Abstract
Background and Aims
Maintaining disease remission improves outcomes for pregnant women with Crohn’s disease (CD). As symptoms may correlate poorly with disease activity in the gravid state, we investigated the utility of bowel sonography during pregnancy to assess disease activity.
Methods
We conducted a prospective observational cohort study of pregnant women with CD undergoing bowel sonography between July 1, 2012, and December 1, 2016. Clinically active disease was defined using standardized clinical indices (Harvey Bradshaw Index >4 for active disease). Sonographic findings were graded as inactive (normal, mild) or active (moderate, severe) by expert radiologists.
Results
There were 91 pregnancies in 82 CD patients. Symptoms were present in 12 pregnancies; however, eight (67%) had sonographic findings of inactive disease, and escalation of therapy was not initiated. Conversely, sonographically active disease in seven asymptomatic pregnancies resulted in four women escalating therapy. The remaining three women declined escalation of therapy, one had a miscarriage, and the other two women had persistently active disease on sonography and endoscopy at one-year postpartum.
Conclusions
Bowel ultrasound may detect subclinical inflammation in asymptomatic pregnant women with CD and stratify CD activity in symptomatic patients. Therefore, bowel sonography should be considered as a useful adjunct for the assessment of the pregnant woman with Crohn’s disease.
Keywords: Crohn’s disease, Pregnancy, Ultrasound
Women with inflammatory bowel disease (IBD) are more likely to experience adverse outcomes in pregnancy including preterm labour, caesarean section and delivering small for gestational age infants (1–4). While these adverse outcomes are more common when disease is active at time of conception or during pregnancy, a large community-based cohort study found a strong association between the presence of IBD and poor pregnancy outcomes even when the disease was symptomatically quiescent (5–9). However, the study design did not allow for assessment of subclinical disease activity, and it has been well documented that there is a poor correlation between symptoms and the presence of inflammation, especially in individuals with Crohn’s disease (CD) (10,11).
Recently, the goals of IBD care have shifted beyond symptom control to objective assessment of disease quiescence (12–14). This is of even more importance in the pregnant woman where the gravid state itself can result in nonspecific gastrointestinal symptoms that may be indistinguishable from a disease flare (15). Due to the perceived and potential risks of therapy on the fetus, confirmation of active inflammation is paramount during pregnancy, particularly before making therapeutic changes (16). However, existing biomarkers have not been established in pregnancy, with the validity of fecal calprotectin in pregnancy being inconsistently reported, while the performance of C-reactive protein (CRP) may be affected by the physiological changes of pregnancy (17). While a limited endoscopic evaluation (e.g., flexible sigmoidoscopy) can be safely performed, often without need for sedation in pregnant women with ulcerative colitis, ileocolonoscopy and diagnostic imaging (e.g., CT) for the assessment of more proximal colonic or small bowel disease activity in CD have to be used with prudence during pregnancy (18).
Bowel ultrasound has been shown to be feasible and accurate in the nonpregnant population with similar accuracy to CT and MRI in detecting inflammation and complications of IBD (19). Its role in the pregnant state has not been studied. In the pregnant population with IBD, the ideal disease monitoring option would confirm or refute that gastrointestinal symptoms are due to underlying disease activity; it would identify subclinical inflammation that would subsequently impact treatment decisions; and the test itself would be safe for both mother and baby. Therefore, the aim of this study was to evaluate the utility of bowel ultrasound and its impact on the clinical management of pregnant women with CD.
MATERIALS AND METHODS
Study Design and Participants
This prospective observational cohort study of women attending a dedicated IBD pregnancy clinic at the University of Calgary, Canada, was undertaken between July 1, 2012, and December 1, 2016. During the study period, there were 91 pregnancies in 82 women with CD. Twelve women with active disease on their initial scan had a bowel sonogram repeated during the pregnancy to document interval change in disease state. The majority of patients had an inflammatory phenotype (51 of 82, 62.2%) with an ileocolonic disease location (38 of 82, 46.3%) and were diagnosed between the ages of 17 and 40 years (62 of 82, 75.6%).
Approval by the Conjoint Health Research Ethics Board (CHREB) of the University of Calgary was obtained. Patients were seen by two physician investigators (authors YL and CHS) following a clinical care pathway with a clinic visit preconception (to facilitate counselling), during each trimester and three-months postpartum. All patients underwent an intrapartum bowel ultrasound. For the purpose of this study, patients were included if they (i) were adults (18 years or older); (ii) had a diagnosis of CD defined by standard endoscopic, radiologic and histologic parameters; and (iii) could provide informed consent. Patients were excluded from this study if they had isolated upper gastrointestinal CD or if there was no corresponding IBD pregnancy clinic visit within the same trimester of the bowel ultrasound examination.
