Abstract
Background
Using published data, we sought to compare outcomes in patients transitioned to either oral fluoroquinolones (FQs) or trimethoprim-sulfamethoxazole (TMP-SMX) vs ß-lactams (BLs) after an initial intravenous (IV) course for gram-negative rod (GNR) bacteremia.
Methods
We conducted a systematic review of PubMed and EMBASE and published IDWeek abstracts. We included studies that reported all-cause mortality and/or infection recurrence in patients transitioned to oral FQ/TMP-SMX and BLs.
Results
Eight retrospective studies met inclusion criteria with data for 2289 patients, of whom 65% were transitioned to oral FQs, 7.7% to TMP-SMX, and 27.2% to BLs. Follow-up periods ranged from 21 to 90 days. All-cause mortality was not significantly different between patients transitioned to either FQ/TMP-SMX or BLs (odds ratio [OR], 1.13; 95% confidence interval [CI], 0.69–1.87). Overall recurrence of infection, either bacteremia or the primary site, occurred more frequently in patients transitioned to oral BLs vs FQs (OR, 2.05; 95% CI, 1.17–3.61). Analysis limited to recurrent bacteremia was similarly suggestive, although limited by small numbers (OR, 2.15; 95% CI, 0.93–4.99). However, based on known pharmacokinetics/pharmacodynamics, prescribed ß-lactam dosing regimens were frequently suboptimal.
Conclusions
In the step-down IV to oral treatment of GNR bacteremia, we found insufficient data regarding outcomes after oral TMP-SMX; however, selection of an FQ over commonly utilized ß-lactam regimens may reduce chances of infection recurrence. Although this may be a class effect, it may simply be the result of inadequate dosing of ß-lactams. Additional investigations are warranted to determine outcomes with TMP-SMX and optimized oral ß-lactam dosing regimens.
Keywords: bacteremia, beta-lactams, fluoroquinolones, gram negative, oral
There is limited data on patient outcomes with different oral antibiotic agents in the IV to oral step-down treatment of gram-negative bacteremias. Using published data, we found increased infection recurrence rates with oral ß-lactams (although frequently used sub-optimally) relative to fluoroquinolones.
Switching from intravenous (IV) to oral antibiotic administration in patients with aerobic gram-negative (GNR) bloodstream infection upon clinical stability has become an increasingly common practice. More highly bioavailable agents such as fluoroquinolones (FQs) and trimethoprim-sulfamethoxazole (TMP-SMX) are often chosen over ß-lactams (BLs) for this purpose, despite a lack of clear evidence for their superiority. This issue has become increasingly important because of the recognition of the risk of adverse effects associated with fluoroquinolones, among which are tendinopathy/tendon rupture, QT prolongation, increased Clostridioides difficile rates including with the 027/BI/NAP1 strain [1], increased colonization and infection rates with multidrug-resistant bacteria [2], dysglycemia [3], and even aortic dissection and aneurysm [4]. The Food and Drug Administration has issued multiple warnings against fluoroquinolone use, and the European Medicines Agency (EMA) states that FQs should not be used in situations where other options are available. The use of TMP-SMX is also often prohibited by concerns for sulfa allergies, renal insufficiency, and/or hyperkalemia.
We conducted a systematic review and meta-analysis to compare patient outcomes, specifically recurrence of infection and all-cause mortality, with the use of FQ/TMP-SMX vs ß-lactams as oral step-down treatment of GNR bacteremia. To the best of our knowledge, this is the first meta-analysis to address this question.
METHODS
Data Sources and Searches
We conducted a systematic search of the PubMed and EMBASE databases from inception to April 15, 2019. For PubMed, we used the following MeSH terms: “gram-negative bacteria,” “gram-negative bacterial infections,” “bacteremia,” “infection/blood,” and “administration, oral” with the appropriate Boolean operators. For EMBASE, we searched for titles and abstracts containing the words “oral” and “enterobacter*,” “gram negative,” “Escherichia,” “Klebsiella,” or “proteus,” and “bacterem*” or “bloodstream” or “blood stream.” The “*” is a wild-card character that enabled search of multiple derivatives of the preceding word. Studies were further filtered to only include human studies. We supplemented our search by examination of reference lists of the eligible studies. To include gray literature in our analysis, we searched for IDWeek abstracts (published in Open Forum Infectious Diseases from 2014 onwards) with the search term “oral bacteremia.”
Study Selection
Articles were considered eligible for inclusion if they evaluated all-cause mortality and/or infection recurrence patients with GNR bacteremia who were transitioned from initial IV course to oral therapy with antibiotics from both groups. If the authors did not publish or report the specific oral antibiotic regimen or relevant outcomes being studied, they were contacted to see if these data were available, and if so, the studies were included in the analysis. Case reports were not included.
