Abstract
Background
The impact of immunosuppression on outcomes in influenza is insufficiently understood. We analyzed the morbidity and mortality of immunocompetent (IC) vs immunosuppressed (IS) patients with influenza A and B in the 2017/2018 season.
Methods
Patients with proven influenza in a German tertiary care hospital were analyzed for hospitalization, intensive care unit (ICU) admission, and mortality. Causes for IS were organ and bone marrow transplantation, AIDS, chemotherapy, and medical immunosuppression.
Results
In total, 227 patients were included in this analysis (IC, n = 118 [52%]; IS, n = 109 [48%]). Hospitalization (71% vs 91%; P < .001) and ICU admission (7% vs 23%; P = .001) were less frequent in the IS compared with the IC group. IC patients had a higher need for invasive ventilation (20% vs 5%; P = .001), vasopressors (19% vs 4%; P < .001), and renal replacement therapy (15% vs 3%; P = .002). Influenza-associated cardiomyopathy was found in 18% of IC vs 2% of IS patients (P < .001). The 30-day in-hospital mortality was 6.6%, 10.2% in the IC group and 2.8% in the IS group (hazard ratio IS group, 0.259; 95% confidence interval [CI], 0.113–0.855; P = .023). Immunosuppression was associated with reduced mortality (odds ratio, 0.25; 95% CI, 0.07–0.91; P = .036).
Conclusions
We observed that IS was not associated with a worse outcome in this influenza cohort. Due to the presence of both confounding and referral and selection bias, the conclusion that immunosuppression reduces mortality cannot be drawn. Prospective studies investigating the influence of baseline immunosuppression on severity of influenza infection are desirable.
Keywords: immunosuppression, influenza, sepsis, cardiomyopathy, extracorporeal life support
The influenza virus is among the most common human respiratory viruses and is associated with significant morbidity [1] and mortality [2] every year. The impact of immunosuppression on outcomes in influenza is insufficiently understood [3].
Many epidemiologic investigations have suggested that chronic immunosuppression places patients at a high risk of severe morbidity and mortality due to influenza infections. After hematopoietic stem cell transplantation (HSCT), patients have recently been reported to develop pneumonia in up to 75% of influenza infections, with mortalities as high as 43% [4]. The largest report so far on HSCT patients infected with influenza observed fatality rates of 23% [5]. Similarly, solid organ transplantation was reported to be associated with high morbidity and mortality in influenza patients [6, 7]. Lung transplant recipients, especially, suffered more severe disease courses compared with liver and kidney transplant patients [8]. Malignant comorbidities treated with chemotherapy such as leukemia and solid tumors were associated with high mortality rates of up to 33% [9] and 11% [10], respectively. Individuals with AIDS were observed to often develop pneumonia due to influenza, which then was associated with increased mortality [11, 12]. On the contrary, there is also a body of evidence describing predominantly mild disease courses in patients infected with influenza after HSCT and solid organ transplantation [13]. Unfortunately, most studies describing the influence of immunosuppression on outcomes in influenza that derive opposing conclusions are compromised by small samples and a lack of robust data directly comparing immunosuppressed (IS) with immunocompetent (IC) patients [14]. A large meta-analysis found the level of evidence supporting general risk factors for influenza-related complications to be rather low [15].
In contrast to the majority of available data so far, we have repeatedly observed during the recent 2017/2018 influenza epidemic that the most severe influenza manifestations, including acute respiratory distress syndrome (ARDS) and acute heart failure due to myocarditis/cardiomyopathy, almost exclusively affect immunocompetent and, surprisingly, not immunosuppressed patients. The aim of the present study was to systematically analyze the morbidity and mortality of IS patients with influenza A and B in the 2017/2018 German epidemic compared with IC patients.
METHODS
Study Population
This was a retrospective single-center observational study performed in a tertiary care hospital in Germany from September 2017 to May 2018. First, adult patients with influenza infection were identified from electronic medical records. Patients who did not give consent to analysis of their personal data for research purposes were excluded from final analysis. The need for ethical approval was waived due to retrospective and noninterventional nature of this analysis. All data were anonymized before analysis. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Definition of Immunosuppression
Individuals were defined as chronically immunosuppressed if they had received HSCT or solid organ transplantation, if they suffered from AIDS, or if they were treated with immunosuppressive medication for rheumatoid disease or chemotherapy due to active cancer. HSCT was performed a median (interquartile range [IQR]) of 6 (3–11.5) months before influenza infection, and a majority of HSCT patients, at 71%, still received IS medication. Steroid use of ≥5 mg of prednisone equivalent for >14 days was defined as immunosuppression. Steroid use of ≥5 mg of prednisone equivalent for >14 days was defined as immunosuppression. A steroid pulse was defined as a temporary prednisone equivalent dose of ≥1 mg/kg. All other individuals were categorized as immunocompetent. One patient received short-term steroid treatment for exacerbated chronic obstructive pulmonary disease (COPD) days before diagnosis of influenza and was therefore not classified as immune-compromised.
Detection of Influenzavirus RNA
Influenza was diagnosed by polymerase chain reaction (PCR) from both nasopharyngeal swab (mainly in stable patients) and broncho-alveolar lavage (BAL; mainly in intubated patients and lung transplant recipients). The Panther Fusion Flu A/B/RSV assay was performed on the Panther Fusion system (Hologic, San Diego, CA). This system includes fully automated nucleic acid extraction, reverse transcription, and real-time multiplex PCR to identify and differentiate influenzavirus A and B RNA.
Outcome Measures
Severity of disease was measured by admission to the hospital and to the ICU, by the need for invasive ventilation, vasopressors, and renal replacement therapy (RRT), and by extracorporeal life support techniques such as extracorporeal membrane oxygenation (ECMO; veno-venous [VV] and veno-arterial [VA] cannulation techniques) and Impella (Abiomed) micro-axial flow-pump. Cardiac manifestation of influenza was defined as a significant and acute increase of troponin T, NT-proBNP, and/or acute reduced left ventricular function on transthoracic echocardiography, combined with hemodynamic instability in the absence of a true coronary pathology. Overall 30-day mortality and ICU mortality were recorded. Neuraminidase (NA) inhibitors were administered only in individuals with onset of symptoms within the last 48 hours, irrespective of disease severity.
