Abstract
Human monogenic diabetes, caused by mutations in genes involved in beta cell development and function, has been a challenge to study because multiple mouse models have not fully recapitulated the human disease. Here, we use genome edited human embryonic stem cells to understand the most common form of monogenic diabetes, MODY3, caused by mutations in the transcription factor HNF1A. We found that HNF1A is necessary to repress an alpha cell gene expression signature, maintain endocrine cell function, and regulate cellular metabolism. In addition, we identified the human-specific long non-coding RNA, LINKA, as an HNF1A target necessary for normal mitochondrial respiration. These findings provide a possible explanation for the species difference in disease phenotypes observed with HNF1A mutations and offer mechanistic insights into how the HNF1A gene may also influence type 2 diabetes.
Introduction
Diabetes is characterized by the disruption of glucose homeostasis due to abnormal insulin secretion and/or responsiveness (Polonsky 2012). The two most common forms, type 1 and type 2, are associated with eventual loss of the insulin-secreting beta cell, which can occur early (type 1) or late (type 2) in disease progression. Type 1 diabetes is an autoimmune disorder where the immune system destroys beta cells while type 2 diabetes is a metabolic syndrome with defects in insulin responsiveness and eventual beta cell failure. (Katsarou et al. 2017; DeFronzo et al. 2015). Elucidating the molecular mechanisms that lead to diabetes is challenging due to the polygenic nature of this disease (Fuchsberger et al. 2016; Grarup et al. 2014), the influence of environmental factors and the interaction of multiple organ systems (Carlsson et al. 2012; Knowler et al. 2002). Another type of diabetes, monogenic diabetes, also known as maturity-onset diabetes of the young (MODY) accounts for ~5% of reported cases in children. MODY is most often characterized by heterozygous dominant mutations in genes important for pancreatic β-cell development and function (Nyunt et al. 2009; Colclough et al. 2013; Owen 2018; Hattersley & Patel 2017)
The most common form of monogenic diabetes, MODY3, is caused by heterozygous mutations in the transcription factor Hepatic Nuclear Factor 1 alpha (HNF1A) (Fajans & Bell 2011). Greater than 400 different mutations in this gene have been described in over 1200 families (Colclough et al. 2013; Ellard 2000; Harries et al. 2006; Yamagata 1996). Patients with HNF1A mutations show β-cell dysfunction and hyperglycemia due to insufficient insulin release in response to increased blood glucose levels (Byrne et al. 1996). Significant efforts have been made to understand the pathophysiology of MODY3 (Fajans & Bell 2011), but these efforts have been limited by the unavailability of patient samples (Skelin et al. 2010).
Mouse models have been used to study the role of HNF1A, but do not fully mimic the human disease phenotype. Mice with heterozygous mutations in HNF1A are healthy (Pontoglio et al. 1998) and mice with homozygous null mutations can have a diabetic phenotype, but with variability dependent on genetic background (Garcia-gonzalez et al. 2016). Another approach has been overexpression of a dominant negative form of HNF1A with phenotypes from this model being comparable to the HNF1A null model and include hyperglycemia, impaired insulin secretion, abnormal expression of genes related to β-cell function and loss of β-cell mass by apoptosis (Bonner et al. 2010; Pontoglio et al. 1998; Wobser et al. 2002; Servitja et al. 2009). Evidence suggests that HNF1A may regulate insulin transcription and genes involved in β-cell replication (Akpinar et al. 2005; Wang et al. 2000).
Due to disease phenotype differences between rodents and humans with HNF1A mutations, a human model system is desirable. The use of human pluripotent stem cells and in vitro differentiation protocols that mimic in vivo pancreatic development are well suited to interrogate monogenic diseases of the pancreas. A number of relevant pancreatic phenotypes due to mutations in GATA4, GATA6, PDX1, and RFX6 have been described (Tiyaboonchai et al. 2017; Shi et al. 2017; Zhu et al. 2014; Zeng et al. 2016). This study uses genetically modified embryonic stem cells (ESCs) for human in-vitro disease modeling to understand the role of HNF1A in pancreatic development and beta cell function. Specifically, we used the CRISPR-CAS9 system to genetically modify ESCs to ablate one or two alleles of HNF1A and differentiated these stem cell lines into pancreatic beta-like cells. Our data suggest that HNF1A plays an essential role in endocrine cell development as loss of HNF1A leads to increased expression of alpha cell markers including glucagon and decreased expression of PAX4, a critical transcription factor regulating beta cell development. Additionally, we observed impaired insulin expression and secretion as well as metabolic defects in glycolysis and mitochondrial respiration, which may be partially explained by downregulation of a human-specific lncRNA, LINKA. Cells deficient in this lncRNA display a similar defect in mitochondrial respiration. The defects in cellular respiration are typical of type 2 diabetes and may provide an explanation for multiple GWAS studies which link HNF1A to type 2 diabetes in addition to MODY (Voight et al. 2010; Fuchsberger et al. 2016; Mishra et al. 2017; Estrada et al. 2014). These studies highlight the utility of using a human model system to dissect endocrine cell development and function.
Results
HNF1A expression during the generation of beta-like cells in vitro.
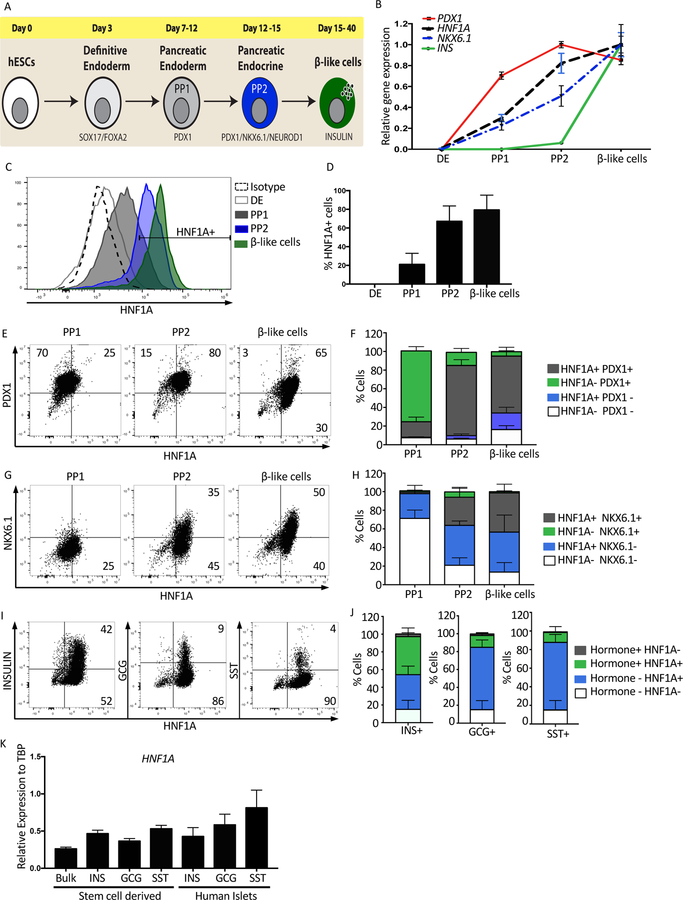
To examine the role of HNF1A in pancreatic development, two different ESC lines were used. The Mel1 reporter line with GFP expressed from the INS locus (INS-GFP)(Micallef et al. 2012) and the H1 line (Thomson 1998). Pancreatic differentiation was performed using the protocol developed by Rezania et al. 2014, with minor modifications. Four stages of pancreatic development were examined: definitive endoderm (DE) characterized by co-expression of CXCR4 and CD117 at day 3 of differentiation, pancreatic endoderm or pancreatic progenitor one (PP1) defined by expression of PDX1+ cells between days 7–12, pancreatic progenitor two (PP2) defined by co-expression of PDX1 and NKX6.1 between days 12–15, and beta-like cells defined by expression of the pancreatic hormone insulin between days 15–40 (Figure 1A and S1A, B). To establish the kinetics of HNF1A expression, mRNA and protein were examined during these four stages of pancreatic differentiation. Both HNF1A and NKX6.1 transcript levels were low but detectable at PP1 and continued to increase through the next two stages of differentiation (Figure 1B and S2L). Using intracellular flow cytometry, the percentage of HNF1A+ cells was similar to the mRNA expression levels (Figure 1C, 1D and S2M). HNF1A was also co-expressed with specified markers for PP1, PP2 and pancreatic hormones (PDX1, NKX6.1, INS, GCG, and SST) (Figure 1E–J and S2N–P). To compare HNF1A transcript levels between in vitro-derived beta-like cells and primary endocrine cells, adult human islets and end stage in-vitro differentiation cultures were sorted into three populations based upon expression of the pancreatic hormones SST, GCG or INS. HNF1A transcript levels were comparable between all of these populations (Figure 1K and S1D–G).
Figure 1: HNF1A expression during the generation of beta-like cells in vitro.
Mel1 ESCs were differentiated into pancreatic endocrine cells and analyzed as described below.
(A) Schematic representation of the protocol used to derive beta-like cells from ESCs.
(B) Relative mRNA expression quantified by qRT-PCR. Time-course quantification to determine the expression kinetics of HNF1A compared to PDX1, NKX6.1 and INS during pancreas differentiation (n=3 per sample).
(C) Intracellular flow cytometry of HNF1A protein during pancreas differentiation. Definitive endoderm (DE), pancreas progenitor 1 (PP1), pancreas progenitor 2 (PP2) and beta-like cells (n=4 per cell line).
(D) Quantification of HNF1A expressing cell population (percentage) during pancreas differentiation in C (n=4 per cell line).
