Abstract
Tetracyclines are well established antibiotics but show phototoxicity as a side effect. Antimicrobial photodynamic inactivation uses nontoxic dyes combined with harmless light to destroy microbial cells by reactive oxygen species. Tetracyclines (demeclocycline and doxycycline) can act as light-activated antibiotics by binding to bacterial cells and killing them only upon illumination. The remaining tetracyclines can prevent bacterial regrowth after illumination has ceased. Antimicrobial photodynamic inactivation can be potentiated by potassium iodide. Azide quenched the formation of iodine, but not hydrogen peroxide. Demeclotetracycline (but not doxycycline) iodinated tyrosine after light activation in the presence of potassium iodide. Bacteria are killed by photoactivation of tetracyclines in the absence of oxygen. Since topical tetracyclines are already used clinically, blue light activation may increase the bactericidal effect.
Keywords: : antimicrobial photodynamic inactivation, bacteria, photochemical mechanisms, potassium iodide, singlet oxygen, tetracyclines
Background: discovery of the tetracyclines
Although Alexander Fleming discovered penicillin as early as 1928, the antibiotic era did not begin in earnest until 1939–1940 when Howard Florey and his team developed production methods using microbial fermentation, and first demonstrated that penicillin could be used to cure bacterial infections in humans. The Second World War served to kick start mass production of penicillin on an industrial scale to treat the Allied troops fighting in Europe. By the early 1940s, antibiotic discovery was progressing apace, as described by microbiologist Rene Dubos [1], and an interesting array of compounds had been isolated from actinomycetes growing in the soil (as discussed by Waksman et al. [2]). It had become evident that environmental microbial cells could produce a variety of natural products, and potential antibiotics could be discovered in the most unlikely places [3].
In 1938, the president of Cyanamid, William B Bell had put forward a new mission statement, thus marking the entry of Cyanamid into the new field of antibiotic discovery. Cyanamid built new laboratories in Pearl River, NJ, USA and hired 71-year-old Benjamin Minge Duggar as a consultant to run their soil-screening department. Duggar had collected soil samples from all over the world, and had isolated actinomycetes or ‘ultra-molds’ as he called them. The isolated strains were assayed for antibiotic activity against a panel of Gram-positive and Gram-negative bacteria. One sample yielded a strain with unusual yellow-colored colonies that inhibited the growth of all tested bacteria with high activity. The species was named Streptomyces aureofaciens, and the compound was called ‘aureomycin’ [4]. The efforts at Cyanamid were then expanded, bringing in RD McCormick to manufacture the compound in commercial quantities. In 1948, aureomycin was approved by the US FDA and went on to save numerous lives of patients suffering from a wide range of infectious diseases. One of the first of these patients was 5-year-old Toby Hockett, who was cured from an abdominal infection that he contracted after a surgical operation for a ruptured appendix [5].
In 1950, Alexander Finlay and his team at Charles Pfizer Co., Inc., obtained thousands of soil samples from around the world, from which they isolated the actinomycete species, Streptomyces rimosus [6]. This strain produced another different yellow compound with better water solubility, which was called Terramycin. It received FDA approval in 1950, and was again used to treat a variety of infectious bacterial diseases [7]. However, it turned out to be difficult to determine the chemical structures of either aureomycin or Terramycin, until Karl Brunings at Pfizer, collaborating with Robert Woodward at Harvard University, suggested that both compounds possessed a DCBA naphthacene core possessing similar functional groups [8]. The structure was finally determined in 1954 by the Pfizer-Woodward group in a landmark paper [9] after which the core ring structure possessed by this new family of antibiotics became known as the ‘tetracyclines’ (TCs). Lloyd Conover working at Pfizer investigated chemical modification of the TCs, and showed that the C7 chlorine atom in aureomycin could be removed (using palladium metal and hydrogen) to give a compound named simply ‘tetracycline’ [10]. This compound received FDA approval in 1954. Stephens et al. synthesized an analog with remarkably high activity and good stability, called ‘doxycycline’ (DOTC) that received FDA approval in 1967 [11]. DOTC is still widely used today with activity against a broad spectrum of community-acquired bacterial infections.
Lederle scientists produced a new TC they called demeclocycline [12]. Church et al. produced [13] an analog with a C7-dimethylamino group called minocycline (MINO) that received FDA approval in 1971.
TC antibiotics bind to the bacterial ribosomes, thus preventing the binding of tRNA to the aminoacyl–mRNA complex, and inhibiting bacterial protein synthesis. TCs principally bind to the 30S ribosomal subunit in the ribosomal translation complex. Gram-negative bacteria are thought to take up TCs via the OmpF and OmpC porin channels, because the TCs can form complexes with positively charged cations (probably magnesium ions) [14]. Once taken up inside the bacteria, the metal ion–TC complex can dissociate to liberate the uncharged TC compound, which can then diffuse through the lipid bilayers of the inner (cytoplasmic) membrane in an energy-dependent manner, driven by the proton gradient across the membrane [15]. Similarly, the uncharged (more lipophilic TCs) are probably transferred intact across the cytoplasmic membrane of Gram-positive bacteria. The species which binds to the ribosome, is probably also a magnesium–TC complex [16]. However, TCs do bind reversibly to the ribosome, which explains why tetracylines are bacteriostatic antibiotics in their mode of action (rather than bactericidal).
TCs have also been found to inhibit matrix metalloproteinases (enzymes produced by the host in cancer or inflammation). This mechanism does not influence their antibiotic effects, but does explain the anti-inflammatory effects of TCs. This anti-inflammatory activity has led to many studies on chemically modified TCs (such as incyclinide [17] for the treatment of skin diseases [rosacea and acne] [18], diabetes and even cancer [19]). However, these studies have not as yet led to any widely approved treatments.
Antimicrobial photodynamic inactivation
Photodynamic therapy (PDT) relies on the excitation of certain dyes known as photosensitizers (PS) by visible light [20]. The electron in the lowest unoccupied molecular orbital is raised up to the first excited singlet state, when some of these molecules can undergo a ‘spin-flip’ to produce the excited triplet state. The long-lived triplet state can interact with molecular oxygen by two different pathways. The type II pathway involves energy transfer from the excited triplet PS to ground state triplet oxygen, to produce excited state singlet oxygen. The second pathway is called type I, and involves an electron transfer reaction from the triplet PS to ground state oxygen, to initially produce superoxide radical anion, followed by hydrogen peroxide and hydroxyl radicals. All these different reactive oxygen species (ROS) can attack biomolecules including, lipids, proteins and nucleic acids, producing cell damage and cell death.
PDT was accidentally discovered in 1900 by its effect on destroying a microorganism, Paramecia, that had been incubated in a solution of the dye, acridine orange, and then exposed to light [21].
However, PDT was mainly developed as a cancer therapy [22], when various compounds based on porphyrins were found to localize in tumors when injected intravenously [23]. Not only could tumors be detected by their red fluorescence when examined under ultra violet A (UVA) excitation [24], but the tumors could also be destroyed when excited by red light that could penetrate into tissue [25]. Starting in the 1990s PDT began to be explored as an antimicrobial therapy, that came to be called antimicrobial photodynamic inactivation (aPDI) [26]. It was realized that nearly all PS (including those used to treat cancer) could be used to kill Gram-positive bacteria and fungi, but special methods needed to be used to kill Gram-negative bacteria, because of their highly impermeable outer membrane structure excluded most PS with neutral or anionic charges [27]. These special methods included the use of additional agents to disrupt the outer membrane (polymyxin nonapeptide [27] or EDTA [27]), the attachment of anionic PS to cationic polymers (such as poly-L-lysine [28] or polyethyleneimine [28]) or the use of PS with pronounced cationic charges [29]. A large range of cationic PS have now been designed including cationic porphyrins [30], cationic phthalocyanines [31], cationic bacteriochlorins [29,32], cationic phenothiazinium dyes [33] and cationic fullerenes [34]. A high quantum yield of singlet oxygen or other ROS is also desirable for an efficient PS.
The use of aPDT to treat localized infections relies on designing PS with selectivity for bacterial cells compared with host mammalian cells, the use of a relatively short drug light interval (minutes rather than hours) and applying the PS topically to the infected tissue (rather than injecting the PS intravenously as is done to treat cancer) [35]. Several studies have reported the use of aPDT to treat wound and burn infections in animal models [36–38], and clinical trials have been performed in periodontitis [39], oral candidiasis [40] and infected diabetic wounds [41].
Phototoxicity of tetracyclines: a troubling side effect?
Not long after TCs were first used clinically as antibiotics, reports of photosensitivity occurring as a side effect began to emerge starting in 1960 [42,43]. Of all the TCs in clinical use, demeclocycline (DMCT) appeared to be one of the most likely to cause photosensitivity [44] and photo-onycholysis [45]. Schorr and Monash [46] confirmed this by intradermal injection of DMCT and TC into human volunteers followed by exposure of the skin to sunlight filtered through window glass, thus establishing that UVA wavelengths were mainly responsible. In 1964 Chang and Weinstein [47] showed that phototoxicity could be produced in human cell cultures that had been incubated with TCs and subsequently illuminated with UVA light. Today phototoxicity is still a troubling problem among travelers to tropical countries who are taking DOTC as prophylaxis against malaria [48].
Hasan and Khan undertook studies designed to elucidate the photochemical mechanism by which TCs could mediate phototoxicity [49]. They concluded that photoactivation of TCs produced ‘singlet δ dioxygen’ (type II photochemical mechanism) and this ROS was responsible for the skin phototoxicity reported as a side effect. They calculated that the singlet oxygen quantum yields were as follows: DMCT = 0.08; TC = 0.05; and MINO = 0.00. The lack of singlet oxygen production by MINO reported by these workers, was in agreement with the clinical lack of photosensitivity produced by this particular TC.
Hasan and Khan [49] reported that DMCT, DOTC and TC were not photostable because they could be bleached by doses of UVA as low as 4 J/cm2, while MINO was more photostable by comparison. However, Niu et al. reported that TC [50] was more photostable than was reported by Hasan and Khan [49]. Perhaps differences in the precise wavelengths emitted by the different light sources employed could explain the different results in the photostability studies and photobleaching rates.
Tetracyclines as PS for aPDI
Previous studies
Goldman et al. asked [51] whether TCs could be photoincorporated into Escherichia coli ribosomes, and used this approach to identify which proteins acted as the binding sites (types of photoaffinity labeling) [52]. They found that three separate processes occurred during this procedure: photoincorporation of native TCs; photoincorporation of TC photoproducts; and light-independent incorporation of TC photoproducts. There was a previous report in 1987 describing the use of tetracylines as antimicrobial PS by Martin et al. [53]. Martin found that several different TCs generated superoxide when activated by UVA light (320–400 nm). The order of activity was DOTC > demeclocycline > TC > oxytetracycline. A similar order of effectiveness was found for killing E. coli in M9 medium + glucose, with DOTC producing about 4 logs of killing at 20 μM after 60 min exposure to 0.12 mW/cm2 of UVA light.
There have only been three additional reports showing that TCs can mediate aPDI that we can trace. In the late 1970s a group from Venezuela reported that photoactivated tetracyclines could exert a virucidal effect. Esparza et al. [54] reported that a solution of DMCT at 100 μg/ml added to a suspension of Venezuelan equine encephalitis virus and illuminated with a ‘daylight type fluorescent light’ for 90 min could give six log10 steps of virus inactivation as judged by a plaque-forming assay in Vero cells. The same group reported [55] that other viruses (vesicular stomatitis virus, herpes simplex virus and poliovirus) could be inactivated, and that other TCs (DOTC and oxytetracycline) were also effective, and that the process required the presence of oxygen.
Choi et al. [56] used TC and UVA light to kill Clostridium difficile (an intestinal anaerobic bacterium responsible for many deaths). They also tested the combination of TC with low MW chitosan in an attempt to boost the penetration of the TC into the bacterial cells. They found that the triple combination (TC at 0.1 mg/ml, chitosan at 0.0125% and UVA 30 min of 2.5 mW/cm2) killed three logs of C. difficile colony-forming unit, while the single or double combination treatments killed at most one log.
Tetracyclines act as antibacterial PS in aPDI studies
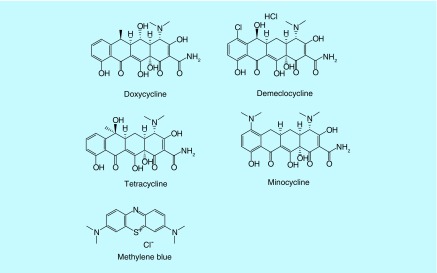
We recently [57] showed that TCs could function as dual-action light-activated antibiotics when they were excited either by UVA (360 nm) or blue (415 nm) light. We tested four different TCs, DMCT, DOTC, TC and MINO whose structures are shown in Figure 1 and whose absorption spectra and excitation light source emission spectra are shown in Figure 2. We compared them in some experiments with the traditional phenothiazinium salt compound, methylene blue (MB) whose structure is also shown in Figure 1.
Figure 1. . Chemical structures of four tetracyclines together with that of methylene blue.
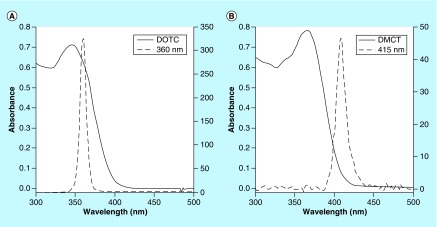
Figure 2. . Overlap of absorption spectra and light source.
Absorption spectra (left axis) of doxycycline (A) and demeclotetracycline (B) at 50 μM in phosphate-buffered saline with emission spectra of the UV A 360 nm and blue 415 nm light sources (right axis).
DMCT: Demeclocycline; DOTC: Doxycycline.
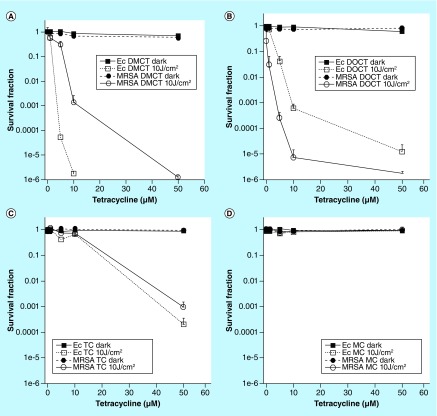
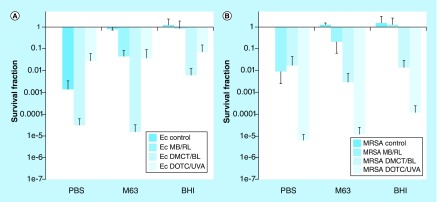
Figure 3 shows the effects of these four different TCs incubated with either Gram-positive methicillin-resistant Staphylococcus aureus (MRSA) bacteria or Gram-negative E. coli bacteria in phosphate-buffered saline and activated either by 10 J/cm2 of UVA light at 360 nm (DOTC and TC) or blue light at 415 nm (DMCT and minocycline [MC]). It can be seen in Figure 3 that both DMCT and DOTC were highly effective (DMCT being a little better) and could eradicate both the bacterial populations (>6 logs of killing) at concentrations of 50 μM (or even lower). TC only had moderate activity, while MC was completely inactive.
Figure 3. . Antimicrobial photodynamic inactivation of two strains of bacteria by four different tetracyclines.
Bacterial cells were incubated for 30 min in phosphate-buffered saline with stated concentration of tetracycline and exposed to 10 J/cm2 of the appropriate light. (A) Demeclotetracycline and blue light; (B) DOCT and UV A light; (C) tetracycline and UV A; (D) MC and blue light.
DMCT: Demeclocycline; DOCT: Doxycycline; Ec: Escheria coli; MC: Minocycline; MRSA: Methicillin-resistant Staphylococcus aureus; TC: Tetracyclines.
Reproduced from [57].
Binding of tetracycline PS to bacteria
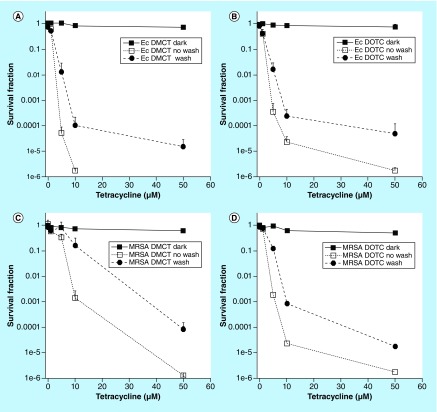
In order to test whether any PS binds to the bacterial cells, it is possible to incubate the cells with the PS and then centrifuge the suspension leaving excess (non bound) TC in solution, and then resuspend the pellet before light delivery to the suspension. This procedure is called a ‘wash’. Figure 4 shows that both DMCT and DOTC substantially bound to both E. coli and MRSA because the killing after a wash was only slightly less than the killing without a wash. This result was expected considering that TCs are known to bind to bacterial ribosomes [58]. TCs are therefore fundamentally different to the vast majority of alternative antibacterial PS. The mechanism of uptake of most antibacterial PS relies on their cationic charge binding to anionic bacterial cells, and being able to carry out the self-promoted uptake pathway [59].
Figure 4. . Effect of a wash-step on antimicrobial photodynamic inactivation with tetracyclines.
Bacterial cells were incubated for 30 min in phosphate-buffered saline with stated concentration of tetracycline and either exposed (or not ‘dark’) to 10 J/cm2 of the appropriate light (‘no wash’) or centrifuged, and resuspended in fresh phosphate-buffered saline and exposed to 10 J/cm2 (‘wash’). (A) Escherichia coli UTI89, demeclotetracycline and BL; (B) E. coli UTI89, DOCT and UV A; (C) MRSA, demeclotetracycline and BL; (D) MRSA, doxycycline and UV A.
DMCT: Demeclocycline; DOTC: Doxycycline; Ec: Escheria coli; MRSA: Methicillin-resistant Staphylococcus aureus; UVA: Ultraviolet A.
Reproduced from [57].
Photoactivated tetracyclines kill bacteria in growth media
We found that in contrast to other well-established antimicrobial PS such as MB, which was only effective in phosphate-buffered saline, and did not mediate photoinactivation in growth medium (either M63 or protein-rich brain heart infusion [BHI]), TCs were localized inside the bacterial cells and were therefore able to kill bacteria in both types of growth media [57]. The aPDI activity was not affected by the protein present in the medium as shown in Figure 5. The DOTC and UVA light combination was better at killing MRSA, while E. coli was killed more by DMCT and blue light. However, in both media the TCs killed three to four logs even in BHI, while MB and red light did not produce any killing of either species in BHI. The reason for this difference was attributed to the specific binding of the tetracylines to the bacterial ribosomes, while the binding of MB to the bacterial cell walls was disrupted by the protein present in the medium.
Figure 5. . Effect of different incubation media on antimicrobial photodynamic inactivation.
Bacterial cells were incubated for 30 min in one of three different media with demeclotetracycline or doxycycline at 10 μM, or with methylene blue at 8 μM (for Escherichia coli UTI89) or 4 μM (for MRSA), and exposed to 10 J/cm2 (taking 14 min) of the appropriate light (360 nm for doxycycline; 415 nm for demeclotetracycline; 660 nm for methylene blue) (A). Escherichia coli UTI89 (B) .
BHI: Brain heart infusion; BL: Blue light; DMCT: Demeclocycline; DOTC: Doxycycline; Ec: Escheria coli; MB: Methylene blue; MRSA: methicillin-resistant Staphylococcus aureus; PBS: Phosphate-buffer solution; RL: Red light.
Reproduced from [57] no permission necessary.
Tetracyclines are dual action antibacterials
When conventional PS plus light is used to kill bacterial cells, it is accepted that when the light is switched off, the killing effect is effectively at an end, and bacterial regrowth will then occur, if there are any surviving bacterial cells left. Therefore, in order to successfully treat an infection in an animal model using regular aPDT, it would be necessary to repeat the treatment at intervals (perhaps every day) to kill the bacterial cells that have regrown in the infected tissue. However, as each successive aPDT treatment is carried out in an infected wound, the killing effect diminishes, perhaps because the scab thickness in the wound increases with time. In fact, this problem of bacterial regrowth after PDT has been an enduring problem in animal studies of wound infections. With the use of genetically engineered bacteria that emit bioluminescence and a low-light imaging camera, it is relatively easy to observe this regrowth, while with traditional culturing methods it might have been missed.
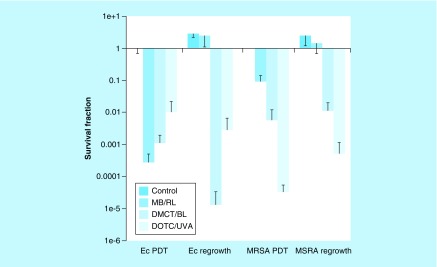
When TCs plus light were used to kill a few logs of bacteria in growth medium (M63), and then the surviving bacteria were added to fresh growth medium, the remaining antibiotic left in the suspension was sufficient to prevent any regrowth after the end of the illumination period for both DMCT and DOTC, and even allowed some extra bacterial killing to take place in the case of E. coli as shown in Figure 6 [57]. This was not the case when MB was used as the PS, where for both E. coli and MRSA, complete regrowth took place. These experiments highlighted the dual action of TCs so that they can function as PS and as antibiotics at the same time.
Figure 6. . Effect of regrowth in medium after antimicrobial photodynamic inactivation in phosphate-buffered saline.
Bacterial cells (Escherichia coli or MRSA was incubated in phosphate-buffered saline with demeclotetracycline or doxycycline at 5 μM, or with methylene blue at 8 μM (for E. coli UTI89) or 4 μM (for MRSA), and exposed to 10 J/cm2 of the appropriate light (BL for demeclotetracycline; UV A for doxycycline; red light for methylene blue). The samples were assayed for colony-forming unit and then added to fresh growth medium and incubated overnight, and the colony-forming units were determined again.
BL: Blue light; DMCT: Demeclocycline; DOTC: Doxycycline; Ec: Escheria coli; MB: Methylene blue; MRSA: Methicillin-resistant Staphylococcus aureus; PDT: Photodynamic therapy; RL: Red light; UVA: Ultra violet A.
Reproduced from [57].
Light activation can increase the antibiotic activity of tetracyclines
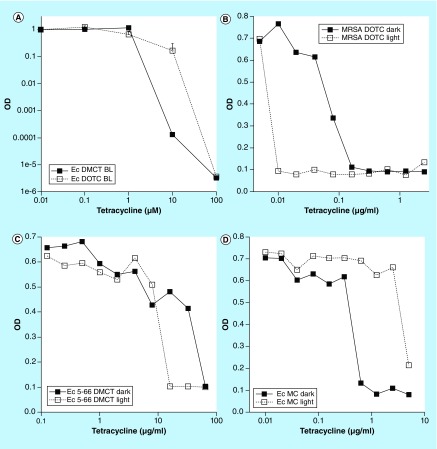
Antibiotic activity is traditionally measured as the minimum inhibitory concentrations (MIC) values expressed in microgram per milliliter. MICs are usually measured by a broth microplate dilution assay. We compared the MICs of TCs in the dark, and under a constant illumination with blue light at a power density of 0.5 mW/cm2 lasting for 16 h. Initially we showed that BL was able to activate both DMCT and DOTC to kill E. coli (Figure 7A). We were then able to show that the MIC values of TCs in the dark were higher (less effective), than when measured under 0.5 mW/cm2 of blue light. Examples of the MIC curves measured in light and dark are shown in Figure 7B, MRSA with DOTC; 7C, E. coli (5-66 resistant) with DMCT; 7D, E. coli (urinary tract infection [UTI] 89) with MC. The size of the improvement was largest with the TC-resistant E. coli strain. However, for some unknown reason not every strain showed an improvement in the MIC values under blue light illumination (Table 1).
Figure 7. . Minimum inhibitory concentration determination in light and dark.
(A) Initially we tested whether both demeclotetracycline and doxycycline could be activated with BL. Next a standard broth microdilution assay (16 h) was conducted with incubation either in dark or exposed to 0.5 J/cm2 BL. Three examples are given. (B) Methicillin-resistant Staphylococcus aureus with doxycycline; (C) Escherichia coli (5-66 resistant) with demeclotetracycline; (D) E. coli (urinary tract infection 89) with MC.
BL: Blue light; DMCT: Demeclocycline; DOTC: Doxycycline; Ec: Escheria coli; MC: Minocycline.
Reproduced from [57].
Table 1. . Light and dark MIC values for Escherichia coli, methicillin-resistant Staphylococcus aureus, two tetracycline resistant E. coli strains using four tetracyclines.
| Microorganism | Tetracycline | Dark MIC (μg/ml)† | Light MIC (μg/ml) | p-value |
|---|---|---|---|---|
| Escherichia coli UTI | DMCT | 0.16–0.32 | 0.16–032 | n.s. |
| DOTC | 0.625 | 0.625 | n.s. | |
| TC | 1.25 | 1.25 | n.s. | |
| MC | 0.32–0.625 | >5 | <0.0001 | |
| MRSA | DMCT | 0.08 | 0.02–0.04 | 0.0001 |
| DOTC | 0.08–0.16 | 0.01–0.02 | 0.0003 | |
| TC | 0.16–0.32 | 0.08–0.16 | 0.018 | |
| MC | 0.04 | 0.04 | n.s. | |
| E. coli 5-66 | DMCT | 64 | 16–32 | 0.0002 |
| DOTC | 16 | 4–8 | 0.0134 | |
| E. coli 3-62 | DMCT | 64 | 16–32 | 0.0004 |
| DOTC | 16–32 | 8 | 0.0134 |
p-values determined by two-tailed unpaired t-test.
As is customary values are given in microgram per milliliter. In order to compare with concentrations given in micrometer in Figure 3, it should be noted that 1 μg/ml is equivalent to 2.25 μM for DOTC and 2 μM for DMCT.
DMCT: Demeclocycline; DOTC: Doxycycline; MC: minocycline; MIC: Minimum inhibitory concentration; MRSA: Methicillin-resistant Staphylococcus aureus; n.s: Not significant; TC: Tetracycline; UTI: Urinary tract infection.
Potentiation of aPDI by addition of potassium iodide & photochemical mechanisms
Studying the potentiation of aPDI by addition of KI can provide useful information on the photochemical mechanisms, and there is a real possibility that iodide potentiation could also be used clinically. It is of considerable interest to elucidate the precise photochemical mechanisms of light-activated TCs, as there have been conflicting reports in the literature, with one report emphasizing type II singlet oxygen [49] and another report emphasizing type I ROS including hydroxyl radicals [53].
Potentiation of aPDI by addition of potassium iodide
In recent years the Hamblin laboratory has published several papers describing the potentiation of aPDI by the addition of a range of simple, nontoxic inorganic salts [60]. These salts have included potassium iodide [61], potassium bromide [62], sodium thiocyanate [63], sodium selenocyanate [64] and sodium azide [65].
Perhaps out of all these different salts KI is the most useful because it is an approved therapy given by oral administration for cutaneous fungal infections and has very low toxicity [66]. We showed that KI could potentiate aPDI using MB as the PS that was excited by red light, against Gram-positive bacteria, Gram-negative bacteria and fungi [67]. We went on to show that addition of KI could also potentiate aPDI mediated by functionalized fullerenes [68]. We next investigated photocatalysis mediated by titanium dioxide nanoparticles with the addition of KI [69]. Both of these compounds (fullerenes and TiO2) are known to be able to catalyze photoinduced electron transfer reactions (type I photochemical mechanism). However, we then discovered that KI could also potentiate aPDI carried out by PS that are well known to produce singlet oxygen (type II photochemical mechanism), such as Photofrin [61], Rose Bengal [70] and the porphyrin TPPS4 [71]. The mechanisms for this potentiation effect is suggested to involve addition of singlet oxygen to iodide anion to form peroxyiodide, which subsequently decomposes into free molecular iodine and hydrogen peroxide [61]. The bacterial killing is probably due to attack of the microbial cells by a mixture of extracellular-free iodine (I2/I3-), and also reactive iodine radicals (I•/I•2) depending on the degree of binding of the PS to the microbial cells. KI potentiation of the aPDT microbial killing effect was shown to increase the efficacy of in vivo treatment of localized infections established in animal models, such as burn infections [72], wound infections [70] and oral candidiasis [73].
Potentiation of tetracycline-mediated aPDI by addition of KI
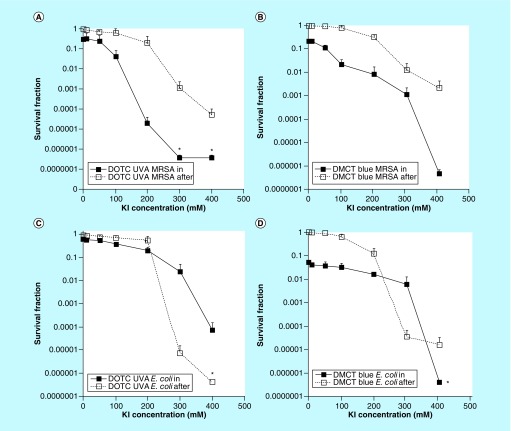
In order to test whether aPDI mediated by TCs could be potentiated by addition of KI, we selected an aPDI dose (combination of TC concentration in micromolar and light fluence in Joule per square centimeter), which only produced a relatively small amount of bacterial killing (1–2 logs), in order to be able to observe a large number of logs of potentiation. We eventually decided on 3 μM of DOTC combined with 10 J/cm2 of 360 nm UVA light and 3 μM of DMCT combined with 10 J/cm2 of 451 nm blue light [74]. The main experimental variable was the KI concentration, which was increased from zero all the way to 400 mM. In order to distinguish between killing by long-lived iodine species (free molecular iodine, I2) and short-lived iodine species (iodine radicals) we compared the ‘in’ format where all the ingredients (bacteria, TC and KI) were present together during the illumination period, with the ‘after’ format, where TC and KI are illuminated together, and the bacteria are not added until immediately after the light has been switched off.
Figure 8 shows the results. As can be seen without any KI only about one log of killing was obtained using the ‘in’ format, while as expected there was no killing using the ‘after’ format. For MRSA, eradication was achieved at 300 or 400 mM KI using the ‘in’ format, while using the ‘after’ format there was significantly less killing. For E. coli and especially for DOTC there was more killing in the ‘after’ format, but again eradication was achieved at 400 mM KI concentration.
Figure 8. . Potentiation of antimicrobial photodynamic inactivation by addition of KI.
Bacteria (10(8) colony-forming unit/ml), tetracyclines (3 μM), exposed to 10 J/cm2 of UVA or blue light with the addition of different concentrations of KI. Cells were either present during light (in format), centrifuged before addition of KI and light (spin format), or added after light (after format). Controls (light alone or light + KI) showed no killing (data not shown). (A) Gram-positive MRSA with doxycycline excited by UVA; (B) MRSA with demeclotetracycline excited by blue light; (C) Gram-negative E. coli with doxycycline excited by UVA light; (D) E. coli with demeclotetracycline excited by blue light.
DMCT: Demeclocycline; DOTC: Doxycycline; KI: Potassium iodide; MRSA: Methicillin-resistant Staphylococcus aureus; UVA: Ultraviolet light.
Reproduced from [74].
Mechanisms, oxygen-independent photoinactivation & effect of azide
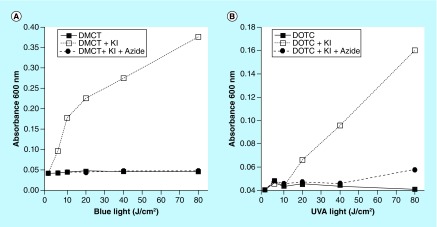
We used the phenomenon of potentiation of aPDI by addition of KI in order to explore the photochemical mechanisms carried out by photoactivated TCs. As mentioned above, one of the main bactericidal species is free molecular iodine, and this can be measured by spectroscopic determination of the absorbance at 600 nm. Figure 9 shows a light dose-dependent increase in iodine production by both DMCT and DOTC in the presence of 400 mM KI, which was almost completely quenched by addition of 50 mM sodium azide, therefore suggesting the involvement of singlet oxygen.
Figure 9. . Production of iodine (measured as blue starch complex).
Wells contained 50-μM tetracyclines, 400-mM KI, with and without 50-mM azide, excited by 10 J/cm2 of UV A or blue light. Aliquots were removed, added to starch indicator and absorbance read at 600 nm.
DMCT: Demeclocycline; DOTC: Doxycycline; UVA: Ultraviolet light.
Reproduced from [74].
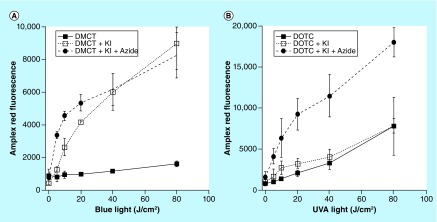
Whenever singlet oxygen carries out a 2-electron oxidation, hydrogen peroxide (H2O2) is produced, and this can be conveniently measured by the Amplex Red reaction. Figure 10 shows the production of H2O2 mediated by increasing light activation of TCs in the presence of 400 mM KI, with or without the further addition of 50-mM azide. In the case of DMCT addition of KI increased the amount of H2O2 that was produced, while when azide was also added, the H2O2 was initially further increased, while later it matched KI alone. In the case of DOTC, KI did not produce any increase in H2O2, but addition of azide produced a large increase. These data suggest that singlet oxygen is chemically quenched by azide as well as iodide.
Figure 10. . Production of hydrogen peroxide measured by Amplex Red assay.
Wells contained 50-μM tetracylines, 400-mM KI, with or without 50 mM azide. Aliquots were withdrawn after each incremental dose of light and added to Amplex Red reagent. (A) Demeclotetracycline excited by blue light; (B) doxycycline excited by UV A light.
DMCT: Demeclocycline; DOTC: Doxycycline; KI: Potassium iodide; UVA: Ultraviolet light.
Reproduced from [74].
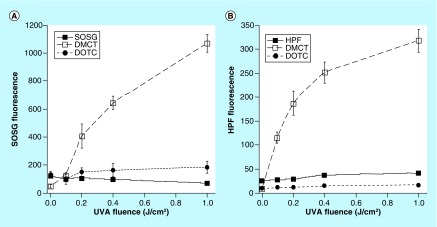
However, the results did not make complete sense at this point and we decided to use two fluorescent probes for different kinds of ROS to gain further information on the involvement of types 1 and 2 photochemical mechanisms. These probes were hydroxyphenyl fluorescein for hydroxyl radicals, and singlet oxygen sensor green (SOSG) for singlet oxygen [75]. We excited both TCs with UVA light because it has been shown that these probes (particularly SOSG) can be activated by blue light alone without any additional PS. The results are shown in Figure 11. Only DMCT excited by UVA light activated both probes, hydroxyphenyl fluorescein in Figure 11A and SOSG in Figure 11B. When DOTC was excited by UVA light there was almost zero activation of either probe.
Figure 11. . Activation of reactive oxygen species fluorescent probes by photoexcited tetracyclines.
Wells contained tetracyclines 10 μM and probes 10 μM in phosphate-buffered saline. Each experimental group contained four wells that were illuminated simultaneously with UV A light in sequential doses of 0–1.0 J/cm2.
DMCT: Demeclocycline; DOTC: Doxycycline; HPF: Hour post fertilization; SOSG: Singlet oxygen sensor green; UVA: Ultra violet A.
Reproduced from [74].
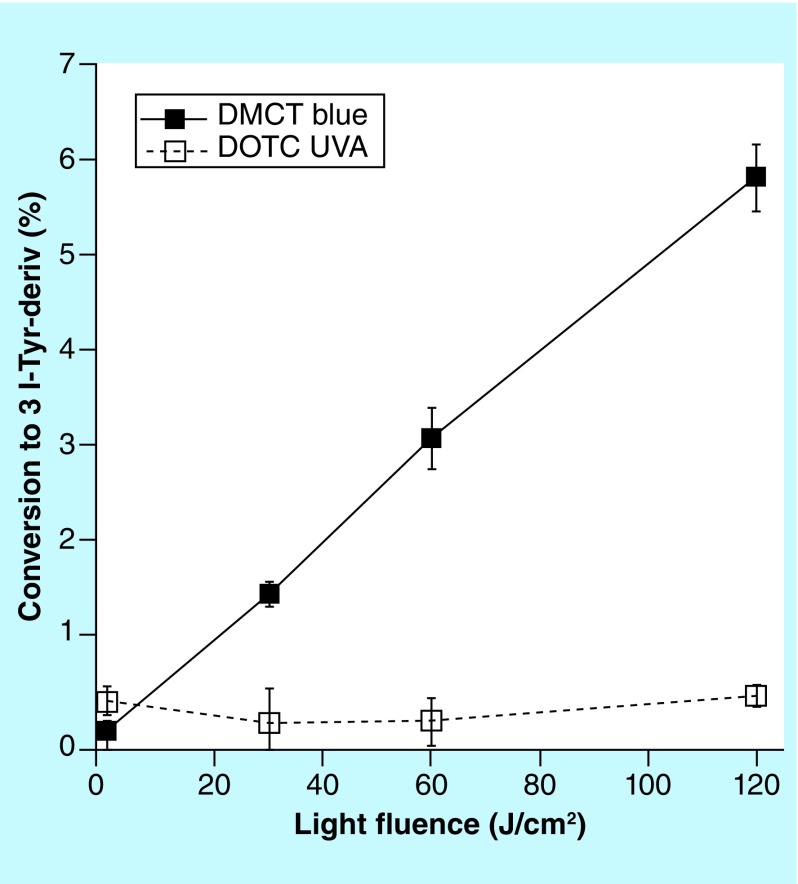
The iodination of tyrosine is a chemical assay for reactive iodine species that can be used in experiments where aPDI is potentiated by addition of KI. This assay is best carried out using N-acetyl-L-tyrosine ethyl ester as the substrate, because the reaction product, N-acetyl-3-iodo-L-tyrosine ethyl ester can be readily quantified by LC–MS. Figure 12 shows that although there was a light dose-dependent increase in the iodinated tyrosine product when 100-μM DMCT plus 400-mM KI was activated by high doses of blue light (up to 120 J/cm2), no iodinated tyrosine product was detected using 100-μM DOTC concentration (and even with 500-μM DOTC; data not shown) plus 400-mM KI and 120-J/cm2 UVA light.
Figure 12. . Iodination of tyrosine.
Solutions contained 100-μM tetracyclines, 400-mM KI, 10-mM N-acetyl-L-tyrosine ethyl ester, and aliquots were removed for LC–MS after aliquots of light had been delivered.
DMCT: Demeclocycline; DOTC: Doxycycline; UVA: Ultra violet A.
Reproduced from [74].
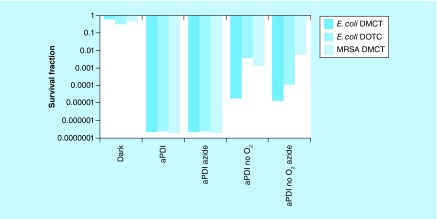
Taking into consideration all the various mechanistic studies presented above, we arrived at the conclusion that the bacterial killing caused by light-activated TCs involved three different mechanisms (which may all be operating to some extent at the same time). These mechanisms are likely to be affected to very different extents by the addition of KI. These three mechanisms are proposed to be: formation of singlet oxygen by a type 2 process acting on the excited triplet state of the TC molecule; formation of type 1 ROS (hydrogen peroxide and hydroxyl radicals) probably again arising from the excited triplet state of the TC molecule; direct formation of covalent bonds between the photoexcited TC (singlet or triplet state) and the proteins comprising the bacterial ribosomes. It is likely that mechanism A would be the one that was most likely to be strongly potentiated by addition of KI. It is possible that mechanism B may also be able to be potentiated by addition of KI. On the other hand, it is unlikely that mechanism C would be much affected by addition of KI. Moreover, mechanisms A and B will be strictly dependent on the presence of oxygen, but there remains a possibility that mechanism C could be oxygen independent. We addressed this question by comparing aPDI mediated by TCs in the presence and absence of oxygen. We had previously shown that it was possible to carry out oxygen-independent aPDI in the presence of sodium azide (50 mM), when using two different PS structures that were both able to carry out type 1 photochemical mechanisms (MB [65] and fullerenes [76]). Therefore, we compared aPDI using a high TC concentration (100 μM) in the presence and absence of air and both with and without addition of sodium azide (50 mM). Figure 13 shows the results. At 100-μM concentrations both DMCT activated with 10 J/cm2 of blue light, and DOTC activated with 10 J/cm2 of UVA light eradicated both E. coli and MRSA (MRSA was only tested with DMCT). When 50-mM azide was added, complete eradication was still achieved showing that the killing was not completely dependent on singlet oxygen. When all the oxygen was replaced with nitrogen, we still achieved substantial degrees of killing; 5 logs with E. coli plus DMCT and blue light; 2.5 logs with E. coli plus DOTC and UVA light, and 3 logs with MRSA plus DMCT and blue light. When 50-mM azide was added in the absence of oxygen, the logs of killing remained the same or even increased (E. coli plus DOTC and UVA light).
Figure 13. . Oxygen independent antimicrobial photodynamic inactivation and effect of azide.
Sealed cuvette contained cells (10(8) colony-forming unit/ml. Tetracyclines (100 μM), with or without azide (50 mM) and bubbled with N2/Ar for 30 min before 10 J/cm2 of UV A or blue light was delivered.
aPDI: Antimicrobial photodynamic inactivation; DMCT: Demeclocycline; DOTC: Doxycycline; MRSA: Methicillin- resistant Staphylococcus aureus.
Reproduced from [74].
Conclusion
In conclusion both DMCT and DOTC have been shown to function as dual-action antibacterial compounds. They act as antibiotics in the dark, and as PS under illumination with blue or UVA light. The photochemical mechanisms involved are complex in nature, and are likely to include type-2 generation of singlet oxygen, type-1 generation of radical intermediates and a direct oxygen-independent photoaffinity labeling of ribosomes. The aPDI activity of TCs can be strongly potentiated (up of five extra logs of killing) by addition of KI with concentrations in the range of 200–400 mM.
Future perspective
Taken together, our data presented above concerning the photochemical mechanisms of light-activated TCs, combined with previous reports of photoaffinity labeling of bacterial ribosomes [52], suggest that the photoinactivation of bacteria mediated by TCs could not be fully explained by simple aPDI mediated by ROS. Moreover, photodynamic oxidization of iodide anions giving free iodine, which could act as a simple disinfectant, likewise could not explain all of the results. Specifically, we propose the possibility of direct and permanent photochemical damage to bacterial ribosomes. Although this photochemical damage to ribosomes could easily be mediated by generation of ROS, it could also be an oxygen-independent process. In this case, a covalent bond could be formed between the excited TC molecule itself and the amino acids contained in proteins making up the 30S ribosomal subunit [52]. There is a little-known publication from 1969 [77] reporting that isolated ribosomes derived from E. coli were highly sensitive to photodynamic inactivation by Rose Bengal in the presence of oxygen. The authors attributed this sensitivity of the bacterial ribosomes to oxidation of certain amino-acid residues in the ribosomal proteins by singlet oxygen, as opposed to oxidation of the guanine nucleic acid bases in the RNA. We investigated the possibility of direct ribosomal photoinactivation, by testing whether TC-mediated aPDI could kill bacteria in the absence (as well as the presence) of oxygen. When we used comparatively high concentrations of both DMCT and DOTC (100 μM) we were able to kill between 3 and 5 logs of bacteria after delivering 10 J/cm2 of light, even in a nitrogen/argon atmosphere (no oxygen), and moreover the killing was not noticeably quenched by azide. These results suggest that the TCs were able to penetrate into the cells and bind to the bacterial ribosomes. Thereafter, covalent bonds could be formed after illumination leading to bacterial death, even in the absence of oxygen. Of course, both the oxygen-dependent and -independent mechanisms could operate at the same time when oxygen was present. There have been only a few previous reports of oxygen-independent photoinactivation of microbial cells (N.B. it is accepted that the term ‘photodynamic’ should be reserved for those instances where oxygen is definitely involved). A covalent dyad between a porphyrin and a C60 fullerene was able to carry out oxygen-independent photoinactivation, reportedly due to photoinduced charge separation [78]. The phenothiazinium dye, MB, was able to carry out photoinactivation of bacteria in the absence of oxygen, provided 50 mM azide was added to the suspension [65]. Similar results were obtained with two different decacationic-functionalized fullerenes, but again only when sodium azide was added [76]. In the latter two cases, the mechanism was explained by photocatalyzed electron transfer between the excited PS and azide anions, producing azidyl radicals that could possibly damage bacterial cells.
One important question that remains to be addressed, is whether the photoactivation of TCs can be effective to kill bacteria in biofilm models. Further work is necessary to answer this question taking into account the fact that short wavelength light (UVA and blue) may have difficulties in penetrating sufficiently deeply into the biofilms.
We very recently showed [74] that a superficial wound infection in a mouse model caused by bioluminescent E. coli could be treated by topical application of DMCT and irradiation with blue light. Importantly, bacterial regrowth did not occur after completion of the light irradiation, likely owing to the continued antibiotic activity exerted by the TC remaining in the wound.
We believe that clinical applications of light-activated tetracyclines could be tested in the near future. Although UVA is unlikely to be damaging to tissue at these fluences and power densities, in reality, blue light activation may be more acceptable than UVA. The addition of blue light irradiation could be considered in certain clinical situations, where TCs are routinely used for infections that are accessible to light delivery. Moreover, topical addition of KI could also be tested clinically, because even high concentrations of KI (saturated solutions of 8 M) are nontoxic.
Executive summary.
Doxycycline and demeclotetracycline activated by UV A and blue light, respectively, are potent broad-spectrum photosensitizers for antimicrobial photodynamic inactivation (aPDI).
Tetracyclines bind to the ribosomes inside bacterial cells.
Tetracyclines mediate aPDI in growth medium in the presence of protein, in contrast to conventional photosensitizers.
Tetracyclines are dual function in nature, killing bacteria in the presence of light and preventing regrowth afterwards in the dark.
Blue light can decrease the MIC values of tetracyclines.
The aPDI activity of tetracyclines is strongly potentiated by addition of potassium iodide.
The photochemical mechanisms are complex and may include type-I, type-II and a direct oxygen-independent mechanism.
Light activation of tetracyclines (with or without addition of KI) may have clinical applications.
Footnotes
Financial & competing interests disclosure
MR Hamblin was supported by US NIH grants R01AI050875 and R21AI121700. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Dubos RJ, Hotchkiss RD. The production of bactericidal substances by aerobic sporulating bacilli. J. Exp. Med. 73(5), 629–640 (1941). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waksman SA, Geiger WB, Reynolds DM. Strain specificity and production of antibiotic substances: VII. Production of actinomycin by different actinomycetes. Proc. Natl Acad. Sci. USA 32(5), 117–120 (1946). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson M. The Million Dollar Bugs. GP Putnam and Sons, NY, USA: (1969). [Google Scholar]
- 4.Duggar BM. Aureomycin; a product of the continuing search for new antibiotics. Ann. NY Acad. Sci. 51(2), 177–181 (1948). [DOI] [PubMed] [Google Scholar]
- 5.Nelson ML, Levy SB. The history of the tetracyclines. Ann. NY Acad. Sci. 1241(1), 17–32 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Finlay AC, Hobby GL. et al. Terramycin, a new antibiotic. Science 111(2874), 85 (1950). [DOI] [PubMed] [Google Scholar]
- 7.King EQ, Lewis CN, Welch H. et al. Clinical observations on the use of terramycin hydrochloride. J. Am. Med. Assoc. 143(1), 1–4 (1950). [DOI] [PubMed] [Google Scholar]
- 8.Stephens CR, Conover LH, Hochstein FA. Terramycin. VIII. Structure of aureomycin and terramycin. J. Am. Chem. Soc. 74(19), 4976–4977 (1952). [Google Scholar]
- 9.Stephens CR, Conover LH, Pasternak R. The structure of aureomycin. J. Am. Chem. Soc. 76(13), 3568–3575 (1954). [Google Scholar]
- 10.Conover LH, Moreland WT, English AR. Terramycin. XI. Tetracycline. J. Am. Chem. Soc. 75(18), 4622–4623 (1953). [Google Scholar]
- 11.Stephens CR, Beereboom JJ, Rennhard HH. 6-Deoxytetracyclines. IV. Preparation, C-6 stereochemistry, and reactions. J. Am. Chem. Soc. 85(17), 2643–2652 (1963). [Google Scholar]
- 12.McCormick JRD, Sjolander NO, Hirsch U. A new family of antibiotics: the demethyltetracyclines. J. Am. Chem. Soc. 79(16), 4561–4563 (1957). [Google Scholar]
- 13.Church RF, Schaub RE, Weiss MJ. Synthesis of 7-dimethylamino- 6-demethyl-6-deoxytetracycline (minocycline) via 9-nitro-6-demethyl-6-deoxytetracycline. J. Org. Chem. 36(5), 723–725 (1971). [DOI] [PubMed] [Google Scholar]
- 14.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65(2), 232–260 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikaido H, Thanassi DG. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: tetracyclines and fluoroquinolones as examples. Antimicrob. Agents Chemother. 37(7), 1393–1399 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chopra I, Hawkey PM, Hinton M. Tetracyclines, molecular and clinical aspects. J. Antimicrob. Chemother. 29(3), 245–277 (1992). [DOI] [PubMed] [Google Scholar]
- 17.Viera MH, Perez OA, Berman B. Incyclinide. Drugs Future 32(3), 209–214 (2007). [Google Scholar]
- 18.Dreno B, Bettoli V, Ochsendorf F. et al. European recommendations on the use of oral antibiotics for acne. Eur. J. Dermatol. 14(6), 391–399 (2004). [PubMed] [Google Scholar]
- 19.Richards C, Pantanowitz L, Dezube BJ. Antimicrobial and non-antimicrobial tetracyclines in human cancer trials. Pharmacol. Res. 63(2), 151–156 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 1(4), 279–293 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raab O. Uber die wirkung fluoreszierender stoffe auf infusorien. Z. Biol. 39, 524–546 (1900). [Google Scholar]
- 22.Agostinis P, Berg K, Cengel KA. et al. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 61(4), 250–281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figge FH, Weiland GS, Manganiello LO. Affinity of neoplastic, embryonic and traumatized tissues for porphyrins and metalloporphyrins. Proc. Soc. Exp. Biol. Med. 68(3), 640–641 (1948). [DOI] [PubMed] [Google Scholar]
- 24.Rassmussan-Taxdal DS, Ward GE, Figge FH. Fluorescence of human lymphatic and cancer tissues following high doses of intravenous hematoporphyrin. Cancer 8(1), 78–81 (1955). [DOI] [PubMed] [Google Scholar]
- 25.Diamond I, Granelli SG, McDonagh AF, Nielsen S, Wilson CB, Jaenicke R. Photodynamic therapy of malignant tumours. Lancet 2(7788), 1175–1177 (1972). [DOI] [PubMed] [Google Scholar]
- 26.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3(5), 436–450 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik Z, Ladan H, Nitzan Y. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J. Photochem. Photobiol. B 14(3), 262–266 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Hamblin MR, O'Donnell DA, Murthy N. et al. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J. Antimicrob. Chemother. 49(6), 941–951 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Huang YY, Mroz P. et al. Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob. Agents Chemother. 54(9), 3834–3841 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marciel L, Mesquita MQ, Ferreira R. et al. An efficient formulation based on cationic porphyrins to photoinactivate Staphylococcus aureus and Escherichia coli. Future Med. Chem. 10(15), 1821–1833 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Vecchio D, Dai T, Huang L, Fantetti L, Roncucci G, Hamblin MR. Antimicrobial photodynamic therapy with RLP068 kills methicillin-resistant Staphylococcus aureus and improves wound healing in a mouse model of infected skin abrasion PDT with RLP068/Cl in infected mouse skin abrasion. J. Biophotonics 6(9), 733–42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang L, Krayer M, Roubil JG. et al. Stable synthetic mono-substituted cationic bacteriochlorins mediate selective broad-spectrum photoinactivation of drug-resistant pathogens at nanomolar concentrations. J. Photochem. Photobiol. B 141, 119–127 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wainwright M, Antczak J, Baca M, Loughran C, Meegan K. Phenothiazinium photoantimicrobials with basic side chains. J. Photochem. Photobiol. B 150, 38–43 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Tegos GP, Demidova TN, Arcila-Lopez D. et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 12(10), 1127–1135 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma SK, Mroz P, Dai T, Huang YY, St Denis TG, Hamblin MR. Photodynamic therapy for cancer and for infections: what is the difference? Isr. J. Chem. 52(8–9), 691–705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Silva ZS, Jr, Bussadori SK, Fernandes KP, Huang YY, Hamblin MR. Animal models for photodynamic therapy (PDT). Biosci. Rep. 35(6), pii: e00265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avci P, Karimi M, Sadasivam M, Antunes-Melo WC, Carrasco E, Hamblin MR. In-vivo monitoring of infectious diseases in living animals using bioluminescence imaging. Virulence 9(1), 28–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J. Photochem. Photobiol. B. 81(1), 15–25 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javed F, Salehpoor D, Al-Dhafeeri T. et al. Is adjunctive photodynamic therapy more effective than scaling and root planing alone in the treatment of periodontal disease in hyperglycemic patients? A systematic review. Photodiagnosis Photodyn. Ther. 22, 1–6 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Scwingel AR, Barcessat AR, Nunez SC, Ribeiro MS. Antimicrobial photodynamic therapy in the treatment of oral candidiasis in HIV-infected patients. Photomed. Laser Surg. 30(8), 429–432 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Morley S, Griffiths J, Philips G. et al. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobial therapy. Br. J. Dermatol. 168(3), 617–624 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Falk MS. Light sensitivity due to demethylchlortetracycline. Report of four cases. J. Am. Med. Assoc. 172, 1156–1157 (1960). [DOI] [PubMed] [Google Scholar]
- 43.Morris WE. Photosensitivity due to tetracycline derivative. J. Am. Med. Assoc. 172, 1155–1156 (1960). [DOI] [PubMed] [Google Scholar]
- 44.Saslaw S. Demethylchloretetracycline phototoxicity. Report of a case. N. Engl. J. Med. 264, 1301–1302 (1961). [DOI] [PubMed] [Google Scholar]
- 45.Orentreich N, Harber L, Tromovitch TA. Photosensitivity and photo-onycholysis due to demethylchlortetracycline. Arch. Dermatol. 83, 730–737 (1961). [DOI] [PubMed] [Google Scholar]
- 46.Schorr WF, Monash S. Photo-irradiation studies of two tetracyclines. Tetracycline and demethylchlortetracycline. Arch. Dermatol. 88, 440–444 (1963). [DOI] [PubMed] [Google Scholar]
- 47.Chang TW, Weinstein L. Photosensitization of human cell cultures by demethylchloretetracycline. Proc. Soc. Exp. Biol. Med. 116, 509–512 (1964). [DOI] [PubMed] [Google Scholar]
- 48.Goetze S, Hiernickel C, Elsner P. Phototoxicity of doxycycline: a systematic review on clinical manifestations, frequency, cofactors, and prevention. Skin Pharmacol. Physiol. 30(2), 76–80 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Hasan T, Khan AU. Phototoxicity of the tetracyclines: photosensitized emission of singlet δ dioxygen. Proc. Natl Acad. Sci. USA 83(13), 4604–4606 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niu J, Li Y, Wang W. Light-source-dependent role of nitrate and humic acid in tetracycline photolysis: kinetics and mechanism. Chemosphere 92(11), 1423–1429 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Goldman RA, Hasan T, Hall CC, Strycharz WA, Cooperman BS. Photoincorporation of tetracycline into Escherichia coli ribosomes. Identification of the major proteins photolabeled by native tetracycline and tetracycline photoproducts and implications for the inhibitory action of tetracycline on protein synthesis. Biochemistry 22(2), 359–368 (1983). [DOI] [PubMed] [Google Scholar]
- 52.Hasan T, Goldman RA, Cooperman BS. Photoaffinity labeling of the tetracycline binding site of the Escherichia coli ribosome. The uses of a high intensity light source and of radioactive sancycline derivatives. Biochem. Pharmacol. 34(7), 1065–1071 (1985). [DOI] [PubMed] [Google Scholar]
- 53.Martin JP, Jr, Colina K, Logsdon N. Role of oxygen radicals in the phototoxicity of tetracyclines toward Escherichia coli B. J. Bacteriol. 169(6), 2516–2522 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esparza J, Pina CI, Novo E. Photoinactivation of Venezuelan equine encephalitis virus mediated by tetracyclines. Antimicrob. Agents Chemother. 10(1), 176–178 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novo E, Esparza J. Tetracycline-mediated photodynamic inactivation of animal viruses. J. Gen. Virol. 45(2), 323–329 (1979). [DOI] [PubMed] [Google Scholar]
- 56.Choi S, Lee H, Yu J, Chae H. In vitro augmented photodynamic bactericidal activity of tetracycline and chitosan against clostridium difficile KCTC5009 in the planktonic cultures. J. Photochem. Photobiol. B 153, 7–12 (2015). [DOI] [PubMed] [Google Scholar]
- 57.He Y, Huang YY, Xi L, Gelfand JA, Hamblin MR. Tetracyclines function as dual-action light-activated antibiotics. PLoS ONE 13(5), e0196485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aleksandrov A, Simonson T. Binding of tetracyclines to elongation factor Tu, the Tet repressor, and the ribosome: a molecular dynamics simulation study. Biochemistry 47(51), 13594–13603 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob. Agents Chemother. 44(3), 522–527 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamblin MR. Potentiation of antimicrobial photodynamic inactivation by inorganic salts. Expert Rev. Anti Infect. Ther. 15(11), 1059–1069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang L, Szewczyk G, Sarna T, Hamblin MR. Potassium iodide potentiates broad-spectrum antimicrobial photodynamic inactivation using Photofrin. ACS Infect. Dis. 3(4), 320–328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu X, Huang YY, Kushida Y, Bhayana B, Hamblin MR. Broad-spectrum antimicrobial photocatalysis mediated by titanium dioxide and UVA is potentiated by addition of bromide ion via formation of hypobromite. Free Radic. Biol. Med. 95, 74–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.St Denis TG, Vecchio D, Zadlo A. et al. Thiocyanate potentiates antimicrobial photodynamic therapy: in situ generation of the sulfur trioxide radical anion by singlet oxygen. Free Radic. Biol. Med. 65, 800–810 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang L, Xuan W, Zadlo A, Kozinska A, Sarna T, Hamblin MR. Antimicrobial photodynamic inactivation is potentiated by the addition of selenocyanate: possible involvement of selenocyanogen? J. Biophotonics 11(8), e201800029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang L, St Denis TG, Xuan Y. et al. Paradoxical potentiation of methylene blue-mediated antimicrobial photodynamic inactivation by sodium azide: role of ambient oxygen and azide radicals. Free Radic. Biol. Med. 53(11), 2062–2071 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandhu K, Gupta S. Potassium iodide remains the most effective therapy for cutaneous sporotrichosis. J. Dermatolog. Treat. 14(4), 200–202 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Vecchio D, Gupta A, Huang L. et al. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob. Agents Chemother. 59(9), 5203–5212 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Dai T, Wang M, Vecchio D, Chiang LY, Hamblin MR. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: in vitro and in vivo studies. Nanomedicine 10(4), 603–614 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang YY, Choi H, Kushida Y, Bhayana B, Wang Y, Hamblin MR. Broad-spectrum antimicrobial effects of photocatalysis using titanium dioxide nanoparticles are strongly potentiated by addition of potassium iodide. Antimicrob. Agents Chemother. 60(9), 5445–5453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen X, Zhang X, Szewczyk G. et al. Potassium iodide potentiates antimicrobial photodynamic inactivation mediated by Rose Bengal: in vitro and in vivo studies. Antimicrob. Agents Chemother. 61(7), pii: e00467-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang L, El-Hussein A, Xuan W, Hamblin MR. Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J. Photochem. Photobiol. B 178, 277–286 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang L, Wang M, Dai T. et al. Antimicrobial photodynamic therapy with decacationic monoadducts and bisadducts of [70]fullerene: in vitro and in vivo studies. Nanomedicine 9(2), 253–266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freire F, Ferraresi C, Jorge AO, Hamblin MR. Photodynamic therapy of oral Candida infection in a mouse model. J. Photochem. Photobiol. B 159, 161–168 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xuan W, He Y, Huang L. et al. Antimicrobial photodynamic inactivation mediated by tetracyclines in vitro and in vivo: photochemical mechanisms and potentiation by potassium iodide. Sci. Rep. 8(1), 17130 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price M, Reiners JJ, Santiago AM, Kessel D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochem. Photobiol. 85(5), 1177–1181 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin R, Wang M, Huang YY. et al. Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: oxygen independent photokilling in presence of azide and new mechanistic insights. Free Radic. Biol. Med. 79, 14–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garvin RT, Julian GR, Rogers SJ. Dye-sensitized photooxidation of the Escherichia coli ribosome. Science 164(3879), 583–584 (1969). [DOI] [PubMed] [Google Scholar]
- 78.Milanesio ME, Alvarez MG, Rivarola V, Silber JJ, Durantini EN. Porphyrin-fullerene C60 dyads with high ability to form photoinduced charge-separated state as novel sensitizers for photodynamic therapy. Photochem. Photobiol. 81(4), 891–897 (2005). [DOI] [PubMed] [Google Scholar]