Abstract
Introduction:
In spite of chlamydia screening recommendations, U.S. testing coverage continues to be low. This study explored the cost-effectiveness of a patient-directed, universal, opportunistic Opt-Out Testing strategy (based on insurance coverage, healthcare utilization, and test acceptance probabilities) for all women aged 15–24 years compared with current Risk-Based Screening (30% coverage) from a societal perspective.
Methods:
Based on insurance coverage (80%); healthcare utilization (83%); and test acceptance (75%), the proposed Opt-Out Testing strategy would have an expected annual testing coverage of approximately 50% for sexually active women aged 15–24 years. A basic compartmental heterosexual transmission model was developed to account for population-level transmission dynamics. Two groups were assumed based on self-reported sexual activity. All model parameters were obtained from the literature. Costs and benefits were tracked over a 50-year period. The relative sensitivity of the estimated incremental cost-effectiveness ratios to the variables/parameters was determined. This study was conducted in 2014–2015.
Results:
Based on the model, the Opt-Out Testing strategy decreased the overall chlamydia prevalence by > 55% (2.7% to 1.2%). The Opt-Out Testing strategy was cost saving compared with the current Risk-Based Screening strategy. The estimated incremental cost-effectiveness ratio was most sensitive to the female pre–opt out prevalence, followed by the probability of female sequelae and discount rate.
Conclusions:
The proposed Opt-Out Testing strategy was cost saving, improving health outcomes at a lower net cost than current testing. However, testing gaps would remain because many women might not have health insurance coverage, or not utilize health care.
Introduction
Chlamydia screening has been recommended for sexually active adolescents since 1990, and currently the U.S. Preventive Services Task Force and the Centers for Disease Control and Prevention (CDC) recommend screening of all sexually active women younger than 25 years.1,2 Despite these recommendations, screening coverage of sexually active women has been low.3–6 Coverage is especially low for adolescents, and a recent study7 found that 37.9% of sexually active women aged 15–25 years reported a chlamydia test in the past year, with rates lowest among those patients with the highest chlamydia prevalence: those aged 15–19 years. Data reported by the National Committee for Quality Assurance show that chlamydia screening coverage rates among sexually active women aged 16–24 years are low (less than 42% among those aged 16–20 years enrolled in commercial insurance plans), though they have improved from rates that prevailed in the early 2000s: Before 2008, annual screening rates for commercially insured sexually active women aged 16–20 years were 36% or less.8 Medicaid HMO chlamydia screening rates have always been higher than commercial chlamydia screening rates but have declined somewhat in recent years, to 51% in 2013.8
Adolescents are a priority population for sexually transmitted infection prevention and control for several reasons. Disease burden has been highest in this population, and among chlamydia cases reported to CDC, the largest number have been in female adolescents.9,10 Other studies11,12 have found high prevalence of infection among female adolescents. Overall, the prevalence of infection was 6.8% among those aged 14–19 years. Optimal protection of the reproductive health of young women requires annual testing to detect infection, which is usually asymptomatic but if untreated can ascend to cause pelvic inflammatory disease (PID).2,13 Both acute PID and subclinical PID can result in damage to fallopian tubes, with repeat episodes causing a greater risk of tubal damage and adverse outcomes of infertility and ectopic pregnancy.13
Many barriers have been identified that prevent universal annual screening of sexually active adolescents, but few studies have identified affordable, sustainable solutions to overcome these barriers. Identifying adolescents who have been sexually active in the past year and those who should be screened is difficult.14–16 Even among providers who are skillful, comfortable, and experienced at taking a sexual history, obtaining accurate information can be difficult. One percent of patients reporting zero lifetime partners in computer-assisted self-interviews tested positive for chlamydia, and in a recent study, even among adolescents who reported abstinence, cases of chlamydia and gonorrhea were found.16–18
Novel chlamydia screening strategies with high patient and provider acceptance could improve adherence to existing screening recommendations. A universal Opt-Out Testing strategy for chlamydia might improve screening coverage of women aged 15–24 years, a population with a high prevalence of chlamydial infection, and protect their long-term reproductive health. The current standard practice of Risk-Based Screening requires taking a sexual history to identify sexually active women who should be tested for chlamydia, which is a barrier to screening.14,15 This study determined the cost and effectiveness of the proposed Opt-Out Testing strategy when compared with the existing Risk-Based Screening approach for young women aged 15–24 years from a societal perspective.
Methods
The Chlamydia Opt-Out Testing Strategy
The proposed Opt-Out Testing strategy would target all young women within the high-risk age group covered by U.S. Preventive Services Task Force and CDC guidelines (15–24 years) without regard to their reported sexual activity. Thus, all young women aged 15–24 years would be eligible for testing unless their record is flagged at check-in as having had a negative test within the past 12 months, or they declined to be tested. It was assumed that women eligible for Opt-Out Testing would be those with health insurance who have at least one clinical encounter in a year.