Data were collected prospectively, including age at time of conception, gestational age, body mass index (BMI) at time of first clinic consult and disease phenotyping as defined by the Montreal Classification (20). Disease activity indices were employed, with active disease defined as a Harvey Bradshaw Index (HBI) of >4. Patients were booked routinely for an intrapartum bowel ultrasound, preferably before the end of the second trimester (to decrease the risk of the gravid uterus precluding views of the bowel). Patients with active disease on index bowel ultrasound may have received additional ultrasound evaluations during the pregnancy to evaluate interval disease stability as per routine clinical care. For the purpose of this study, we only included the first sonographic assessment for analysis. There was no patient in this study who had an initial normal baseline bowel ultrasound at the beginning of pregnancy who then underwent an interval ultrasound for suspected active disease.
All patients were scanned in an ultrasound facility within our tertiary institution, which has a strong focus on the evaluation of the bowel in IBD with sonographers and radiologists with a high level of acquired skill and expertise in IBD imaging. Bowel ultrasound scans were performed by sonographers but with results interpreted and finalized by radiologists. Sonographic findings were graded as inactive (quiescent, mild) or active (moderate, severe) disease based on a composite assessment of bowel wall thickness, inflammatory fat and blood flow on color Doppler imaging (21). Quality of the sonographic assessment was defined as the ability of the radiologist to visualize the extent of the diseased bowel segment and to grade the disease activity. This was categorized into either optimal or suboptimal quality. Complicated disease behaviour—including the presence of a stricture, fistula, perforation or inflammatory mass—was also documented. Laboratory markers (complete blood count, CRP, erythrocyte sedimentation rate [ESR] and serum albumin) performed within one month of the clinic visit or bowel ultrasound were also recorded.
Clinical Outcomes
The primary outcome of the study was to report the ability of bowel ultrasound to detect subclinical inflammation in pregnant women with CD who were clinically asymptomatic. The proportion of pregnancies in which there was discordance between clinical and sonographic findings was recorded. Secondary outcomes included the proportion of pregnancies in which bowel sonography did not reveal evidence of inflammation in an otherwise symptomatic patient. The resultant changes to clinical management in both scenarios (inflammation in asymptomatic patients or lack of inflammation in symptomatic patients) are described, and clinical factors which may have contributed to discordance between clinical and ultrasound findings were investigated. This included a priori clinical factors: BMI, Montreal classification, gestational age and history of bowel surgery.
Data Analysis
Statistical analysis was performed using SPSS 19.0 statistical software (IBM Corporation, Armonk, NY). Baseline patient characteristics were analyzed using standard descriptive statistics; medians with interquartile ranges (IQR) were calculated for continuous data, and proportions were calculated for categorical data. Univariate analysis for characteristics predictive of sonographic disease activity were performed. Variables for the univariate analysis were chosen a priori. This includes history of bowel surgery, body mass index (BMI), HBI score, and laboratory markers (hemoglobin level, serum total white cell count, platelet count, C-reactive protein level [CRP], serum albumin level and erythrocyte sedimentation rate). Nonparametric continuous variables were performed using the Mann-Whitney U test, and categorical data were calculated with the Fisher exact test. A P value <0.05 was considered to be statistically significant.
RESULTS
Details of the Study Cohort
Ninety-one pregnancies were identified in 82 CD patients during the study period. Baseline demographic details are summarized in Table 1.
Table 1.
Baseline demographics of subjects
| Crohn’s disease | |
|---|---|
| Patients (n) | 82 |
| Pregnancies (n) | 91 |
| Age at conception (year*, IQR) | 31 (29–34) |
| BMI at time of sonography (kg/m2*, IQR) | 24.3 (21.7–27.6) |
| Gestational age at time of sonography (weeks*, IQR) | 20.1 (13.2–24.6) |
| Time between sonography and clinical assessment (weeks*, IQR) | 2.3 (1.0–4.7) |
| Montreal classification | |
| Age at diagnosis, (n) | |
| A1 (less than 17 years old) | 20 (24.4%) |
| A2 (17 to 40 years old) | 62 (75.6%) |
| A3 (more than 40 years old) | 0 |
| Location of disease, (n) | |
| L1 (ileal) | 25 (30.5%) |
| L2 (colonic) | 19 (23.2%) |
| L3 (ileocolonic) | 38 (46.3%) |
| Disease behaviour, (n) | |
| B1 (inflammatory) | 51 (62.2%) |
| B2 (stricturing) | 10 (12.2%) |
| B3 (fistulizing) | 21 (25.6%) |
| p (+ perianal involvement) | 28 (34.1%) |
| History of bowel surgery, (n) | 27 (32.9%) |
| HBI score at sonography* (IQR) | 0 (0–2) |
| Sonographic findings, (n) | |
| Normal scan | 67 (73.6%) |
| Mild disease | 13 (14.3%) |
| Moderate disease | 4 (4.4%) |
| Severe disease | 7 (7.7%) |
| Sonographic assessment quality, (n) | |
| Optimal | 84 (92.3%) |
| Suboptimal | 7 (7.7%) |
IQR, Interquartile Ratio; HBI, Harvey Bradshaw Index; BMI, body mass index; *median
In keeping with the clinical care pathway, most bowel sonographic examinations were performed before the end of the second trimester (76 of 91, 83.5%), with 15 of 91 (16.5%) in the early third trimester. The median gestational age at time of the bowel ultrasound was 20.1 weeks (IQR: 13.2–24.6 weeks). The median interval between clinic consultation (at which time patient assessment and laboratory work was performed) and bowel sonography was 2.3 weeks (IQR 1.0–4.7). Most patients were in clinical remission with a median Harvey Bradshaw Index score of zero (IQR 0–2). Only 13 percent (12 of 91) of patients were clinically active with HBI score >4. The majority (80 of 91, 87.9%) of the sonographic assessments confirmed inactive disease. However, of the 79 patients who were thought to be in clinical remission, seven (8.9%) had active disease on sonographic assessments. [Table 2]
Table 2.