Data Collection and Quality Assessment
The primary outcomes assessed were all-cause mortality (per individual study protocol follow-up period) and infection recurrence rate (same genus and species) in patients with GNR bacteremia who were transitioned from initial IV therapy to either an oral FQ/TMP-SMX or a ß-lactam. The quality of the included studies was assessed using the Newcastle-Ottawa scale. Two researchers (C.P. and V.T.) independently extracted the data from eligible studies into separate spreadsheets and performed quality assessment of the included studies; discrepancies were resolved by consensus or by a third author (M.H.).
Data Analysis
We calculated pooled odds ratios (ORs) and 95% confidence intervals (CIs) for comparison. We used Cochrane RevMan5 software for statistical analysis, which uses I2 and chi-square (χ 2 or Chi2) to evaluate the statistical heterogeneity among studies, and Egger’s test for publication bias. We selected a random-effects model (DerSimonian) for meta-analysis.
RESULTS
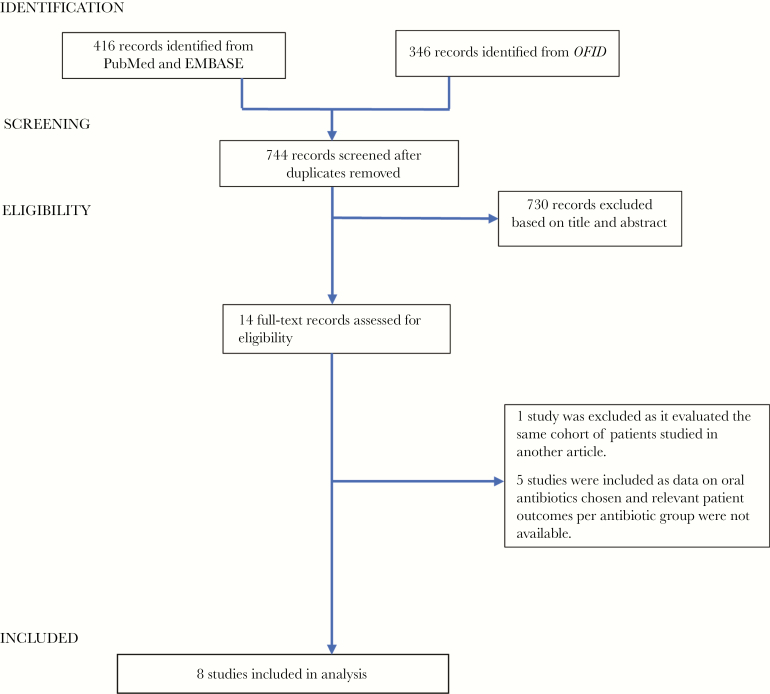
Our initial search yielded 762 records for review (73 articles from PubMed, 343 articles from EMBASE, and 346 articles from Open Forum Infectious Diseases). After removing duplicates and excluding articles by their titles and abstracts, we included 14 articles for full-text review. Of these, 1 study was excluded as it evaluated the same cohort of patients studied in another article, and 5 other studies were excluded because data on oral antibiotics chosen and patient outcomes per antibiotic group were not available. Eight studies were thus included in our analysis (Figure 1) [5–12].
Figure 1.
Search algorithm.
Description of Studies Included
All 8 included studies were retrospective cohort analyses of adult patients hospitalized in the United States; no studies with pediatric patients were identified. The types of patients varied and included those initially admitted to the ICU, those with chronic kidney disease, diabetes mellitus, cirrhosis, and immunosuppressed patients including transplant recipients. Only 1 study [7] excluded neutropenic patients. One study was a multicenter analysis [8], and the remainder were single-center studies. Two studies included only cases of urosepsis [6, 12], whereas the remaining 6 included uncomplicated infections from a variety of sources, including urinary (1510 of 2289, or 66% of all infections in total), intraabdominal/biliary, central line infections, skin and soft tissue infections, pneumonia, and unclear sources. Only 1 study included Escherichia coli infections [10], but the rest examined bloodstream infections due to Enterobacteriaceae in general. Most studies [5, 8–10] reported patient outcomes with FQ and TMP-SMX collectively (“high bioavailability group”) vs the oral ß-lactams (“low bioavailability group”), whereas 2 [7, 11] only examined FQs vs ß-lactams. Most studies reported recurrences of infection, defined as symptomatic infection with the same organism (genus and species) at the primary site of infection with or without bacteremia, but 2 studies looked only at recurrent bacteremias [8, 12]. One study reported all-cause mortality at 21 days [12], 5 at 30 days [6–10], and 2 at 90 days [5, 11]. All 8 authors provided additional information that was not reported in the published paper or abstract. A summary of the studies included is in Table 1.
Table 1.