Data Collection and Statistical Analysis
Data were collected using electronic medical records including the patient data monitoring system (PDMS) m-life. We used GraphPad Prism 7 (La Jolla, CA) and IBM SPSS Statistics (version 25.0; IBM Corp., Armonk, NY) for data analysis and graph generation. Categorical variables are shown as numbers and percentages. Continuous variables are shown as median and 25%–75% quartiles, unless indicated otherwise. For comparisons, the chi-square test and Mann-Whitney U test were used accordingly. Normal distribution was graphically evaluated and using the Shapiro-Wilk test. Univariate and multivariate logistic regressions were conducted to identify factors influencing mortality due to influenza infection. All variables that proved statistical significance on univariate regression analysis were subsequently entered into the multivariate regression analysis. Additional possible confounding variables (age, congestive heart failure, COPD, diabetes mellitus [DM], and chronic kidney disease [CKD]) were also included in the multivariate regression analysis. Survival data were analyzed by log-rank test and visualized by Kaplan-Meier curves. All reported P values are 2-sided unless indicated otherwise; P values <.05 were considered statistically significant.
RESULTS
Overall Cohort
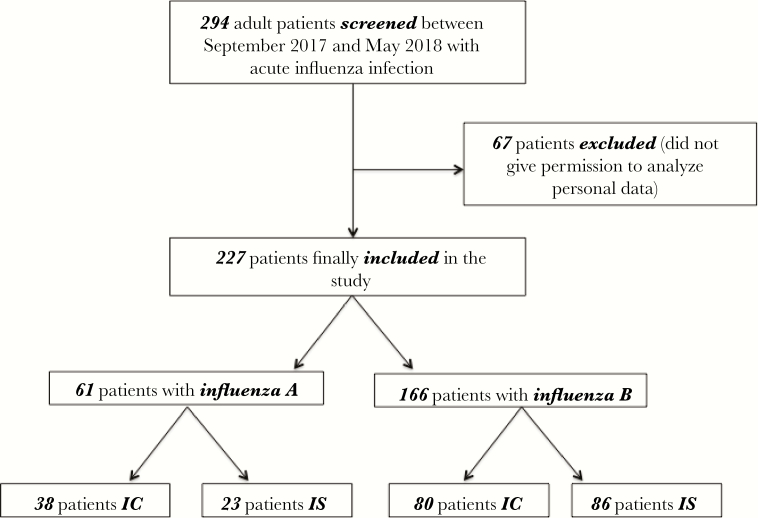
A total of 294 adult patients with influenza infection were identified from electronic medical records. After exclusion of 67 patients, who did not give permission to analyze their personal data, 227 patients diagnosed with influenza were finally enrolled into the study. Of these, 61 (27%) patients were diagnosed with influenza A and 166 (73%) with influenza B. A flowchart in accordance to the CONSORT statement is shown in Figure 1.
Figure 1.
Flowchart of study participants. Flowchart demonstrates inclusion of patients into the retrospective analysis. Abbreviations: IC, immunocompetent; IS, immunosuppressed.
Baseline demographic and clinical characteristics of the overall cohort are demonstrated in Table 1. Fourty-seven percent of the patients were female, and the median age (IQR) was 62 (49–72) years. The most common medical comorbidities were chronic kidney disease (CKD) in 73 (32%), diabetes in 42 (19%), and cardiac disease in 94 (42%) patients. Eighty-eight (39%) patients received an NA inhibitor. The majority of patients were treated inpatient, and 15% needed to be admitted to the ICU. Those patients received mechanical ventilation, vasopressors, and renal replacement therapy in about two-thirds of cases. Three percent of patients received VV-ECMO support for refractory ARDS, and 3.5% received advanced cardiac support techniques, for example, VA-ECMO and/or Impella for stabilization of influenza-associated acute heart failure.
Table 1.
Baseline Demographic and Clinical Characteristics and Outcome Measures for the Overall Cohort
| Category | Overall Cohort | |||
|---|---|---|---|---|
| All | IC | IS | P | |
| (n = 227) | (n = 118) | (n = 109) | ||
| Age, median (IQR), y | 62 (49–72) | 67 (54–77) | 58 (48–65) | <.001 |
| Female gender, No. (%) | 106 (46.7) | 46 (39) | 60 (55) | .015 |
| Comorbidities, No. (%) | ||||
| Adipositas | 26 (11.5) | 15 (12.7) | 11 (10.1) | .536 |
| Diabetes | 42 (18.5) | 20 (16.9) | 22 (20.2) | .531 |
| Coronary artery disease | 45 (19.8) | 28 (23.7) | 17 (15.6) | .125 |
| Congestive heart failure | 49 (21.6) | 32 (27.1) | 17 (15.6) | .035 |
| COPD | 25 (11) | 14 (11.9) | 11 (10.1) | .67 |
| Asthma | 12 (5.3) | 5 (4.2) | 7 (6.4) | .462 |
| CF | 3 (1.3) | 1 (0.8) | 2 (1.8) | .515 |
| Chronic kidney disease | 73 (32.2) | 29 (24.6) | 44 (40.4) | .011 |
| Chronic renal replacement therapy | 10 (4.4) | 2 (1.7) | 8 (7.3) | .038 |
| Immunosuppression, No. (%) | 109 (48) | 0 (0) | 109 (100) | <.001 |
| HTX | 1 (0.4) | - | 1 (0.9) | |
| LuTX | 22 (9.7) | - | 22 (20.2) | |
| NTX | 20 (8.8) | - | 20 (18.3) | |
| LTX | 2 (0.9) | - | 2 (0.9) | |
| HSCT | 17 (7.5) | - | 17 (22) | |
| Cancer | 25 (11) | - | 25 (11) | |
| Rheumatoid disease | 21 (9.3) | - | 21 (19.3) | |
| AIDS | 2 (0.9) | - | 2 (1.8) | |
| Steroids baseline, No. (%) | 68 (30) | 1 (0.8) | 67 (61.5) | <.001 |
| Neuraminidase inhibitor, No. (%) | 88 (38.8) | 48 (40.7) | 40 (36.7) | .539 |
| Steroid pulse, No. (%) | 10 (4.4) | 7 (5.9) | 3 (2.8) | .243 |
| Hospital admission, No. (%) | 184 (81.1) | 107 (90.7) | 77 (70.6) | <.001 |
| ICU admission, No. (%) | 35 (15.4) | 27 (22.9) | 8 (7.3) | .001 |
| Invasive ventilation, No. (%) | 28 (12.3) | 23 (19.5) | 5 (4.6) | .001 |
| Norepinephrin, No. (%) | 26 (11.5) | 22 (18.6) | 4 (3.7) | <.001 |
| Renal replacement therapy, No. (%) | 20 (8.8) | 17 (14.4) | 3 (2.8) | .002 |
| Antibiotic treatment, No. (%) | 93 (41) | 49 (41.5) | 44 (40.4) | .859 |
| Pneumonia, No. (%) | 90 (39.6) | 48 (40.7) | 42 (38.5) | .741 |
| Cardiac manifestation, No. (%) | 23 (10.1) | 21 (17.8) | 2 (1.8) | <.001 |
| Aspergillus superinfection, No. (%) | 6 (2.6) | 3 (2.5) | 3 (2.8) | .922 |
| Extracorporeal life support systems, No. (%) | 15 (6.6) | 14 (11.9) | 1 (0.9) | .002 |
| Vv ECMO | 7 (3.1) | 6 (5.1) | 1 (0.9) | .07 |
| Va ECMO | 3 (1.3) | 3 (2.5) | 0 (0) | .094 |
| Impella | 5 (2.2) | 5 (4.2) | 0 (0) | .03 |
| Mortality, No. (%) | 15 (6.6) | 12 (10.2) | 3 (2.8) | .023 |
| ICU mortality, No. (%) | 10 (28.6) | 9 (33.3) | 1 (12.5) | .248 |
Abbreviations: CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; HSCT, hematopoetic stem cell transplantation; HTX, heart transplantation; IC, immunocompetent patients; ICU, intensive care unit; IQR, interquartile range; IS, immunosuppressed patients; LTX, liver transplantation; LuTx, lung transplantation; NTX, kidney transplantation; Va ECMO, veno-arterial extracorporeal membrane oxygenation; Vv ECMO, veno-venous extracorporeal membrane oxygenation. Statistical significant P values are bolded.