Concordance of sonographic findings in CD pregnancies (n = 91)
| Sonographic assessment | |||
|---|---|---|---|
| Inactive (80) | Active (11) | ||
| Clinical assessment (HBI) | Remission (79) | 72 | 7 |
| Active disease (12) | 8 | 4 | |
HBI: Harvey Bradshaw Index
The median BMI at time of bowel sonography was 24.3 kg/m2 (IQR 21.7–27.6), and despite the presence of a gravid uterus, the majority of sonographic evaluations (84 of 91, 92.3%) were of optimal quality with clear visualization of the bowel segments. For the remaining seven sonographic evaluations with suboptimal quality, there was no significant trend with trimester of pregnancy (trimester 1, n = 0; trimester 2, n = 6; trimester 3, n = 1); or location of disease (L1, n = 2; L2, n = 1; L3, n = 5).
Outcome of Cases with Discordant Clinical and Sonographic Findings
Discordance between clinical and sonographic findings occurred in 15 of 91 (16.5%) pregnancies in women with CD, as summarized in Table 2.
There were eight pregnancies in which the mothers were symptomatic but had sonographic findings of inactive disease. These individuals were reassured, and no further investigations or escalation of therapy was provided or required. Symptoms resolved spontaneously during subsequent follow-up visits; therefore, repeat ultrasounds were not organized. Within six months following delivery, one of the eight patients had flare requiring a change in biologic due to antidrug antibody. The eight symptomatic patients with inactive sonographic findings had a lower median HBI score and a higher median BMI compared with the four who were symptomatic with disease activity on sonography (6.5 versus 10.5, P = 0.048, and 25.8 versus 20.0 kg/m2, P = 0.02, respectively). (Table 3). Despite the elevated BMI, all were reported as having good quality scans.
Table 3.
Characteristics of pregnancies with clinically active disease as defined by an HBI of >4.
| HBI > 4 | Sonographically inactive disease (n = 8) | Sonographically active disease (n = 4) | P value |
|---|---|---|---|
| History of bowel surgery | 3 (37.5%) | 0 | 0.26 |
| BMI* (kg/m2, IQR) | 25.8 (25.0–33.0) | 20.0 (18.3–22.0) | 0.02 |
| HBI* (IQR) | 6.5 (6.0–11.5) | 10.5 (6.0–23.5) | 0.048 |
| Hemoglobin* (g/L) | 121 (116–131) | 115 (103– 123) | 0.55 |
| Total white cell count* (x109/L) | 8.4 (6.9–11.5) | 9.2 (6.2–10.8) | 1.00 |
| Platelet count* (x 109/L) | 262 (226–281) | 335 (241–421) | 1.00 |
| CRP *(mg/L, IQR) | 8.4 (5.4–10.3) | 13.8 (3.4–26.9) | 1.00 |
| Albumin level* (g/L) | 33 (30–34) | 34 (33–35) | 0.40 |
| ESR* (mm/h) | 20 (18–26) | 39 (28.5–52) | 0.21 |
*median; HBI, Harvey Bradshaw Index; BMI, body mass index; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate
Conversely, seven asymptomatic CD patients had sonographically active disease. Four of the patients underwent significant changes to their management; one was commenced on biologic therapy, the second was commenced on antibiotics for a sealed perforation with subsequent biologic initiation postpartum, the third was continued on biologic therapy to term (week 37) rather than cessation in the mid-third trimester, and the fourth was commenced on corticosteroids. The remaining three patients received close monitoring with repeat clinic consultations and repeat sonographic assessments. One patient underwent an obstetric ultrasound within the same month, which reported a subchorionic hemorrhage, and subsequently had a miscarriage. At one-year postpartum, the remaining two patients who declined therapy had persistently active disease on sonography and endoscopy. The seven patients who were asymptomatic but with active sonographic findings were noted to have significantly higher biochemical inflammatory markers compared with the 72 patients who were asymptomatic with inactive sonographic findings. This included lower levels of hemoglobin and serum albumin and higher levels of C-reactive protein and ESR (Table 4). Disease behaviour, location and history of bowel surgery were not associated with an increased risk of subclinical inflammation (P > 0.05).
Table 4.