Summary of Included Studies
| Study | Study Design | Patient Characteristics | Patient (n) and PO Regimens | Source of Infection | Follow-up Period, d | Median (or Mean) Length of IV Therapy FQ/TMP-SMX vs BLs, if Reported | Median (or Mean) Length of Total Therapy FQ/TMP-SMX vs BLs, if Reported | 30- or 90-d All-Cause Mortality, Died/Total | Recurrent Infection All Recurrence/Total (in Parentheses: Recurrent BSI Only) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FQ/TMP-SMX | BLs | FQ/TMP-SMX | BLs | ||||||||
| Kutob 2016 | Retrospective cohort | Mean age 63; 40% male; 55% white; 37% DM; 3% cirrhosis; 9.4% IC; mean Pitt score 1.5 | n = 362; FQ = 257; T/S = 28; BL 77 | UTI: 70.2% | 90 | 4.65 vs 4.8 | 13.8 vs 13.9 | (9/285) | (3/77) | 12/285 (5) | 7/77 (3) |
| Others: 29.8% | |||||||||||
| Sessa 2018 | Retrospective cohort, abstract | Mean age 70; 39% male; mean CCI 2.15; mean Pitt score 1.2 | n = 208; FQ = 49; T/S = 8; BL = 151 | UTI: 77.8% | 30 | (4.60 vs 4.67) | (13.98 vs 12.93) | 0/57 | 0/151 | 3/57 (1) | 14/151 (3) |
| IA: 16.3% | |||||||||||
| Others: 5.7% | |||||||||||
| Rieger 2018b | Retrospective cohort | Median age 64; 46% male; 49% white; 79% DM; 18% liver disease; 7% metastatic cancer; median CCI 6 | n = 114; FQ = 74; T/S = 10; BL = 30 | UTI: 100% | 30 | 4 | 14.4 vs 14 | 2/84 | 0/30 | 2/84 (2) | 1/30 (1) |
| Mercuro 2018 | Retrospective cohort | Mean age 70.8; 51% male; 43% DM; 5% cirrhosis; 5% transplant hx; excluded neutropenia; median CCI 3; median Pitt score 1 | n = 224; FQ = 140; BL = 84 | UTI: 70.85% | 30 | 3 | 14 | 1/140 | 1/84 | 3/140 (0) | 5/84 (2) |
| IA: 21% | |||||||||||
| Others: 6.95% | |||||||||||
| Fong 2018 | Retrospective cohort, abstract | Median age 70; 54% male; 65% white; 33.5% DM; 6.3% cirrhosis; 25% IC; median CCI 5; median Pitt score 2 | n = 173; FQ = 114; BL = 59 | UTI: 57% | 90 | 4 vs 5 | 16 vs 16 | (4/114) | (1/59) | 5/114 (1) | 4/59 (2) |
| IA: 22.5% | |||||||||||
| Others: 20.5% | |||||||||||
| Gumbleton 2018 | Retrospective cohort, abstract | Mean age 65.7; 33% male; mean Pitt score 1.4 | n = 205; FQ = 108; T/S = 11; BL = 86 | UTI: 80% | 30 | 3.6 vs 4.36 | 14.3 vs 13.6 | 1/119 | 2/86 | 0/119 (0) | 3/86 (1) |
| IA: 11% | |||||||||||
| Others: 9% | |||||||||||
| Tamma 2019 | Retrospective, propensity-matched cohort, multicenter study | Median age 59; 52% male; 49% white; 25% DM; 6% cirrhosis; 59% IC; median Pitt score 2 | n = 739; FQ = 518; T/S = 99; BL = 122 | UTI: 40.2% | 30 | 3 | 15 | 68/617 | 15/122 | (4)/617a | (0)/122a |
| IA: 20.1% | |||||||||||
| CLABSI: 18.4% | |||||||||||
| Others: 6.7% | |||||||||||
| Thurber 2019 | Retrospective cohort | Median age 73; 40% male; 93% white; 21% IC; median CCI 5 | n = 264; FQ = 229; T/S = 21; BL = 14 | UTI: 100% | 21 | 3 | 14 | 0/250 | 0/14 | (4)/250a | (0)/14a |
Abbreviations: BL, ß-lactams; BSI, bloodstream infection; CCI, Charlson Comorbidity Index; CLABSI, Central line associated blood stream infection; DM, Diabetes mellitus; FQ, fluoroquinolone; IA, intraabdominal; IC, immunocompromised; PO, oral; T/S or TMP-SMX, Trimethropim-Sulfamethoxazole; UTI, urinary tract infection.
aRecurrent bacteremias only recorded.
bAuthors provided their deidentified data for our review and analysis. As only UTI patients were included, patients de-escalated to moxifloxacin were removed from our analysis.