The 2 cohorts of IC and IS patients varied in terms of demographic characteristics and important clinical outcome parameters (Table 1). IS patients were younger and more often female and more frequently had CKD but less frequently had cardiac disease. IC and IS patients received comparable treatments with NA inhibitors. However, IC patients had a significantly more severe course of influenza infection, as indicated by a higher likelihood of hospital and ICU admission. IC patients were 3 times as likely to be admitted to the ICU (23% vs 7%; P = .001). Consequently, IC patients had a 4–5 times higher need for invasive ventilation (20% vs 5%; P = .001), vasopressor support (19 vs 4%; P < .001), and renal replacement therapy (15% vs 3%; P = .002). Importantly, only 2% of IS patients compared with 18% of IC patients had an influenza-associated cardiac manifestation (P < .001). Corresponding to more severe pulmonary and cardiac manifestations, IC patients required extracorporeal life support significantly more often than IS patients (12% in IC vs only 1% in IS; P = .002). The proportion of additional antibiotic treatment and secondary pneumonia was not different between IC and IS patients, neither in the overall cohort nor in the influenza A and B subcohorts (Tables 1–3).
Table 2.
Baseline Demographic and Clinical Characteristics and Outcome Measures for Influenza A
| Category | Influenza A | |||
|---|---|---|---|---|
| All | IC | IS | P | |
| (n = 61) | (n = 38) | (n = 23) | ||
| Age, median (IQR), y | 62 (48–70) | 63 (46–74) | 62 (49–67) | .38 |
| Female gender, No. (%) | 24 (39.3) | 10 (26.3) | 14 (60.9) | .007 |
| Comorbidities, No. (%) | ||||
| Adipositas | 10 (16.4) | 7 (18.4) | 3 (13) | .582 |
| Diabetes | 7 (11.5) | 3 (7.9) | 4 (17.4) | .259 |
| Coronary artery disease | 11 (18) | 8 (21.1) | 3 (13) | .43 |
| Congestive heart failure | 12 (19.7) | 9 (23.7) | 3 (13) | .311 |
| COPD | 7 (11.5) | 4 (10.5) | 3 (13) | .765 |
| Asthma | 3 (4.9) | 2 (5.3) | 1 (4.3) | .873 |
| CF | 0 (0) | 0 (0) | 0 (0) | - |
| Chronic kidney disease | 13 (21.3) | 8 (21.1) | 5 (21.7) | .949 |
| Chronic renal replacement therapy | 0 (0) | 0 (0) | 0 (0) | - |
| Immunosuppression, No. (%) | 23 (37.7) | 0 (0) | 23 (100) | <.001 |
| HTX | 1 (1.6) | - | 1 (4.3) | |
| LuTX | 3 (4.9) | - | 3 (13) | |
| NTX | 3 (4.9) | - | 3 (13) | |
| LTX | 0 (0) | - | 0 (0) | |
| HSCT | 5 (8.2) | - | 5 (21.7) | |
| Cancer | 7 (11.5) | - | 7 (30.4) | |
| Rheumatoid disease | 4 (6.6) | - | 4 (17.4) | |
| AIDS | 0 (0) | - | 0 (0) | |
| Steroids baseline | 10 (16.4) | 0 (0) | 10 (43.5) | <.001 |
| Steroid pulse, No. (%) | 4 (6.6) | 3 (7.9) | 1 (4.3) | .588 |
| Neuraminidase inhibitor, No. (%) | 21 (34.4) | 13 (34.2) | 8 (34.8) | .964 |
| Hospital admission, No. (%) | 51 (83.6) | 33 (86.8) | 18 (78.3) | .38 |
| ICU admission, No. (%) | 14 (23) | 13 (34.2) | 1 (4.3) | .007 |
| Invasive ventilation, No. (%) | 13 (21.3) | 12 (31.6) | 1 (4.3) | .012 |
| Norepinephrin, No. (%) | 11 (18) | 10 (26.3) | 1 (4.3) | .031 |
| Renal replacement therapy, No. (%) | 9 (14.8) | 9 (23.7) | 0 (0) | .011 |
| Antibiotic treatment, No. (%) | 29 (47.5) | 19 (50) | 10 (43.5) | .621 |
| Pneumonia, No. (%) | 29 (47.5) | 19 (50) | 10 (43.5) | .621 |
| Cardiac manifestation, No. (%) | 10 (16.4) | 9 (23.7) | 1 (4.3) | .048 |
| Aspergillus superinfection, No. (%) | 4 (6.6) | 3 (7.9) | 1 (4.3) | .588 |
| Extracorporeal life support systems, No. (%) | 5 (8.2) | 5 (13.2) | 0 (0) | .127 |
| Vv ECMO | 4 (6.6) | 4 (10.5) | 0 (0) | .107 |
| Va ECMO | 0 (0) | 0 (0) | 0 (0) | - |
| Impella | 1 (1.6) | 1 (2.6) | 0 (0) | .433 |
| Mortality, No. (%) | 5 (8.2) | 5 (13.2) | 0 (0) | .074 |
| ICU mortality, No. (%) | 5 (35.7) | 5 (38.5) | 0 (0) | .497 |
Abbreviations: CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; HSCT, hematopoetic stem cell transplantation; HTX, heart transplantation; IC, immunocompetent patients; ICU, intensive care unit; IQR, interquartile range; IS, immunosuppressed patients; LTX, liver transplantation; LuTx, lung transplantation; NTX, kidney transplantation; Va ECMO, veno-arterial extracorporeal membrane oxygenation; Vv ECMO, veno-venous extracorporeal membrane oxygenation. Statistical significant P values are bolded.
