Abstract
Measuring early tau accumulation is important in studying aging and Alzheimer disease and is only as accurate as the signal-to-noise ratio of the tracer. Along with aggregated tau in the form of neurofibrillary tangles, 18F-flortaucipir has been reported to bind to neuromelanin, monoamine oxidase, calcifications, iron, leptomeningeal melanocytes, and microhemorrages. Although 18F-flortaucipir successfully differentiates healthy controls (HCs) from subjects with Alzheimer disease, variability exists in the cortical signal in amyloid-negative HCs. We aimed to explore the relationship between off-target binding signal and variability in the cortical signal in HCs. Methods: Subjects (n = 139) received 11C-Pittsburgh compound B (PIB) and 18F-flortaucipir PET scans and a magnetization-prepared rapid gradient echo MRI scan. PET frames were realigned and coregistered to the MR images, which were segmented using FreeSurfer. In amyloid-negative HCs (n = 90; age range, 21–94 y), 7 nonspecific or off-target binding regions were considered: caudate, pallidum, putamen, thalamus, cerebellar white matter, hemispheric white matter, and choroid plexus. These regions of interest were assigned to 3 similarly behaving groups using principle components analysis, exploratory factor analysis, and Pearson correlations for caudate, putamen, and pallidum (also correlated with age); thalamus and white matter; and choroid plexus. In amyloid-negative HCs with 11C-PIB and 18F-flortaucipir scans, correlations were calculated between white and gray matter before and after partial-volume correction. Results: The correlation between white and gray matter disappeared after partial-volume correction in 11C-PIB (r2 = 0) but persisted for 18F-flortaucipir (r2 = 0.27), demonstrating that the correlation between white and gray matter signal in 18F-flortaucipir is not solely due to partial-volume effects. A linear regression showed that off-target signal from putamen and thalamus together explained 64% of the variability in the cortical signal in amyloid-negative HCs (not seen in amyloid-positive HCs). Variability in amyloid-negative HCs but not amyloid-positive HCs correlated with white matter signal (unrelated to partial-volume effects) and age-related off-target signal (possibly related to iron load). Conclusion: The noise in the 18F-flortaucipir measurement could pose challenges when studying early tau accumulation.
Keywords: tau, 18F-flortaucipir PET, off-target binding
Accumulation of the tau protein as neurofibrillary tangles and the amyloid-β protein as plaques is the hallmark of Alzheimer disease and also occurs in some healthy controls (HCs). Studying these proteins in vivo is important in understanding aging and Alzheimer disease. The typical pattern of tau deposition in brain aging and dementia has been well established in autopsy studies (1). The use of PET with radiopharmaceuticals that bind to tau permits longitudinal in vivo investigations of tau deposition across the life span.
18F-flortaucipir (also known as T807 and 18F-AV-1451) is a PET tracer that binds with high affinity to paired helical filament tau in neurofibrillary tangles (2,3). 18F-flortaucipir scans differentiate between HC and Alzheimer disease subjects and parallel Braak stage neurologic tau progression (4–8). 18F-flortaucipir scans have shown that tau accumulation is mostly restricted to the medial temporal lobe in HCs (9), unless cortical amyloid-β is present, when the tracer is found in isocortical regions (5–7). Yet there is wide variability in 18F-flortaucipir SUV ratios (SUVRs) from amyloid-negative HCs (HCAβ−) in regions outside the medial temporal lobes (10). Although 18F-flortaucipir demonstrates great promise as a clinical and research tool, off-target binding (OFF), or 18F-flortaucipir signal where aggregated tau is not expected, may complicate image interpretation (11,12). We hypothesized that variability in 18F-flortaucipir signal seen in cortical regions outside the medial temporal lobes in HCAβ− could be related to OFF.
Examples of 18F-flortaucipir OFF include binding in caudate, putamen, pallidum, and thalamus (4,13) seen in HCs, where tau accumulation should not occur until late Alzheimer disease stages. These regions also have 18F-flortaucipir tracer kinetics different from the kinetics in cortical regions (13–16), further supporting the idea that the ligand binds to targets other than pathologic tau in these regions.
Iron accumulation and age correlate with 18F-flortaucipir in the basal ganglia in HCs (17) making iron or ferritin a possible source of 18F-flortaucipir OFF. 18F-flortaucipir also binds to neuromelanin, which increases with age (18,19). Neuromelanin plays a role in the sequestration of iron and is found in lower concentrations in the cerebellum than in cortical regions (20). Although 3H-flortaucipir binds to monoamine oxidase A (MAO-A) and B (MAO-B) in vitro (21), in vivo studies have yet to replicate 18F-flortaucipir binding to MAO-A or MAO-B (22). Both exist in lower concentrations in the cerebellum than the cortex (23). Also, there was no significant difference in the 18F-flortaucipir OFF in the basal ganglia between Parkinson disease patients receiving MAO-B inhibitors and those who were not (22). Many targets have been postulated to explain the binding of 18F-flortaucipir in the choroid plexus (ChPlex) (19,24), although a difference in 18F-flortaucipir-ChPlex between African American and Caucasian subjects suggests 18F-flortaucipir could be binding to neuromelanin (25). Lastly, variability in hemispheric white matter (HemiW) signal was reported in HCs as minor displaceable binding (19), leading us to classify white matter (WM) as an OFF region.