(E) Flow cytometry plots of HNF1A versus PDX1 at PP1, PP2 and beta-like cell stages (n=4 per cell line).
(F) Cell quantification for PDX1 and HNF1A cell populations in E (n=4 per cell line).
(G) Flow cytometry plots of HNF1A versus NKX6.1 at PP1, PP2 and beta-like cell stages (n=4 per cell line).
(H) Cell quantification for NKX6.1 and HNF1A cell populations in G (n=4 per cell line).
(I) Flow cytometry plots of end stage differentiation cultures, examining expression of pancreatic hormones Insulin (INS), Glucagon (GCG) and Somatostatin (SST) with HNF1A (n=4 per cell line).
(J) Cell quantification for pancreatic hormones INS, GCG and SST with HNF1A in I (n=4 per cell line).
(K) Relative HNF1A mRNA expression in bulk and hormone sorted cells from in vitro differentiation of stem cells compared to human islets sorted for pancreatic hormones INS, SST or GCG (n=3 per sample).
See also Figure S1.
Deficiency of HNF1A influences pancreatic hormone expression.
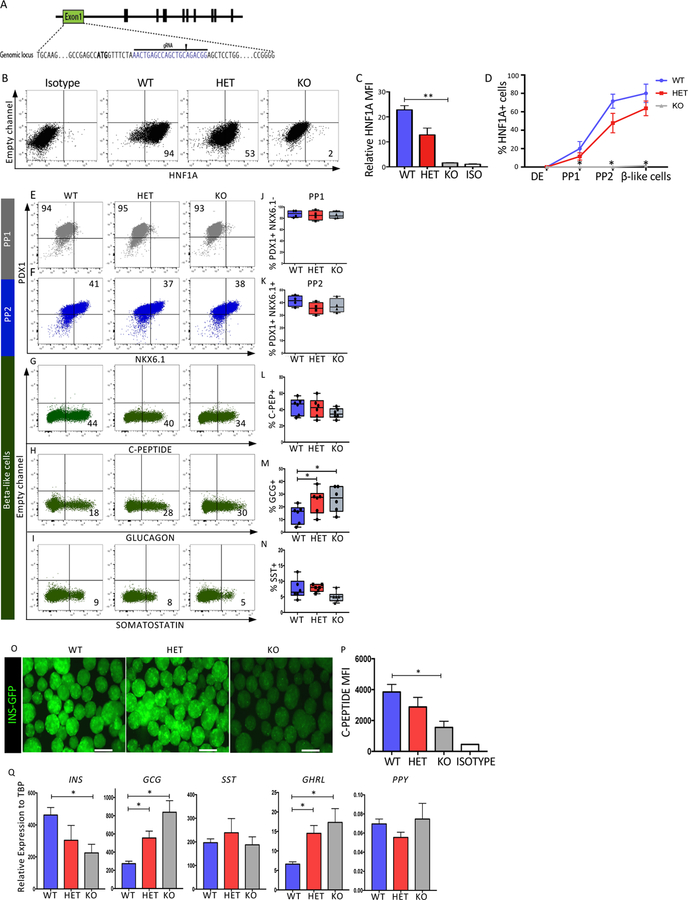
To examine the role of HNF1A during pancreas differentiation, heterozygous (HET) and homozygous knockout (KO) HNF1A mutations were created in the Mel1 INS-GFP and H1 lines using the CRISPR-CAS9 technology. A guide RNA was designed in exon 1 of HNF1A, generating a deletion and premature termination in one or both alleles of HNF1A (Figure 2A and Figure S2A–C). To quantify HNF1A protein levels in wild-type (WT), HET and KO lines, immunoblots and intracellular flow cytometry were performed using beta-like cells. HNF1A protein levels were reduced in the HET and absent in the KO (Figures 2B–D, S2D–E and S2Q–S). We examined the percentage of HNF1A+ cells during pancreatic differentiation and noted similar kinetics between WT and HET with reduction of HNF1A+ cell population in HET and complete absence in KO cell population throughout the differentiation (Figure 2D and S2S). To examine the impact of HNF1A loss on pancreatic differentiation, marker analyses were performed during the PP1, PP2, and beta-like cell stages of differentiation. At the PP1 stage of differentiation, PDX1 expression levels were comparable between all cell lines (Figure 2E, J and S2T). At the PP2 stage of differentiation, the PDX1+NKX6.1+ population was also comparable between all cell lines (Figure 2F, K and S2U). At the beta-like cell stage of differentiation, cells were stained for C-peptide (C-PEP), glucagon (GCG) or somatostatin (SST). The C-PEP+ population ranged from between 30–60% in the Mel1 lines (Figure 2G, 2L) and between 39–80% in the H1 lines (Figure S2V) with no significant differences in the mutant lines. The GCG+ population was increased approximately two-fold in the HET and KO cells of both Mel1 (Figure 2H, M) and H1 (Figure S2W) lines. SST was unaffected in the Mel1 line (Figure 2I, N) while the H1 HET showed an increase in the SST+ population (Figure S2X).
Figure 2: Deficiency of HNF1A influences pancreatic hormone expression.
Mel1 ESCs were genome edited and examined as described below.
(A) CRISPR-CAS9 based strategy to generate HNF1A heterozygous (HET) and Knock-out (KO) ESCs. ATG is bold and gRNA highlighted.
(B–C) Representative intracellular flow cytometric analysis of HNF1A performed at beta-like cell stage using the HNF1A allelic series (n=3 per cell line). (B) Representative dot plots of HNF1A expression. (C) Quantification of HNF1A by mean fluorescence intensity (MFI) from B.
(D) Quantification of the HNF1A cell population (percentage) during pancreatic differentiation (n=3 per cell line).
(E–N) Flow cytometry quantification of specific proteins during pancreatic differentiation using HNF1A WT, HET, and KO lines. (E,J) Quantification of PDX1+ cells at PP1 (n=4 per genotype). (F,K) Quantification of PDX1+ NKX6.1+ cells at PP2 (n=4 per genotype).
(G,L) Quantification of C-peptide+ cells at end stage differentiation (n=6 per genotype).
(H,M) Quantification of glucagon+ cells at end stage differentiation (n=6 per genotype).
(I,N) Quantification of somatostatin+ cells at end stage differentiation (n=6 per genotype).
(O) Fluorescence microscopy at end stage of differentiation showing INS-GFP fluorescence in the HNF1A allelic series (bar size 300µM)(n=3 per sample).
(P) Quantification of C-Peptide MFI at end stage differentiation of beta-like cells from G (n=3 per sample).
(Q) qRT-PCR quantification for pancreatic hormones INS, GCG, SST and GHRL at the end of pancreatic differentiation in bulk cells (n=3 per sample).
For all statistical analyses: * P<0.05, **P<0.01. See also Figure S2.
The comparable percentage of C-PEP+ cells in the KO lines was surprising considering that HNF1A KO in mouse models displayed a reduction in beta cell mass and a decrease in insulin expression. By analyzing the Mel1 INS-GFP cell line, the KO line had lower GFP fluorescence intensity (Figure 2O). In addition, C-PEP staining was reduced in the Mel1 and H1 KO lines (Figure 2P and S2Z). INS mRNA levels were also significantly reduced in the KO lines, but not the HET lines (Figure 2Q and S2Y). Analysis of other pancreatic hormones showed that GCG and GHRL transcript levels were upregulated in the HET and KO of both genetic backgrounds while SST and PPY transcript levels were comparable between all lines of each genetic background (Figure 2Q and S2Y). To confirm findings from the stem cell differentiation cultures, we used the EndoC-BH1 and EndoC-BH3 cell lines (Ravassard et al. 2011 and Scharfmann & Pechberty 2014) in which indel mutations were generated in HNF1A using the CRISPR/CAS9 technology (Figure S5E, F and S4I). The EndoC-BH1 HNF1A KO line displayed a decrease in insulin expression (Figure S5G). These data suggest that HNF1A is an important regulator of endocrine hormone gene expression.
Ablation of HNF1A drives pancreatic endocrine differentiation toward cells with a more alpha cell gene expression signature.
