Abstract
The Zika virus (ZIKV) genome, its negative-strand viral proteins, and virus-like particles were detected in placenta-derived mesenchymal cells (MSCs), indicating that ZIKV persists after virus clearance from maternal blood and can be rescued by in vitro cultivation. We report for the first time the presence of replication-competent ZIKV in MSCs from an asymptomatic woman who acquired infection during pregnancy.
Keywords: Zika virus, vertical transmission, placenta, mesenchymal cells, viral reservoir
Replication-competent ZIKV was rescued from placenta-derived mesenchymal cells (MSCs), isolated from a woman who acquired the infection during pregnancy, cleared viremia and delivered a newborn with no signs of infection. Our findings suggest a possible role of placental MSCs as ZIKV reservoir in vertical transmission.
Zika virus (ZIKV) is one of the most significant emerging arboviruses in the Americas, given its widespread infection. During the 2013–2014 outbreak in French Polynesia, a link between Guillain-Barre syndrome (GBS) and ZIKV infection was reported [1]. In the 2015–2016 outbreak, Brazil reported the highest number of ZIKV infections worldwide and the highest number of cases associated with microcephaly and other birth defects [2]. Due to the association between ZIKV and increased risk of fetal insults (reported as up to 35% of infants with confirmed infection), particularly those affecting the brain, on February 1, 2016, the World Health Organization declared ZIKV to be a Public Health Emergency of International Concern [3].
ZIKV has been detected in brain tissue samples from stillborn infants and from placental tissue obtained from pregnancy loss as well as in animal models [4–6]. Moreover, ZIKV can be detected in maternal blood up to 84 days after symptom onset, suggesting that persistent viremia may occur as a consequence of immune impairment or viral replication in the fetus or placenta, which may act as a virus reservoir [7–9]. The placenta is the unique barrier between the maternal and fetal compartments, preventing the spread of maternal infections to the fetus. Despite significant research efforts, the mechanisms used by ZIKV to cross the placental barrier and reach the fetus remain to be defined. ZIKV is able to replicate in a broad range of primary placental cells [fetal-derived trophoblast progenitor cells (cytotrophoblasts, CTBs) and Hofbauer cells, HCs)], representing a possible source of prolonged viremia and transplacental transmission [10, 11]. Mesenchymal stromal/stem cells (MSCs) are important in successful pregnancy outcome [12] but, on the other hand, are susceptible to viral infections, leading to vertical transmission. In a recent study of a GBS case with spontaneous abortion immediately after ZIKV infection, virus-harboring MSCs were observed in both the maternal and fetal sides of the placenta, confirming their possible contribution in vertical transmission [13].
A major unresolved issue is whether vertical transmission occurs in asymptomatic ZIKV-infected women.
We report an in-depth characterization of ZIKV infection in a placenta collected at delivery (38th week of gestation) of an asymptomatic woman who acquired ZIKV infection between weeks 15 and 17 of gestation. The woman was viremic for about 10 weeks and cleared the infection from blood 12 weeks before the delivery. The newborn showed no signs of ZIKV infection, and the whole placenta tissue and cord blood were negative to extensive virological examination (Supplementary Tables 1 and 2). Fresh biopsies from decidua, chorionic villi, and amniochorionic membranes were tested for the presence of ZIKV RNA by reverse transcription polymerase chain reaction (RT-PCR). All samples were negative. We wondered whether MSCs, known to be highly permissive to viral infections and possible players in vertical transmission, might harbor ZIKV. Therefore, we isolated and expanded in vitro MSCs from placenta tissues (decidua, chorionic villi, and amniochorionic membranes) and assessed the presence of ZIKV using different approaches.
Surprisingly, in vitro culture of placenta-derived MSCs revealed that a minute proportion of these cells harbor replication-competent ZIKV, which upon subsequent passages progressively vanished.
Decidua-derived MSCs (dd-MSCs), as well as those of fetal origin (chorionic villi–derived and amniochorionic membrane–derived) were characterized by flow cytometry. dd-MSCs, defined as CD90+CD105+, represented 2.6% among CD45-negative cells and expressed CD73 MSC marker at low intensity (mean fluorescence intensity (MFI), 582) (Supplementary Figure 1A). After 3 weeks of in vitro culture, dd-MSCs reached 97.0% purity and expressed CD73 marker at a high intensity (MFI, 8848) (Supplementary Figure 1B). A similar cytometric profile was observed in MSCs of fetal origin. The typical fibroblast-like morphology was also observed for dd-MSCs after 3 weeks of in vitro culture (Figure 1A).