Table 3.
Baseline Demographic and Clinical Characteristics and Outcome Measures for Influenza B
| Category | Influenza B | |||
|---|---|---|---|---|
| All | IC | IS | P | |
| (n = 166) | (n = 80) | (n = 86) | ||
| Age, median (IQR), y | 62 (49–73) | 68 (55–77) | 57 (47–65) | <.001 |
| Female gender, No. (%) | 82 (49.4) | 36 (45) | 46 (53.5) | .274 |
| Comorbidities, No. (%) | ||||
| Adipositas | 16 (9.6) | 8 (10) | 8 (9.3) | .879 |
| Diabetes | 35 (21.1) | 17 (21.3) | 18 (20.9) | .96 |
| Coronary artery disease | 34 (20.5) | 20 (25) | 14 (16.3) | .164 |
| Congestive heart failure | 37 (22.3) | 23 (28.7) | 14 (16.3) | .054 |
| COPD | 18 (10.8) | 10 (12.5) | 8 (9.3) | .508 |
| Asthma | 9 (5.4) | 3 (3.8) | 6 (7) | .359 |
| CF | 3 (1.8) | 1 (1.3) | 2 (2.3) | .603 |
| Chronic kidney disease | 60 (36.1) | 21 (26.3) | 39 (45.3) | .01 |
| Chronic renal replacement therapy | 10 (6) | 2 (2.5) | 8 (9.3) | .066 |
| Immunosuppression, No. (%) | 86 (51.8) | 0 (0) | 86 (100) | <.001 |
| HTX | 0 (0) | - | 0 (0) | |
| LuTX | 19 (11.4) | - | 19 (22.1) | |
| NTX | 17 (10.2) | - | 17 (19.8) | |
| LTX | 2 (1.2) | - | 2 (2.3) | |
| HSCT | 12 (7.2) | - | 12 (14) | |
| Cancer | 18 (10.8) | - | 17 (19.8) | |
| Rheumatoid disease | 17 (10.2) | - | 17 (19.8) | |
| AIDS | 2 (1.2) | - | 2 (2.3) | |
| Steroids baseline, No. (%) | 58 (34.9) | 1 (1.3) | 57 (66.3) | <.001 |
| Neuraminidase inhibitor, No. (%) | 67 (40.4) | 35 (43.8) | 32 (37.2) | .391 |
| Hospital admission, No. (%) | 133 (80.1) | 74 (92.5) | 59 (68.6) | <.001 |
| ICU admission, No. (%) | 21 (12.7) | 14 (17.5) | 7 (8.1) | .07 |
| Steroid pulse, No. (%) | 6 (3.6) | 4 (5) | 2 (2.3) | .356 |
| Invasive ventilation, No. (%) | 15 (9) | 11 (13.8) | 4 (4.7) | .041 |
| Norepinephrin, No. (%) | 15 (9) | 12 (15) | 3 (3.5) | .01 |
| Renal replacement therapy, No. (%) | 11 (6.6) | 8 (10) | 3 (3.5) | .092 |
| Antibiotic treatment, No. (%) | 64 (38.6) | 30 (37.5) | 34 (39.5) | .788 |
| Pneumonia, No. (%) | 61 (36.7) | 29 (36.3) | 23 (37.2) | .898 |
| Cardiac manifestation, No. (%) | 13 (7.8) | 12 (15) | 1 (1.2) | .001 |
| Aspergillus superinfection, No. (%) | 2 (1.2) | 0 (0) | 2 (2.3) | .17 |
| Extracorporeal life support systems, No. (%) | 10 (6) | 9 (11.3) | 1 (1.2) | .01 |
| Vv ECMO | 3 (1.8) | 2 (2.5) | 1 (1.2) | .518 |
| Va ECMO | 3 (1.8) | 3 (3.8) | 0 (0) | .07 |
| Impella | 4 (2.4) | 4 (5) | 0 (0) | .036 |
| Mortality, No. (%) | 10 (6) | 7 (8.8) | 3 (3.5) | .147 |
| ICU Mortality, No. (%) | 5 (23.8) | 4 (28.6) | 1 (14.3) | .429 |
Abbreviations: CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; HSCT, hematopoetic stem cell transplantation; HTX, heart transplantation; IC, immunocompetent patients; ICU, intensive care unit; IQR, interquartile range; IS, immunosuppressed patients; LTX, liver transplantation; LuTx, lung transplantation; NTX, kidney transplantation; Va ECMO, veno-arterial extracorporeal membrane oxygenation; Vv ECMO, veno-venous extracorporeal membrane oxygenation. Statistical significant P values are bolded.