Consequently, expected testing coverage was the product of insurance coverage (80%19); healthcare utilization rate (i.e., the proportion with at least one clinical encounter each year [83%11,20]); and the proportion accepting to test. Studies21,22 have generally shown a high acceptance for sexually transmitted infection testing among young women. Although the acceptability in a clinical encounter is unknown, a test acceptance probability of 75% was used in the base case analysis for the sexually active group and 5% for the sexually inactive group. Thus, the expected testing coverage for the sexually active group was approximately 50% (i.e., 0.80 × 0.83 × 0.75) and that for the sexually inactive group was determined using the same formula−−3.3% (i.e., 0.80 × 0.83 × 0.05). Also, the expected testing coverage would be approximately 63% when the test acceptance probability is increased to 95% (i.e., 0.80 × 0.83 × 0.95).
Model Summary
To account for population-level transmission dynamics, a basic dynamic compartmental transmission model that included two groups based on self-reported sexual activity in the past 12 months, with 72%7 being sexually active (high sexual activity group, within the range reported in a previous study--model assumption) and 28% inactive (low sexual activity group--model assumption) was developed. It was assumed that those classified as “inactive” had some sexual activity, albeit low. This provision was included because of studies showing that some patients reporting zero partners had chlamydia or gonorrhea; for example, 1.0% of women reporting zero lifetime partners in the National Health and Nutrition Examination Survey between 1999 and 2002 tested positive for chlamydia.16–18
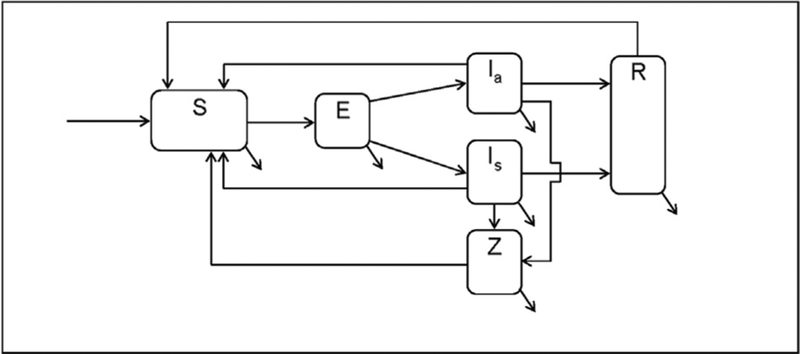
Figure 1 depicts a schematic of the deterministic population-based compartmental model of heterosexual chlamydia transmission that is based on previously published models.23–26 A constant population size of 100,000 (half men, half women24,27) was assumed with approximately 10% entry–exit rate, because the age cohort examined consisted of 10-year birth cohorts (aged 15–24 years).26 All model parameters were obtained from the literature (Table 1). The model was calibrated to produce a pre–opt out prevalence (with existing screening coverage of 30%) close to the national burden for women aged 15–24 years in the U.S. (approximately 3.21%10,26). Technical details of the model are presented in the Appendix (available online).
Figure 1.
Schematic for exploring the cost-effectiveness of an Opt-Out Testing strategy for chlamydia screening.
Note: Infected individuals move into the exposed (E, incubation compartment). From E, they move to either the infectious asymptomatic (Ia or infectious symptomatic (Is) based on the probability of being symptomatic and the duration of incubation. Infected persons may recover naturally and move to the infection-conferred immunity compartment R, be treated and move back to the susceptible compartment S, or develop chlamydia-associated complications and enter the sequelae compartment Z.
S, susceptible; E, exposed; Ia, infectious asymptomatic; Is, infectious symptomatic; R, infection-conferred immunity; Z, sequelae.
Table 1.
Parameters Used to Assess the Health and Economic Outcomes of an Opt-Out Chlamydia Testing Strategy
| Values (range) | ||||
|---|---|---|---|---|
| Parameter | Symbol/name | Male | Female | Source |
| Duration of symptomatic infection (days) | 1/qs | 14 (10–21) | 28 (10–35) | Owusu-Edusei et al. (2013),23 Gift et al. (2008),24 Owusu-Edusei et al. (2015)26 |
| Duration of asymptomatic infection (days) | 1/qa | 182.5 (120–240) | 365 (240–480) | Owusu-Edusei et al. (2013),23 Gift et al. (2008),24 Owusu-Edusei et al. (2015)26 |
| Incubation period (days) | 1/ω | 14 (7–21) | 14 (7–21) | Owusu-Edusei et al. (2013),23 Gift et al. (2008),24 Owusu-Edusei et al. (2015)26 |
| Duration of sequelae (days) | 1/δ | 21 (10–30) | 60 (45–75) | Gift et al. (2008),24 Owusu-Edusei et al. (2015)26 |
| Probability of sequelae (%) | p | 2 (0–5) | 15 (10–20) | Gift et al. (2008),24 Owusu-Edusei et al. (2015),26 Papp et al. (2014)47 |
| Per-partnership transmission probability (%) | β | 70 (25–80) | 68 (25–80) | Schachter et al. (2005)48 |
| Probability of symptomatic infection (%) | ξ | 50 (20–80) | 20 (10–50) | Owusu-Edusei et al. (2013),23 Gift et al. (2008)24 |
| Average No. of partners in the last year, high sexual-activity | c | 6.00 (4.00–8.00) | 4.24 (4.00–5.00) | Model assumption |
| Average No. of partners in last year, low sexual-activity | c | 0.05 (0.02–0.10) | 0.05 (0.02–0.10) | Model assumption |