Figure 1.
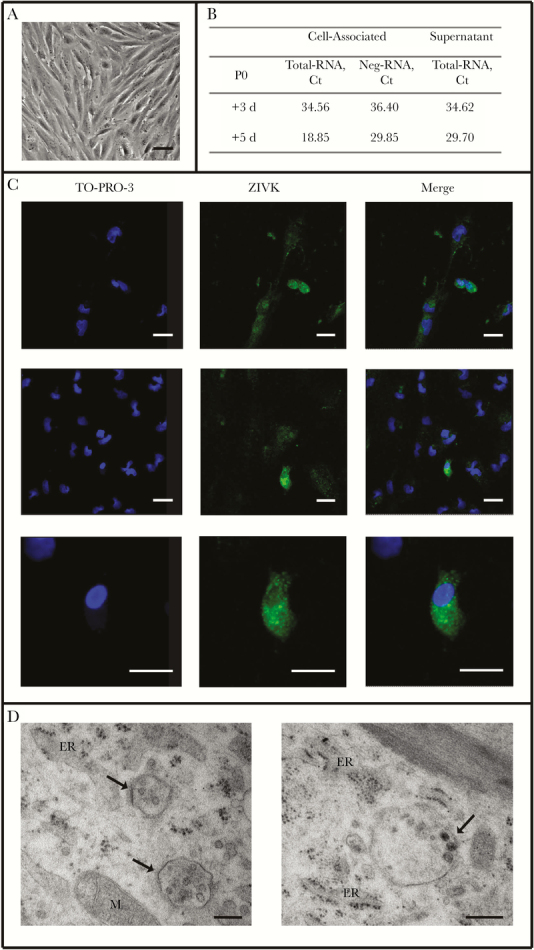
Characterization of Zika virus (ZIKV) infection of decidua-derived mesenchymal cells (dd-MSCs). A, Morphology of dd-MSCs (P0, 21 days post expansion (DPE)) observed by light microscope. Scale bar = 500 μm. B, ZIKV total-RNA and neg-RNA quantification in dd-MSCs cells and culture supernatants sampled at different days of P0 culture. C, Immunolocalization by confocal microscopy of the ZIKV glycoprotein, detected by anti-pan-flavi-specific IgG antibody (ZIKV, green) on dd-MSCs (day 3 of P0). Left panels: nuclear staining; middle panels: ZIKV-specific staining; right panels: merged images. The 2 higher rows show different microscopy fields; the lower row shows a higher magnification of the central row pictures. A dotted immunolabeling can be observed in the cytoplasm of positive cells. Scale bar = 100 μm. D, Ultrastructural analysis of dd-MSCs (day 3 of P0), assessed by transmission electron microscopy. The cultured cells show virus-like structures measuring approximately 40–50 nm in diameter in cytoplasmic vesicles close to the rough endoplasmic reticulum (arrows). The morphologic characteristics of the virus-like structures are consistent with viral particles of the Flaviviridae family. Scale bars = 200 μm. Abbreviations: ER, endoplasmic reticulum; M, mitochondrion.
The presence of ZIKV RNA in dd-MSCs (P0) was evaluated in cells and supernatants collected after 3 and 5 days of culture (Figure 1 B). ZIKV RNA was detected both in cells and in supernatants after 3 days and increased after 5 days. Neg-RNA was found in cells at day 3 (Ct: 36.40) and increased at day 5 (Ct: 29.85), suggesting active replication of ZIKV genome in these cells. The partial NS5 sequence of ZIKV RNA harbored by dd-MSCs was identical to that of ZIKV RNA detected in the mother’s serum at the time of acute infection (Supplementary Figure 2).