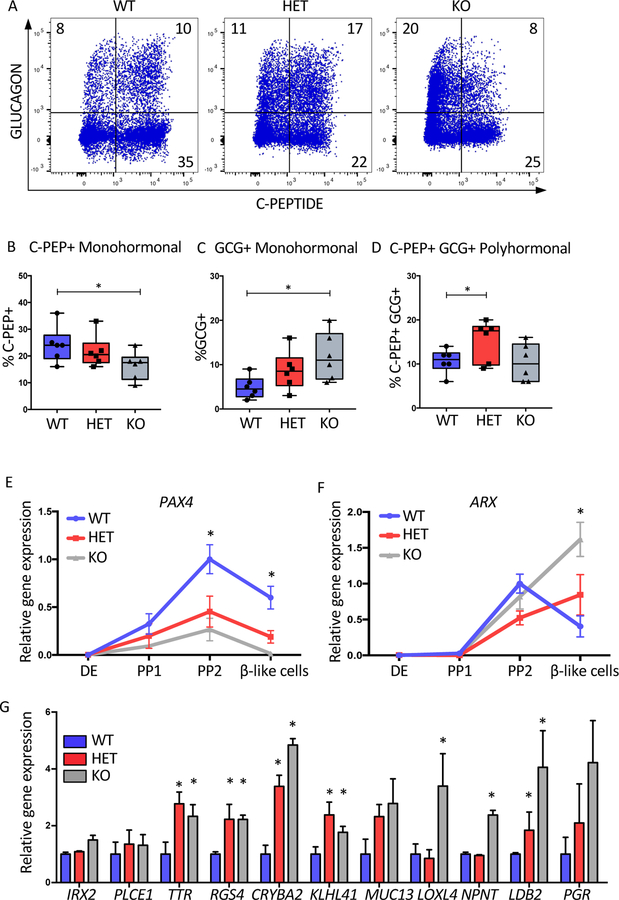
The increased GCG expression observed in both the Mel1 and H1 KO cell lines was interesting, particularly in the context of polyhormonal cells that can arise during pancreatic differentiation. In order to examine these subpopulations, co-expression of GCG, C-PEP and SST was examined using flow cytometry. Analyses focused on GCG and C-PEP as SST was present at low levels, that mostly co-stained with C-PEP even in WT cells (Figure S1A–B). With the loss of one or two alleles of HNF1A, both monohormonal GCG+ and double positive GCG+CPEP+ cell populations were increased in the HET and KO cells, with a significantly reduced monohormonal C-PEP+ population in the KO line (Figure 3A–D and S3A–D). To determine if changes in cell proliferation could be influencing these results, KI67 was examined but no differences were seen in the INS+ or GCG+ subpopulations (Figure S3G–L). In mouse models, the transcription factors PAX4 and ARX have well described roles in driving beta and alpha cell fates, respectively (Collombat et al. 2003; Courtney et al. 2013). We hypothesized that loss of HNF1A may lead to upregulation of ARX and/or downregulation of PAX4 during pancreatic differentiation, leading to the observed differences in hormone expression in the mutant lines. We examined mRNA expression of PAX4 and ARX during the differentiation. At the PP2 stage, PAX4 is expressed exclusively in HNF1A+ cells (Figure S1H–J) and had significantly lower expression in HET and KO PP2 and beta-like cells in both genetic backgrounds (Figure 3E and S3E). ARX was upregulated at the beta-like cell stage in the HNF1A KOs (Figure 3F and S3E). Due to the increased GCG+ population in the HNF1A mutants, we determined if this difference would encompass a wider gamut of alpha cell specific genes. We quantified mRNA levels of a set of alpha cell specific genes reported previously (Muraro et al. 2016). In addition to GCG and ARX, which we had already examined, we found seven of these genes were significantly upregulated in the HNF1A KO (TTR, RGS4, CRYBA2, KLHL41, LOXL4, NTPN and LDB2,) (Figure 3G and S3F). When HNF1A was knocked out in the EndoC-BH1 cell line, some alpha cell genes were upregulated (CRYBA2, KLHL41, NTPN) while others were downregulated (MUC13, TTR, PGR) (Figure S5K) suggesting that HNF1A may be regulating most of these genes indirectly via abnormal development. Overall, these data suggest that HNF1A inactivation impacts endocrine cell development probably via PAX4 down-regulation and subsequent up-regulation of ARX expression, which ultimately leads to de-repression of alpha cell genes.
Figure 3: Ablation of HNF1A drives pancreatic endocrine differentiation toward cells with a more alpha cell gene expression signature.
Mel1 HNF1A WT, HET and KO ESCs were differentiated into pancreatic endocrine cells and analyzed as described below.
(A) Flow cytometry of C-Peptide versus GLUCAGON at the end stage differentiation cultures (n=6 per sample).
(B–D) Quantification of pancreatic hormone expressing cells from A (n=6 per sample).
(B) C-Peptide+ (C-PEP+) monohormonal cells. (C) GLUCAGON+ (GCG+) monohormonal cells. (D) C-Peptide+ GLUCAGON+ (C-PEP+GCG+) cell population.
(E–F) mRNA time-course analysis during pancreatic differentiation at DE, PP1, PP2 and beta-like cell stages (n=3 per sample). (E) PAX4 mRNA expression. (F) ARX mRNA expression.
(G) Relative gene expression of alpha cell specific genes at end stage of differentiation in HNF1A allelic series (n=3 per sample).
For all statistical analyses: * P<0.05. See also Figure S3.
HNF1A is required for optimal insulin secretion and cellular respiration in stem cell derived beta-like cells.
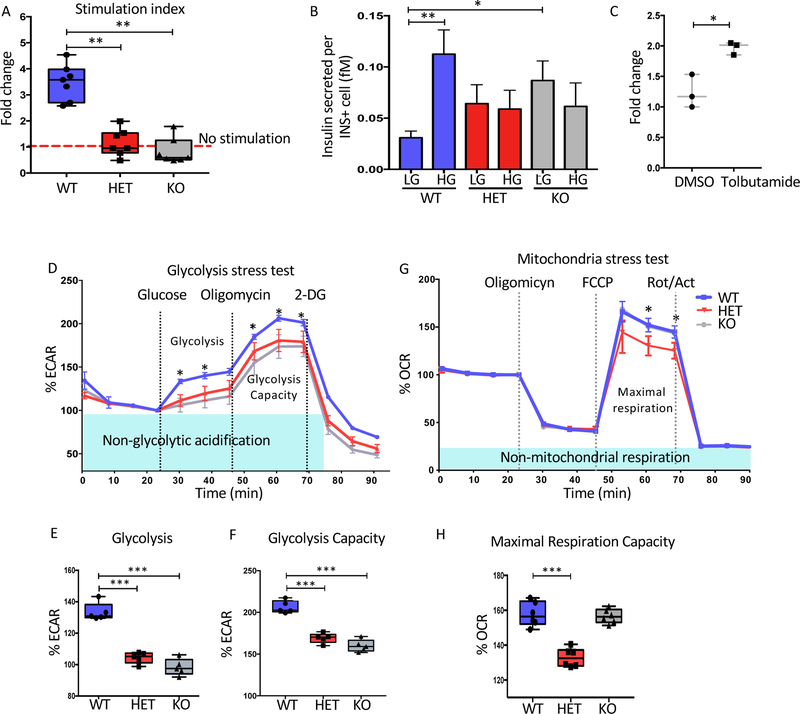
Because patients with HNF1A heterozygous mutations display hyperglycemia, we sought to determine the function of the beta-like cells lacking HNF1A. We assayed glucose-stimulated insulin secretion (GSIS). Both the HET and KO cell lines did not respond to high glucose while the WT cell lines increased their insulin secretion approximately 2–4 fold (Figure 4A and S4A). The lack of stimulation which was also noted in a MODY3 patient (Haliyur et al. 2019), may be due to inadequate GSIS from a normal basal secretion or elevated basal secretion. We found that both mutant beta-like cells had a trend towards increased basal secretion which did not reach statistical significance along with a failure to increase secretion at higher levels of glucose (Figure 4B and S4B). Both phenotypes were seen in the MODY3 patient’s islets (Haliyur et al. 2019). Sulfonylureas are used to improve insulin secretion in HNF1A patients. When tolbutamide was added in addition to glucose, the HNF1A KO cell secretion could be partially rescued with a stimulation index of approximately 2-fold (Figure 4C and S4C). However, CRISPR mediated mutation of HNF1A for 7 days in the EndoC-BH3 cell line displayed similar GSIS to the control (Figure S4I–K) suggesting that short term loss of HNF1A does not lead to GSIS defects. Interestingly, HNF1A deficient EndoC-BH3 cells could not be maintained in culture, precluding long term studies (data not shown).
Figure 4: HNF1A is needed for optimal insulin secretion and cellular respiration in stem cell derived beta-like cells.
Mel1 HNF1A WT, HET and KO ESCs were differentiated into pancreatic endocrine cells and analyzed as described below.
(A–C) Glucose stimulation insulin secretion (GSIS) in HNF1A allelic series at end stage of differentiation (n=4 per sample). (A) Stimulation index of insulin secreted showing fold change 20mM over 2mM glucose. (B) Quantification of insulin secreted per insulin positive cell in femtomoles (fM), low glucose (LG) 2mM and high glucose (HG) 20mM. (C) Stimulation index of insulin secreted in HNF1A KO cell line comparing LG to HG or HG plus 20uM Tolbutamide (n=3 per sample).
(D–F) Glycolysis stress test for HNF1A allelic series (n=3 per sample). (D) Glycolytic profile of HNF1A allelic series showing as a extracellular acidification rate (ECAR) normalized at basal levels. (E) Glycolysis quantification. (F) Glycolysis capacity quantification.
(G–H) Mitochondria stress test (n=3 per sample). (G) Mitochondrial respiration profile of HNF1A allelic series obtained using oligomycin, FCCP and Rotenone/actinomycin (Rot/Act). (H) Quantification of maximal respiration capacity.
For all statistical analyses: * P<0.05, **P<0.01, ***P<0.001. See also Figure S4.
To determine why the HNF1A mutants presented with abnormal insulin secretion, upstream pathways critical for GSIS including glycolysis and mitochondrial respiration were analyzed using the extracellular flux assay. To measure glycolysis in sorted INS-GFP+ cells, extracellular acidification rate (ECAR) was examined in the presence of glucose to activate glycolysis which increases ECAR due to lactate production, oligomycin to block ATP resulting in maximum glycolysis due to glycolytic reserve and the glucose analog 2-deoxyglucose (2-DG) to return back to the non-glycolytic level (Teslaa & Teitell 2014). Both HET and KO cells had significantly lower glycolysis or ECAR (Figure 4D,E) and glycolysis capacity (Figure 4D,F) compared to WT cells. To measure mitochondrial function in sorted INS-GFP+ cells, oxygen consumption rate (OCR) was calculated in the presence of oligomycin to block ATP, the uncoupling agent FCCP to reverse the hyperpolarized state and recover membrane potential, and two electron transport chain inhibitors rotenone and actinomycin to stop mitochondrial respiration returning OCR to the lowest state (Dranka et al. 2011). The HET line displayed lower oxygen consumption while the KO line was similar to WT (Figure 4G,H). Both the glycolysis and HET specific mitochondrial defect were present in the HNF1A mutants on a second genetic background (Figure S4D–H). Confirmation of these phenotypes was seen in the EndoC-BH3 HNF1A mutant cell line (Figure S4L–P). These data suggest that the levels of HNF1A expression play critical roles in glucose metabolism and mitochondrial function in endocrine cells.