Quality Assessment of Studies
Quality assessment of the included articles is shown in Supplementary Table 1. Two studies scored the maximum 9 points in the Newcastle-Ottawa scale, 3 scored 8 points, and the remaining 3 scored 6 points.
Antibiotic Regimens
The studies included a total of 2289 patients, among whom 1666 (72.8%) were transitioned from IV therapy to orally administered FQ/TMP-SMX: 1489 or 65% received an FQ (most commonly ciprofloxacin or levofloxacin), and 177 or 7.7% received TMP-SMX, whereas 623 (27.2%) received oral ß-lactams. Median (or mean) duration of IV therapy ranged from 3 to 5 days, whereas total duration of therapy (IV and oral combined) ranged from 13.6 to 16 days. Four studies [5, 9–11] reported separate data for comparators and found that patients who received FQ/TMP-SMX and ß-lactams received similar durations of IV and total length of therapy. Combined testing of available data similarly shows no difference in length of IV or total duration of antibiotic therapy between FQ/TMP-SMX and ß-lactams (Supplementary Table 2).
Some studies reported the most commonly used dosing of the oral antibiotics. Sessa et al. did not list the regimens used but did report the number of patients in each cohort who received subtherapeutic doses, which they defined as being doses less than the maximum recommended dose of antibiotic for the respective renal functions. A summary is provided in Table 2.
Table 2.
Dosing Regimens of Antibiotics
| Study | Antibiotic | Dose | Frequency, % of (n) |
|---|---|---|---|
| Kutob (most common regimens reported) | Amoxicillin/clavulanic acid (n = 30) | 875/125 mg q12h | 70 |
| 500/125 mg q8h | 30 | ||
| Amoxicillin (n = 12) | 500 mg q8h | 83 | |
| Cephalexin (n = 16) | 500 mg q6h | 56 | |
| Levofloxacin (n = 106) | 500 mg q24h | 48 | |
| 750 mg q24h | 33 | ||
| Ciprofloxacin (n = 151) | 500 mg q12h | 84 | |
| TMP-SMX (n = 28) | 800/160 mg q12h | 100 | |
| Mercuro (doses for normal renal function only) | Amoxicillin/clavulanic acid (n = 25) | 875/125 mg q12h | 92 |
| 500/125 mg q12h | 4 | ||
| 500/125 mg q8h | 4 | ||
| Amoxicillin (n = 8) | 1000 mg q8h | 50 | |
| 500 mg q8h | 37.5 | ||
| 500 mg q12h | 12.5 | ||
| Cephalexin (n = 11) | 500 mg q6h | 82 | |
| 500 mg q8h | 9 | ||
| 500 mg q12h | 9 | ||
| Levofloxacin (n = 29) | 500 mg q24h | 13.7 | |
| 750 mg q24h | 82.7 | ||
| Ciprofloxacin (n = 56) | 500 mg q12h | 91 | |
| 750 mg q12h | 7.1 | ||
| Tamma | Amoxicillin/clavulanic acid (n = 38) | 500–1000 mg q 8–12h | N.A. |
| Cephalexin (n = 16) | 500 mg q 6h | N.A. | |
| Cefpodoxime (n = 17) | 200–400 mg q12h | N.A. | |
| Ciprofloxacin (n = 337) | 500–750 mg q12h | N.A. | |
| Levofloxacin (n = 171) | 500–750 mg q24h | N.A. | |
| Moxifloxacin (n = 10) | 400 mg q24h | N.A. | |
| TMP-SMX (n = 99) | 160–320 mg q6–12h | N.A. | |
| Sessa | Subtherapeutic dosing: | No. (%) | |
| FQ/TMP-SMX | 2/57 (3.51) | ||
| ß-lactams | 45/151 (29.8) |
Abbreviations: FQ, fluoroquinolone; TMP-SMX, trimethoprim-sulfamethoxazole.
All-Cause Mortality
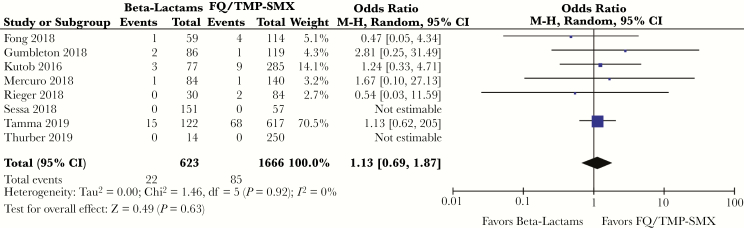
During the follow-up period, 85 of 1666 (5.1%) patients transitioned to FQ or TMP-SMX, and 22 of 623 (3.5%) patients who received an oral ß-lactam died from any cause. There was no significant difference in odds of dying in patients deescalated to either group of oral antibiotics (OR, 1.13; 95% CI, 0.69–1.87; P = .63) (Figure 2).
Figure 2.