ZIKV RNA and neg-RNA were also detected in dd-MSCs at the subsequent passage (P1), although at lower levels than in P0 dd-MSCs (Supplementary Table 3). The presence of both total- and neg-RNA was also observed in MSCs of fetal origin, at levels comparable to those observed in dd-MSCs (Supplementary Table 3). The kinetics of ZIKV RNA (both total and negative strand) in the in vitro MSC cultures, showed a decrease of Ct values after 5-7 days of culture, suggesting a viral replicative activity, as the Ct values decreased during the days of culture, peaking at days 5–7 (Figure 1 B). However, when frozen P1 dd-MSCs were thawed and cultured, ZIKV RNA was detected only in the thawed cells (Ct, 30) and was lost upon subsequent culture, suggesting that the ZIKV-infected cells were not able to survive the freezing procedure.
Confocal microscopy was performed to assess the expression of ZIKV glycoprotein in P0 dd-MSCs (Figure 1 C). An intense dotted cytoplasmic positivity (SD) was revealed in 2.9% (1.8%) of total dd-MSCs.
Transmission electron microscopy, used to visualize viral particles in the cytoplasm of dd-MSCs, showed virus-like particles in cytoplasmic vesicles with morphologic characteristics consistent with the Flaviviridae family (Figure 1 D).
Here, we demonstrate for the first time ZIKV persistence in MSCs isolated from apparently healthy placenta tissues (Supplementary Results) after the full-term delivery of a woman who contracted ZIKV infection between the 15th and 17th weeks of gestation and cleared the infection from blood 12 weeks before the delivery. The virus was harbored by a minute fraction (around 2%–3%) of MSCs of both maternal and fetal origin and was evidenced only after in vitro MSC expansion, whereas, due to the low frequency of infected MSCs, it was undetectable when the whole fresh tissue biopsies were analyzed.
ZIKV infection of MSCs was supported by multiple findings: viral genome and its neg-RNA, ZIKV proteins, virus-like particles in cytoplasmic vesicles consistent with the maturation pathway of most flaviviruses. These findings indicate that replication-competent virus persists in a limited number of these cells for a long time after virus clearance from the maternal blood and can be rescued by in vitro cultivation. The presence and intracellular localization of virus-like particles and viral proteins suggest that ZIKV is present in these cells in the native form, and the presence of neg-RNA suggests active genome replication. Notably, it seems that ZIKV-infected MSCs are labile, as ZIKV-RNA, which is detected after freezing, is lost upon subcultivation.
The susceptibility of MSCs to ZIKV infection is well demonstrated both in vitro [11] and in vivo. ZIKV-infected MSCs can contribute to reservoir establishment and vertical transmission and, on the other hand, can directly participate in the pathogenesis of neurological disorders [14]. Rabelo et al. recently reported a ZIKV case associated with GBS and spontaneous abortion in which, different from the case here described, maternal and fetal placenta MSCs were found infected in concomitance with the viremic phase [13]. To our knowledge, there is no previous in vivo evidence of ZIKV persistence in MSCs a long time after viral clearance from blood, which indeed clearly emerges from our study. It is intriguing that ZIKV remains so long within cells with a very low replication level. Persistence of other viruses in immune-privileged organs (eg, eyes, placenta, fetal brain, testis) has been reported [15], and previous studies performed on other arboviruses, including West Nile virus and chikungunya virus, have also reported persistence of arbovirus RNA in various tissues [16]. The balance among immune surveillance, tolerance signals, and MSC restriction factors could modulate ZIKV replication and persistence. From the present study, it is not possible to assess whether ZIKV-infected MSCs may actually ignite virus spread to the fetus or if they merely represent a locked sanctuary of infection without pathogenetic relevance. In fact, no apparent ZIKV transmission to the fetus could be demonstrated; in addition, it is unknown whether MSC infection in apparently healed pregnant woman is a general finding or is restricted to anecdotal findings. Overall, despite no sign of ZIKV infection in the fetus, replication-competent ZIKV persisted in placenta-derived MSCs, suggesting their possible role in the complex network of vertical transmission. These findings highlight several interesting issues from a pathogenetic and clinical standpoint involving basic studies aimed to identify persistence and restriction factors of ZIKV infection in MSC and a new awareness of clinicians about the possibilities of hidden unsuspected ZIKV infection in PCR-negative placenta biopsies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Giuseppe Ippolito and Alimuddin Zumla are members of the PANDORA-ID-NET Consortium, which is funded by the European and Developing Countries Clinical Trials Partnership (EDCTP2) programme (EDCTP Reg/Grant RIA2016E-1609), which is supported under Horizon 2020. Gary Kobinger, Alimuddin Zumla, and Giuseppe Ippolito are members of the International Public Health Crisis Group (IPHCG).