Disruption of HNF1A leads to abnormal expression of genes related to beta cell function, development and diabetes.
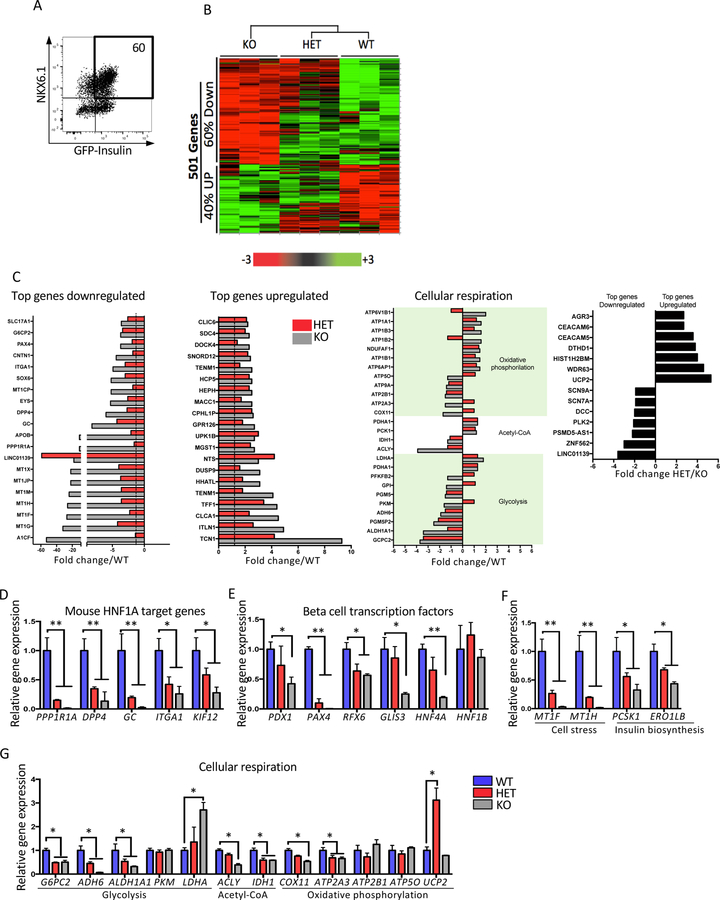
To understand the mechanism by which loss of HNF1A leads to the functional phenotypes described above, genome wide gene expression analyses were performed. Sorted INS-GFP+NKX6.1+ beta-like cells were used (Figure 5A) and 501 genes were differentially expressed between the WT and mutant cells (Figure 5B). Of these genes, 60% were downregulated and 40% were upregulated in the KO cell line. Of the top downregulated genes, a number of them have been reported in the HNF1A KO mouse model such as PPP1R1A, DPP4, GC, ITGA1 and KIF12 (Figure 5C). These particular downregulated genes were validated in mutant beta-like cells derived from Mel1 and H1 genetic backgrounds and also using the EndoC-BH1 cell line mutant for HNF1A (Figure 5D, S5A and S5H). Five critical beta cell transcription factors, of which only HNF4A and PDX1 have been reported in mouse models (Horikawa 2018; Hattersley & Patel 2017), were also downregulated in the mutant lines (Figure 5E, S5B, and S5I). Metallothionein genes encode intracellular metal binding proteins that can function as potent antioxidants in pancreatic beta cells to confer resistance to oxidative stress, apoptosis and diabetes development (Cai 2004; Nygaard et al. 2015; Guo et al. 2017). Two of these genes (MT1F and MT1H) and two other genes involved in insulin biosynthesis (PCSK1 and ERO1LB) were validated using HNF1A mutant cells from both genetic backgrounds but were unaffected in the EndoC-BH1 cell line mutant for HNF1A (Figure 5F, S5C, and S5J). In assessing the top upregulated genes (Figure 5C), the top ten were not known regulators in the pancreas and eight of the ten genes were expressed in intestine (Uhlen et al. 2015), suggesting a possible role for HNF1A in repressing intestine fate. These data suggest functional roles for HNF1A in early and late stages of pancreatic development.
Figure 5: Disruption of HNF1A leads to an abnormal expression of genes related with beta cell function, development and diabetes.
Mel1 HNF1A WT, HET and KO ESCs were differentiated into pancreatic endocrine cells and analyzed as described below.
(A–B) HNF1A allelic series genome profiling using purified INS-GFP+ NKX6.1+ cells at end stage of differentiation (n=3 per sample). (A) Flow cytometry plot for sorting double positive INS-GFP+ NKX6.1+ cells. (B) 501 differential expressed genes presented in a heat map plot obtained using a microarray analysis from samples sorted in A.
(C) Differential expressed genes shown in the following categories: top downregulated genes, top upregulated genes, cellular respiration and genes differentially expressed in HET compared to KO.
(D–G) qRT-PCR gene expression validation of a subset of gene targets in B (n=3 per sample). (D) Mouse HNF1A target genes. (E) Pancreas transcription factors. (F) cell stress and insulin biosynthesis. (G) Cellular respiration.
For all statistical analyses: * P<0.05, **P<0.01. See also Figure S5.
To corroborate the observed metabolic phenotypes in the HNF1A HET and KO beta-like cells (Figure 4, S4A–H, and S4L–P), genes involved in cellular respiration (glycolysis, Acetyl-CoA, and oxidative phosphorylation) were identified from the microarray dataset (Figure 5C). In the glycolysis pathway, a number of genes were validated (Figure 5G, S5D and S5L). The known genes G6PC2, a regulator of glucose cycle with implications in insulin secretion (Li et al. 2009) and LDHA which decreases insulin secretion and deregulates mitochondrial metabolism (Ainscow et al. 2000) were decreased and increased in the mutant lines, respectively. Two new target genes, ADH6 and ALDH1A1, were decreased in the mutant lines. Genes involved in the Acetyl-CoA pathway, ACLY and IDH1, important in lipid metabolism and production of NADPH (Calvert et al. 2017; Beckner et al. 2010) were identified. Genes involved in oxidative phosphorylation included downregulation of COX11 and ATP2A3 and upregulation of UCP2 (Taneera et al. 2014; Olsson et al. 2011; Zhang et al. 2001). These data suggest that HNF1A plays an important role in regulating cellular respiration in beta-like cells.
As an additional confirmation of these gene expression studies, we compared our dataset to a recently published study where a single MODY3 patient’s pancreatic endocrine cells were examined (Haliyur et al. 2019). First, from the top 100-downregulated genes in our data set, we found 55 of these genes were downregulated in beta cells of the MODY3 patient (Figure S5M). Focusing on the 21 downregulated genes that were validated by qRT-PCR (Figure 5D–G), we found 16 (~75%) of them were downregulated in the MODY3 patient’s beta cells (Figure S5N). These included the critical transcription factors PAX4, RFX6, and HNF4A, the MT genes and almost all of the targets related to cellular respiration. While data from only a single patient is available, it is very encouraging to see that many of the important human specific HNF1A targets from the stem cell system were also present in this dataset.
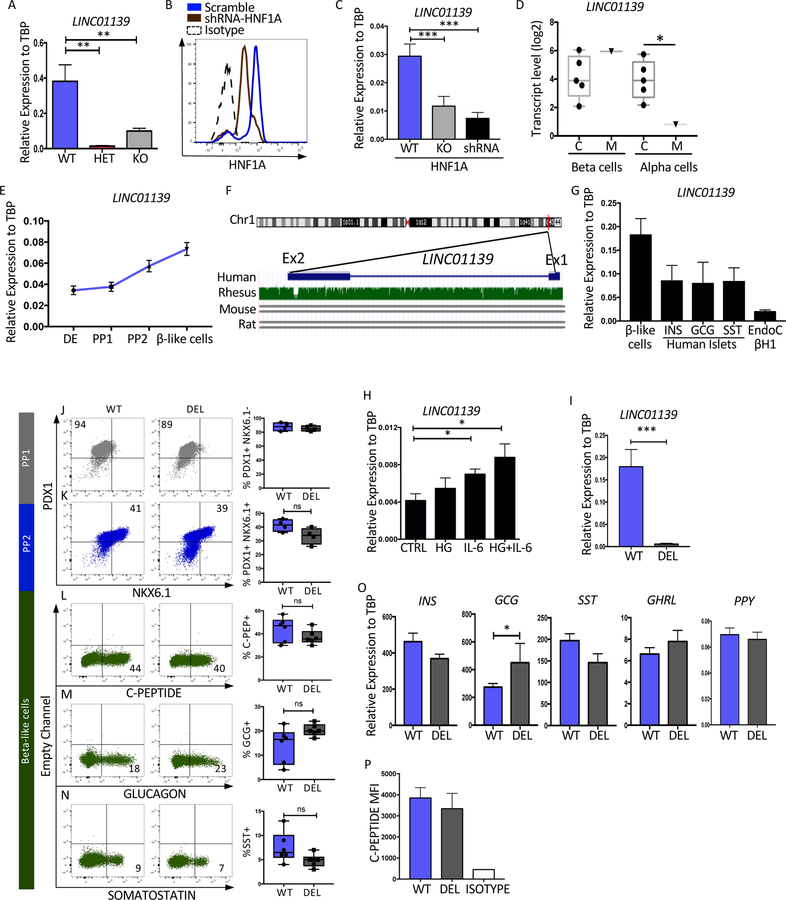
The human specific lncRNA, LINC01139 (LINKA), is a downstream target of HNF1A.