Characteristics of pregnancies with clinically inactive disease as defined by an HBI of ≤ 4.
| HBI ≤4 | Inactive sonographic findings (n = 72) | Active sonographic findings (n = 7) | P-value |
|---|---|---|---|
| History of bowel surgery | 25 (34.7%) | 2 (28.6%) | 0.55 |
| BMI* (kg/m2, IQR) | 24.4 (22.0–27.7) | 22.5 (21.0–27.4) | 0.71 |
| HBI* (IQR) | 0 (0–1) | 0 (0–3) | 0.42 |
| Hemoglobin* (g/L, IQR) | 123 (120–131) | 111 (99–126) | 0.02 |
| Total white cell count* (x10*9/L, IQR) | 9.0 (7.9–11.1) | 9.2 (8.4–10.6) | 0.85 |
| Platelet level*(x 10*9/L, IQR) | 244 (201–294) | 297 (216–306) | 0.14 |
| CRP* (mg/L, IQR) | 4.0 (1.8–6.9) | 16.4 (9.2–68.0) | 0.003 |
| Albumin level* (g/L, IQR) | 30 (28–34) | 28 (26–29) | 0.01 |
| ESR* (mm/hr, IQR) | 20 (13–31) | 42 (33–97) | 0.01 |
*median; HBI, Harvey Bradshaw Index; BMI, body mass index; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate
Role of Biochemical Monitoring
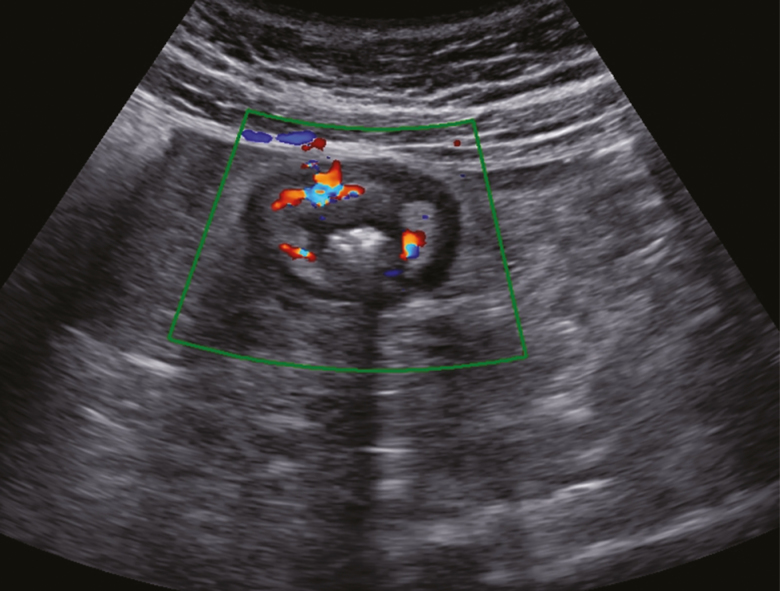
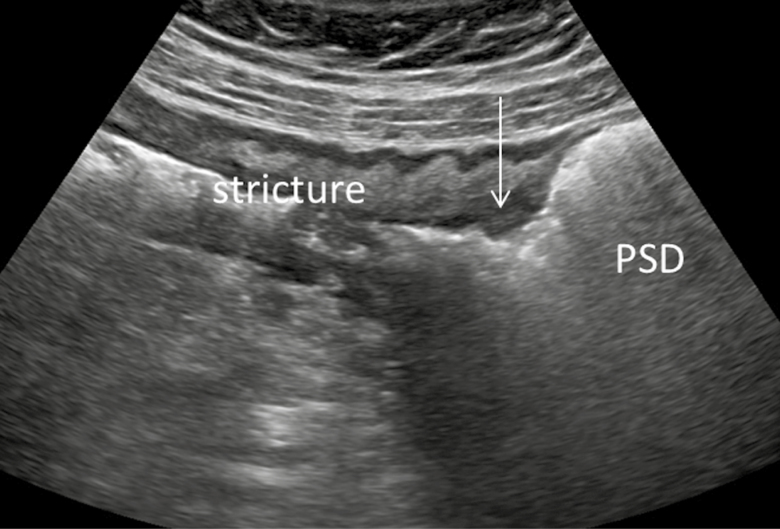
The majority of the patients (84 of 91, 92.3%) had a CRP level performed within the same month of the ultrasound scan. Of these, 58 had a normal CRP (defined by the laboratory as a CRP <8mg/mL), while 26 had an elevated CRP. A normal CRP corresponded to quiescent disease (defined by an HBI score of <4) in the majority, (53 of 58, 91.4%) of the patients. However, a single asymptomatic patient (1 of 53, 0.02%) with a normal CRP had sonographic evidence of active disease and had a miscarriage (as described previously) in the setting of a subchorionic hemorrhage. Furthermore, of five symptomatic patients with a normal CRP, two had sonographically active disease (including a phlegmon and severe ileitis) (Figure 1 and 2). Of the 26 women with an elevated CRP, seven (27%) were symptomatic, two of whom had sonographically active disease. Unfortunately, fecal calprotectin testing was not available at time of the study precluding correlation between this and the CRP, HBI and the ultrasound (Table 5).
Figure 1.
An asymptomatic pregnant patient with active Crohn’s disease on bowel sonography. Terminal ileum in axial view. The bowel wall is thickened with surrounding inflammatory fat. Doppler observed increased vascularity in the bowel wall reflective of active inflammation.
Figure 2.
Bowel sonography of the same patient. Terminal ileum in long axis view. There is a strictured segment with prestenotic dilation (PSD), as indicated by the arrow. Distal bowel is on the left and the proximal bowel is on the right
Table 5.
Change in laboratory results per trimester for CD pregnancies with clinically and sonographically quiescent disease* (n = 72)
| Trimester 1 (n = 16) | Trimester 2 (n = 44) | Trimester 3 (n = 12) | P value** | |
|---|---|---|---|---|
| Hemoglobin*** (g/L) | 130 | 122 | 122 | 0.05 |
| Total white cell count*** (x10*9/L) | 7.8 | 9.2 | 11.7 | 0.06 |
| Platelet level*** (x 10*9/L) | 293 | 244 | 220 | 0.24 |
| CRP level*** (mg/L) | 4.0 | 4.0 | 4.1 | 0.65 |
| Albumin level*** (g/L) | 37 | 30 | 28 | <0.001 |
| ESR*** (mm/h) | 18 | 20 | 25 | 0.19 |
*Defined by HBI score ≤4 and inactive bowel sonography.
**by Kruskal Wallis test
***median
DISCUSSION
This prospective observational study demonstrates the utility of bowel sonography during pregnancy in patients with CD. Bowel sonography in addition to an existing routine clinical assessment may lead to changes in the management of pregnant women with CD by detecting subclinical inflammation, resulting in modification of therapy. It also allows for ongoing noninvasive monitoring of disease activity (including assessing response to treatment) and avoids inappropriate therapy in those who have symptoms in the absence of objective inflammation.
In a meta-analysis, the pooled prevalence of irritable bowel syndrome (IBS) in patients with CD was 41.0% (95% CI, 28.0–56.0%) (22). Consistent with other studies that report on patients with significant symptoms in the absence of active disease, two-thirds of our cohort who had a HBI > 4 had no significant corresponding inflammation on sonographic examination (22–24). Furthermore, clinical scores and, specifically, the items in the HBI (general well-being, abdominal pain, number of liquid stools per day, presence of an abdominal mass and extraintestinal manifestations) may be altered or are difficult to interpret in the context of pregnancy. Given that current clinical practice guidelines stress the importance of treating active disease in pregnancy, confirmation of active disease using objective measures is a priority (25). Therefore, in these symptomatic patients without objective evidence of disease activity, we were able to avoid unnecessary use of corticosteroids, immunosuppressants or escalation of existing therapy. In contrast, of 79 CD pregnancies with clinically quiescent disease, seven (8.9%) showed evidence of active inflammation on sonography.
Our study demonstrates the utility of bowel sonography over routine laboratory testing during pregnancy in CD. While the hemoglobin, albumin, CRP and ESR may be useful adjuncts in assessing inflammation, these biomarkers do not provide detailed information on the severity or location of disease nor do they aid in determining complications such as strictures, fistulae or abscesses, which may influence therapeutic decision-making. While hemoglobin and albumin often decrease in patients with active IBD, reliability in pregnancy is poor due to hemodilution from plasma expansion. C-reactive protein and ESR increase during pregnancy, which may limit their utility as objective markers of inflammation (17). Additionally, validated cutoffs differentiating active from inactive disease in the setting of pregnancy have not been established. While CRP and ESR levels were significantly higher in the seven asymptomatic patients with active disease on ultrasound than in asymptomatic patients with inactive disease, bowel ultrasound provided the benefit of confirming the presence, severity and location of active disease. Conversely, there were symptomatic patients with normal CRP levels with sonographically active disease. These scenarios underscore the limited utility of CRP in the setting of pregnancy.
While our study lacked fecal calprotectin data, which has been shown to be more reliable than clinical symptoms in the nonpregnant state, the utility of fecal calprotectin in pregnancy has so far been inconsistently reported (26–29). A single published study demonstrated the correlation of fecal calprotectin with the physician global assessment in 46 pregnant individuals with IBD (26). The following three studies have been published in abstract form. This includes a small study of 17 patients which suggested that fecal calprotectin correlated with symptom-based disease activity, but this is contradicted by two larger studies of 33 and 75 patients, respectively, that reported fecal calprotectin correlated poorly with disease activity during pregnancy (27–29). Therefore, more data is required before fecal calprotectin can be used as a biomarker of choice in pregnant women with IBD. Overall, hematological and biochemical biomarkers are either not reliable indicators of inflammation in IBD or have not yet been consistently validated in pregnancy. Furthermore, biomarkers cannot determine the location of the inflammation, which may have therapeutic implications, nor detect stricturing or penetrating complications in CD.
Ileocolonoscopy has long been the gold standard for the diagnosis and assessment of disease activity in individuals with IBD. Guidelines suggest that colonoscopy should be performed only if strongly indicated and if it would change clinical management (18, 30). Furthermore, patient preference should be considered because ileocolonoscopy is an invasive test, requires preparation and may involve sedation (31).
All forms of cross-sectional imaging (CT and MRI) have similar accuracy to ileocolonoscopy in detecting inflammation in CD and have the added ability to detect stenosing and penetrating complications (32). However during pregnancy, a CT scan that involves high-dose ionizing radiation should only be performed when there is no diagnostic alternative. Gadolinium should be avoided if an MRI is performed because of theoretical risks to the fetus (33). Therefore, nonionizing bowel ultrasound should be considered an important first choice diagnostic modality in pregnant women with CD. In contrast, imaging is not the preferred diagnostic modality for pregnant patients with UC; the latter can safely undergo limited endoscopic evaluation (14,18,34).
Limitations of our study include generalizability because bowel sonography is not currently available in all gastroenterology centers, and althouth it is used routinely for the assessment of individuals with IBD in Europe, this modality is not yet widely available across Canada and other geographic regions. Our tertiary institution has a strong focus on the evaluation of the bowel in IBD and is run by multiple proficient and experienced radiologists and sonographers. Fortunately, the uptake of this diagnostic modality and expertise is increasing (35). Being operator-dependent, we acknowledge issues surrounding potential inter- and intra-observer variability. Sonographers and radiologists were not blinded to the patient’s clinical status at time of ultrasound scan. However, because all pregnant women with IBD underwent an intrapartum bowel ultrasound irrespective of clinical activity (i.e., the presence or absence of symptoms) referral bias should be minimized accordingly. Our centre is a tertiary referral centre with a specialized preconception and pregnancy IBD clinic, where patients are counselled to have disease optimized before and during pregnancy. Therefore, while our clinic may see more complex cases prior to conception, disease optimization may result in lesser disease activity during pregnancy itself. Our study may then underestimate the benefits of bowel sonography because active disease may be more prevalent in less controlled settings.
Further limitations include the absence of fecal calprotectin data in this cohort. The utility of this biomarker has been inconsistently reported in the setting of pregnancy, as outlined previously. If future studies demonstrate the utility of fecal calprotectin in identifying subclinical inflammation in pregnant women with IBD, it may be a useful adjunct in determining which individuals should proceed onto ultrasound, with the ultrasound then helping define the location and severity of disease (27–29).
Because routine colonoscopy is not indicated for disease surveillance in pregnancy, we were not able to compare ultrasound with colonoscopy in women with CD. This may be particularly relevant in women with symptoms who had a normal ultrasound. However, as only one of the eight women were diagnosed with a flare of CD within six months of delivery, it suggests that most patients did not have untreated CD throughout their pregnancy.
In summary, this novel study demonstrates the utility of bowel sonography in pregnant women with Crohn’s disease. By objectively and noninvasively assessing inflammation in this specialized cohort, sonography may improve management decisions that may result in favourable outcomes for both mother and baby.
Acknowledgements
Author’s Contributions: YL contributed to the conception of research project, recruitment of study subjects, drafting of manuscript and interpretation of the data, critical revision of the final manuscript. HHS contributed to the drafting of manuscript, data collection, analysis and interpretation of the data. EEA and NS contributed to the data collection. DT contributed to the data analysis. RW, MP, KLN, GGK, RP and SRW contributed to the interpretation of data and critical revision of manuscript. CS contributed to the conception of research project, recruitment of study subjects, data collection, interpretation of data and critical revision of manuscript. All authors made a substantial contribution to the concept and design of the work. All authors approved the final version of the article and are accountable for its content.
This manuscript, including the related data and tables has previously been presented as an oral free paper at the Asian Organisation of Crohn’s and Colitis (AOCC) 2017 scientific meeting.
Conflicts of Interest
YL is a consultant for Janssen, Abbvie, Shire, Takeda, Actavis and Ferring and a speaker for Janssen and Abbvie. HHS is an advisory board member for Ferring and Janssen and a speaker for Takeda. RW has received speaker fees from Takeda, Janssen and a Travel grant from Abbvie. KLN is a consultant and speaker for Abbvie and Janssen and sits on the advisory boards for Pfizer, Abbvie, Janssen and Takeda. She has received investigator-initiated study funds from AbbVie and educational grants from Janssen. GGK is a speaker for Janssen, Abbvie and Pfizer and has received grants from Merck, Abbvie, Shire and Glaxo-Smith Kline. RP is a consultant for AbbVie, ActoGeniX, AGI Therapeutics, Alba Therapeutics Albireo, Alfa Wasserman, Amgen, AM-Pharma BV, Anaphore, Aptalis, Astellas, Athersys, Atlantic Healthcare, BioBalance, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Celek, Cellerix, Cerimon, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cytokine Pharmasciences, Eagle, Eisai Medical Research, Elan, EnGene, Eli Lilly, Enteromedics, Exagen Diagnostics, Ferring, Flexion Therapeutics, Funxional Therapeutics, Genentech, Genzyme, Gilead, Given Imaging, GlaxoSmithKline, Human Genome Sciences, Ironwood, Janssen, KaloBios, Lexicon, Lycera, Meda, Merck & Co., Merck Research Laboratories, MerckSerono, Millennium, Nisshin Kyorin, Novo Nordisk, NPS Pharmaceuticals, Optimer, Orexigen, PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProtAb, Purgenesis Technologies, Receptos, Relypsa, Salient, Salix, Santarus, Shire Pharmaceuticals, Sigmoid Pharma, Sirtris (a GSK company), S.L.A. Pharma (UK), Targacept, Teva, Therakos, Tillotts, TxCell SA, UCB Pharma, Vascular Biogenics, Viamet and Warner Chilcott UK. Speaker: Abbvie, Aptalis, AstraZeneca, Ferring, Janssen, Merck, Prometheus, Shire, Takeda. Advisory Boards: Abbvie, Abbott, Amgen, Aptalis, AstraZeneca, Baxter, Biogen Idec, Eisai, Ferring, Genentech, Janssen, Merck, Shire, Elan, Glaxo-Smith Kline, Hospira, Pfizer, Bristol-Myers Squibb, Takeda, Cubist, Celgene, Salix. Research/Educational Support: Abbvie, Ferring, Janssen, Shire and Takeda. SRW has receied ultrasound equipment support from Philips, Siemens and Samsung and research support from Lantheus Medical Imaging. CHS is a consultant for Janssen, Abbvie, Shire, Takeda, Actavis, Ferring and Pfizer and is a speaker for Janssen, Abbvie, Takeda and Shire.
References
- 1. Cornish J, Tan E, Teare J, et al. . A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut 2007;56(6):830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Getahun D, Demissie K, Marcella SW, et al. . The impact of changes in preterm birth among twins on stillbirth and infant mortality in the United States. J Perinatol 2014;34(11):823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyd HA, Basit S, Harpsøe MC, et al. . Inflammatory Bowel Disease and Risk of Adverse Pregnancy Outcomes. PLoS One 2015;10(6):e0129567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shand AW, Chen JS, Selby W, et al. . Inflammatory bowel disease in pregnancy: a population-based study of prevalence and pregnancy outcomes. BJOG 2016;123(11):1862–70. [DOI] [PubMed] [Google Scholar]
- 5. Bengtson MB, Martin CF, Aamodt G, et al. . Inadequate Gestational Weight Gain Predicts Adverse Pregnancy Outcomes in Mothers with Inflammatory Bowel Disease: Results from a Prospective US Pregnancy Cohort. Dig Dis Sci 2017;62(8):2063–9. [DOI] [PubMed] [Google Scholar]
- 6. Deepak P, Stobaugh DJ. Maternal and foetal adverse events with tumour necrosis factor-alpha inhibitors in inflammatory bowel disease. Aliment Pharmacol Ther 2014;40(9):1035–43. [DOI] [PubMed] [Google Scholar]
- 7. Bortoli A, Pedersen N, Duricova D, et al. . Pregnancy outcome in inflammatory bowel disease: Prospective European case-control ECCO-EpiCom study, 2003–2006. Aliment Pharmacol Ther 2011;34(7):724–34. [DOI] [PubMed] [Google Scholar]
- 8. de Lima A, Zelinkova Z, Mulders AG, et al. . Preconception Care Reduces Relapse of Inflammatory Bowel Disease During Pregnancy. Clin Gastroenterol Hepatol 2016;14(9):1285–1292.e1. [DOI] [PubMed] [Google Scholar]
- 9. Mahadevan U, Sandborn WJ, Li DK, et al. . Pregnancy outcomes in women with inflammatory bowel disease: A large community-based study from Northern California. Gastroenterology 2007;133(4):1106–12. [DOI] [PubMed] [Google Scholar]
- 10. Torp R, Jelness Jorgensen LP, Henriksen M, et al. . Fecal calprotectin may differentiate between IBS and IBD symptoms in IBD patients. Scand J Gastroenterol 2009;44:31. [Google Scholar]
- 11. Targownik LE, Sexton KA, Bernstein MT, et al. . The association between symptom burden and inflammatory activity in IBD. Gastroenterology 2013;144(5):S766. [Google Scholar]
- 12. Bouguen G, Levesque BG, Feagan BG, et al. . Treat to target: A proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol 2015:1042–50.e2. [DOI] [PubMed] [Google Scholar]
- 13. Colombel JF, Panaccione R, Bossuyt P, et al. . Superior endoscopic and deep remission outcomes in adults with moderate to severe Crohn’s disease managed with treat to target approach versus clinical symptoms: Data from calm. Gastroenterology 2017;152(5):S155. [Google Scholar]
- 14. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110(9):1324–38. [DOI] [PubMed] [Google Scholar]
- 15. Audu BM, Mustapha SK. Prevalence of gestrointestinal symptoms in pregnancy. Niger J Clin Pract 2006;9(1):1–6. [PubMed] [Google Scholar]
- 16. Mountifield RE, Prosser R, Bampton P, et al. . Pregnancy and IBD treatment: This challenging interplay from a patients’ perspective. J Crohns Colitis 2010;4(2):176–82. [DOI] [PubMed] [Google Scholar]
- 17. de Oliveira LC, Franco-Sena AB, Rebelo F, et al. . Factors associated with maternal serum C-reactive protein throughout pregnancy: A longitudinal study in women of Rio de Janeiro, Brazil. Nutrition 2015;31(9):1103–8. [DOI] [PubMed] [Google Scholar]
- 18. de Lima A, Zelinkova Z, van der Woude CJ. A prospective study of the safety of lower gastrointestinal endoscopy during pregnancy in patients with inflammatory bowel disease. J Crohns Colitis 2015;9(7):519–24. [DOI] [PubMed] [Google Scholar]
- 19. Maconi G, Sampietro GM, Parente F, et al. . Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn’s disease: A prospective comparative study. Am J Gastroenterol 2003;98(7):1545–55. [DOI] [PubMed] [Google Scholar]
- 20. Satsangi J, Silverberg MS, Vermeire S, et al. . The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006;55(6):749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medellin-Kowalewski A, Wilkens R, Wilson A, et al. . Quantitative contrast-enhanced ultrasound parameters in crohn disease: Their role in disease activity determination with ultrasound. AJR Am J Roentgenol 2016;206(1):64–73. [DOI] [PubMed] [Google Scholar]
- 22. Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: Systematic review and meta-analysis. Am J Gastroenterol 2012;107(10):1474–82. [DOI] [PubMed] [Google Scholar]
- 23. Spiller R, Major G. IBS and IBD: Separate entities or on a spectrum?Nat Rev Gastroenterol Hepatol 2016;13(10):613–21. [DOI] [PubMed] [Google Scholar]
- 24. Simrén M, Axelsson J, Gillberg R, et al. . Quality of life in inflammatory bowel disease in remission: The impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol 2002;97(2):389–96. [DOI] [PubMed] [Google Scholar]
- 25. Nguyen GC, Seow CH, Maxwell C, et al. ; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology The toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016;150(3):734–757.e1. [DOI] [PubMed] [Google Scholar]
- 26. Julsgaard M, Hvas CL, Gearry RB, et al. . Fecal calprotectin is not affected by pregnancy: clinical implications for the management of pregnant patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23(7):1240–6. [DOI] [PubMed] [Google Scholar]
- 27. Huang V, Bal J, Foshaug RR, et al. . Fecal calprotectin is elevated with clinical disease activity during pregnancy in women with Inflammatory Bowel Disease. J Crohns Colitis 2015;9(Suppl1):S215–6. [Google Scholar]
- 28. Shitrit A, Granovsky-Grisaru S, Adar T, et al. . LImitations in using fecal Calprotectin as a biomarker of IBD disease activity duing Pregnancy. J Crohns Colitis 2015;9(suppl_1):S196. [Google Scholar]
- 29. Kanis SL, de Lima A, Van Oorschot V, Van Der Woude CJ. Su1802 fecal calprotectine is a poor predictor of IBD relapse during pregnancy. Gastroenterology 2017;150(4):S556. [Google Scholar]
- 30. Cappell MS. Risks versus benefits of gastrointestinal endoscopy during pregnancy. Nat Rev Gastroenterol Hepatol 2011;8(11):610–34. [DOI] [PubMed] [Google Scholar]
- 31. Buisson A, Gonzalez F, Poullenot F, et al. . Comparative acceptability and perceived clinical utility of monitoring tools: A nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23(8):1425–33. [DOI] [PubMed] [Google Scholar]
- 32. Panes J, Ricart E. Can we monitor a patient with inflammatory bowel disease and adapt treatment without endoscopy?Curr Drug Targets 2018;19(7):777–81. [DOI] [PubMed] [Google Scholar]
- 33. Masselli G, Derchi L, McHugo J, et al. ; ESUR Female Pelvic Imaging Subcommittee Acute abdominal and pelvic pain in pregnancy: ESUR recommendations. Eur Radiol 2013;23(12):3485–500. [DOI] [PubMed] [Google Scholar]
- 34. Travis SP, Schnell D, Feagan BG, et al. . The impact of clinical information on the assessment of endoscopic activity: Characteristics of the Ulcerative Colitis Endoscopic Index of Severity [UCEIS]. J Crohns Colitis 2015;9(8):607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dietrich CF. Significance of abdominal ultrasound in inflammatory bowel disease. Dig Dis 2009;27(4):482–93. [DOI] [PubMed] [Google Scholar]