Financial support. This study was supported by funds from the Italian Ministry of Health (Ricerca Corrente, line 1, RF-2016-02364155 and GR-2016-02362110) and from the European Union’s Horizon 2020 Research and Innovation Programme European Virus Archive (EVAg, 653316).
Potential conflicts of interest. The authors declare no conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. V.B. and E.C. performed MSC culture and characterization; E.L., F.C., L.B., and F.C. performed virological analysis; A.B. and F.D.N. performed placenta histological examination; L.F. and R.N. performed microscopy examination; V.D.A., A.G., L.M., G.L., and F.N. performed clinical management; C.A. and C.C. designed the study; C.C., V.B., F.C., and C.A. drafted the manuscript; C.C., A.Z., G.K., C.A., G.I., and M.R.C. revised the manuscript. All authors approved the final version of the manuscript.
References
- 1. Wikan N, Smith DR. Zika virus: history of a newly emerging arbovirus. Lancet Infect Dis 2016; 16:e119–26. [DOI] [PubMed] [Google Scholar]
- 2. Ministério da Saúde. Boletins epidemiológicos—Secretaria de Vigilância em Saúde Available at: http://portalarquivos2.saude.gov.br/images/pdf/2018/janeiro/10/2017-046-Publicacao.pdf. Accessed 2 November 2018.
- 3. de Araújo TVB, Ximenes RAA, Miranda-Filho DB, et al. ; investigators from the Microcephaly Epidemic Research Group; Brazilian Ministry of Health; Pan American Health Organization; Instituto de Medicina Integral Professor Fernando Figueira; State Health Department of Pernambuco Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis 2018; 18:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martines RB, Bhatnagar J, de Oliveira Ramos AM, et al. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet 2016; 388:898–904. [DOI] [PubMed] [Google Scholar]
- 5. Schaub B, Monthieux A, Najihoullah F, et al. Late miscarriage: another Zika concern? Eur J Obstet Gynecol Reprod Biol 2016; 207:240–1. [DOI] [PubMed] [Google Scholar]
- 6. Martinot AJ, Abbink P, Afacan O, et al. Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell 2018; 173:1111–22.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schaub B, Vouga M, Najioullah F, et al. Analysis of blood from Zika virus-infected fetuses: a prospective case series. Lancet Infect Dis 2017; 17:520–7. [DOI] [PubMed] [Google Scholar]
- 8. Suy A, Sulleiro E, Rodó C, et al. Prolonged Zika virus viremia during pregnancy. N Engl J Med 2016; 375:2611–3. [DOI] [PubMed] [Google Scholar]
- 9. Reagan-Steiner S, Simeone R, Simon E, et al. Evaluation of placental and fetal tissue specimens for Zika virus infection - 50 states and District of Columbia, January-December, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tabata T, Petitt M, Puerta-Guardo H, et al. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016; 20:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tabata T, Petitt M, Puerta-Guardo H, et al. Zika virus replicates in proliferating cells in explants from first-trimester human placentas, potential sites for dissemination of infection. J Infect Dis 2018; 217:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu W, Morschauser A, Zhang X, et al. Human placenta-derived adherent cells induce tolerogenic immune responses. Clin Transl Immunology 2014; 3:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rabelo K, Souza LJ, Salomão NG, et al. Placental inflammation and fetal injury in a rare Zika case associated with Guillain-Barré syndrome and abortion. Front Microbiol 2018; 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beys-da-Silva WO, Rosa RL, Santi L, et al. Zika virus infection of human mesenchymal stem cells promotes differential expression of proteins linked to several neurological diseases. Mol Neurobiol 2019; 6:4708–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winchester SA, Varga Z, Parmar D, Brown KE. Persistent intraocular rubella infection in a patient with Fuchs’ uveitis and congenital rubella syndrome. J Clin Microbiol 2013; 51:1622–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Appler KK, Brown AN, Stewart BS, et al. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS One 2010; 5:e10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