To summarize, 18F-flortaucipir OFF has been reported in caudate, putamen, pallidum, thalamus, ChPlex, and HemiW. In 18F-flortaucipir imaging in HCAβ−, a range of 0.5 SUVR units has been reported in the cortex (10). OFF could account for variability in cortical 18F-flortaucipir in HCAβ−. We explored the relationship between OFF and cortical binding in HCAβ− and amyloid-positive HCs (HCAβ+) to better understand the variability in the 18F-flortaucipir SUVRs.
MATERIALS AND METHODS
Subjects
HC subjects (n = 139; Table 1) were recruited from the Berkeley Aging Cohort Study for 18F-flortaucipir scans (132 of 139 subjects also had a 11C-Pittsburgh compound B (PIB) scan to determine amyloid status). Berkeley Aging Cohort Study eligibility required that subjects be living independently in the community, perform normally on neuropsychologic examinations, be taking no medications that interfere with cognition, have no medical conditions that affect cognition, and have no contraindications to MRI or PET examinations. The Institutional Review Board of Lawrence Berkeley National Laboratory approved this study; all subjects gave written informed consent.
TABLE 1.
Subjects with No Significant Difference Between HCAβ− and HCAβ+ in MMSE or Education
| Age range (y) | n | Sex (M/F) | MMSE | Education (y) | Amyloid group | Has 11C-PIB scan (n) |
| 20–35 | 14 | 12/2 | 29 ± 1.2* | 15.5 ± 1.4** | Aβ− | 9 |
| 35–55 | 5 | 4/1 | 29.2 ± 1.8 | 17.2 ± 1.8 | Aβ− | 3 |
| 55–75 | 24 | 10/14 | 29.0 ± 1.2 | 17.8 ± 1.7 | Aβ− | 24 |
| 75–95 | 47 | 18/29 | 29.0 ± 1.0 | 16.9 ± 1.8 | Aβ− | 47 |
| 55–95 | 49 | 20/29 | 28.5 ± 1.4 | 16.5 ± 1.8 | Aβ+ | 49 |
One subject missing MMSE.
**Two subjects missing years of education.
MMSE = Mini-Mental State Examination.
PET Acquisition
At Lawrence Berkeley National Laboratory, 11C-PIB and 18F-flortaucipir were synthesized at the Biomedical Isotope Facility; subjects were scanned on a Siemens Biograph TruePoint PET/CT. CT was performed at the start of each emission scan for attenuation correction. For 11C-PIB, subjects were injected with 555 MBq at the start of the emission scan. Subjects were scanned for 90 min, and frames were binned as 4 × 15, 8 × 30, 9 × 60, 2 × 180, 10 × 300, and 2 × 600 s. For 18F-flortaucipir, subjects were injected with 370 MBq, and the emission scan was acquired 75–115 min after injection. Data were collected in list mode, allowing 80–100 min to be reconstructed as four 5-min frames. PET data were reconstructed using an ordered-subset expectation maximization algorithm with attenuation and scatter correction and a 4-mm gaussian kernel.
MRI Acquisition
The subjects received a high-resolution T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) scan (repetition time/echo time, 2,110/3.58 ms; flip angle, 15°; 1 × 1 × 1 mm resolution) on a 1.5-T Siemens Magnetom Avanto scanner at Lawrence Berkeley National Laboratory. MRI data were used to define regions of interest (ROIs) for image analysis.
Data Processing
MPRAGE images were segmented using FreeSurfer, version 5.3 (http://surfer.nmr.mgh.harvard.edu/). All coregistration and realignment steps were performed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). 18F-flortaucipir individual frames were realigned to create a mean, which was then coregistered to the subject’s MPRAGE image.
All 18F-flortaucipir analyses used SUVRs with an inferior cerebellar gray matter reference region (26), except for a SUV analysis examining the stability of the cortex, OFF ROIs, and reference region. Data underwent partial-volume correction (PVC) using a geometric transfer matrix approach (26,27) using a combination of FreeSurfer ROIs, extracortical hot spots, and SPM12 segmentations for cerebrospinal fluid, skull, and meninges. Both non-PVC and PVC data were analyzed. Mean SUVRs of HemiW, cerebellar WM (CereW), caudate, pallidum, putamen, thalamus, ChPlex, and cortical ROIs (grouped as Braak-stage ROIs (28)) and whole cortex (composite of Braak-stage ROIs) were calculated. Braak I was defined as FreeSurfer-segmented entorhinal cortex. II was hippocampus. III was parahippocampal gyrus, fusiform gyrus, amygdala, and lingual gyrus. IV was middle and inferior temporal cortices, temporal pole, insula, and anterior, posterior, and isthmus cingulate gyrus. V was frontal cortex, lateral occipital cortex, parietal cortex, precuneus, banks of superior temporal sulcus, accumbens, and superior and transverse temporal cortices. VI was pericalcarine, precentral, paracentral, and postcentral gyrus, and cuneus.
For 11C-PIB data, the first 5 min of data were summed and subsequent frames were realigned to the first 5 min of data. Data were coregistered to the MPRAGE image. To determine whether subjects were HCAβ+ or HCAβ−, Logan graphical analysis distribution volume ratios (29) were calculated (35–90 min; reference region was cerebellar gray matter). The 11C-PIB index was calculated using the weighted mean of FreeSurfer-derived prefrontal, parietal, lateral temporal, and cingulate cortices. HCs without a 11C-PIB scan and under 50 y old or with a 11C-PIB distribution volume ratio of less than 1.065 were considered HCAβ− (30,31).
Statistical Comparisons
Associations Between OFF ROIs and Age in HCAβ−
Pearson correlations (r2) were calculated on 18F-flortaucipir SUVRs to determine which OFF ROIs were correlated with each other or with age. Age was included in these analyses because possible targets for OFF (iron, MAO-B) are correlated with age. OFF ROIs were caudate, pallidum, putamen, thalamus, HemiW, CereW, and ChPlex. The grouping of OFF ROIs was also examined with exploratory factor analysis and principle components analysis. These analyses were done only in the HCAβ− subjects (n = 90; non-PVC and PVC) to minimize the likelihood of on-target binding of 18F-flortaucipir to tau, especially outside the medial temporal lobe. As expected, exploratory factor analysis and principle components analysis confirmed the grouping patterns revealed by Pearson correlations; these ROIs were grouped together for future analyses.
HCAβ+ > HCAβ−
Differences between HCAβ− (aged 55–95 y) and HCAβ+ for 18F-flortaucipir SUVRs in Braak ROIs and OFF ROIs were tested using analysis of covariance (controlling for age and sex). Partial η2, the portion of variance accounted for by the amyloid status, was calculated.
Correlations Between Age/OFF ROIs and Braak ROIs
To define the proportion of OFF that might be included in cortical ROIs, Pearson correlations (r2) were calculated between 18F-flortaucipir SUVRs in individual OFF ROIs (and age) and whole cortex, Braak I, II, III/IV, and V/VI ROIs. This was done separately in HCAβ− and HCAβ+ groups. Significance of correlations were Bonferroni-corrected for multiple comparisons.
Multiple Linear Models in HCAβ−
Multiple linear models were fit using SUVRs from an ROI from each of the OFF ROI groups to model the variability in the whole-cortex and individual Braak ROIs. This resulted in an adjusted r2 as well as βs and P values for OFF ROIs for each model. This was done separately in HCAβ− and HCAβ+ groups.
Is Cortical Variability Being Driven by Variability in the Reference Region?
Without arterial sampling data, it is difficult to conclude whether the variability in the cortical SUVRs is due to actual cortical variability or to variability in the reference region. To explore this, we calculated the mean, SD, and coefficient of variation of 18F-flortaucipir SUVs in OFF ROIs and Braak-ROIs in HCAβ−.
Is Cortical Variability Being Driven by WM Variability and Partial-Volume Effects (PVEs)?
Because we found that 18F-flortaucipir signal in cortex and HemiW are correlated, we investigated whether this was related to PVE, shared OFF, or both by comparing WM and cortical signal in 11C-PIB and 18F-flortaucipir SUVR images before and after PVC in the same subjects (HCAβ− subjects; n = 83). Since PVEs should be similar for 11C-PIB and 18F-flortaucipir, comparison of the effect of PVC for these 2 tracers should provide some evidence for how much of the binding is related to PVE and how much is related to shared binding to targets.
For this comparison, 11C-PIB scans were processed using an approach similar to the one applied to the 18F-flortaucipir data. 11C-PIB SUVRs were calculated from 50 to 70 min, the data underwent PVC using the same set of ROIs as for 18F-flortaucipir, and inferior cerebellar gray matter was the reference region. Means of HemiW, CereW, and cortex were calculated with and without PVC. Pearson correlations were calculated between cortical tracer binding and HemiW and CereW for 18F-flortaucipir and 11C-PIB SUVRs (non-PVC and PVC).
RESULTS
High Variability of 18F-Flortaucipir Retention in HCAβ−
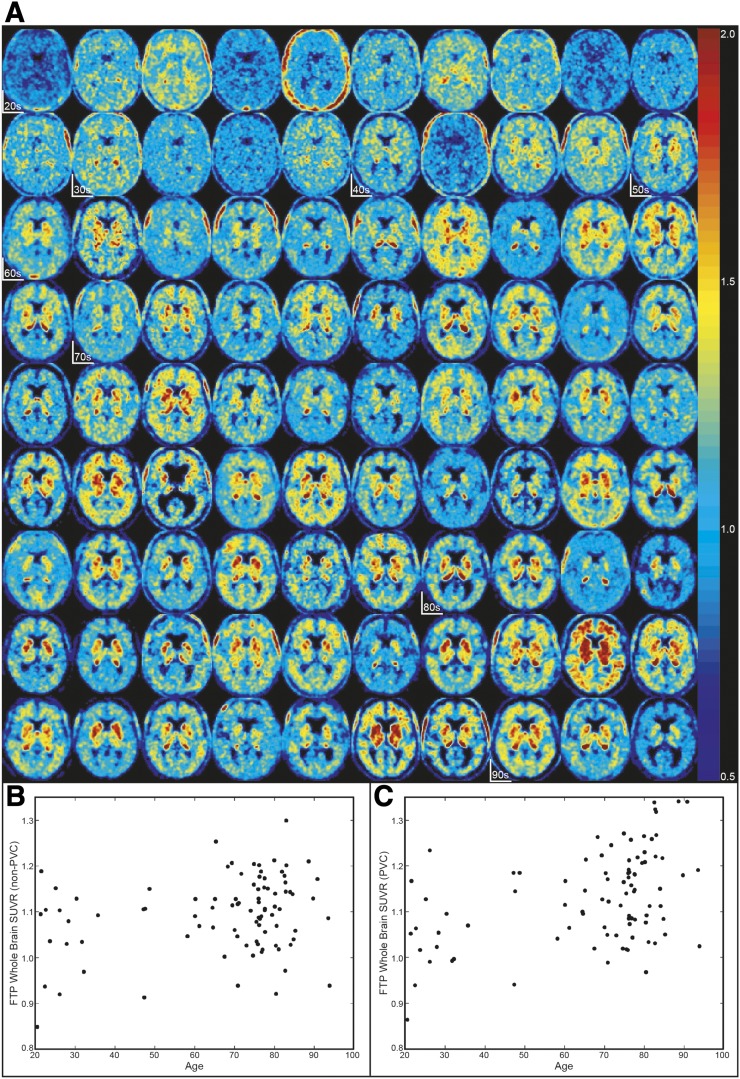
Figure 1 demonstrates the variability seen across HCAβ− subjects. Even in subjects younger than 40 y, who should have no global cortical tau accumulation (32), the whole cortical SUVR shows considerable variability, ranging from 0.85 to 1.19 (non-PVC) and 0.86 to 1.23 (PVC).
FIGURE 1.
High variability in HCAβ−. (A) 18F-flortaucipir SUVR images in Montreal Neurological Institute space in order of age. Start of each age decade is marked. (B) Age vs. non-PVC whole-cortex SUVR (r2 = 0.06, P < 0.05). (C) Age vs. PVC whole-cortex SUVR (r2 = 0.18, P < 0.001).
Associations Between OFF ROIs and with Age in HCAβ−
Pearson intercorrelations between all OFF ROI SUVRs and between each OFF ROI and age in all HCAβ− subjects are shown for both non-PVC and PVC (Table 2) data. Age and 18F-flortaucipir binding in caudate, pallidum, and putamen are highly correlated with one another (non-PVC, r2 > 0.4; PVC, r2 > 0.5) and were also grouped into one factor (exploratory factor analysis) and component (principle components analysis) (Supplemental Tables 1–2; supplemental materials are available at http://jnm.snmjournals.org). SUVR in thalamus is weakly correlated with caudate, putamen, and pallidum but more strongly correlated with HemiW and CereW. Thalamus and WM were also grouped together using exploratory factor analysis and principle components analysis. The higher correlations seen in HemiW with caudate, pallidum, and putamen for non-PVC data are most likely due to PVEs, demonstrated by lower r2 for PVC correlations. Lastly, 18F-flortaucipir binding in ChPlex significantly correlated with thalamus before PVC and with nothing after PVC. Results from Pearson correlations, principle components analysis, and exploratory factor analysis support the 3 different groups of OFF ROIs: age, caudate, pallidum, and putamen (the age-related group); thalamus, HemiW, and CereW; and ChPlex.
TABLE 2.
Positive Correlations Between Age and OFF ROIs
| Parameter | Caudate | Pallidum | Putamen | Thalamus | HemiW | CereW | ChPlex |
| Age | |||||||
| Non-PVC data | 0.42* | 0.64* | 0.68* | 0.30* | 0.13 | 0.15** | 0.03 |
| PVC data | 0.57* | 0.55* | 0.71* | 0.12 | 0.00 | 0.01 | 0.12 |
| Caudate | |||||||
| Non-PVC data | 0.72* | 0.77* | 0.67* | 0.58* | 0.55* | 0.11 | |
| PVC data | 0.59* | 0.83* | 0.41* | 0.13 | 0.18* | 0.08 | |
| Pallidum | |||||||
| Non-PVC data | 0.92* | 0.69* | 0.55* | 0.56* | 0.07 | ||
| PVC data | 0.66* | 0.40* | 0.21* | 0.25* | 0.10 | ||
| Putamen | |||||||
| Non-PVC data | 0.68* | 0.52* | 0.51* | 0.06 | |||
| PVC data | 0.38* | 0.10 | 0.13 | 0.08 | |||
| Thalamus | |||||||
| Non-PVC data | 0.79* | 0.79* | 0.21* | ||||
| PVC data | 0.61* | 0.66* | 0.02 | ||||
| HemiW | |||||||
| Non-PVC data | 0.83* | 0.11 | |||||
| PVC data | 0.83* | 0.01 | |||||
| CereW | |||||||
| Non-PVC data | 0.09 | ||||||
| PVC data | 0.02 |
P < 0.001, corrected for multiple comparisons.
**P < 0.01, corrected for multiple comparisons.
HCAβ+ > HCAβ−
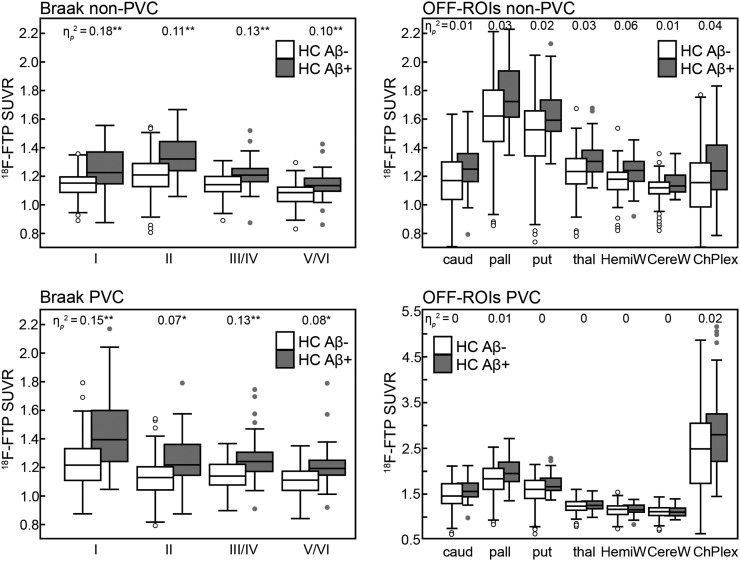
Figure 2 shows the comparison between HCAβ− and HCAβ+ for Braak and OFF ROIs. There was no significant difference in 18F-flortaucipir SUVR OFF ROIs between HCAβ+ and HCAβ− when controlling for age and sex (non-PVC and PVC; amyloid status partial η2 ≤ 0.06). There was a significant difference in 18F-flortaucipir SUVR for all Braak ROIs between HCAβ+ and HCAβ− subjects.
FIGURE 2.
Comparison between HCAβ− and HCAβ+ in Braak and OFF ROIs with and without PVC. Central mark indicates median. Top and bottom of box indicate 75th and 25th percentiles, respectively. Whisker extent indicates median ± 1.57(75th percentile – 25th percentile)/square root(number subjects). C represents subjects beyond whisker extent. Significance of P < 0.005 (*) and P < 0.001 (**) denoted for ηp2.
Age/OFF-to-Braak ROIs
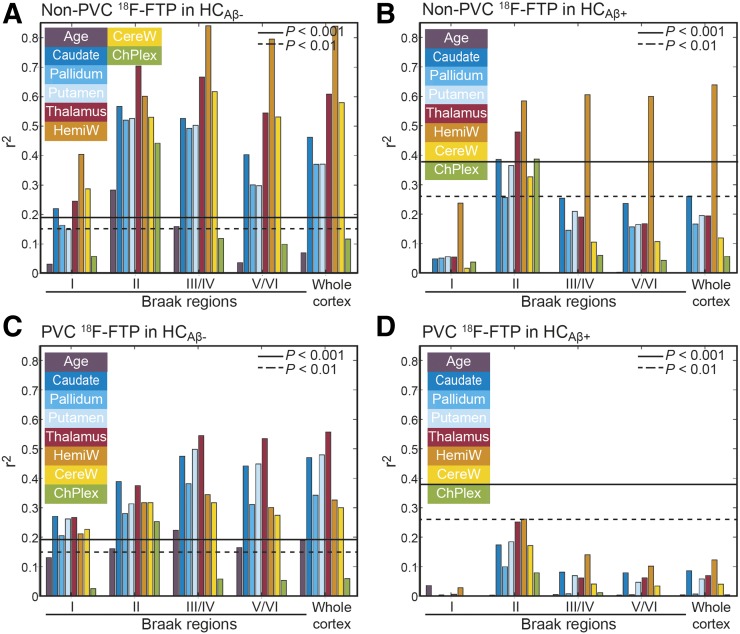
We next investigated relationships between age, OFF ROIs, and cortical 18F-flortaucipir binding by performing Pearson correlations for non-PVC (Figs. 3A and 3B) and PVC SUVRs (Figs. 3C and 3D) in HCAβ− (Figs. 3A and 3C) and HCAβ+ (Figs. 3B and 3D). Within HCAβ− subjects, correlations of cortical binding with OFF ROIs decrease after PVC but remain significant, demonstrating that PVEs alone cannot explain the correlations. All correlations in HCAβ+ are lower than in HCAβ− subjects. In HCAβ+, Braak II (hippocampus) has the highest correlations with OFF ROIs; however, none are significant after PVC. Predictably, in HCAβ+, HemiW has significant correlations with Braak II–VI before PVC, but nothing is significant after PVC.
FIGURE 3.
Pearson r2 between 18F-flortaucipir SUVR in cortical regions, age, and OFF ROIs. P values were Bonferroni-corrected.
Multiple Linear Models
Whole cortex showed the strongest correlations after PVC with putamen (from the age-related OFF ROIs) and thalamus (from the WM and thalamus OFF ROIs). Therefore, we used putamen, thalamus, and ChPlex as independent measures and whole cortex and Braak ROIs as dependent measures with a series of multiple linear models to explore how well OFF accounted for cortical variability in HCAβ− subjects. The βs and adjusted r2 are reported in Table 3 for Braak I, II, III/IV, V/VI, and whole cortex. The βs are the weights for putamen, thalamus, and ChPlex in the linear model resulting in the r2, with the Braak ROI or whole cortex as the dependent variable. For example, the linear equation for PVC whole cortex is (0.1 × putamen) + (0.33 × thalamus) + (0.01 × ChPlex). To put the βs into perspective, the mean SUVR in HCAβ− subjects for putamen was 1.47 ± 0.29 (non-PVC) and 1.54 ± 0.35 (PVC), thalamus was 1.24 ± 0.18 (non-PVC and PVC), and ChPlex was 1.15 ± 0.22 (non-PVC) and 2.78 ± 0.94 (PVC). A lower amount of variability was explained in Braak I (adjusted r2 = 0.30 and 0.22 for non-PVC and PVC) than in other Braak regions. ChPlex was only significant when explaining variability in the signal for the hippocampus (Braak II). Thalamus was significant (P < 0.01) in explaining SUVR for all Braak ROIs. Putamen was significant (P < 0.001) in explaining Braak III/IV, V/VI, and whole cortex (PVC), and Braak II (non-PVC, P < 0.01).
TABLE 3.
Series of Multiple Linear Models (Run in HCAβ−) with Flortaucipir SUVRs in Cortical ROIs as Dependent Measure and SUVRs in Putamen, Thalamus, and ChPlex as Independent Measures
| Dependent | Independent-variable β | Adjusted | ||
| variable | Putamen | Thalamus | ChPlex | r2 |
| Braak I non-PVC | −0.02 | 0.34* | 0 | 0.22** |
| Braak I PVC | 0.16 | 0.38* | 0.01 | 0.30** |
| Braak II non-PVC | 0.14* | 0.39** | 0.26** | 0.82** |
| Braak II PVC | 0.07 | 0.42** | 0.07** | 0.55** |
| Braak III/IV non-PVC | 0.03 | 0.38** | −0.01 | 0.66** |
| Braak III/IV PVC | 0.11** | 0.31** | 0.01 | 0.64** |
| Braak V/VI non-PVC | −0.07 | 0.49** | −0.03 | 0.55** |
| Braak V/VI PVC | 0.09** | 0.33** | 0.01 | 0.61** |
| Whole-cortex non-PVC | −0.04 | 0.46** | −0.02 | 0.60** |
| Whole-cortex PVC | 0.10** | 0.33** | 0.01 | 0.64** |
P < 0.01.
**P < 0.001.
Multiple linear models were also performed in HCAβ+ (data not shown). Only the linear model for Braak II resulted in a significant (P < 0.001) adjusted r2 (non-PVC = 0.59, PVC = 0.31); thalamus and ChPlex significantly contributed to the model.
Is Cortical Variability Being Driven by Variability in the Reference Region?
Whether the cortical variability was driven by reference region in 18F-flortaucipir was explored by calculating the coefficient of variation of SUVs in HCAβ− subjects, shown in Supplemental Table 3. Coefficient of variation was similar across regions but was smallest in the inferior cerebellar gray matter; the mean and SD of SUV were smallest in the inferior cerebellar gray matter, leading us to believe that inferior cerebellar gray matter is a sufficient reference region and is not the cause of the cortical variability.
Is Cortical Variability Being Driven by WM Variability and PVEs?
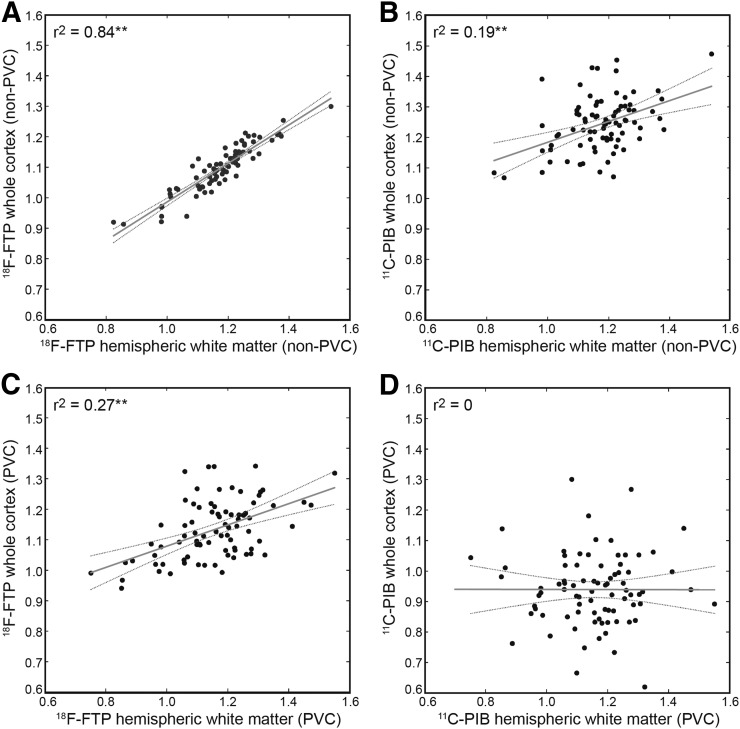
The PVEs of WM on cortical gray matter were explored by comparing the correlation between WM (HemiW and CereW) and cortical gray matter in 18F-flortaucipir SUVR before and after PVC. We decided to examine both HemiW and CereW because CereW does not suffer from PVEs from whole cortex. To gauge the degree of correlation expected from PVEs, the analysis was also done in 11C-PIB SUVRs in the same subjects. The analysis was limited to HCAβ− subjects to minimize any on-target binding. Figure 4 shows the corresponding HemiW versus mean of whole cortex for the 83 subjects. 11C-PIB correlations between HemiW and whole cortex changed from 0.19 to 0 as a result of PVC (CereW correlations with cortex also went from 0.19 to 0), demonstrating that PVC can remove the correlation between white and gray matter signal. However, PVC did not remove the correlations between white and gray matter signal in 18F-flortaucipir; HemiW to whole cortex was r2 = 0.84 for non-PVC and decreased to 0.27 as a result of PVC (18F-flortaucipir CereW to whole cortex went from r2 = 0.57 to 0.23 after PVC). All correlations between gray and white matter were significant at P < 0.001 except for PVC 11C-PIB.
FIGURE 4.
Correlations between HemiW and whole-cortex SUVRs: non-PVC 18F-flortaucipir (A), non-PVC 11C-PIB (B), PVC 18F-flortaucipir (C), and PVC 11C-PIB (D). Gray line = linear model fit; dashed gray lines = 95% confidence interval. **P < 0.001.
DISCUSSION
18F-flortaucipir is a useful tracer to examine pathologic tau load differences between HCs and subjects with Alzheimer disease. However, in HCAβ−, where tau paired helical filaments are unlikely to be found in substantial numbers in the neocortex (33,34), there is a variability in cortical signal (10) that makes measuring early tau deposition challenging. We showed that 64% of the cortical signal variability in our HCAβ− subjects can be explained by signal from OFF ROIs (Table 3). Furthermore, the mean cortical 18F-flortaucipir variability in HCAβ− subjects has an SUVR range of 0.5 SUVR units and is similar in HCAβ− subjects younger than 40 y old (these subjects have a low likelihood of having neocortical tau (33,34)). Similar variability in HCAβ− 18F-flortaucipir cortical signal was also shown in 422 HCAβ− subjects (10).
There is a possibility that there is little variability in Braak ROI SUVRs in HCAβ− subjects and that the variability seen stems from the reference region. In an attempt to determine whether variability in the reference region could be driving this, we calculated SUVs for HCAβ−. The reference region, inferior cerebellar gray matter, had the lowest coefficient of variation of SUVs of all the ROIs. The inferior cerebellar gray matter is no more or less stable than other regions. It has been shown that the cerebellum has a similar distribution volume (calculated using arterial sampling data) between HCs and subjects with Alzheimer disease (16), implying a certain level of stability. However, it has also been shown that the distribution volume calculated from arterial input function correlates with age (with different slopes) both in the cerebellum and in cortical regions (15).
The 3 OFF ROI groups were an age-related group (which included caudate, pallidum, and putamen), a WM group (which included thalamus), and ChPlex. The age-related group could be driven by binding to neuromelanin or iron. Iron load (quantified using MRI R2*) has been shown to correlate with 18F-flortaucipir binding in the basal ganglia and age in HCs and Alzheimer disease patients (17); additionally, in HCs, pallidum and putamen have been reported to have higher iron than other ROIs, which we also observed for 18F-flortaucipir in our HCAβ− cohort. The age-related OFF contribution to variability in cortical signal is relatively small.
The WM group of OFF ROIs contributed more to the variability in the cortical signal than the age-related group in a multiple linear regression. Correlations between CereW and Braak ROIs were similar to correlations between HemiW and Braak ROIs after PVC. CereW and Braak ROIs are not proximal and therefore do not contribute to each other’s PVEs, implying that the correlations between WM and Braak ROIs are not driven solely by PVEs. We further explored the possibility that PVEs drive the cortical signal variability in 18F-flortaucipir by looking at the relationship between WM and whole cortex in 18F-flortaucipir and 11C-PIB scans before and after identical PVC approaches. We showed that 11C-PIB WM signal could be removed from cortical signal using PVC, but a correlation between WM and cortical signal persisted after PVC in 18F-flortaucipir scans. This suggests that the correlation between the WM and gray matter signal is a real feature of 18F-flortaucipir binding properties and not the result of PVEs. WM explains substantial variance of signal in the cortex of the HCAβ−. It is unclear what 18F-flortaucipir could be binding to in WM, although the lipophilic nature of β-sheet binding tracers may explain this finding.
ChPlex did not significantly correlate with any OFF ROI or age, except thalamus before PVC (not significant after PVC). ChPlex significantly correlated only with hippocampus non-PVC SUVR in HCAβ− and HCAβ+ and PVC SUVR in HCAβ− and significantly contributed only to the multiple linear model predicting hippocampal SUVR. Although ChPlex makes quantification of hippocampus SUVR challenging, the OFF in this region does not correlate with other OFF ROIs or Braak regions outside the hippocampus. Along with being steeped in PVEs, the hippocampus is also known to have automated segmentation problems (8). Segmentation inaccuracies would negatively impact the precision of non-PVC and PVC quantification. Hippocampus 18F-flortaucipir signal should be interpreted with caution.
PVEs are not the only source of the variability in 18F-flortaucipir signal in HCAβ−, nor did our PVC algorithm create correlations where none existed. The correlations between cortex and WM existed in both non-PVC and PVC data, and most correlations between cortical and OFF ROIs decreased as a result of PVC. The agreement between non-PVC and PVC results leads us to conclude that the OFF in the cortical regions is not an artifact of data analysis.
Focusing on only the HCAβ− subjects, OFF ROIs account for less of the signal variance in Braak I (entorhinal cortex) than other Braak regions. Because entorhinal cortex is the site of earliest accumulation of tau in HCs, this is most likely due to true binding of 18F-flortaucipir to tau in subjects in this region. In contrast, more than 50% of cortical signal variability could be explained by OFF ROIs in HCAβ− subjects.
In HCAβ+ subjects, OFF ROIs were only significant predictors of hippocampal 18F-flortaucipir signal. There is a significant difference between HCAβ+ and HCAβ− subjects’ Braak ROI SUVRs but no significant difference in OFF ROI SUVRs. When OFF ROIs were correlated to Braak ROIs in HCAβ+ subjects, there were few significant correlations in the non-PVC data (save hippocampus, HemiW, and caudate) and no significant correlations after PVC. These 2 results lead us to conclude that 18F-flortaucipir is binding to tau in HCAβ+ subjects. The ratio of on-target binding to OFF in HCAβ+ subjects seems to be high enough that the OFF ROI correlations are not significant. However, an interesting future direction would be to quantify the relative amounts of on-target binding and OFF in HCAβ+ subjects.
It is beyond the scope of this paper to analyze the variability of the OFF ROI and Braak signal within subjects, longitudinally. It has been shown that the use of HemiW as a reference region for longitudinal tau data reduced variability and enhanced discrimination between diagnostic cohorts (35). The success of the HemiW reference region could be partly related to covariance of HemiW and gray matter, and therefore normalizing by HemiW would remove that white-matter–related OFF signal from the gray matter.
CONCLUSION
Although 18F-flortaucipir has been shown to track tau deposition in the brain, OFF increases variability in the cortical signal in HCAβ− subjects. There are 3 main ROI groups with related OFF signal: an age-related group including the caudate, pallidum, and putamen; a WM-related group including the thalamus; and lastly the ChPlex. This variability is correlated to a signal likely related to iron deposition, as well as the amount of 18F-flortaucipir measured in the WM. PVEs do not completely account for the variability in the cortical signal. The variability in signal in HCs related to OFF should be considered when studying the earliest deposition of tau using 18F-flortaucipir. Either the sample size should be sufficient to account for the variability across subjects, or possibly, controlling for measurements of OFF when correlating 18F-flortaucipir measurements with other neuroimaging or cognitive measure could result in improved sensitivity.
DISCLOSURE
This research was supported by NIH grant AG034570. Suzanne Baker serves as a consultant to Genentech. William Jagust serves as a consultant to Bioclinica, Genentech, Novartis, and Biogen. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
KEY POINTS
QUESTION: Is the variability in cortical binding of flortaucipir related to off-target binding in PET scans in amyloid-negative healthy controls?
PERTINENT FINDINGS: Sixty-four percent of the variability in the flortaucipir cortical signal in subjects with little to no tau can be explained by off-target signal in the putamen (age-related) and in the thalamus (lipophilicity-related). These correlations are no longer significant in subjects with cortical tau accumulation.
IMPLICATIONS FOR PATIENT CARE: Measuring the earliest deposition of tau using flortaucipir may be challenging due to cortical signal variability in amyloid-negative healthy controls.
REFERENCES
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl). 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 2.Chien DT, Bahri S, Szardenings AK, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–468. [DOI] [PubMed] [Google Scholar]
- 3.Xia CF, Arteaga J, Chen G, et al. [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013;9:666–676. [DOI] [PubMed] [Google Scholar]
- 4.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz AJ, Yu P, Miller BB, et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain. 2016;139:1539–1550. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Benzinger TL, Su Y, et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between beta-amyloid and tauopathy. JAMA Neurol. 2016;73:1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ossenkoppele R, Rabinovici G, Smith R. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz SA, Gordon BA, Mishra S, et al. Widespread distribution of tauopathy in preclinical Alzheimer’s disease. Neurobiol Aging. 2018;72:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowe VJ, Wiste HJ, Senjem ML, et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain. 2018;141:271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sander K, Lashley T, Gami P, et al. Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 2016;12:1116–1124. [DOI] [PubMed] [Google Scholar]
- 12.Barrio JR. The irony of PET tau probe specificity. J Nucl Med. 2018;59:115–116. [DOI] [PubMed] [Google Scholar]
- 13.Baker SL, Lockhart SN, Price JC, et al. Reference tissue-based kinetic evaluation of 18F-AV-1451 for tau imaging. J Nucl Med. 2017;58:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shcherbinin S, Schwarz AJ, Joshi A, et al. Kinetics of the tau PET tracer 18F-AV-1451 (T807) in subjects with normal cognitive function, mild cognitive impairment, and Alzheimer disease. J Nucl Med. 2016;57:1535–1542. [DOI] [PubMed] [Google Scholar]
- 15.Barret O, Alagille D, Sanabria S, et al. Kinetic modeling of the tau PET tracer 18F-AV-1451 in human healthy volunteers and Alzheimer disease subjects. J Nucl Med. 2017;58:1124–1131. [DOI] [PubMed] [Google Scholar]
- 16.Golla SSV, Timmers T, Ossenkoppele R, et al. Quantification of tau load using [18F]AV1451 PET. Mol Imaging Biol. 2017;19:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JY, Cho H, Ahn SJ, et al. Off-target 18F-AV-1451 binding in the basal ganglia correlates with age-related iron accumulation. J Nucl Med. 2018;59:117–120. [DOI] [PubMed] [Google Scholar]
- 18.Marquié M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol Commun. 2016;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zecca L, Bellei C, Costi P, et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc Natl Acad Sci USA. 2008;105:17567–17572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermeiren C, Motte P, Viot D, et al. The tau positron-emission tomography tracer AV-1451 binds with similar affinities to tau fibrils and monoamine oxidases. Mov Disord. 2018;33:273–281. [DOI] [PubMed] [Google Scholar]
- 22.Hansen AK, Brooks DJ, Borghammer P. MAO-B inhibitors do not block in vivo flortaucipir([18F]-AV-1451) binding. Mol Imaging Biol. 2018;20:356–360. [DOI] [PubMed] [Google Scholar]
- 23.Tong J, Meyer JH, Furukawa Y, et al. Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab. 2013;33:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquie M, Verwer EE, Meltzer AC, et al. Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson’s case. Acta Neuropathol Commun. 2017;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CM, Jacobs HIL, Marquie M, et al. 18F-flortaucipir binding in choroid plexus: related to race and hippocampus signal. J Alzheimers Dis. 2018;62:1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief. 2017;15:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rousset OGMY, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39:904–911. [PubMed] [Google Scholar]
- 28.Maass A, Landau S, Baker SL, et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157:448–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logan J, Volkow ND, Wang GK, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. [DOI] [PubMed] [Google Scholar]
- 30.Mormino EC, Brandel MG, Madison CM, et al. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2012;59:1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, et al. Existing Pittsburgh compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Bergen JMG, Li X, Quevenco FC, et al. Low cortical iron and high entorhinal cortex volume promote cognitive functioning in the oldest-old. Neurobiol Aging. 2018;64:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. [DOI] [PubMed] [Google Scholar]
- 34.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. [DOI] [PubMed] [Google Scholar]
- 35.Southekal S, Devous MD, Sr, Kennedy I, et al. 18F-flortaucipir quantitation using a parametric estimate of reference signal intensity (PERSI). J Nucl Med. 2018;59:944–951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.