As shown above, only HET cells displayed a mitochondrial respiration defect (Figure 4G–H and S4G–H), suggesting an interesting dosage effect of HNF1A on mitochondrial function. To identify putative gene targets, the microarray gene expression datasets for the HET and KO cells were compared to WT. Those genes that were upregulated or downregulated to a greater extent in the HET versus KO cells were identified (Figure 5C). The top downregulated target was a lncRNA, LINC01139, designated LINKA (Figure 5C). This was confirmed by qRT-PCR in Mel1 mutant cells but it was unaffected in the H1 genetic background (Figure 6A and S6A). However, LINKA was downregulated in EndoC-BH1 cells treated with either HNF1A-shRNA or CRISPR-CAS9 targeting HNF1A (Figure 6B and 6C). Analyzing the dataset from the MODY3 patient (Haliyur et al. 2019) revealed that LINKA is downregulated in MODY3 alpha cells but not beta cells (Figure 6D). During differentiation of WT cells, LINKA expression was shown to increase in PP2 and beta-like cells (Figure 6E and S6B). LINKA is encoded on chromosome one of human and primates without homology to mouse or rat genomes (Figure 6F). Confirmation of human LINKA expression in other human beta cells was shown in primary islets, sorted for the three main endocrine hormone expressing cell types, and in EndoC-BH1 cells (Figure 6G). In addition, LINKA was expressed during human fetal pancreatic development in endocrine cells at week 18 (Figure S6K).
Figure 6: The human specific lncRNA, LINC01139 (LINKA), is a downstream target of HNF1A.
(A) qRT-PCR mRNA quantification of LINC01139 in HNF1A allelic series (Mel1), purified INS-GFP+ end stage beta-like cells (n=3 per sample).
(B–C) The EndoC-bH1 cell line was treated with or without HNF1A shRNA or CRISPR-CAS9 targeting HNF1A (KO) as described in Figure S5E (n=3 per sample). (B) Flow cytometry analysis of HNF1A in control or shRNA treated cells. (C) LINC01139 relative mRNA levels in WT, shRNA or KO cells.
(D) LINC01139 transcript levels in sorted beta cells and alpha cells from healthy pancreas donor controls referred to as C compared to cells from a single MODY3 mutant donor referred to as M (Haliyur et al 2019) (Control n=5, MODY3 n=1).
(E) Time-course LINC01139 mRNA quantification during pancreatic differentiation (n=3 per sample).
(F) LINC01139 gene homology, modified from UCSC genome browser comparing Human vs Rhesus, mouse and rat.
(G) LINC01139 mRNA quantification comparing purified stem cell derived beta-like cells with human islets sorted for INS, GCG, or SST and EndoC-bH1 cells (n=3 per sample).
(H) LINC01139 mRNA quantification in EndoC-bH1 cell line treated with different beta cell stressors: high glucose (HG)(100mM), Interleukin 6 (IL-6)(50ng/ul) or both (n=4 per sample).
(I) qRT-PCR quantification for LINC01139 mRNA in wild-type Mel1 line (WT) and deleted (DEL) LINC01139 cell line differentiated into end stage differentiation cultures.
(J–N) Pancreatic differentiation of LINC WT and LINC01139 DEL cell line with quantification of specific proteins by flow cytometry. (J) Quantification of PDX1+ cells at PP1 (n=4 per sample). (K) Quantification of PDX1+ NKX6.1+ cells at PP2 (n=4 per sample). (L) Quantification of C-peptide+ cells at end of the differentiation (n=6 per sample). (M) Quantification of glucagon+ cells at end of the differentiation (n=6 per sample). (N) Quantification of somatostatin+ cells at end of the differentiation (n=6 per sample).
(O) qRT-PCR mRNA quantification of pancreatic hormones INS, GCG, SST and GHRL at the end of the pancreatic differentiation (n=3 per sample).
(P) Quantification of C-Peptide Mean Fluorescence Intensity (MFI) at the end of the differentiation protocol (n=3 per sample).
For all statistical analysies: * P<0.05, **P<0.01, ***P<0.001. See also Figure S6.
LINKA has been shown to be a positive regulator of AKT signaling in cancer cells (Lin A et al 2017), but has not been studied in the context of pancreatic beta cells. Considering this link to AKT, we hypothesized that LINKA may be involved in stress signaling in beta cells. To test this hypothesis, EndoC-BH1 cells were exposed to different stressors including high glucose (HG), the cytokine IL-6 or both. LINKA expression was increased in response to IL-6 or HG+IL6 (Figure 6H) suggesting a putative role in stress responses of pancreatic beta cells.
To interrogate the role of LINKA in pancreas differentiation and beta-like cell function, LINKA deficient cells were generated using the CRISPR-CAS9 technology in both Mel1 and H1 cell lines (Figure 6I and S6C). During pancreatic differentiation, LINKA deficient and WT lines generated comparable PP1 and PP2 cell populations (Figure 6J–K and S6D–E). Analyses of C-PEP+, SST+, or GCG+ endocrine cells were also comparable in the Mel1 background (Figure 6L–N), but GCG+ and SST+ cells were slightly increased in the H1 background (Figure S6F–H). Transcript levels of GCG was slightly upregulated only in the Mel1 background (Figure 6O and S6I). C-peptide protein levels were similar in both genetic backgrounds (Figure 6P and S6J). These data show that besides having a minor effect on glucagon expression, LINKA loss had little impact on the differentiation of endocrine cells.
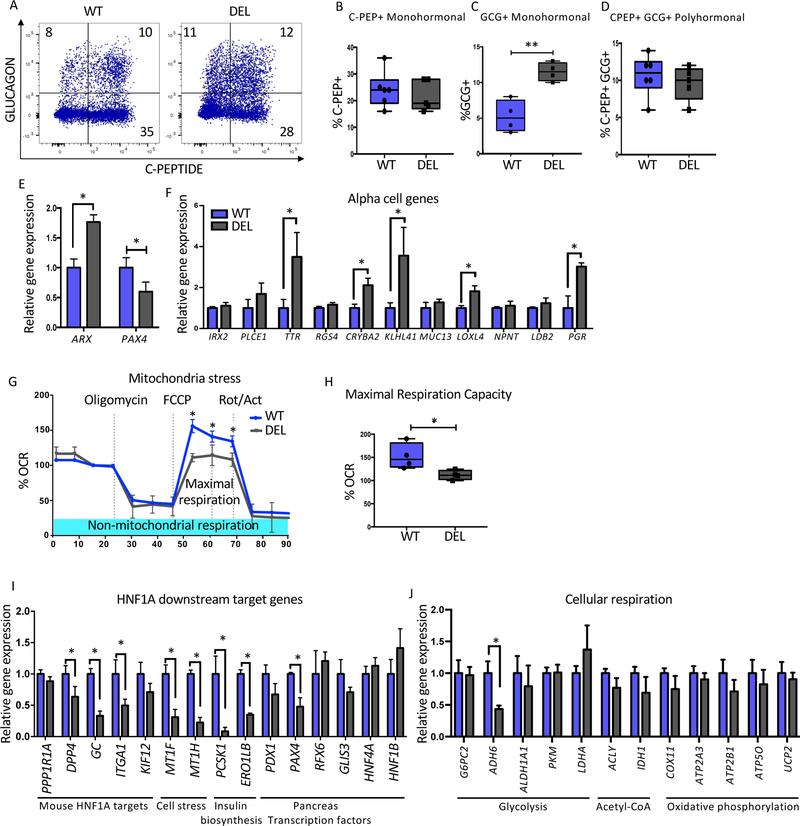
Lack of LINKA mimics a subset of the phenotypes found in HNF1A mutant cells.
Because LINKA had an effect on glucagon expression, studies similar to those using HNF1A mutant lines (Figure 3) were performed. LINKA deficient cells displayed an increase in GCG+ monohormonal cells in both genetic backgrounds (Figure 7A, 7C, S7A and S7C) while a decrease in monohormonal C-PEP+ cells were seen only in the H1 background (Figure 7B and S7B). Gene expression analyses in beta-like cells showed increased ARX and decreased PAX4 expression in the Mel1 background (Figure 7E), but these results were not recapitulated in the H1 background (Figure S7E). Expression levels of the alpha specific genes, TTR, CRYBA2, KLHL41, LOXL4 and PGR, were upregulated in LINKA mutant lines of both genetic backgrounds (Figure 7F and S7F). Overall, these studies suggest that LINKA loss may impact endocrine cell development but to a less severe degree compared to the HNF1A mutant cell lines.
Figure 7: Lack of LINKA mimics a subset of the phenotypes found in HNF1A mutant cells.
Mel1 LINC01139 WT and deletion (DEL) ESCs were differentiated into pancreatic endocrine cells and analyzed as described below.
(A) Flow cytometry analysis of C-Peptide versus GLUCAGON at the end stage of pancreas differentiation (n=6 per WT and n=5 per LINC DEL cell line).
(B–D) Quantification of pancreatic hormone expressing cells. (B) Percent C-Peptide+ (C-PEP+) monohormonal cells. (C) Percent GLUCAGON+ (GCG+) monohormonal cells.