Odds ratio, all-cause mortality, ß-lactams vs fluoroquinolone/trimethoprim-sulfamethoxazole. Abbreviations: CI, confidence interval; FQ, fluoroquinolone; TMP-SMX, trimethoprim-sulfamethoxazole.
Recurrence
Recurrence was defined in studies as subsequent infection, either bacteremia or at the primary site, with the same organism (genus and species, with no description of susceptibility profiles). Of the 1666 patients deescalated to FQ/TMP-SMX, recurrence within the bloodstream or at the infection source was documented in 33 patients (1.98%). In the ß-lactam group, 34 of the 623 patients had recurrences (5.46%). Although no individual study found a significant difference in recurrence between the antibiotic groups, collectively there was an increased frequency of overall recurrences in patients transitioned to ß-lactam antibiotics (OR, 2.06; 95% CI, 1.18–3.61; P = .01) (Supplementary Figure 1).
An analysis limited to recurrent bacteremia was also performed, with data available from all 8 studies. Seventeen of 1666 patients transitioned to FQ/TMP-SMX (1%) and 12 of 623 patients transitioned to ß-lactams (1.9%) had recurrent bacteremia (OR, 2.12; 95% CI, 0.92–4.87; P = .08) (Supplementary Figure 2). The point estimate shows an approximately 2-fold increase in recurrent bacteremia in the oral ß-lactam group, but the 95% CI crossed 1 with a wide confidence interval likely resulting from the small sample size.
Given the small number of patients who received TMP-SMX, we performed a sensitivity analysis comparing recurrence in patients who received FQ or TMP-SMX, vs ß-lactams (Supplementary Table 3).
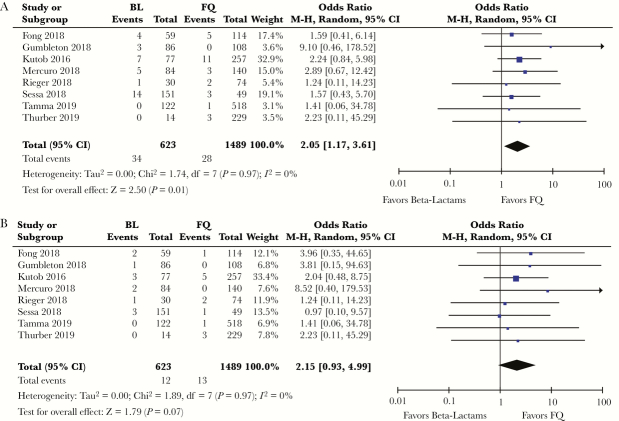
There were 1489 patients who received FQs, among whom 28 (1.88%) had recurrent infections and 13 (0.88%) had recurrent bacteremia. The above findings were thus replicated in the ß-lactam vs FQ analysis; that is, there was increased frequency of overall recurrences in patients transitioned to ß-lactam antibiotics (OR, 2.05; 95% CI, 1.17–3.61; P = .01) (Figure 3A) and an approximately 2-fold increase in recurrent bacteremia in the ß-lactam group, with the CI crossing 1 due to the small sample size (OR, 2.15; 95% CI, 0.93–4.99; P = .07) (Figure 3B).
Figure 3.
A, Odds ratio (OR), overall recurrence of infection, ß-lactams (BLs) vs fluoroquinolones (FQs). B, OR, recurrent bacteremia, BL vs FQ. Abbreviation: CI, confidence interval.
Importantly, however, these findings were not seen in the ß-lactam vs TMP-SMX analysis. Five of 177 patients (2.8%) had recurrent infections (OR, 0.96; 95% CI, 0.30–3.06; P = .95), and 4 (2.2%) had recurrent bacteremias (OR, 0.53; 95% CI, 0.15–1.91; P = .33). Analysis was limited by the small number of patients in this group.
Other Outcomes
Five studies [6, 7, 9–11] compared infection-related readmission rates (per study protocol follow-up time period) between patients transitioned to ß-lactams vs FQ/TMP-SMX. Combined analysis shows an odds ratio of 1.82 (95% CI, 0.85–3.89; P = .12) (Supplementary Figure 3) for readmission in patients who received ß-lactams, mirroring the previous finding of increased recurrences in the ß-lactam group.
Only 2 studies compared the emergence of CDI or multidrug-resistant organisms (MDROs) in the comparator groups [7, 11]. Although limited by small numbers, neither study found a difference in the incidence of C. difficile colitis between comparator groups (Fong, 3% BLs vs 1% FQ; P = .27; Mercuro, 2.4% BLs vs 4.3% FQs; P = .71). Neither study found a difference in the emergence of MDROs between groups (Fong, 0% BLs vs 2.6% FQ; Mercuro, 8.3% BLs vs 10.7% FQ; P = .56). Combined analysis for these outcomes was limited by the small numbers.