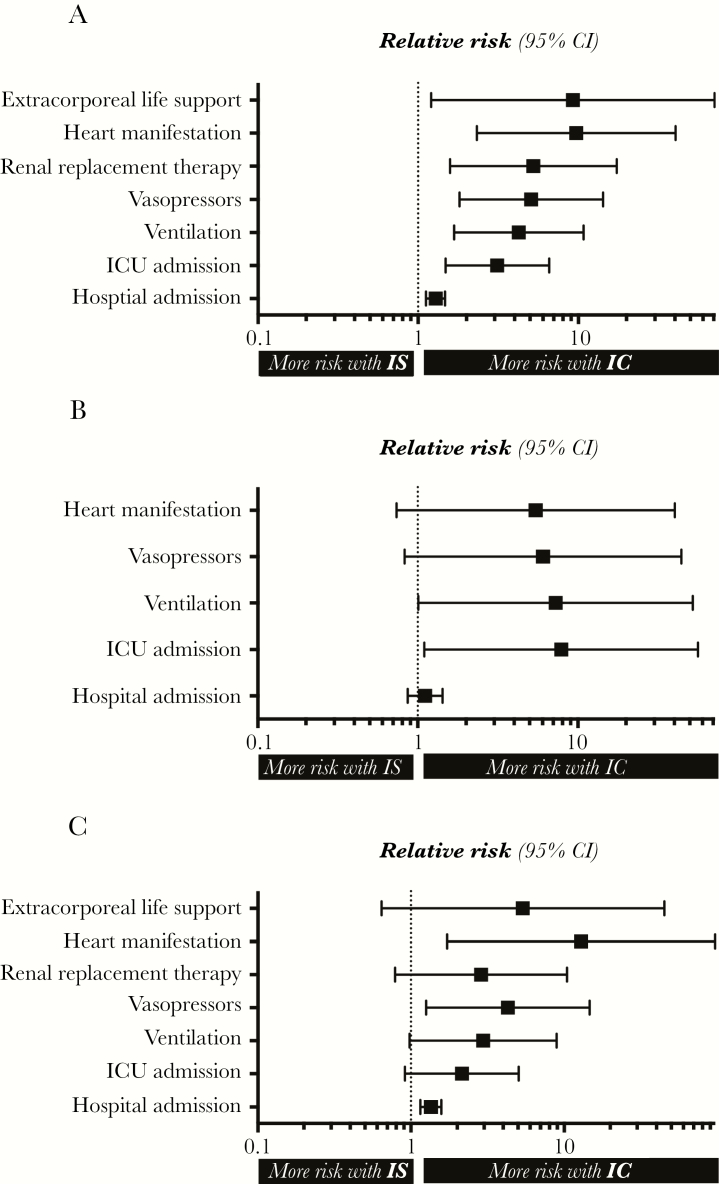
Figure 2 summarizes the most important outcome parameters as Forest plots, displaying relative risks (95% confidence intervals) of IC compared with IS patients for the overall (Figure 2A), influenza A (Figure 2B), and influenza B (Figure 2C) cohorts.
Figure 2.
Important outcome parameters. Forest plots demonstrate relative risk (95% confidence intervals) of immunocompetent compared with immunosuppressed patients for the overall (A), the influenza A (B) and influenza B (C) cohorts. Abbreviations: CI, confidence interval; IC, immunocompetent; ICU, intensive care unit; IS, immunosuppressed.
Influenza A Cohort
Table 2 demonstrates the corresponding characteristics for influenza A patients only. Thirty-eight percent of these patients were IS. ICU admission was about 9 times as likely for IC patients as for IS patients (P = .007). Correspondingly, more IS patients needed invasive ventilation, vasopressors, and RRT. Nine patients (24%) with influenza A who were immunocompetent had cardiac manifestations, whereas only 1 IS patient (4%; P = .043) showed cardiac involvement. Five (13%) IC patients, but none of the IS patients, diagnosed with influenza A needed extracorporeal life support.
Influenza B Cohort
Eighty-six patients (52%) diagnosed with influenza B were on maintenance IS, whereas 80 patients were IC (48%). The clinical characteristics of influenza B patients are demonstrated in Table 3. Again, the clinical course of the disease was more severe in IC patients. Seventy-four IC (93%) and only 59 IS patients (69%) were admitted to the hospital (P < .001). IC patients were more than twice as likely as IS patients to be admitted to the ICU, 3 times as likely to be invasively ventilated (P = .041), and 4 times more vasopressor dependent (P = .01). IC patients had an influenza-associated acute cardiac manifestation significantly more often (15 vs 1%; P = .001). Nine (12%) IC patients but only 1 (1%) of the IS patients diagnosed with influenza B needed extracorporeal life support (P = .01).
30-Day Mortality and ICU Mortality
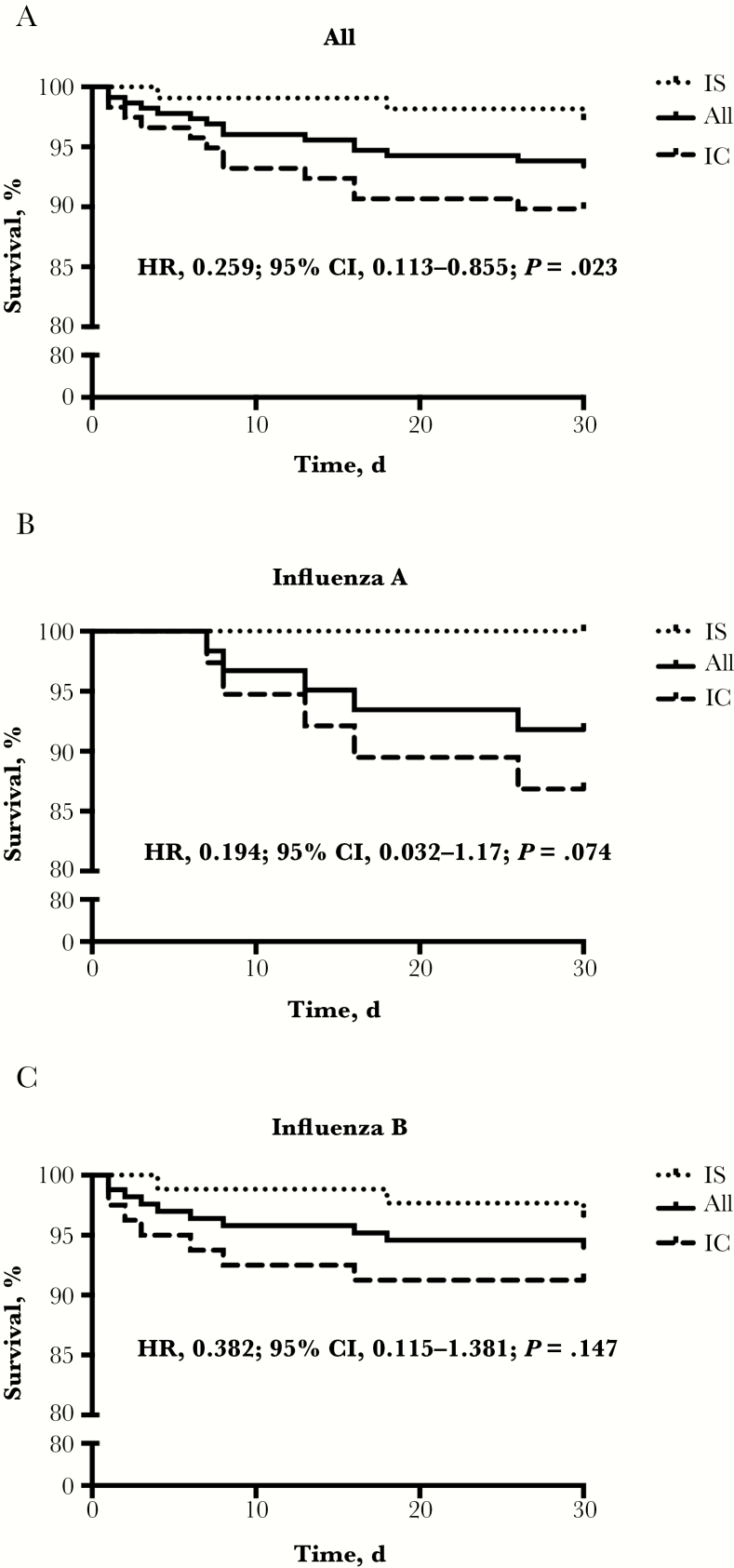
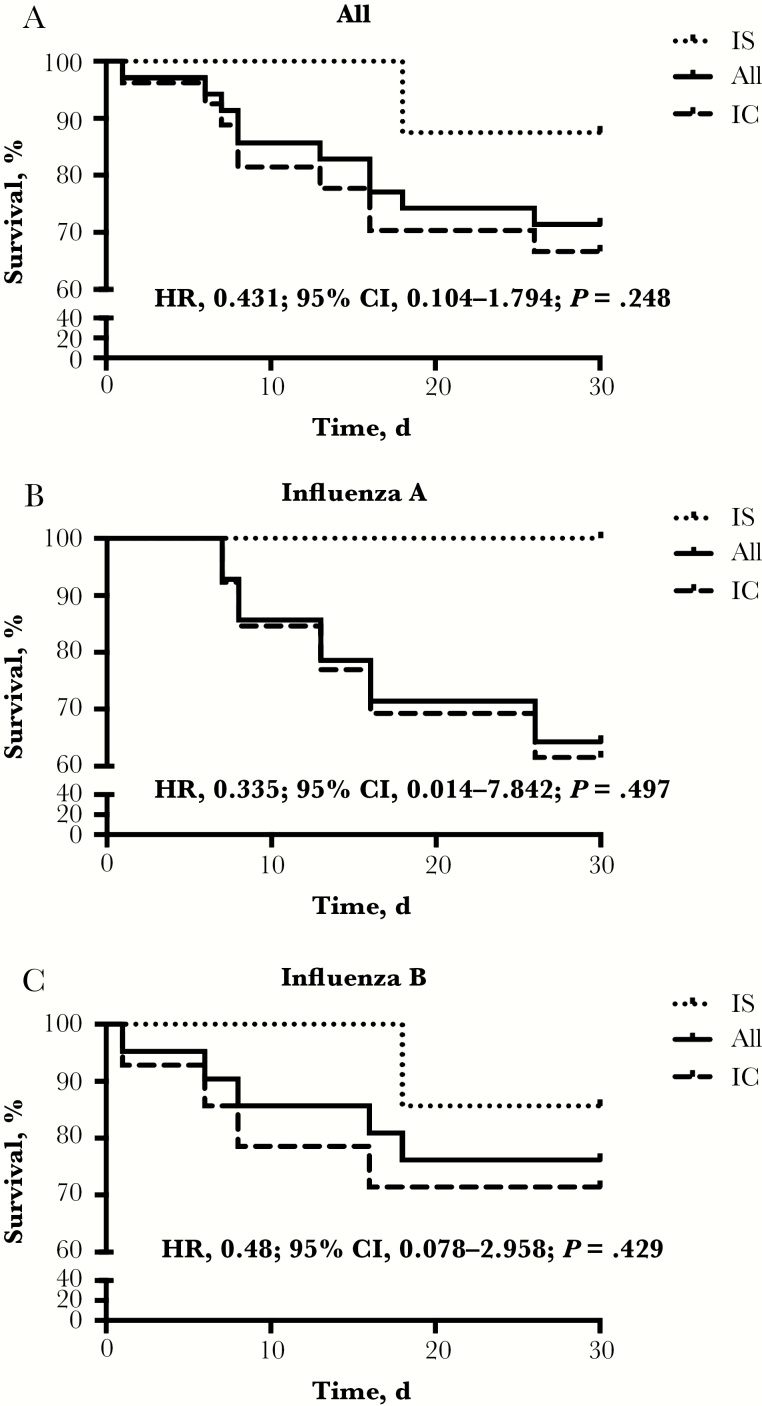
Thirty-day mortality was 6.6% in the overall cohort, 10.2% in the IC group, and 2.8% in the IS group (hazard ratio [HR] IS group, 0.259; 95% confidence interval [CI], 0.113–0.855; P = .023) (Figure 3A). ICU mortality was 28.6% for all patients, 33.3% for IC and 12.5% for IS patients (P = .248) (Figure 4A). A similar trend toward better survival in IS patients was seen in the influenza A and B subgroups. Five IC patients with influenza A (13.2%), and none in the IS group (0%), died (P = .074) (Figure 3B). Correspondingly, ICU mortality was 39% in IC and 0% in IS patients (P = .497) (Figure 4B). The total mortality for influenza B–infected patients was 6%, 9% for IC patients and 4% for IS patients (P = .147) (Figure 3C). ICU mortality of IC patients was 29% vs 14% for IS patients (P = .429) (Figure 4C). Of the 3 IS patients, 2 died of ARDS (1 of the 2 had additional septic shock) and 1 due to graft-vs-host disease progression after HSCT. IC patients died of ARDS (n = 4), ARDS and septic shock (n = 4), ARDS and combined septic and cardiogenic shock (n = 2), isolated cardiogenic shock (n = 1), and massive intestinal bleeding (n = 1). Autopsy was performed in only 1 patient in whom ARDS as the primary cause of death was confirmed. In 2 IC patients with cardiogenic shock, myocardial biopsy was performed while the patients were still alive, and in both, diffuse myocarditis was confirmed.
Figure 3.
Thirty-day survival. Kaplan-Meier graphs showing the 30-day survival course in the overall cohort (A), as well as in influenza A patients (B) and B patients (C) only. Compared are immunosuppressed patients with immunocompetent patients. Abbreviations: CI, confidence interval; HR, hazard ratio; IC, immunocompetent; IS, immunosuppressed.
Figure 4.
Intensive care unit (ICU) survival. Kaplan-Meier graphs showing the 30-day ICU survival course in the overall cohort (A), as well as in influenza A patients (B) and B patients (C) only. Compared are immunosuppressed patients with immunocompetent
patients. Abbreviations: CI, confidence interval; HR, hazard ratio; IC, immunocompetent; IS, immunosuppressed.
Predictors of Mortality
Table 4 shows significant predictors of mortality in the overall influenza cohort. Although a significant age difference was seen between IC and IS patients, age was not different between surviving and nonsurviving patients (median [IQR], 62 [49–72] years in surviving vs 63 [47–74] years in nonsurviving patients; P = .577). Being on maintenance IS was associated with a reduced likelihood of death (odds ratio [OR], 0.25; 95% CI, 0.07–0.91; P = .036) on univariate regression analysis. In multivariate regression analysis, cardiac manifestation (OR, 13.75; 95% CI, 3.84–49.42; P < .001) and pneumonia (OR, 10.23; 95% CI, 1.19–87.83; P = .034) were the only predictors of mortality.
Table 4.
Predictors of Mortality for the Overall Cohort
| Univariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Survivors (n = 212) | Nonsurvivors (n = 15) | P | OR | 95% CI | P | OR | 95% CI | P |
| Immunosuppression | 106 (50) | 3 (20) | .025 | 0.25 | 0.07–0.91 | .036 | |||
| Steroid medication at baseline | 68 (32.1) | 0 (0) | .009 | - | - | - | |||
| ICU admission | 25 (11.8) | 10 (66.7) | <.001 | 14.96 | 4.73–47.33 | <.001 | |||
| Invasive ventilation | 18 (8.5) | 10 (66.7) | <.001 | 21.56 | 6.64–69.95 | <.001 | |||
| Norepinephrin | 16 (7.5) | 10 (66.7) | <.001 | 24.51 | 7.47–80.4 | <.001 | |||
| Cardiac manifestation | 13 (6.1) | 10 (66.7) | <.001 | 30.62 | 9.12–102.8 | <.001 | 13.75 | 3.84–49.24 | <.001 |
| Renal replacement therapy | 11 (5.2) | 9 (60) | <.001 | 27.41 | 8.27–90.83 | <.001 | |||
| Extracorporeal life support | 3 (1.4) | 8 (53.3) | <.001 | 79.62 | 17.32–366.13 | <.001 | |||
| Pneumonia | 76 (35.8) | 14 (93.3) | <.001 | 25.05 | 3.23–194.23 | .002 | 10.23 | 1.19–87.83 | .034 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
DISCUSSION
This study compared the morbidity and mortality of influenza A and B patients in the 2017/2018 epidemic who were either immunocompetent or immunosuppressed in a German tertiary care hospital.
Surprisingly, in this present study, influenza-associated disease severity was in general lower in IS patients, as indicated by less frequent hospitalization and ICU admission compared with the IC group. Additionally, mechanical ventilation, vasopressors, and renal replacement therapy were less frequently needed in IS than IC patients. Acute heart failure due to influenza—mostly, but not exclusively subtype B—was observed in 17.8% of IC but only 1.8% of IS patients. As influenza-associated ARDS and acute cardiac decompensation were more frequent in IC patients, these individuals needed advanced extracorporeal life support, such as ECMO and/or Impella, about 12 times more often. Importantly, not only influenza-associated morbidity but also mortality was lower in IS patients. In accordance, immunosuppression was a significant determinant of reduced mortality on univariate regression analysis.
In fact, most of the existing evidence from the literature points toward the opposite, that is, increased morbidity and mortality in influenza-infected IS patients [4–7, 9, 11, 12]. However, mild disease severity has also been described in IS patients infected with influenza [13], and most studies describing the influence of immunosuppression on outcomes in influenza are compromised in general of a small sample size and lack of comparative data directly analyzing IS vs IC patients [14].
Influenza infection, especially in cases of associated organ failure such as ARDS, AKI, and/or acute cardiac disease, is often associated with a hyperinflammatory phenotype, most likely the consequence of a dysregulated host response to influenza similar to sepsis [16, 17]. On this basis, immunosuppressive treatment strategies to dampen the overwhelming (and therefore harmful) immune response have been on trial in severe influenza disease for years, with both positive [18, 19] and negative effects [20, 21] on disease severity being reported. The most recent evidence might point toward a rather harmful effect [22], and a current meta-analysis advises against general use of corticosteroids in the treatment of severe influenza [23].
One could speculate, however, that baseline maintenance immunosuppression established before onset of infection would be able to mitigate influenza-associated excessive inflammatory reaction and subsequent multi-organ failure from the very beginning. Immunosuppressive strategies in modulating the host response in response to influenza infection, such as the CXC chemokine receptor 2 antagonist danirixin, are currently being evaluated in clinical trials [24]. In this study, acute influenza-associated cardiac manifestations were commonly observed in IC, but surprisingly not in IS patients. At the same time, cardiac manifestation was the strongest independent predictor of mortality on multivariate regression. Acute myocardial infarction and myocarditis with acute cardiac decompensation are known complications of influenza infection [25] and were observed with unusual frequency during the 2017/2018 influenza epidemic [26]. Cardiac manifestations have been recently described as a significant predictor of influenza-associated mortality [27]. Systemic hyperinflammation is a supposed putative mechanism of influenza-associated myocarditis [28]. The role of immunosuppression in viral myocarditis is uncertain due to low-quality evidence—it appears not to reduce mortality but may improve cardiac function [29]. It is possible that baseline immunosuppression might have been responsible in part for a lower incidence of acute cardiac manifestations in IS patients.
The strengths of this study are its large sample size, the high percentage of immunosuppressed individuals (almost 50%), and its comparative design. This study, however, has important limitations—namely its retrospective and observational nature. The majority of influenza B patients represents another limitation of the study. As cardiac manifestations have been recently described mainly with influenza B infections [30], the results of our investigation might not be transferable to more classic influenza A–dominated influenza seasons. Observational studies are potentially susceptible to bias by multiple uncontrolled confounding factors. The fact that both groups are equally sized is a strong argument for the presence of selection bias. However, with Hannover Medical School representing a leading transplant center (about 140 lung, 200 kidney, and 140 liver transplantations per year) it is inevitable that transplant patients contribute in a major proportion to this single-center cohort. It is noticeable that both cohorts differed in some important baseline characteristics, including younger age, more females, and a lower prevalence of preexisting coronary artery disease and heart failure in IS patients. Although older patients in general are at higher risk of dying from influenza [27], in this cohort, age was comparable between surviving and nonsurviving patients. The fact that IS patients are educated in disease awareness often leads to early medical consultation, which might have introduced a referral bias. Furthermore, it is possible that the generally more favorable prognosis of women in infectious diseases [31] influences outcomes—although sex-specific risk assessments for influenza are sparse and, where available, ambiguous [32]. At least in theory, all the positive effects on influenza-associated morbidity and mortality could have been independent of the fact that patients were immunocompromised. Less chronic cardiac morbidity was contrasted in IS patients by significantly more CKD, which might have been a result of the widespread and long-term use of nephrotoxic medications such as calcineurin inhibitors and nonsteroidal anti-inflammatory drugs in this population.
In conclusion, this observational investigation surprisingly shows that maintenance of immunosuppression did not lead to worse outcomes in this influenza cohort. Due to the presence of both confounding and referral and selection bias within this study, the conclusion that immunosuppression reduces mortality cannot be drawn. However, an important clinical implication could be that immunosuppression should not be withheld in such a population. Future larger-scale multicenter comparative studies investigating the influence of baseline immunosuppression on the severity of influenza infection are highly desirable.
Acknowledgments
Financial support. S.D. is supported by the German Research Foundation (DFG; DA1209/4-3). M.M.H. and J.B. are supported by the DFG (KFO311 TP1).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Thompson WW, Shay DK, Weintraub E, et al. . Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–40. [DOI] [PubMed] [Google Scholar]
- 2. Thompson WW, Shay DK, Weintraub E, et al. . Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–86. [DOI] [PubMed] [Google Scholar]
- 3. Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis 2009; 9:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weigt SS, Gregson AL, Deng JC, et al. . Respiratory viral infections in hematopoietic stem cell and solid organ transplant recipients. Semin Respir Crit Care Med 2011; 32:471–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Marrow Transplant 2001; 7:5S–7S. [DOI] [PubMed] [Google Scholar]
- 6. Kumar D, Michaels MG, Morris MI, et al. ; American Society of Transplantation H1N1 Collaborative Study Group Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis 2010; 10:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med 2007; 28:222–42. [DOI] [PubMed] [Google Scholar]
- 8. Vilchez RA, McCurry K, Dauber J, et al. . Influenza virus infection in adult solid organ transplant recipients. Am J Transplant 2002; 2:287–91. [DOI] [PubMed] [Google Scholar]
- 9. Yousuf HM, Englund J, Couch R, et al. . Influenza among hospitalized adults with leukemia. Clin Infect Dis 1997; 24:1095–9. [DOI] [PubMed] [Google Scholar]
- 10. Schepetiuk S, Papanaoum K, Qiao M. Spread of influenza A virus infection in hospitalised patients with cancer. Aust N Z J Med 1998; 28:475–6. [DOI] [PubMed] [Google Scholar]
- 11. Radwan HM, Cheeseman SH, Lai KK, Ellison IR. Influenza in human immunodeficiency virus-infected patients during the 1997–1998 influenza season. Clin Infect Dis 2000; 31:604–6. [DOI] [PubMed] [Google Scholar]
- 12. Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med 2001; 161:441–6. [DOI] [PubMed] [Google Scholar]
- 13. Ljungman P, Andersson J, Aschan J, et al. . Influenza A in immunocompromised patients. Clin Infect Dis 1993; 17:244–7. [DOI] [PubMed] [Google Scholar]
- 14. Memoli MJ, Athota R, Reed S, et al. . The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis 2014; 58:214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mertz D, Kim TH, Johnstone J, et al. . Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 2013; 347:f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis 2014; 14:57–69. [DOI] [PubMed] [Google Scholar]
- 17. Lin GL, McGinley JP, Drysdale SB, Pollard AJ. Epidemiology and immune pathogenesis of viral sepsis. Front Immunol 2018; 9:2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Annane D. Pro: the illegitimate crusade against corticosteroids for severe H1N1 pneumonia. Am J Respir Crit Care Med 2011; 183:1125–6. [DOI] [PubMed] [Google Scholar]
- 19. Quispe-Laime AM, Bracco JD, Barberio PA, et al. . H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med 2010; 36:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brun-Buisson C, Richard JC, Mercat A, et al. ; REVA-SRLF A/H1N1v 2009 Registry Group Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med 2011; 183:1200–6. [DOI] [PubMed] [Google Scholar]
- 21. Kim SH, Hong SB, Yun SC, et al. . Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med 2011; 183:1207–14. [DOI] [PubMed] [Google Scholar]
- 22. Moreno G, Rodríguez A, Reyes LF, et al. ; GETGAG Study Group Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med 2018; 44:1470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lansbury L, Rodrigo C, Leonardi-Bee J, et al. . Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev 2019; 2:CD010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts G, Chen S, Yates P, et al. . Randomized, double-blind, placebo-controlled study of the safety, tolerability, and clinical effect of danirixin in adults with acute, uncomplicated influenza. Open Forum Infect Dis 2019; XXX(X):XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwong JC, Schwartz KL, Campitelli MA. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018; 378:2540–1. [DOI] [PubMed] [Google Scholar]
- 26. Harris JE, Shah PJ, Korimilli V, Win H. Frequency of troponin elevations in patients with influenza infection during the 2017–2018 influenza season. Int J Cardiol Heart Vasc 2019; 22:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pawelka E, Karolyi M, Daller S, et al. . Influenza virus infection: an approach to identify predictors for in-hospital and 90-day mortality from patients in Vienna during the season 2017/18. Infection. In press. [DOI] [PubMed] [Google Scholar]
- 28. Teijaro JR, Walsh KB, Cahalan S, et al. . Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 2011; 146:980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen HS, Wang W, Wu SN, Liu JP. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev 2013; CD004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hékimian G, Jovanovic T, Bréchot N, et al. . When the heart gets the flu: fulminant influenza B myocarditis: a case-series report and review of the literature. J Crit Care 2018; 47:61–4. [DOI] [PubMed] [Google Scholar]
- 31. Schurz H, Salie M, Tromp G, Hoeal EG, Kinnear CJ, Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics 2019; 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gabriel G, Arck PC. Sex, immunity and influenza. J Infect Dis 2014; 209(Suppl 3):S93–9. [DOI] [PubMed] [Google Scholar]