(D) Percent C-Peptide+ GLUCAGON+ (C-PEP+GCG+) cells.
(E) PAX4 and ARX mRNA expression at end of pancreatic differentiation in bulk cells (n=3 per sample).
(F) Relative gene expression of alpha cell specific genes at the end stage of differentiation in LINC01139 DEL and WT cell lines (n=3 per sample).
(G–H) Mitochondria stress test using oligomycin, FCCP and Rotenone/actinomycin (Rot/Act) in LINC01139 DEL and WT cell lines (n=3 per sample). (G) Mitochondrial respiration profile. (H) Quantification of maximal respiration capacity comparing WT and LINC01139 DEL cell lines (n=3 per genotype).
(I–J) mRNA quantification of HNF1A downstream target genes between WT and LINC01139 DEL cells using purified INS-GFP+ NKX6.1+ at end stage differentiation samples (n=3 per sample).
For all statistical analysis: * P<0.05. See also Figure S7.
With a putative role of LINKA in the stress response pathway, mitochondrial respiration was analyzed comparing WT and deficient (DEL) beta-like cells using the extracellular flux assay (Figure 7G and S7G). The LINKA deficient cells in both genetic backgrounds displayed a decrease in maximal respiration capacity to a similar extent as seen in the HNF1A HET cells (Figure 7H, S7H and 4G). To identify HNF1A target genes that may be regulated by LINKA, gene expression analysis of sorted LINKA deficient INS-GFP+NKX6.1+ cells were compared to WT cells. A subset of HNF1A mouse target genes including DPP4, GC, and ITGA1 were identified. In addition, genes related to cell stress (MT1F, MT1H), insulin biosynthesis (PCSK1, ERO1LB), pancreas development (PAX4) and glycolysis (ADH6) were also impacted (Figure 7I–J). Downregulation of DPP4, GC, MT1F, PCSK1 and ADH6 were recapitulated in the H1 genetic background (Figure S7I–J). These data suggest that LINKA may regulate a subset of HNF1A target genes that have implications in cellular respiration.
Discussion:
Despite the fact that mutations in HNF1A are a relatively common cause of monogenic diabetes, the mechanism by which HNF1A contributes to disease pathogenesis in human beta cells remains unclear. This is in part due to the failure of mouse models to mimic the human phenotype and the difficulty in accessing patient samples. In this study, we developed a human stem cell model to study the molecular mechanisms by which HNF1A regulates both beta cell development and function. The use of multiple isogenic ESC lines combined with the immortalized human beta cell lines EndoC-BH1&3 was critical, as we identified specific phenotypes in HNF1A mutants independent of genetic background or model system. These included the downregulation of important beta cell transcription factors, genes related to beta cell stress, insulin biosynthesis and cellular respiration (Figures 2Q, S2Y, 5D–G, and S5A–D). In addition, functional defects in both glycolysis and mitochondrial function were also seen in all lines (Figures 4 and S4), providing a potential mechanism for both the MODY3 phenotype as well as the link between HNF1A and type 2 diabetes.
In this study, we found that even a complete loss of HNF1A did not impair the generation of pancreatic progenitors but was necessary for proper endocrine cell development. First, HNF1A KO cells displayed downregulation of insulin mRNA and protein content which was similar to observations reported in mouse models due to direct regulation of insulin transcription (Akpinar et al. 2005; Wang et al. 1998; Brial et al. 2015). Second, HNF1A HET and KO cell lines upregulated expression of the hormones ghrelin and glucagon (Figures 2Q) with a decrease of insulin monohormonal and increase in glucagon monohormonal cells (Figure 3B–C). The increase in alpha cells found in the stem cell model is consistent with the findings observed in a human MODY3 patient where alpha cell mass was higher than the non-diabetic samples (Haliyur et al. 2019). The downregulation of PAX4 which we hypothesize could be contributing to the increase in alpha cell markers was also seen in the MODY3 patient (Figure S5N). The phenotypes described here appear to be human specific, as rodent Hnf1a models do not have an increase in alpha cells. However, inactivation of Pax4 in mice does have similar phenotypes to the HNF1A mutant stem cell model. Pax4 mutant mice have a dramatic increase in the ghrelin and glucagon cell populations with loss of most insulin+ cells (Collombat et al. 2003). Our human stem cell model also displays an increase in GCG and GHRL expression but still generates beta-like cells. This could be explained by the fact that HNF1A mutants still transiently express PAX4 with significant downregulation beginning at the PP2 stage (Figure 3E). This low level expression may be sufficient for beta cell generation, or alternatively, PAX4 could have distinct roles in mouse and humans in regard to beta cell development. In the mouse, it was also shown that PAX4 is a repressor of ghrelin (Wang et al. 2008) and ARX expression, where overexpression of PAX4 resulted in decreased glucagon+ cells (Spijker et al. 2013; Collombat et al. 2003; Gage et al. 2014). PAX4 transcript is exclusively expressed in the HNF1A+ cell population during pancreatic differentiation (Figure S1H–J) opening the possibility that HNF1A may play a role in directly regulating PAX4 expression. In addition, it was shown previously that HNF1A binds the human PAX4 promoter (Smith et al. 2003).
We found that inactivation of HNF1A in the EndoC-BH3 cell line did not impair insulin secretion (Figure S4J–K) but loss of HNF1A in the human stem cell system did impair secretion (Figure 4A). These differences may suggest that HNF1A is needed during endocrine development to generate a functional beta cell and loss at the beta cell stage may have a less severe phenotype. Future studies utilizing a beta cell specific knockout of HNF1A could address this possibility. The HNF1A mutant ESC derived beta-like cells in addition to having defects in glucose responsiveness also may have a higher basal insulin secretion though this did not reach statistical significance (Figure 4B). Both of these phenotypes were seen in the MODY3 patient’s islets (Haliyur et al. 2019). Moreover, It had been reported that neonatal MODY3 patients could surprisingly present with hyperinsulinism at birth, which would then resolve and transition to diabetes later in life (Stanescu et al. 2012; Petra et al. 2011).
Looking at the differentially expressed genes that could help to explain the insulin secretion phenotype, we found known HNF1A target genes (PPP1R1A, DPP4, GC, ITGA1, KIF12, HNF4A, G6PC2) (Servitja et al. 2009) but also discovered some novel HNF1A target genes that were also dysregulated in human MODY3 beta cells (RFX6, PAX4, MT1F, MT1H, ERO1LB, ALDH1A1, ACLY, IDH1 and ATP2A3) (Figure S5N) (Haliyur et al. 2019) and a subset of genes unique to our data sets (PDX1, PCSK1, ADH6, LDHA, COX11, UCP2). Several of these genes are known causes of MODY such as PAX4 (Jo et al. 2011), RFX6 (Chandra et al. 2014; Piccand et al. 2014), and PDX1 (Sachdeva et al. 2009) or known regulators of insulin secretion such as the insulin processing enzyme PCSK1 (Stijnen et al. 2016), and ERO1LB which plays an essential role in proinsulin protein folding and maturation. Mice deficient in Ero1lb are hyperglycemic with impaired insulin secretion (Zito et al. 2010). Another gene that could lead to compromised insulin secretion, LDHA, was upregulated in the HNF1A KO cell line as well as in type 2 diabetes (Marselli et al. 2010). LDHA is a so called ‘disallowed gene’ with low expression in healthy mature beta cells (Lemaire et al. 2016; Pullen et al. 2014). Examination of a larger list of 26 disallowed genes in our microarray dataset, showed 9 of these genes were upregulated and 4 downregulated in HNF1A mutant beta like cells (Figure S5O). When LDHA is upregulated, it impacts metabolism and impairs insulin secretion (Ainscow et al. 2000; Pullen et al. 2014; Lemaire et al. 2016). LDHA upregulation seems to be a common feature of diabetic beta cells as similar upregulation was observed in different diabetic models such as in GK rats (Homo-Delarche et al. 2006), Sprague-Dawley rats (Jonas et al. 1999) and db/db mice (Ye et al. 2016). Overall, we present evidence suggesting that the lack of HNF1A leads to the generation of abnormal beta-like cells with increased glucagon expression and defective insulin secretion explained in part by deregulation of beta cell genes important for beta cell function and development.
Data presented here also demonstrate that HNF1A is necessary for normal beta cell metabolism. Beta-like cells derived from HNF1A mutant stem cells display both decreased glycolysis and mitochondrial respiration (Figure 4D–H). Genomics data suggested that HNF1A regulates genes at different steps in the cellular respiration pathway such as G6CP2 and LDHA, well known for regulating glycolysis, ATP production and insulin secretion (Li et al. 2009; Ainscow et al. 2000; Lemaire et al. 2016). Similar target genes have been reported in Hnf1a mouse models (Wang et al. 1998; Servitja et al. 2009). It is interesting that HNF1A impacted the regulation of metallothionein (MT) family genes such as MT1F and MT1H which when examined in the MODY3 patient dataset were also seen to be downregulated (Figure S5N). These genes are important for cell protection under glucotoxicity and lipotoxicity stress, a recurrent phenomenon in type 2 diabetes (Guo et al. 2017; Poitout et al. 2011). Surprisingly, when we examined oxygen consumption in beta-like cells, we found a defect in maximal respiration capacity only in the HNF1A HET but not in the KO cell lines. A complex gene regulatory network could explain this phenotype, where HNF1A may have both positive and negative effects on genes required for proper cellular respiration. We hypothesized that such targets would be downregulated to a greater extent in HET versus KO genotypes. We found one such target, LINKA, that displayed a more severe downregulation of mRNA in HET over KO beta-like cells. When this lncRNA was disrupted by genome editing, the mitochondrial defect from HNF1A HET beta-like cells was phenocopied. Overall, these metabolic phenotypes present possible contributing mechanisms for both MODY3 as well as the known association between SNPs in the HNF1A gene and type 2 diabetes (Voight et al. 2010; Fuchsberger et al. 2016; Mishra et al. 2017; Estrada et al. 2014).