DISCUSSION
Several studies have indicated the safety and benefits of oral step-down therapy after an initial IV antibiotic course for the treatment of gram-negative bacteremia [13]. Fluoroquinolones are the most frequent choice for this purpose given their favorable pharmacokinetics and pharmacodynamics (PK/PD), that is, excellent bioavailability that enables high serum levels, coupled with concentration-dependent inhibition and killing. TMP-SMX is also often employed as it is known to be highly bioavailable, although its optimal PD parameter most related to bacterial kill has not been clearly determined (ie, whether it exhibits concentration- or time-dependent killing).
Given the adverse effects of fluoroquinolones and TMP-SMX, the use of other oral options, specifically the oral ß-lactams, has gained attention for step-down therapy in the treatment of GNR bacteremia after an initial IV course, clearance of bacteremia, and source control. Should we preferentially employ FQs or TMP-SMX if the bacterial burden has presumably decreased?
Our findings suggest that mortality is not significantly different with use of FQ/TMP-SMX vs ß-lactams in the step-down treatment of uncomplicated GNR bacteremias. We did find, however, that overall recurrence of infection occurred more frequently with ß-lactams when compared with FQs. Notably, previous RCTs have similarly demonstrated inferior clinical and microbiologic cure rates with ß-lactams relative to FQs in the treatment of uncomplicated cystitis [14, 15].
Recurrence of bacteremia analysis failed to show a statistically significant difference between comparator groups; however, the wide confidence interval suggests that this may have been due to a small sample size, and findings remain compatible with the conclusion that the frequency of recurrent bacteremia was greater in the ß-lactam group, with most values within the comparability index being consistent with this conclusion [16]. It is worth emphasizing that these conclusions were not applicable to TMP-SMX on sensitivity analysis due to the limited number of patients.
A possible explanation for this finding is that the more frequent dosing required with oral ß-lactams leads to poorer compliance. Alternatively, the suboptimal dosing of oral ß-lactams noted in these studies could also account for or contribute to the increased recurrences.
A Note on Oral ß-Lactam Dosing
In treating serious infections with oral antibiotics, clinicians should use PK/PD principles to carefully select antibiotics and their oral dosing in order to achieve pharmacodynamic target attainment and therapeutic outcomes similar to those achieved with IV administration.
Despite the frequent notion that they have low bioavailability, certain ß-lactams do in fact have excellent absorption. For instance, amoxicillin has up to 92%, amoxicillin/clavulanate 60%, cephalexin up to 100%, and cefaclor up to 95% bioavailability, whereas ciprofloxacin has 85% and TMP-SMX has 90% bioavailability [17, 18]. But bioavailability is not the entire answer; the concentration of antibiotic necessary to inhibit an organism is also of critical importance [19–21].
ß-lactams exhibit time-dependent inhibition and killing, and it is generally recommended to target a free drug concentration time greater than the minimum inhibitory concentration (MIC; fT > MIC) of >50% for penicillins and 60% for cephalosporins. Two methods of optimizing pharmacodynamics are increasing doses and dosing more frequently [20]. Cunha [17] recommends, for example, that amoxicillin be dosed at 1 g every 8 hours, and cephalexin at 1 g every 6 hours, when treating serious infections—both in contrast to commonly used longer dosing intervals. Based on various dosing regimens and various MICs, Mogle et al. calculated the probability of achieving the target fT > MIC for various antibiotic regimens [21]. They similarly concluded that cephalexin dosed at 1 g every 6 hours is most likely to achieve these targets, whereas high-dose amoxicillin and amoxicillin/clavulanate should be used and dosed every 8 hours, and ideally only when MICs are known to be sufficiently low to allow pharmacodynamic target attainment. Importantly, many laboratories report only categoric interpretations of susceptibility (ie, sensitive [S], intermediate [I], or resistant [R]), and not actual MICs [22], and even if an organism may be categorically susceptible, its MIC, albeit within the range considered susceptible, may still be too high to achieve the targeted fT > MIC [21]. Thus, taking into account pharmacodynamic considerations, whether currently frequently used ß-lactam dosing regimens provide effective antibacterial therapy in patients with GNR bacteremia can be questioned.
Table 2 shows the various regimens of oral ß-lactams that were used in the studies included in our analysis. As can be seen, more frequent dosing is required, that is, every 6–8 hours, vs every 12–24 hours for FQ or TMP-SMX, possibly leading to lesser compliance and thus more recurrences. Conversely, these prescribed regimens still fall short of the ideal in dose and frequency based on previously discussed PK/PD principles. Sessa reports that while only 3.51% of patients in the FQ/TMP-SMX group had suboptimal dosing, 29.8% were underdosed in the ß-lactam group. In patients with normal renal function, the Mercuro study shows that only 50% of patients transitioned to amoxicillin and none in the cephalexin group received the high doses previously discussed. The dosing regimens in the Kutob study likewise show that the majority of patients did not receive optimal ß-lactam dosing.
Hence, although our study suggests the potential superiority of FQ as a step-down therapy over ß-lactams (if we assume compliance), the more apt conclusion is that this may be due to suboptimally dosed ß-lactam regimens. It is possible that higher and more frequent dosing of oral ß-lactams may prove equal to FQs. On the other hand, patient inconvenience and adverse effects may also possibly decrease compliance and negate any benefit. Therapeutic drug monitoring of ß-lactams may also be helpful, as it is increasingly becoming important in optimizing dosing, especially when dealing with patients with renal insufficiency, alterations in volume of distribution, etc.
A Note on TMP-SMX—Limitations of Our Study and Dosing Considerations
Four of 8 studies included in our analysis grouped patients transitioned to FQ and TMP-SMX in 1 group (“high-bioavailability group”) and compared their outcomes with those who received ß-lactams. Overall, we found that only a small portion of patients received TMP-SMX across included studies, only 177 of 2289 patients in total. Subsequent sensitivity analysis limiting evaluation to TMP-SMX vs ß-lactams failed to show any difference in recurrence between comparator groups. Due to such small numbers, definitive conclusions regarding TMP-SMX are hard to draw based on our review.
Nevertheless, there are data in support of the use of TMP-SMX as step-down therapy in patients with GNR bacteremia. A recent propensity-matched study of 101 patients with ESBL or AmpC-positive Enterobacteriaciae bacteremia showed similar clinical outcomes (mortality and rate of relapse) in those treated with non-IV antibiotics vs carbapenems [23]. Definitive treatment with oral TMP-SMX was used in 59.5% of patients in the non-IV antibiotic group, after a median of 2.5 days of appropriate IV therapy. All but 1 patient was dosed with TMP-SMX 160/800 mg orally twice daily.
Unlike with FQs and ß-lactams, there are insufficient data to determine the optimal PD parameter for efficacy of TMP-SMX, as data are conflicting on whether it exhibits time- or concentration-dependent killing [24, 25]. Autmizguine et al. utilized free TMP concentrations above the MIC for >50% of the dosing interval (fT > MIC > 50%) as a surrogate PD target for TMP efficacy; simulations of oral TMP-SMX 12/60 mg/kg/d in those aged 6–21 years showed similar exposures to those in adults dosed at 320/1600 mg (double strength) orally every 12 hours, and achieving >90% PD target attainment against bacteria with an MIC of 1 mcg/mL. Higher doses would be expected to be necessary for isolates with an MIC at the CLSI breakpoint of 2 mcg/mL [22, 26], but the use of such high doses should be used cautiously due to potential dose-limiting concentration-related toxicities, that is, renal tubular obstruction, hyperkalemia, and myelosuppression [25, 26], especially in the elderly and those with kidney injury/disease, populations with an increased half-life due to decreased renal secretion.
Further studies are thus required to determine the optimal TMP-SMX dose in bacteremia and to determine patient outcomes relative to other oral antibiotic options.
Our analysis is limited by the retrospective nature of all included studies, with the associated potential for bias and confounders. There was full reliance on the accuracy of medical records, and patient compliance with oral regimens was presumed. Moreover, the isolation of the same organism from the primary site of infection (eg, urine) was defined as a recurrence in most studies, but whether this truly represents a recurrent infection or instead indicates persistent colonization after clearance of infection cannot be distinguished, although the recurrent bacteremia data cannot be discounted. Finally, most included studies followed patients for fewer than 90 days. Consensus recommendation for follow-up for GNR bacteremia is 90 days [27], only 2 studies had this follow-up period, and most were limited to 30 days.
In summary, in the IV-to-oral step-down treatment of GNR bacteremias, our study indicates superiority of FQs over commonly utilized, real-world, suboptimal doses of oral ß-lactams. However, the lack of available evidence regarding TMP-SMX in this role and the likely suboptimal dosing of oral ß-lactam regimens utilized in common practice beget the need for further investigation. A prospective randomized trial utilizing optimized dosing regimens for various oral antibiotic classes would be most useful in further evaluating these findings.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to convey our sincerest gratitude to Drs. Majdi Al-Hasan, Nicole Bohm, Karen Fong, Ryan Gumbleton, Julia Sessa, Pranita Tamma, Kristina Thurber, and Minkey Wungwattana for their encouragement and generous provision of supplementary data that allowed for the (otherwise impossible) completion of this study.
Financial support. The authors received no specific funding for this work.
Potential conflicts of interest. All authors have no potential conflicts of interest to disclose. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dingle KE, Didelot X, Quan TP, et al. . Modernising Medical Microbiology Informatics Group Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017; 17:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nseir S, Ader F, Marquette CH, Durocher A. Impact of fluoroquinolone use on multidrug-resistant bacteria emergence [in French]. Pathol Biol (Paris) 2005; 53:470–5. [DOI] [PubMed] [Google Scholar]
- 3. Aspinall SL, Good CB, Jiang R, et al. . Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis 2009; 49:402–8. [DOI] [PubMed] [Google Scholar]
- 4. Lee CC, Lee MT, Chen YS, et al. . Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Intern Med 2015; 175:1839–47. [DOI] [PubMed] [Google Scholar]
- 5. Kutob LF, Justo JA, Bookstaver PB, et al. . Effectiveness of oral antibiotics for definitive therapy of gram-negative bloodstream infections. Int J Antimicrob Agents 2016; 48:498–503. [DOI] [PubMed] [Google Scholar]
- 6. Rieger KL, Bosso JA, MacVane SH, et al. . Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017; 37:1479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdown therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus β-lactams. Int J Antimicrob Agents 2018; 51:687–92. [DOI] [PubMed] [Google Scholar]
- 8. Tamma PD, Conley AT, Cosgrove SE, et al. . Antibacterial Resistance Leadership Group Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med 2019; 179:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gumbleton R, Ott J, Townsend M, et al. . Treatment failure rates in patients receiving low versus high oral bioavailability antibiotics for gram-negative bacteremia. J Am Coll Clin Pharm 2018; 1:220. [Google Scholar]
- 10. Sessa J, Conn KM, Avery L. 1037. Effect of oral step-down therapy on readmission rates in Escherichia coli bacteremia. Open Forum Infect Dis 2018; 5(Suppl 1):S310. [Google Scholar]
- 11. Fong K, Dubrovskaya Y, Siegfried J, et al. . 1072. Streamlining to oral β-lactam vs fluoroquinolone as definitive therapy for Enterobacteriaceae bacteremia. Open Forum Infect Dis 2018; 5(Suppl 1):S321. [Google Scholar]
- 12. Thurber KM, Arnold JR, Narayanan PP, et al. . Comparison of intravenous and oral definitive antibiotic regimens in hospitalized patients with gram-negative bacteremia from a urinary tract infection. J Glob Antimicrob Resist. 2019;18:243-248. [DOI] [PubMed] [Google Scholar]
- 13. Al-Hasan MN, Rac H, Transition from intravenous to oral antimicrobial therapy in patients with uncomplicated and complicated bloodstream infections. Clin Microbiol Infect. In press. [DOI] [PubMed] [Google Scholar]
- 14. Hooton TM, Scholes D, Gupta K, et al. . Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA 2005; 293:949–55. [DOI] [PubMed] [Google Scholar]
- 15. Hooton TM, Roberts PL, Stapleton AE. Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial. JAMA 2012; 307:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019; 567:305–7. [DOI] [PubMed] [Google Scholar]
- 17. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am 2006; 90:1197–222. [DOI] [PubMed] [Google Scholar]
- 18. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis 1997; 24:457–67. [DOI] [PubMed] [Google Scholar]
- 19. Levison ME, Levison JH. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am 2009; 23:791–815, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Care Clin 2011; 27:77–93. [DOI] [PubMed] [Google Scholar]
- 21. Mogle BT, Beccari MV, Steele JM, et al. . Clinical considerations for oral beta-lactams as step-down therapy for Enterobacteriaceae bloodstream infections. Expert Opin Pharmacother 2019; 20:903–7. [DOI] [PubMed] [Google Scholar]
- 22. CLSI. Performance Standards for Antimicrobial Susceptibility Testing . 29th ed. CLSI document M100-S29. Wayne, PA: Clinical and Laboratory Standards Institute; 2019;54:189-196. [Google Scholar]
- 23. Meije Y, Pigrau C, Fernández-Hidalgo N, et al. . Non-intravenous carbapenem-sparing antibiotics for the definitive treatment of bacteremia due to Enterobacteriaceae-producing ESBL or AmpC β-lactamase: a propensity score study. Int J Antimicrob Agents. In press. [DOI] [PubMed] [Google Scholar]
- 24. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother 2017; 62:e01813-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown GR. Cotrimoxazole - optimal dosing in the critically ill. Ann Intensive Care 2014; 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siber GR, Gorham CC, Ericson JF, Smith AL. Pharmacokinetics of intravenous trimethoprim-sulfamethoxazole in children and adults with normal and impaired renal function. Rev Infect Dis 1982; 4:566–78. [DOI] [PubMed] [Google Scholar]
- 27. Harris PNA, McNamara JF, Lye DC, et al. . Proposed primary endpoints for use in clinical trials that compare treatment options for bloodstream infection in adults: a consensus definition. Clin Microbiol Infect 2017; 23:533–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.