| Proportion sexually activity (%, reported) | p_active | 81.5 (75–90) | 72 (65–80) | Tao et al. (2012)7 |
| Pre-opt-out annual screening coverage, high sexual-activity (%)a | s | – | 30 | Owusu-Edusei et al. (2013),23 Owusu-Edusei et al. (2015)26 |
| Pre-opt-out annual screening coverage, low sexual-activity (%)a | s | – | 0 | Model assumption |
| Insurance coverage (%)b | p_ins | – | 80 (70–90) | Cohen et al. (2014)19 |
| Utilization (% having at least one encounter)b | p_enc | – | 83 (70–95) | Cohen et al. (2014)19 |
| Uptake, high sexual activity (% accepting test) | p_acc | – | 75 (70–100) | Model assumption43,44 |
| Probability of post-screening treatment (%) | p_rx_sc | 80 (50–99) | 80 (50–99) | Owusu-Edusei et al. (2013),23 Owusu-Edusei et al. (2015)26 |
| Probability of treatment, symptomatic (%) | p_rx | 89 (80–100) | 89 (80–100) | Owusu-Edusei et al. (2015),26 Chernesky et al. (2005)49 |
| Test sensitivity (%) | Sens | 95 (90–100) | 95 (90–100) | Owusu-Edusei et al. (2015),26 Broad et al. (2013)50 |
| Test specificity (%) | Spec | 99 (95–100) | 99 (95–100) | Owusu-Edusei et al. (2015),26 Broad et al. (2013)50 |
| Treatment efficacy (doxycycline, azithromycin) (%) | rx_success | 92 (80–100) | 92 (80–100) | Owusu-Edusei et al. (2013),23 Holland-Hall et al. (2002)51 |
| Health state utility weights | ||||
| Acute infection | qaly_ct | 0.005646 (±50%) | 0.009913 (±50%) | Gift et al. (2008),24 Owusu-Edusei et al. (2015),26 Smith et al. (2008)28 |
| Sequelaec | qaly_seq | 0.009530 (±50%) | 0.497580 (±50%) | Gift et al. (2008),24 Owusu-Edusei et al. (2015)26 |
| Costs (2014 U.S. Dollars) | ||||
| Treatment ($)d | rx_cost | 189.6 (±50%) | 187.4 (±50%) | Gift et al. (2008),24 Owusu-Edusei et al. (2015)26 |
| Sequelae ($)c | seq_cost | 1,369 (±50%) | 4,624 (±50%) | Gift et al. (2008),24 Owusu-Edusei et al. (2015)26 |
| Testing ($) | test_cost | 56 (±50%) | 56 (±50%) | Gift et al. (2008),24 Owusu-Edusei et al. (2015)26 |
| Duration of infection-conferred immunity (years) | 1/n | 1 (0.5–5.0) | 1 (0.5–5.0) | Brunham et al. (2005),25 Owusu-Edusei et al. (2015)26 |
| Entry-exit rate (%) | φ | 10 (5–15) | Model assumption26 | |
| Mixing parameter | ε | 0.50 (0.10–0.90) | Model assumption26 | |
| Discount rate (%) | r | 3 (0–10) | Model assumption26 | |
For the Opt-Out Testing strategy, s equals the product of insurance coverage (p_ins), proportion with at least one encounter (p_enc) and uptake (p_acc – proportion accepting the test)—50% (i.e., 0.80,•0.83, •0.75)for the high sexual activity women and 3.3% (50% (i.e., 0.80, •0.83, •0.05)for the low sexual activity women.
Lower bounds were chosen such that the estimated expected testing coverage would be >30%.
Includes productivity costs or QALYs (where applicable) for epididymitis for males and complications associated with pelvic inflammatory diseases (i.e., chronic pelvic pain, ectopic pregnancy, and infertility) for females.
Includes productivity costs based on the reported youth (16–24 years old) employment rate.26
QALYs, quality-adjusted life-years.
Health outcomes were measured in quality-adjusted life-years (QALYs) estimated using health state utility weights from acute infections and sequelae for both men (epididymitis) and women (PID, including chronic pelvic pain, ectopic pregnancy, and infertility).24,26,28 Three strategies were considered: No Screening (baseline); Risk-Based Screening (current practice, 30% of the sexually active [or high sexual activity] group [i.e., 72%] and 0% of the sexually inactive [or low sexual activity] group [i.e., 28%] women aged 15–24 years screened for chlamydia annually); and Opt-Out Testing (50% of the sexually active/high sexual activity [i.e., 72%] and 5% of the sexually inactive [i.e., 28%] women aged 15–24 years screened for chlamydia annually). All costs were calculated from the societal perspective and included direct medical costs for testing, treatment, and indirect costs for lost productivity.26 All costs were updated to 2014 U.S. dollars using the medical care component of the Consumer Price Index.29 Finally, cumulative cost and effects (QALYs) over a 50-year time frame and analytic horizon were estimated for all strategies and discounted at a 3% annual rate. A 50-year analytic horizon was used for this study primarily because the steady-state (equilibrium) prevalence for Opt-Out Testing was beyond 20–30 years after onset (Figure 2), although the relative incremental cost-effectiveness ratios (ICERs) would have remained pretty much unchanged for shorter analytic horizon. Summary results are presented in ICERs.