The HNF1A target LINKA is a lncRNA present in primates but not in rodent genomes, making it a potential candidate to understand species-specific phenotypes relevant to human disease. LINKA was expressed in human pancreatic endocrine cell types and human fetal pancreas in addition to in vitro derived beta-like cells (Figure 6G and S6K). In addition, it was seen that LINKA was downregulated in a MODY3 patient’s alpha cells but not beta cells (Figure 6D). We hypothesize that loss of LINKA may be contributing to skewing development towards an alpha cell fate with those cells with lowest levels being overrepresented in the alpha cell pool. While the severity of the mitochondrial defect in LINKA null cells was similar to that of the HNF1A HET cells, it only partially mimicked the other phenotypes of the HNF1A mutants, including an increase in alpha cell genes, suggesting that other HNF1A targets are important for the full spectrum of phenotypes observed in MODY3 patients. How LINKA influences mitochondria respiration is unknown, but based on its function in other cell types, the AKT signaling pathway may be a possible candidate. In cancer cell lines, LINKA has been shown to regulate AKT activation by acting as a scaffold to stabilize PIP3 and AKT interactions (Lin et al. 2017). Considering that AKT signaling has been reported to play an essential role in cellular respiration via the PI3K/mTOR pathways (Goo et al. 2012), we speculate that loss of LINKA may lead to diminished AKT activation and impaired mitochondrial function. In addition, we found that LINKA was upregulated when beta cells were treated with beta cell stressors such as high glucose and IL-6 (Figure 6H). These findings suggest that this lncRNA may influence both type 1 and type 2 diabetes and be one potential mechanism for GWAS studies which have linked HNF1A SNPs to both of these diseases (Voight et al. 2010; Fuchsberger et al. 2016; Mishra et al. 2017; Estrada et al. 2014). The de-repression of alpha cell genes observed in both the HNF1A and LINKA mutant lines was reminiscent of defects seen in diabetic islets and highlighted how HNF1A/LINKA could influence this disease (Cinti et al. 2016; John et al. 2018; Spijker et al. 2015)
Overall, our studies suggest that HNF1A regulates beta cell function and cellular metabolism through a subset of genes that contribute to the pathogenesis of MODY3 as well as type 1 and type 2 diabetes. Future studies will be essential to dissect the mechanism by which the novel human HNF1A targets, including LINKA, contributes to diabetes.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Paul Gadue (gaduep@email.chop.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Culture
All the ESC studies were performed using Mel1 and H1 hESCs along with two different immortalized human beta cell lines, EndoC-BH1 and EndoC-BH3.
Human ESCs:
We used two different genetic backgrounds of stem cells: MEL1 and H1 hESCs, these cell lines were cultured on 0.1% gelatin and irradiated Mouse Embryonic Fibroblasts (MEFs). The medium used to maintain cell growth and stemness was DMEM/F12 supplemented with 2mM of glutamine, 15% Knockout Serum Replacement (KSR), 1X NEAA, 0.1mM β-mercaptoethanol and 10ng/ml of bFGF which we called hESC medium. Cells were split when they reach 80% confluence using TrypLE and replate using 1/6 ratio.
EndoC-BH1 and EndoC-BH3 cell lines:
DMEM low glucose was used as a media backbone, then we prepared a stock for 500ml as follows: 10g Bovine serum albumin (BSA) fraction V (Sigma A1470–100g), 1.75µl beta-mercaptoethanol (BME) (Gibco Cat No. 21985023), 0.611g Nicotinamide (Sigma Cat N0636–100g), 55µl (50mg/ml) transferrin (Roche cat: 652202), 3.35µl (1mg/ml) Sodium Selenite and 5ml Pen/Strep (Gibco). Cells were split once they reached 90% confluency and replated on plates coated 24 hours before using DMEM low glucose with 1/100 Matrigel and 10ug/ml fibronectin. Cell splitting was performed using 0.25% trypsin and split at a 1:2 ratio.
METHODS DETAILS
Cell preparation for pancreatic differentiation
Mel1 hESCs were plated on 1/30 Matrigel when cells reach 100% confluency cell differentiation starts. The Mel1 cell line was differentiated in adherent format. H1 hESCs was maintained on gelatin and MEFS and feed with the medium described before. H1 pancreatic differentiation was performed in a embryoid body (EB) format.
Embryoid body generation
Cells were incubated with the accutase solution for 6 min at 37°C, and cells were transferred to a 50ml falcon tube washed twice using 40ml of DMEM-F12. 5.5 million cells were resuspended in 5ml of hESC medium with 1µM of ROCK inhibitor and plate them on one well of 6-well plate of ultra-low attachment cell culture plate. The plate was placed on an orbital shaker set up at 100rpm inside of incubator with 5% CO2 and 37°C. Overnight cells form embryoid bodies, which were fed for two days using hESC medium. After two days, the medium was removed, and it was replaced with pancreatic differentiation medium for day zero. During differentiation protocol until day 14, medium was replaced every 24 hours with fresh medium made during the day. After day 14 cells were fed every day after with fresh medium.
Pancreatic differentiation protocol 1
Day zero medium contains RPMI supplemented with 0.3mM Chir99021, 100µg/ml Activin A and 0.2% fetal bovine serum (FBS). For day 1 medium contains RPMI with 100µg/ml Activin A, 0.03mM Chir99021, 10µg/ml bFGF and 0.2% FBS. For day 2 and 3 the backbone medium was SFD with 100µg/ml Activin A. From day 4–6 the backbone medium was change to DMEM-F12 with 50ng/ml FGF7. Then medium was replaced from day 7–8 with DMEM high glucose (5g/L) supplemented with 1:100 B27 without RA, 1X Glutamax, 0.25mM ascorbic acid, 1:200 ITS-X, 50ng/ml FGF7, 0.5µM SANT-1, 1µM Retinoic Acid, 100nM LDN-193189 and 500nM Phorbol. Medium for day 9–11 consist of DMEM high glucose (5g/L) supplemented with 1:100 B27 without RA, 1X Glutamax, 0. 25mM ascorbic acid, 1:200 ITS-X, 2ng/ml FGF7, 0.5µM SANT-1, 0.1µM Retinoic Acid, 200nM LDN-193189 and 250nM Phorbol. From day 12–14 the backbone medium was change to MCDB131 supplemented with 20mM glucose, 2% FBS, 1X Glutamax, 1:200 ITS-X, 10ug/ml Heparin, 10uM Zinc sulfate, 0.5µM SANT-1, 0.05µM Retinoic Acid, 200nM LDN-193189, 1µM T3 and 10µM ALK5i II. From day 15–28 cells were fed every day after with medium that contains MCDB131 with 20mM glucose, 2% FBS, 1X Glutamax, 1:200 ITS-X, 10ug/ml Heparin, 10uM Zinc sulfate, 200nM LDN-193189, 1µM T3, 10µM ALK5i II and 100nM GSIS XX. From day 29–40 cells were fed every day after with medium that contains MCDB131 with 20mM glucose, 2% FBS, 1X Glutamax, 1:200 ITS-X, 10µg/ml Heparin, 10uM Zinc sulfate, 1µM T3, 10µM ALK5i II, 1mM N-acetyl cysteine, 10µM Trolox and 2µM R428.
Pancreatic differentiation protocol 2 for GSIS
Day zero medium contains RPMI supplemented with 0.3mM Chir99021, 100µg/ml Activin A and 0.2% fetal bovine serum (FBS). For day 1 medium contains RPMI with 100µg/ml Activin A, 0.03mM Chir99021, 10µg/ml bFGF and 0.2% FBS. For day 2 and 3 the backbone medium was SFD with 100µg/ml Activin A. From day 4–6 the backbone medium was change to DMEM-F12 with 50ng/ml FGF7. Then medium was replaced from day 7–8 with DMEM high glucose (5g/L) supplemented with 1:100 B27 without RA, 1X Glutamax, 0.25mM ascorbic acid, 1:200 ITS-X, 50ng/ml FGF7, 0.5µM SANT-1, 1µM Retinoic Acid, 100nM LDN-193189 and 500nM Phorbol. Medium for day 9–11 consist of DMEM high glucose (5g/L) supplemented with 1:100 B27 without RA, 1X Glutamax, 0. 25mM ascorbic acid, 1:200 ITS-X, 2ng/ml FGF7, 0.5µM SANT-1, 0.1µM Retinoic Acid, 200nM LDN-193189 and 250nM Phorbol. From day 12–14 the backbone medium was change to MCDB131 supplemented with 20mM glucose, 2% FBS, 1X Glutamax, 1:200 ITS-X, 10ug/ml Heparin, 10uM Zinc sulfate, 0.5µM SANT-1, 0.05µM Retinoic Acid, 200nM LDN-193189, 1µM T3 and 10µM ALK5i II. From day 15–22 cells were fed every day after with medium that contains MCDB131 with 20mM glucose, 2% FBS, 1X Glutamax, 1:200 ITS-X, 10ug/ml Heparin, 10uM Zinc sulfate, 200nM LDN-193189, 1µM T3, 10µM ALK5i II and 100nM GSIS XX. From day 23–40 cells were fed every day after with medium that contains MCDB131 with 8mM glucose, 2% FBS, 1X Glutamax, 1:200 ITS-X, 10µg/ml Heparin, 10uM Zinc sulfate, 1mM N-acetyl cysteine, 10µM Trolox and 2µ M R428.
CRISPR-CAS9 genetically modified hESCs and EndoC-BH cell lines
We used the CRISPR-CAS9 nuclease system to generate all the different mutations used in this study. To edit hESCs two CRISPR-CAS9 plasmids were used, one carrying the CAS9 fused to a GFP (CAS9-GFP) (addgene plasmid 44720), and the second plasmid was the gRNA empty vector (addgene plasmid 41824). The second plasmid was used to clone different gRNAs according to the gene of interest. 19bp of each gRNA was clone into the empty vector using In-Fusion HD (Clontech Cat No. 639647). The gRNAs used for each DNA region of interest were HNF1A: 5’-AACTGAGCCAGCTGCAGA-3’, LINC01139, gRNA1:5’-TGCGTACCAAAGATGTCGC-3’ and gRNA2: 5’-CCGTATGTAATGATGTCTG-3’.
We performed lipid transfection of DNA plasmids in 30–50% confluent hESCs. Lipid transfection master mix was perform performed by incubating 50µl IMDM, 1ug of DNA of each plasmid and 4µl of DNA In-Stem (MTI Global Stem) and incubated for 30min at room temperature. The 50µl reaction was added drop-wise into one well of a 6-well plate of cells. 24 hours after transfection, GFP+ cells were sorted and replated on 1/3 matrigel with MEFS. GFP expression is lost 48–72 hours after cell transfection due to loss of the plasmid. After 10 days single colonies were picked and screened for indels.
To genome edit EndoC-BH cell lines, we modified the CRISPR-Cas9 lentivirus vector V2-CRISPR from Addgene (Plasmid 52961); the EFS promoter was replaced by a modified rat insulin promoter (RIP) (Hayes HL et al 2016) and it was cloned using the In-Fusion cloning kit (Clontech). The modified V2-RIP-CAS9 vector has a BsmBI restriction site that allows cloning of the HNF1A specific gRNA: 5’-CTGGCTCAGTTTAGAAACCATGG-3’. Lentivirus particles carrying the HNF1A gRNA and CRISPR-CAS9 were used to infect EndoC-BH cell lines.
Rapid DNA isolation and PCR:
Each colony of hESCs that was picked for indel screening was resuspended in 20µl of PCR buffer containing 50ug/ml proteinase K. Then samples were put at 55°C for 1h, 94°C for 10 min. Samples were spin down, and 5µl of the supernatant was used for PCR.
Western Blot
Protein from the cell pellet was quantified using Pierce™ BCA Protein Assay Kit (Thermo Fischer scientific, cat No. 23227) 20ug of protein was load in 4–12% Bis-Tris SDS-polyacrylamide gel (Invitrogen). Samples were transferred into a PVDF membrane (Thermo Fisher) and membrane blocking was performed using 2% nonfat dry milk. Primary antibody incubation was overnight and secondary antibody for one hour. The membrane was washed between antibodies incubation using 1X PBS-T three times each 10min. HRP was detected using Pierce™ TMB Subs trate Kit and membrane was exposed to HyBlot CL autoradiography film (Denville Scientific) to visualize the protein band.
Flow cytometry and cell sorting.
Samples were dispersed into single cells and then fixed using 1.6% PFA incubating at 37°C for 30min. Cells used for flow cytometry analy sis of intracellular antigens were processed using intracellular staining which consists of cell permeabilization in order to allow antibody access to internal antigens. Cells were washed twice using 1X saponin permeabilization buffer, and primary antibody was incubated for 30min. Samples were washed in 1X saponin buffer twice and incubated for 30min using the appropriate secondary antibody. After 30 minutes samples were washed twice using FACS buffer, then samples were resuspended in FACS buffer to be analyzed by flow cytometry.
Cell sorting was performed in two different ways. For live cell sorting, cells were kept in their respective culture medium containing ROCK inhibitor (Cayman chemicals) and then sorted and plated according to their normal culture conditions. To preserve RNA integrity of sorting fixed cells, samples were maintained in the presence of 1/100 dilution of RNAse during fixation, staining and sorting.
RNA isolation and cDNA synthesis
For non-fixed cells, PureLink RNA Micro Kit (Invitrogen Cat No 12183–016) was used, and an indication from manufacturer recommendations was followed. 16µl of RNAse free water was used to resuspend isolated RNA.
For the fixed cells, RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE was used (Ambion Cat No. AM1975) and manufacturer recommendations were followed. 30µl RNAse free water was used to resuspend isolated RNA. 3µl of isolated total RNA was used for cDNA synthesis with SuperScript reverse transcriptase (Invitrogen Cat No 18064–022).
Microarray
Three biological replicates of RNA from end-stage pancreatic differentiation GFP+ NKX6.1+ fixed sorted cells were used to perform a genome profiling using a whole human transcriptome with Affymetrix chip array performed by the Penn Molecular Profiling Facility. The data analysis was performed using XLSTAT software. All the genes and individual genotypes were clustered independently using ascendant hierarchical clustering based on Euclidean distances. Microarray data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-8120.
Lentivirus production
293T cells were grow using 145mm plates using DMEM high glucose (4.5g/L) with 1X glutamine and 5% FBS. Cells were fed twice a week and were used to be infected with lentivirus when cells were 90% confluent. Lentivirus backbone used here was the second generation. 293T cells were transfected using 2 M CaCl2 and HEPES with along with the helper plasmids: G protein of the vesicular stomatitis virus (VSV), Hgpm2, Tat and Rev. Virus was collected 2 days after transfection and lentivirus was used, transducing 50µl of lentivirus soup with 2 µg/mL polybrene (Millipore) and 2ml of medium in one well of 6-well plate.
Glucose stimulation insulin secretion (GSIS)
KRBH ringer buffer was made of three different solutions, solution A: NaCl 107g, solution B: KCl 5.96 g, NaHCO3 32.256g, MgCl2.6H2O 3.25g and solution Buffer C: CaCl2.2H2O 5.168g. For one liter we mixed 50ml of each solution and added 2g BSA + 1.906g HEPES and bring it up to 1L using ddH2O, pH was adjusted 7.4. The buffer was filtered and kept at 37C. Cells were incubated with 2mM glucose for one hour (Starvation) then the 2mM glucose solution was change for new 2mM glucose solution and incubated for 30min (basal glucose) and 20mM D-glucose for 30min and finally 30mM KCL for 30min. Supernatants were collected and keep at −80C until assayed. To quantify insulin secreted on the supernatant, Insulin high range assay kit was used (CisBio) and the plate reader (CLARIOstart) was used to read the fluorescent signal.
Mitochondrial Respiration and glycolysis stress.
End-stage cell differentiation at day 35 were used, cells were sorted for GFP+ cells in the Mel1 line while in the H1 line bulk cells were used. 60,000 cells were plated per well in a 96-well plate using 80µl of growth medium. The next day, cells were washed using 200µl of sea horse medium and then 175µl of the respective medium used for the glycolysis or mitochondrial test. The respective concentrations of the different drugs used for the assay were for the mitochondrial test were 1.25µM oligomycin, 0.75µM FCCP, and 0.5µM Rotenone/Actinomycin. For the glycolysis test, we used 20mM D-glucose, 1.25µM, and 50mM 2-DG. Sample plate was transferred to a Seahorse XFe96 analyzer and results were analyzed using the free software wave.
Single cell analysis
One human fetal pancreas sample at 18 weeks gestational age was obtained from Advance Bioscience Resources (Alameda, CA) at the time of elective termination of pregnancy and after obtaining appropriate informed consent for the use of fetal samples in research. We ensured that the tissue procurement company had provided an informed consent form that is in accordance with state and federal requirements for research use of human fetal tissues. Gestational age was determined by ultrasound, usually using a crown-rump length. Further information on maternal history was not available, but there was no known genetic defect present. The sample was shipped overnight in RPMI/5%FBS/antibiotics medium on ice. We received the sample after 16–20 hrs. Experiments performed on these samples are exempt from IRB evaluation by the both University of Pennsylvania and The Children’s Hospital of Philadelphia, as they are not considered human-subject research. Single fetal pancreatic cells were obtained after 10 minutes dissociation of small pancreatic fragments with TripLE (ThermoFisher). Single cell suspension in RPMI/5%FBS/antibiotics medium was kept on ice and submitted to The Children’s Hospital of Philadelphia Center for Applied Genomics, and then loaded on the 10X Genomics Chromium platform following manufacturer’s protocol. Single cell libraries were obtained using the Chromium™ Single Cell 3’ v2 Reagent Kit. A total of 2292 cells were sequenced, of which 116 expressed pancreatic hormones. Cells were visualized and clustered using the LoupeTM Cell Browser, v2.0.0.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed using GraphPad Prism 6 software. The results are expressed as the mean +/− standard error of the mean. An unpaired two-tailed Student’s t-test for groups with equal variance was performed to determine p values. In figures * P<0.05, **P<0.01 and ***P<0.001.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-HNF1A | Cell Signaling | Cat# D7Z2Q |
| Biotinylated goat polyclonal anti PDX-1/IPF1 | R&D | Cat# BAF2419 |
| Mouse IgG1 monoclonal anti-NKX6.1 | DSHB | Cat# F55A10 |
| Rat monoclonal anti-Somatostatin | Santa Cruz | Cat# sc-47706 |
| