0.028 | ||
| Hemiplegia or paraplegia, % | 21.9 | 14.5 | 0.192 | 13.7 | 14.2 | 0.015 | ||
| Rheumatoid arthritis, % | 11.4 | 13.6 | 0.067 | 5.9 | 5.5 | 0.014 | ||
| Osteoarthritis, % | 65.7 | 59.1 | 0.137 | 59.2 | 57.4 | 0.036 | ||
| Charlson comorbidity index, mean | 3.8 | 2.1 | 0.554 | 2.4 | 2.6 | 0.013 | ||
| Fractures | ||||||||
| Fragility fracture, % | 36.2 | 50.9 | 0.300 | 39.4 | 36.3 | 0.063 | ||
| BMD | ||||||||
| Lumbar spine, T-score | −2.3 | −2.7 | 0.396 | −2.4 | −2.3 | 0.061 | ||
| Total hip, T-score | −1.9 | −2.3 | 0.467 | −2.0 | −1.9 | 0.046 | ||
| Femoral neck, T-score | −2.3 | −2.5 | 0.372 | −2.3 | −2.2 | 0.057 | ||
| Lumbar spine, g/cm2 | 0.79 | 0.74 | 0.402 | 0.77 | 0.78 | 0.059 | ||
| Total hip, g/cm2 | 0.71 | 0.66 | 0.474 | 0.70 | 0.71 | 0.042 | ||
| Femoral neck, g/cm2 | 0.60 | 0.57 | 0.384 | 0.60 | 0.61 | 0.054 | ||
| Osteoporosis agents | ||||||||
| Prior oral BP duration, y | 5.9 | 6.8 | 0.199 | 6.6 | 6.7 | 0.008 | ||
| Prior intravenous BP duration, y | 1.4 | 0.8 | 0.271 | 1.1 | 1.2 | 0.045 | ||
| Glucocorticoids, % | 60.0 | 55.5 | 0.092 | 57.0 | 54.0 | 0.067 | ||
| HRT, % | 36.2 | 46.4 | 0.208 | 44.7 | 41.9 | 0.057 | ||
| Raloxifene, % | 9.5 | 13.6 | 0.129 | 11.9 | 11.3 | 0.016 | ||
| BP washout period, mo | 24.4 | 9.7 | 0.607 | 14.6 | 15.7 | 0.043 | ||
Abbreviation: BP, bisphosphonate; DMAb, denosumab; SMD, standardized mean difference; TPTD, teriparatide.
Differences in BMD change between teriparatide and denosumab
In the weighted analyses, denosumab significantly increased BMD at all three anatomic sites (the lumbar spine, total hip, and femoral neck), whereas teriparatide only significantly increased BMD at the lumbar spine (Table 2). For >2 years, compared with denosumab, teriparatide users had a greater annualized BMD increase at the spine by 1.3% (95% CI 0.02% to 2.7%, P = 0.046) but also a greater annualized BMD loss at the total hip by −2.2% (95% CI −2.9 to −1.5%, P < 0.001) and femoral neck by −1.1% (95% CI −2.1 to −0.1%, P = 0.029).
Table 2.
Difference in Annualized Percentage BMD Change Between Denosumab and Teriparatide Over 2 Years
| Site | Therapy | Mean Annualized BMD Changes From Baseline, % (95% CI) | Difference Between Teriparatide and Denosumab, % (95% CI) | P Value |
|---|---|---|---|---|
| Lumbar spine | Denosumab | 3.1 (2.3, 3.9) | Reference | |
| Teriparatide | 4.4 (3.4, 5.5) | 1.3 (0.02, 2.7) | 0.046 | |
| Total Hip | Denosumab | 1.9 (1.5, 2.4) | Reference | |
| Teriparatide | −0.3 (−0.8, 0.3) | −2.2 (−2.9, −1.5) | <0.001 | |
| Femoral neck | Denosumab | 1.8 (1.2, 2.4) | Reference | |
| Teriparatide | 0.7 (−0.2, 1.5) | −1.1 (−2.1, −0.1) | 0.029 |
Weighted generalized estimating equations were used to compare BMD change in the weighted cohorts. Change in BMD from baseline to follow-up was modeled as a linear term to provide annualized change estimate.
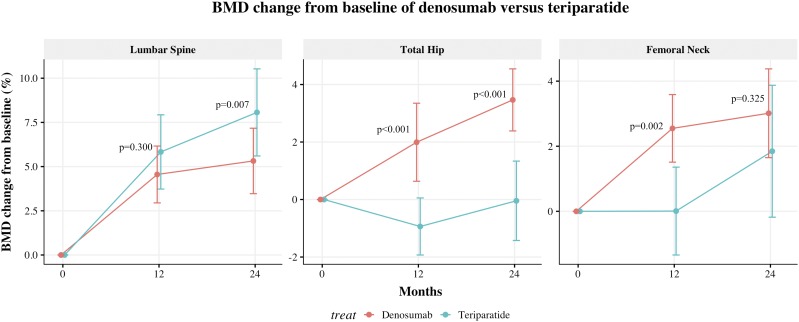
Nonlinear BMD change trajectories for teriparatide and denosumab are shown in Fig. 2. Teriparatide and denosumab demonstrated different changes in BMD; patients who switched to teriparatide showed a nonsignificant trend for greater increases in lumbar spine BMD than denosumab through the first 2 years. However, teriparatide users had BMD loss at the hip (both total hip and femoral neck) in the first year, with no overall change for over 2 years. During the consolidation stage, teriparatide users had a continued BMD response at the lumbar spine through 36 and 48 months, but responses at the hip areas were lower compared with values observed at the lumbar spine (30).
Figure 2.
BMD change trajectories of the switch to denosumab vs teriparatide in patients with prior bisphosphonate use. Teriparatide and denosumab demonstrated different changes in BMD; patients who switched to teriparatide showed a nonsignificant trend for greater increases in lumbar spine BMD than denosumab through the first 2 y. However, teriparatide users had BMD loss at the hip (both total hip and femoral neck) in the first year, with no overall change over 2 y. Time categorized into baseline (−24 to 3 mo), 12 mo (9 to 15 mo), and 24 mo (21 to 27 mo).
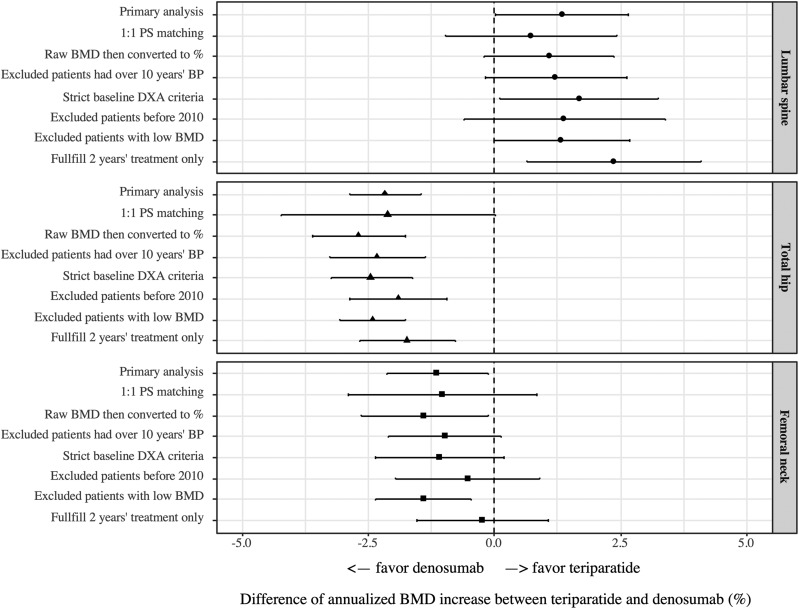
Sensitivity analyses
Effect-size estimates from sensitivity analyses were consistent with the primary analysis (Fig. 3). As most sensitivity analyses only included a subset of the original study population, especially for the 1:1 propensity score-matched analysis, they were less efficient and had wider CIs than the primary analysis. For lumbar spine BMD, differences between the two treatments ranged from 0.7% to 2.4%. For the total hip, teriparatide had a lower annualized BMD increase than denosumab, with estimated differences ranging from −1.7 to −2.7%. At the femoral neck, teriparatide again had lower annualized BMD increases than denosumab, with the estimated difference ranging from −0.2 to −1.4%.
Figure 3.
Sensitivity analyses for the BMD increase differences between denosumab and teriparatide. Effect-size estimates from sensitivity analyses were consistent with the primary analysis at all the three sites (the lumbar spine, total hip, and femoral neck). PS, propensity score.
Discussion
In this observational study of long-term bisphosphonates users, the annualized BMD increase after the switch to teriparatide was 1.3% higher at the lumbar spine and lower by 2.2% at the total hip and 1.1% at the femoral neck compared with the switch to denosumab. Those who switched to teriparatide had a transient loss of hip BMD for the first year, with no overall increase in total hip BMD over 2 years. In patients with long-term bisphosphonate use, our results suggest that clinical decisions to switch to teriparatide should be made with caution, especially for patients at high risk of hip fracture.
In our study, the 2.2% annual difference in total hip BMD between teriparatide and denosumab groups and 1.1% at the femoral neck may suggest a clinically meaningful difference in fracture risk reduction. BMD change is regarded as the most important surrogate for the evaluation of a therapeutic response. A recent meta-analysis of 21 randomized trials showed that changes in hip BMD over 2 years explained 60% to 65% of the treatment-related reduction in fracture risk (31), although only some of the data are from patients with prior bisphosphonate use. More specifically, a 3% increase in hip BMD at 1 year was associated with a 46% reduction in nonvertebral fracture risk (32).
The efficacy of teriparatide and denosumab is different between bisphosphonate-naïve patients and long-term bisphosphonates users. Previous results of randomized clinical trials found that teriparatide increased total hip BMD by 2.6% (6) and denosumab 3.6% at 12 months in treatment-naive patients (26). However, in long-term, bisphosphonate-treated patients, the effect sizes were much smaller: the total hip BMD increase at 12 months after switching to teriparatide was −0.9% and denosumab 2.0% (Fig. 2).
The hip BMD response using teriparatide in prior bisphosphonates users was less than expected. There are possible mechanistic reasons for these findings. With long-term bisphosphonate use, bone turnover is inhibited, and cortical bone is highly mineralized. At cortical sites, such as the hip, teriparatide induces absorption of old bone matrix and apposition of new bone matrix not yet fully mineralized (8, 33, 34). A transient fall in BMD can be seen at the beginning of teriparatide therapy as a result of the resorption of highly mineralized old bone and increased cortical porosity (8, 35, 36). BMD then slowly increases with ongoing treatment as new bone fully mineralizes. In our patients who had a median duration of prior bisphosphonate use of 7 years, BMD gained by new bone mineralization may be offset by old bone resorption for at least the first year. In contrast, denosumab binds and inhibits the receptor activator of the nuclear factor-κB ligand to achieve extensive suppression of bone turnover and increases BMD at all skeletal sites (37). The switch to denosumab increases BMD even after long-term antiresorptive therapy (38). A transition to denosumab from alendronate produced greater BMD increase at all measured anatomic sites and a further reduction in biochemical markers of bone turnover (38).
The poor hip BMD response in patients switching from bisphosphonates to teriparatide highlights the importance of a drug sequence when using anabolic and antiresorptive agents (7, 9, 16, 39–41). Cosman et al. (7) summarized BMD changes at the hip in various published clinical trials investigating the effects of teriparatide when used after an antiresorptive agent. BMD, at the hip, fell below baseline values for the first 12 months after switching, resulting a decrease of −2.7 to −0.3% in total hip BMD, but returned to baseline at 18 months (−1.7%–0.9%) and almost increased above baseline by 24 months (−0.7% to 2.9%) (8–10, 42, 43). Our study showed similar BMD trajectories: hip BMD dropped for the first 12 months and then returned to the baseline level. As the switch to teriparatide in prior bisphosphonate-treated patients does not achieve optimal BMD gain at all sites, and teriparatide can only be used for 24 months, this routinely used strategy needs examination.
To maximize the treatment effect, substantial data suggest use of teriparatide before bisphosphonates (44–46). In one study, teriparatide followed by bisphosphonates, had better BMD gains than bisphosphonates followed by teriparatide (47). Over a period of 19 to 24 months, teriparatide achieved an average gain of ∼3% in the hip area (total hip and femoral neck). After teriparatide, the transition to a bisphosphonate led to a 2% additional increase in the hip area after 1 year (46). We evaluated prescription patterns in our study population and observed that teriparatide followed by bisphosphonates, was rarely used. The most widely used pattern in the last decade at Partners HealthCare was bisphosphonates followed by teriparatide. We examined the BMD increase profile of this pattern and did not identify a relative gain in BMD during the 2-year treatment compared with teriparatide followed with antiresorptive agents in a prior study (46). Thus, in patients who are likely to require more than one drug, previous sequential studies (8–10, 42, 46, 48) and our results suggest initial use of teriparatide, followed by an antiresorptive, as an alternative choice to achieve maximal gains in BMD (41).
The main strength of this study is that we used 14 years of observational data to emulate a randomized trial, comparing the effectiveness of denosumab with teriparatide when an RCT is not available. Whereas theoretically possible, it is unlikely that an RCT will ever be conducted for this question. Thus, results of the current study provide an important piece of information for clinical decisionmaking. This study not only showed a transient decrease for teriparatide in the hip areas but also provided a contrast with denosumab, suggesting that the switch to teriparatide should be made with caution, especially for patients at high risk of hip fracture. We applied several rigorous methods to reduce bias and confounding in both study design and data analysis. First, we used an active-comparator and new-user design to help mitigate confounding by design and facilitate confounding adjustment by the establishment of correct temporality between pretreatment variables and drug exposure (49, 50). Second, we balanced the baseline characteristics between two groups using matching weights, an extension of inverse probability of the treatment weighting method, and estimated the BMD increase with marginal structural models.
Despite these rigorous methods, our study still has limitations. Unlike an RCT, which can balance both the measured and unmeasured confounders—head-to-head comparison with observational data can only balance the measured confounders using statistical approaches—there is a possibility for unmeasured confounding that could create bias. For example, concomitant use of proton pump inhibitors would reduce BMD; if patients who switched to teriparatide were more likely to use proton pump inhibitors, then this would be an unmeasured confounder. However, compared with the effect of bisphosphonate, glucocorticoids, and HRT, the effect of such proton pump inhibitors might be minor. Second, this was a retrospective study using routine clinical data; therefore, not all patients in the source population underwent sufficient numbers of DXA tests to describe BMD changes, leading to the exclusion of over one-half of the study population during the selection process. Current guidelines (1, 51) recommend the same DXA monitoring schedule (1 or 2 years after initiation of osteoporosis drugs) for patients who switched to denosumab or teriparatide. Thus, the risk of selection bias is low. Sensitivity analysis excluding patients who had a baseline DXA > 12 months before switching produced similar results. Third, our primary analysis assumed that patients who switched to denosumab or teriparatide were from the same population, despite teriparatide and denosumab having different marketing dates (2001 and 2010, respectively). An additional sensitivity analysis restricted to a switch after June 2010 reached the same conclusions. Fourth, the various bisphosphonates used in the period before the switch to teriparatide or denosumab have an inherent difference in efficacy, and our study did not have enough power to study the interaction between response and prior bisphosphonate type. Last, we did not evaluate the difference in fracture events as a result of low fracture incidence in the study cohorts. As the evidence on BMD change and fracture risk reduction are based on data using antiresorptive agents, further studies using fracture endpoints are needed to confirm the efficacy difference between teriparatide and denosumab in patients treated with prior bisphosphonates.
Conclusion
Among long-term bisphosphonate users that switched to a different class of osteoporosis treatment, denosumab and teriparatide both increased BMD at the spine, but BMD increases at the total hip and femoral neck were greater in the denosumab group. The switch to teriparatide led to a transient BMD loss at the hip for the first year, but whether this loss affects fracture risk is unknown. In this particular population, our results suggest that the decision of the switch to teriparatide should be made with caution, especially for patients at high risk of hip fracture. Future trials or large observational studies that compare fracture end-points with special focus on the first 2 years after switching are needed to support our findings.
Acknowledgments
Financial Support: This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant NIH-P30-AR072577 (to D.H.S.). H.L. received a scholarship from the Chinese People’s Liberation Army General Hospital and received support from the Young Scientists Fund of the National Natural Science Foundation of China (81702176). K.Y. received financial support for his doctoral study from Harvard T.H. Chan School of Public Health (partially supported by training grants from the companies of Takeda, Pfizer, Bayer, and ASISA) and the Honjo International Scholarship Foundation. S.K.T. received support from the Lupus Foundation of America Career Development Award. D.H.S. received salary support from the National Institutes of Health Grant NIH-K24AR055989.
Author Contributions: H.L. and D.H.S. made a substantial contribution to the concept and design. H.L. and C.X. acquired the data. H.L., S.S.Z., K.Y., S.K.T., S.U.N., B.Z.L., and D.H.S. analyzed and interpreted the data and drafted and revised the article.
Additional Information
Disclosure Summary: D.H.S. has received salary support from Amgen for work unrelated to osteoporosis. B.Z.L. received research funding from Amgen. The remaining authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because the data were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Glossary
Abbreviations:
- BMD
bone mineral density
- BMI
body mass index
- DXA
dual-energy X-ray absorptiometry
- EMR
electronic medical record
- HRT
hormone replacement therapy
- RCT
randomized controlled trial
References and Notes
- 1. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis [published correction appears in Osteoporos Int. 2015;26(7):2045–2047]. Osteoporos Int. 2014;25(10):2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parikh S, Mogun H, Avorn J, Solomon DH. Osteoporosis medication use in nursing home patients with fractures in 1 US state. Arch Intern Med. 2008;168(10):1111–1115. [DOI] [PubMed] [Google Scholar]
- 3. Yusuf AA, Matlon TJ, Grauer A, Barron R, Chandler D, Peng Y. Utilization of osteoporosis medication after a fragility fracture among elderly Medicare beneficiaries. Arch Osteoporos. 2016;11(1):31. [DOI] [PubMed] [Google Scholar]
- 4. Yusuf AA, Cummings SR, Watts NB, Feudjo MT, Sprafka JM, Zhou J, Guo H, Balasubramanian A, Cooper C. Real-world effectiveness of osteoporosis therapies for fracture reduction in post-menopausal women. Arch Osteoporos. 2018;13(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis [published correction appears in N Engl J Med. 2009;361(19):1914]. N Engl J Med. 2009;361(8):756–765. [DOI] [PubMed] [Google Scholar]
- 6. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster J-Y, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH, Oefjord ES, Marcinowska-Suchowierska E, Salmi J, Mulder H, Halse J, Sawicki AZ, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. [DOI] [PubMed] [Google Scholar]
- 7. Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32(2):198–202. [DOI] [PubMed] [Google Scholar]
- 8. Boonen S, Marin F, Obermayer-Pietsch B, Simões ME, Barker C, Glass EV, Hadji P, Lyritis G, Oertel H, Nickelsen T, McCloskey EV; EUROFORS Investigators. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2008;93(3):852–860. [DOI] [PubMed] [Google Scholar]
- 9. Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, Burnett-Bowie S-AM. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386(9999):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cosman F, Wermers RA, Recknor C, Mauck KF, Xie L, Glass EV, Krege JH. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab. 2009;94(10):3772–3780. [DOI] [PubMed] [Google Scholar]
- 11. Cosman F, Nieves JW, Zion M, Garrett P, Neubort S, Dempster D, Lindsay R. Daily or cyclical teriparatide treatment in women with osteoporosis on no prior therapy and women on alendronate. J Clin Endocrinol Metab. 2015;100(7):2769–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cosman F, Keaveny TM, Kopperdahl D, Wermers RA, Wan X, Krohn KD, Krege JH. Hip and spine strength effects of adding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. J Bone Miner Res. 2013;28(6):1328–1336. [DOI] [PubMed] [Google Scholar]
- 13. Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH. Comparison of denosumab vs. bisphosphonates in osteoporosis patients: a meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2019;104(5):1753–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonafede MM, Shi N, Bower AG, Barron RL, Grauer A, Chandler DB. Teriparatide treatment patterns in osteoporosis and subsequent fracture events: a US claims analysis. Osteoporos Int. 2015;26(3):1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng L-I, Durden E, Limone B, Radbill L, Juneau PL, Spangler L, Mirza FM, Stolshek BS. Persistance and compliance with osteroporosis therapies among women in a commercially insured population in the United States. J Manag Care Spec Pharm. 2015;21(9):824–833, 833a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, Burnett-Bowie S-AM, Neer RM, Leder BZ. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382(9886):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandema JW, Zheng J, Libanati C, Perez Ruixo JJ. Time course of bone mineral density changes with denosumab compared with other drugs in postmenopausal osteoporosis: a dose-response-based meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3746–3755. [DOI] [PubMed] [Google Scholar]
- 18. Lyu H, Zhao S, Yoshida K, Tedeschi SK, Xu C, Nigwekar SU, Leder BZ, Solomon DH Comparison of teriparatide and denosumab in patients switching from long-term bisphosphonate use. figshare 2019. Deposited 10 June 2019. 10.6084/m9.figshare.8248682.v3 [DOI] [PMC free article] [PubMed]
- 19. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 20. Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29(9):1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li L, Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat. 2013;9(2):215–234. [DOI] [PubMed] [Google Scholar]
- 22. Yoshida K, Hernández-Díaz S, Solomon DH, Jackson JW, Gagne JJ, Glynn RJ, Franklin JM. Matching weights to simultaneously compare three treatment groups: comparison to three-way matching. Epidemiology. 2017;28(3):387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113(521):390–400. [Google Scholar]
- 24. Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. [DOI] [PubMed] [Google Scholar]
- 25. Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349(13):1216–1226. [DOI] [PubMed] [Google Scholar]
- 26. McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ; AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821–831. [DOI] [PubMed] [Google Scholar]
- 27. Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyu H, Zhao SS, Yoshida K, Tedeschi SK, Xu C, Nigwekar SU, Leder BZ, Solomon DH Comparison of teriparatide and denosumab in patients switching from long-term bisphosphonate use. figshare 2019. Deposited 10 June 2019. 10.6084/m9.figshare.8248709.v5 [DOI] [PMC free article] [PubMed]
- 29. Lyu H, Zhao SS, Yoshida K, Tedeschi SK, Xu C, Nigwekar SU, Leder BZ, Solomon DH Comparison of teriparatide and denosumab in patients switching from long-term bisphosphonate use. figshare 2019. Deposited 10 June 2019. 10.6084/m9.figshare.8248715.v3 [DOI] [PMC free article] [PubMed]
- 30. Lyu H, Zhao SS, Yoshida K, Tedeschi SK, Xu C, Nigwekar SU, Leder BZ, Solomon DH Comparison of teriparatide and denosumab in patients switching from long-term bisphosphonate use. figshare 2019. Deposited 10 June 2019. 10.6084/m9.figshare.8248724.v2 [DOI] [PMC free article] [PubMed]
- 31. Black D, Vittinghoff E, Eastell R Change in BMD as a surrogate for fracture risk reduction in osteoporosis trials: results from pooled, individual-level patient data from the FNIH Bone Quality Project. In: Annual meeting of the American Bone and Mineral Research Society. September 28 – 1 October 2018; Montreal, Quebec. Summary 1070. [Google Scholar]
- 32. Miller PD. Bone density and markers of bone turnover in predicting fracture risk and how changes in these measures predict fracture risk reduction. Curr Osteoporos Rep. 2005;3(3):103–110. [DOI] [PubMed] [Google Scholar]
- 33. Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19(5):745–751. [DOI] [PubMed] [Google Scholar]
- 34. McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165(15):1762–1768. [DOI] [PubMed] [Google Scholar]
- 35. Lindsay R, Krege JH, Marin F, Jin L, Stepan JJ. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int. 2016;27(8):2395–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1-34, PTH 1-84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28(4):736–745. [DOI] [PubMed] [Google Scholar]
- 37. Anastasilakis AD, Polyzos SA, Makras P. Therapy of endocrine disease: denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur J Endocrinol. 2018;179(1):R31–R45. [DOI] [PubMed] [Google Scholar]
- 38. Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man H-S, San Martin J, Bone HG. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25(1):72–81. [DOI] [PubMed] [Google Scholar]
- 39. Leder BZ, Tsai JN, Uihlein AV, Burnett-Bowie S-AM, Zhu Y, Foley K, Lee H, Neer RM. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cosman F. Long-term treatment strategies for postmenopausal osteoporosis. Curr Opin Rheumatol. 2018;30(4):420–426. [DOI] [PubMed] [Google Scholar]
- 41. Leder BZ. Optimizing Sequential and Combined Anabolic and Antiresorptive Osteoporosis Therapy. JBMR Plus. 2018;2(2):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, Rosen CJ. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85(6):2129–2134. [DOI] [PubMed] [Google Scholar]
- 43. Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP; Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide Investigators. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93(10):3785–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18(11):1932–1941. [DOI] [PubMed] [Google Scholar]
- 45. Nishiyama KK, Cohen A, Young P, Wang J, Lappe JM, Guo XE, Dempster DW, Recker RR, Shane E. Teriparatide increases strength of the peripheral skeleton in premenopausal women with idiopathic osteoporosis: a pilot HR-pQCT study. J Clin Endocrinol Metab. 2014;99(7):2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, Halse J, Lindsay R, Dalsky GP, Mitlak BH. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res. 2005;20(9):1507–1513. [DOI] [PubMed] [Google Scholar]
- 47. Kurland ES, Heller SL, Diamond B, McMahon DJ, Cosman F, Bilezikian JP. The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone(1-34)]. Osteoporos Int. 2004;15(12):992–997. [DOI] [PubMed] [Google Scholar]
- 48. Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ; PaTH Study Investigators. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353(6):555–565. [DOI] [PubMed] [Google Scholar]
- 49. Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD, Narula HS, Pessah-Pollack R, Tangpricha V, Wimalawansa SJ, Watts NB. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis — 2016. Endocr Pract. 2016;22(Suppl 4):1–42. [DOI] [PubMed] [Google Scholar]