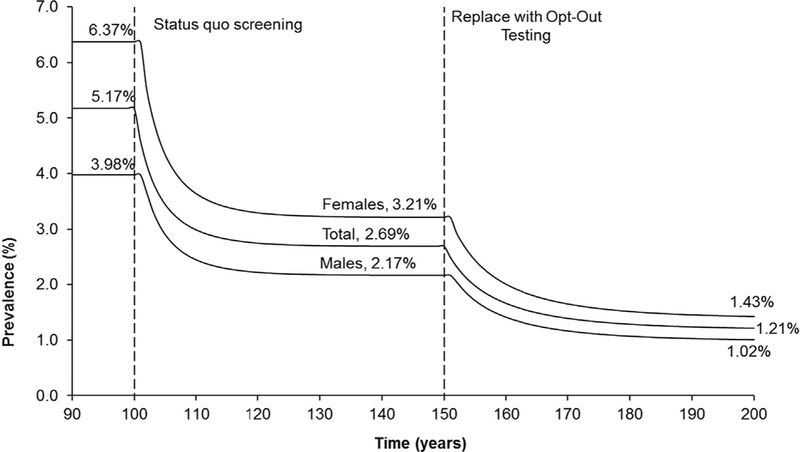
Figure 2.
Time-prevalence chart for the annual Risk-Based Screening and the Opt-Out Testing strategies for females aged 15–24 years old in the U.S.
Note: To avoid clutter by overlaying the estimated prevalence lines for each strategy over the same period and for illustrative purposes, the start of the strategies (Status quo screening and Opt-Out Testing) were separated. When estimating the health and economic outcomes, each strategy was started at the 100-year mark and outcomes were tracked over the 50-year time horizon, which ended at the 150-year mark.
Sensitivity Analyses
Given that the Opt-Out Testing strategy is primarily designed to increase the annual testing coverage, a one-way sensitivity analysis (SA) on the effectiveness of increasing the testing coverage was performed. A multi-way SA was performed in which the focus was on the cost-effectiveness of the Opt-Out Testing strategy when compared with the status quo (i.e., Risk-Based Screening; screening coverage of 30%). Thus, all the variables were varied except the pre–opt out screening coverage of 30%. Given the uncertainties surrounding the numerous parameter values (N=40) that were used in the model, the sensitivity of the results was assessed by conducting a comprehensive SA. First, a more efficient sampling method (Latin hypercube sampling23,24,26,30) was used to create 1,000 random combinations of parameter values by randomly choosing (without replacement) from 1,000 equiprobable parameter value intervals from the ranges provided in Table 1 by assuming uniform distribution for all the variables.23,26 Each simulation was run and checked to make sure that steady state was reached before and after introducing the strategy.23,26 The resulting prevalence (female, male, and total); costs; and QALYs before and after the onset of the Opt-Out Testing strategy were recorded.
Next, to determine the relative impact of the variables/parameters, all the values (i.e., parameter values, prevalence, and ICERs) were ranked to determine the partial rank correlation coefficients (PRCCs).23,26 The PRCCs provided the magnitude of the impact of the referent parameter on the ICER after accounting for the changes (in rank) in the other parameter values. Thus, the PRCC helped to do two important things. First, the PRCC revealed the influential variables/parameters in the model. Second, it allowed the determination of the order of the magnitude of the influence—a hierarchy of influential variables/parameters.23,26,30
Following a previous study,26 it was determined in the preliminary analyses that the equilibrium female pre–opt out prevalence (i.e., under the Risk-Based Screening) was influential in determining the ICER, and that the female pre–opt out prevalence was in turn influenced by the other variables in the model. Consequently, the PRCC was done in two distinct parts. First, the variables that influenced the female pre–opt out prevalence were determined, and then the female pre–opt out prevalence was added in the final PRCC to determine the influential variables/parameters for the ICER.26 This study was conducted in 2014–2015.
Results
Based on the assumptions and the base case parameter values used in this study, total chlamydia prevalence without any screening (No Screening strategy) was 5.2% (women, 6.4%; men, 4.0%), reducing to 2.7% (women, 3.2%; men, 2.2%) when the Risk-Based Screening strategy was introduced (Figure 2). When the Risk-Based Screening strategy (status quo) was replaced with the Opt-Out Testing strategy, the total prevalence decreased further by >55% from 2.7% to 1.2% (Figure 2). Detailed results of the resulting prevalence by sex and sexual activity under each strategy are provided in the Appendix (available online). Over the analytic horizon, the Risk-Based Screening strategy decreased the discounted total cumulative number of sequelae (PID in women and epididymitis in men) by approximately 42% (14,990 to 8,754) compared with No Screening. Replacing the Risk-Based Screening strategy with the Opt-Out Testing strategy decreased the discounted total cumulative sequelae (women and men) further by approximately 37% to 5,527 (Table 2). Based on the health outcomes and the estimated associated costs, the ICER of the Risk-Based Screening strategy was cost saving (Table 2) when compared with the No Screening strategy, and the ICER of the Opt-Out Testing strategy was also cost saving when compared with the status quo Risk-Based Screening strategy (Table 2). In fact, the Opt-Out Testing strategy completely dominated the Risk-Based Screening strategy (i.e., the Opt-Out Testing strategy was more effective and less costly than the current Risk-Based Screening strategy).
Table 2.
Summary Health and Cost Outcomes for a Hypothetical Population of 100,000 Individuals Aged 15–24 Years
| Cumulative no. of sequelae | Incremental | ||||||
|---|---|---|---|---|---|---|---|
| Strategy | Men | Women | Total costa | QALYs lost | Costa | QALYs | $/QALY |
| No screening | 2,268 | 12,722 | 109,016,300 | 7,137 | ref | ref | ref |
| Risk-based screeningb | 1,403 | 7,351 | 88,951,100 | 4,077 | −20,065,200 | 3,060 | Cost-saving |
| Opt-out testing | 913 | 4,614 | 70,843,300 | 2,625 | −18,107,900 | 1,452 | Cost-saving |
Note: Sequelae count were pelvic inflammatory disease in women and epididymitis in men.
All costs are in 2014 U.S. dollars.
Although the Risk-Based Screening strategy was completely dominated, it was not eliminated because it was important to show the results when compared with the proposed Opt-Out Screening strategy.
QALYs, quality-adjusted life-years.
In the one-way SA, when the expected testing coverage for the high sexual activity group was >60%, overall prevalence decreased to zero. Thus, overall prevalence decreased to zero when the test acceptance probability was increased to 95%. A summary of the first part of the multi-way SA is presented in Appendix Table 1 (available online). The top panel provides a summary of the results showing the variables/parameters with the highest impact on the female pre–opt out prevalence in a hierarchical order. The results showed that the most influential variable that impacted the estimated female pre–opt out prevalence was the proportion of women in the high sexual activity category, followed by the duration of infection-conferred immunity; per-partner transmission probability (male to female); duration of asymptomatic infections (female followed by male); proportion of symptomatic infections (female followed by male); pre–opt out annual screening coverage (females); number of partners in the last year for high sexual activity women; the duration of symptomatic infections in women; the probability of post-screening treatment; and the entry–exit rate (Appendix Table 1, available online).
In the lower panel of Appendix Table 1 (available online), the summary results from the second and final part of the SA used to determine the hierarchy of influential parameters/variables of the estimated ICER are shown. The results indicated that the most influential variable impacting the estimated ICER was the female pre–opt out prevalence, probability of sequelae (females), the discount rate, testing coverage for women in the high sexual activity group, and testing cost (Appendix Table 1, available online).
The estimated female pre–opt out prevalence ranged from 0.82% to 5.8%. Based on the range of study parameter values, the estimated ICER ranged from cost saving to $19,974/QALY saved, and >70% of the simulations resulted in cost savings (i.e., the estimated net costs were <$0).
Discussion
This study used a simple population-based heterosexual compartmental model to assess the health and economic outcomes of the Opt-Out Testing strategy for chlamydia screening among a population of sexually active women aged 15–24 years. The model showed that the Opt-Out Testing strategy was very effective at reducing chlamydia prevalence in the population: The overall chlamydia prevalence decreased by more than half. Other dynamic models of chlamydia transmission have found similar results for broadly implemented chlamydia screening of young women.31,32 It was found that the Opt-Out Testing strategy was cost saving when compared with the status quo Risk-Based Screening strategy. In fact, the Opt-Out Testing was more effective and less costly than Risk-Based Screening. The finding that the current screening rate was cost saving for high-morbidity populations is consistent with previous studies that have reported that screening and treatment of infected individuals in high-morbidity settings were cost saving.24,33–37 The principal contribution of this study is the proposal of a mechanism to significantly increase screening rates in young women.
Limitations
This study has many limitations worth noting. First, the transmission model is an oversimplification of real-world events; thus, all limitations associated with models (in general) are applicable. Second, all the study variables were obtained from the literature with varying magnitudes of uncertainty around them. Some data were drawn from different studies conducted in different populations at different times. However, the comprehensive SAs should help to reduce these concerns, although the use of PRCC might result in misleading conclusions about the influence of variables that had non-linear and non-monotonic relationship with the ICERs.38 Owing to lack of data on same-sex sexual behavior and transmission dynamics, this study focused exclusively on heterosexual transmission. Consequently, the model was driven by female parameters, whereas the prevalence among men was determined by the resulting equilibria under varying parameter and intervention assumptions in the model.26 As a result, the estimated status quo prevalence among men was somewhat different from the national estimates for the same age group (2.2% vs 1.7%).10
Because reinfection of treated women by their untreated sex partner is not explicitly accounted for in compartmental models, recent studies20,39 have shown that they potentially overestimate the impact of chlamydia screening. Thus, the cost-effectiveness of testing/screening might have been overestimated. Had this study fully accounted for reinfection of treated women by their untreated partners, the estimated ICERs might be higher (less favorable) than estimated. Because of delays in the development of sequelae and discounting, interventions that treat current infections—even if patients become reinfected—can be cost effective. Owing to lack of data, equal insurance coverage and healthcare utilization rates for both the high and low sexually active individuals was assumed. If high sexually active individuals are less likely to be insured and have lower utilization rates, then the expected testing coverage for the high sexually active women, as determined in this model, would be lower and the estimated ICER would be higher (less favorable).
Because the population comprised one age group (age 15–24 years), the model was not age structured. As a result, the model ignored the impact of potential differences in the transmission dynamics and other estimates (such as test acceptance rates) within that age group. Finally, based on the model, applying a higher current screening coverage (i.e., >30%) would increase the estimated ICER, making the Opt-Out strategy less favorable.
Although some young women currently have screening rates higher than 30% (such as those enrolled in Medicaid HMOs), rates overall (including those who are uninsured) are low. However, at any percentage of current screening coverage, if the effective testing coverage for the Opt-Out strategy is less than the current screening coverage, then the Opt-Out strategy should not be considered.
Universal HIV screening of men and women aged 13–64 years has been recommended since 2006, in communities and populations with an HIV prevalence of 0.1% or greater. Studies40,41 have demonstrated that it resulted in increased HIV screening coverage, although implementation of this recommendation has lagged in some healthcare venues such as emergency depart-ments.42 HIV prevalence is 0.47% in the U.S. among people aged 13–49 years.42 In comparison, the prevalence of chlamydia is very high in the target population for Opt-Out Testing—3.21% among women aged 15–24 years.10 Many providers find conducting a sexual risk assessment difficult, and it is commonly not done at routine visits.14,15,18 Even if a sexual risk assessment is conducted, adolescents may not disclose relevant risk information. Also, adolescents might have limited knowledge of chlamydial infection, and be unaware of its potential long-term adverse outcomes.43,44
Such structural health services strategies should be designed to minimize the efforts required by the office or clinic staff. Other studies45,46 have demonstrated the effectiveness of such structural interventions. Both self-collected vaginal swabs and urine specimens are included as appropriate specimens for testing with U.S. Food and Drug Administration–cleared nucleic acid amplification tests.47 Studies48,49 have found that the sensitivity and specificity of these tests using urine or vaginal swab specimens to be high, and patients have found self-collection of vaginal swabs to be preferred to clinician-collected swabs. Currently, monitoring chlamydia screening coverage is quite challenging, in part because of the difficulty identifying sexually active women who should be tested.50
Conclusions
Using conservative values for the test acceptance probability, this study suggests that implementation of an Opt-Out Testing strategy to screen young women for chlamydia during clinical encounters might substantially increase screening coverage of sexually active young women, and be cost saving. The proposed Opt-Out Testing strategy was highly cost effective under a wide range of assumptions/scenarios because it did not require additional costs over and above testing and treatment. However, although substantially high test acceptance probabilities (>95%51,52) at clinical encounters are achievable, testing gaps would remain because many women might not have health insurance coverage or not utilize healthcare. Further studies are needed to assess the effectiveness of this strategy in clinical settings.
Supplementary Material
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC. Mention of company names or products does not imply endorsement by CDC.
No financial disclosures were reported by the authors of this paper.
Appendix
Supplementary data
Supplementary data associated with this article can be found at http://dx.doi.org/10.1016/j.amepre.2016.01.007.
References
- 1.U.S. Preventive Services Task Force. Screening for chlamydial infection: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;147(2):128–134. 10.7326/0003-4819-147-2-200707170-00172. [DOI] [PubMed] [Google Scholar]
- 2.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Chlamydia screening among sexually active young female enrollees of health plans—United States, 2000–2007. MMWR Recomm Rep. 2009;58(14):362–365. [PubMed] [Google Scholar]
- 4.Hoover K, Tao G, Kent C. Low rates of both asymptomatic chlamydia screening and diagnostic testing of women in U.S. outpatient clinics. Obstet Gynecol. 2008;112(4):891–898. 10.1097/AOG.0b013e318185a057. [DOI] [PubMed] [Google Scholar]
- 5.Hoover K, Tao G. Missed opportunities for chlamydia screening of young women in the United States. Obstet Gynecol. 2008;111(5):1097–1102. 10.1097/AOG.0b013e31816bbe9b. [DOI] [PubMed] [Google Scholar]
- 6.Eugene JM, Hoover KW, Tao G, Kent CK. Higher yet suboptimal chlamydia testing rates at community health centers and outpatient clinics compared with physician offices. Am J Public Health. 2012;102 (8):e26–e29. 10.2105/AJPH.2012.300744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao G, Hoover KW, Leichliter JS, Peterman TA, Kent CK. Self-reported Chlamydia testing rates of sexually active women aged 15–25 years in the United States, 2006–2008. Sex Transm Dis. 2012;39(8): 605–607. 10.1097/OLQ.0b013e318254c837. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Quality Assurance. The State of Health Care Quality 2014. Washington, DC: National Committee for Quality Assurance, 2014. [Google Scholar]
- 9.CDC. Sexually Transmitted Disease Surveillance, 2013. Atlanta, GA: USDHHS, 2014. [Google Scholar]
- 10.Satterwhite CL, Torrone E, Meitis E, et al. Sexually transmitted infections among U.S. women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193. 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 11.Hoover KW, Tao G, Nye MB, Body BA. Suboptimal adherence to repeat testing recommendations for men and women with positive Chlamydia tests in the United States, 2008–2010. Clin Infect Dis. 2013;56(1):51–57. 10.1093/cid/cis771. [DOI] [PubMed] [Google Scholar]
- 12.CDC. CDC. Grand Rounds: Chlamydia prevention: challenges and strategies for reducing disease burden and sequelae. MMWR Morb Mortal Wkly Rep. 2011;60:370–373. [PubMed] [Google Scholar]
- 13.Paavonen J, Westrom L, Eschenbach DA. Pelvic inflammatory disease In: Holmes KK, Sparling PF, Stamm WE, et al. , eds. Sexually Transmitted Diseases. New York: McGraw Hill, 2008:1017–1050. [Google Scholar]
- 14.Lafferty WE, Downey L, Shields AW, Holan CM, Lind A. Adolescent enrollees in Medicaid managed care: the provision of well care and sexual health assessment. J Adolesc Health. 2001;28(6):497–508. 10.1016/S1054-139x(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 15.Wimberly YH, Hogben M, Moore-Ruffin J, Moore SE, Fry-Johnson Y. Sexual history-taking among primary care physicians. J Natl Med Assoc. 2006;98(12):1924–1929. [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal MK, Witt R, Hayes KL, Zaoutis TE, Gerber JS. Clinician adherence to recommendations for screening of adolescents for sexual activity and sexually transmitted infection/human immunodeficiency virus. J Pediatr. 2014;165(2):343–347. http://dx.doi.org/10.1016Aj.jpeds.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiClemente RJ, Sales JM, Danner F, Crosby RA. Association between sexually transmitted diseases and young adults’ self-reported abstinence. Pediatrics. 2011;127(2):208–213. 10.1542/peds.2009-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta SD, Sternberg M, Johnson RE, et al. Gonorrhea and Chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann Intern Med. 2007;147(2):89–96. 10.7326/0003-4819-147-2-200707170-00007. [DOI] [PubMed] [Google Scholar]
- 19.Cohen RA, Martinez ME. Health insurance coverage: early release of estimates from the National Health Interview Survey, Jan-Mar 2014. www.cdc.gov/nchs/nhis/releases.htm. Published 2014. Accessed May 20, 2015.
- 20.Althaus CL, Heijne JC, Roellin A, Low N. Transmission dynamics of Chlamydia trachomatis affect the impact of screening programmes. Epidemics. 2010;2(3):123–131. 10.1016/j.epidem.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Cole J, Hotton A, Zawitz C, Kessler H. Opt-out screening for Chlamydia trachomatis and Neisseria gonorrhoeae in female detainees at Cook County jail in Chicago, IL. Sex Transm Dis. 2014;41(3): 161–165. 10.1097/OLQ.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 22.Fielder RL, Carey KB, Carey MP. Acceptability of sexually transmitted infection testing using self-collected vaginal swabs among college women. J Am Coll Health. 2013;61(1):46–53. 10.1080/07448481.2012.750610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owusu-Edusei Jr., Gift TL, Chesson HW, Kent CK. Investigating the potential public health benefit of jail-based screening and treatment programs for chlamydia. Am J Epidemiol. 2013;177(5):463–473. 10.1093/aje/kws240. [DOI] [PubMed] [Google Scholar]
- 24.Gift TL, Gaydos CA, Kent CK, et al. The program cost and cost-effectiveness of screening men for Chlamydia to prevent pelvic inflammatory disease in women. Sex Transm Dis. 2008;35(11 suppl): S66–S75. 10.1097/OLQ.0b013e31818b64ac. [DOI] [PubMed] [Google Scholar]
- 25.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192(10): 1836–1844. 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 26.Owusu-Edusei K Jr., Chesson HW, Gift TL, Brunham RC, Bolan G. Cost-effectiveness of Chlamydia vaccination programs for young women. Emerg Infect Dis. 2015;21(6):960–968. 10.3201/eid2106.141270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1): 28–41. 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith KJ, Tsevat J, Ness RB, Wiesenfeld HC, Roberts MS. Quality of life utilities for pelvic inflammatory disease health states. Sex Transm Dis. 2008;35(3):307–311. 10.1097/OLQ.0b013e31815b07dd. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Labor. Consumer price indexes—all urban consumers. http://www.bls.gov/cpi/home.htm. Published 2015. Accessed May 15, 2015.
- 30.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model as an example. Int Stat Rev. 1994;62(2):229–243. 10.2307/1403510. [DOI] [Google Scholar]
- 31.Turner KM, Adams EJ, Lamontagne DS, Emmett L, Baster K, Edmunds WJ. Modelling the effectiveness of chlamydia screening in England. Sex Transm Infect. 2006;82(6):496–502. 10.1136/sti.2005.019067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kretzschmar M, Turner KME, Barton PM, Edmunds WJ, Low N. Predicting the population impact of chlamydia screening programmes: comparative mathematical modelling study. Sex Transm Infect. 2009;85(5):359–366. 10.1136/sti.2009.036251. [DOI] [PubMed] [Google Scholar]
- 33.Hu D, Hook 3rd, Goldie SJ. Screening for Chlamydia trachomatis in women 15 to 29 years of age: a cost-effectiveness analysis. Ann Intern Med. 2004;141(7):501–513. 10.7326/0003-4819-141-7-200410050-00006. [DOI] [PubMed] [Google Scholar]
- 34.Kretzschmar M, Welte R, van den Hoek A, Postma MJ. Comparative model-based analysis of screening programs for Chlamydia trachomatis infections. Am J Epidemiol. 2001;153(1):90–101. 10.1093/aje/153.1.90. [DOI] [PubMed] [Google Scholar]
- 35.Welte R, Kretzschmar M, Leidl R, van den Hoek A, Jager JC, Postma MJ. Cost-effectiveness of screening programs for Chlamydia trachomatis: a population-based dynamic approach. Sex Transm Dis. 2000;27 (9):518–529. 10.1097/00007435-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Tuite AR, Jayaraman GC, Allen VG, Fisman DN. Estimation of the burden of disease and costs of genital Chlamydia trachomatis infection in Canada. Sex Transm Dis. 2012;39(4):260–267. 10.1097/OLQ.0b013e31824717ae. [DOI] [PubMed] [Google Scholar]
- 37.Fisman DN, Spain CV, Salmon ME, Goldberg M. The Philadelphia High-School STD Screening Program: key insights from dynamic transmission modeling. Sex Transm Dis. 2008;35(11 suppl):S61–S65. 10.1097/OLQ.0b013e3181802822. [DOI] [PubMed] [Google Scholar]
- 38.Marino S, Hogue IB, Ray CJ, Kirschner DE. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol. 2008;254(1):178–196. 10.1016/j.jtbi.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Low N, Heijne JCM, Kretzschmar M. Use of mathematical modeling to inform Chlamydia screening policy decisions. J Infect Dis. 2009;199 (5):767–768. 10.1086/596744. [DOI] [PubMed] [Google Scholar]
- 40.Momplaisir F, Yehia BR, Harhay MO, Fetzer B, Brady KA, Long JA. HIV testing trends: Southeastern Pennsylvania, 2002–2010. AIDS Patient Care STDs. 2014;28(6):303–310. 10.1089/apc.2014.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West-Ojo T, Samala R, Griffin A, et al. Expanded HIV testing and trends in diagnoses of HIV infection—District of Columbia, 20042008. Morb Mortal Wkly Rep. 2010;59(24):737–741. [PubMed] [Google Scholar]
- 42.Hoover JB, Tao G, Heffelfinger JD. Monitoring HIV testing at visits to emergency departments in the United States: very-low rate of HIV testing. J Acquir Immune Defic Syndr. 2013;62(1):90–94. 10.1097/QAI.0b013e3182742933. [DOI] [PubMed] [Google Scholar]
- 43.Trent M, Millstein SG, Ellen JM. Gender-based differences in fertility beliefs and knowledge among adolescents from high sexually transmitted disease-prevalence communities. J Adolesc Health. 2006;38(3): 282–287. 10.1016/j.jadohealth.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Quach S, Librach C. Infertility knowledge and attitudes in urban high school students. Fertil Steril. 2008;90(6):2099–2106. 10.1016/j.fertnstert.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Burstein GR, Snyder MH, Conley D, et al. Chlamydia screening in a health plan before and after a national performance measure introduction. Obstet Gynecol. 2005;106(2):327–334. 10.1097/01.AOG.0000171119.81704.51. [DOI] [PubMed] [Google Scholar]
- 46.Guy RJ, Ali H, Liu B, et al. Efficacy ofinterventions to increase the uptake of chlamydia screening in primary care: a systematic review. BMC Infect Dis. 2011;11:211 10.1186/1471-2334-11-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papp JR, Schachter J, Gaydos CA, Van Der Pol B. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63(RR-2):1–19. [PMC free article] [PubMed] [Google Scholar]
- 48.Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis. 2005;32(12): 725–728. 10.1097/01.olq.0000190092.59482.96. [DOI] [PubMed] [Google Scholar]
- 49.Chernesky MA, Hook EW 3rd, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005;32(12):729–733. 10.1097/01.olq.0000190057.61633.8d. [DOI] [PubMed] [Google Scholar]
- 50.Broad JM, Manhart LE, Kerani RP, Scholes D, Hughes JP, Golden MR. Chlamydia screening coverage estimates derived using healthcare effectiveness data and information system procedures and indirect estimation vary substantially. Sex Transm Dis. 2013;40(4):292–297. 10.1097/OLQ.0b013e3182809776. [DOI] [PubMed] [Google Scholar]
- 51.Holland-Hall CM, Wiesenfeld HC, Murray PJ. Self-collected vaginal swabs for the detection of multiple sexually transmitted infections in adolescent girls. J Pediatr Adolesc Gynecol. 2002;15(5):307–313. 10.1016/S1083-3188(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 52.Wiesenfeld HC, Lowry DL, Heine RP, et al. Self-collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomoniasis: opportunity to encourage sexually transmitted disease testing among adolescents. Sex Transm Dis. 2001;28(6):321–325. 10.1097/00007435-200106000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.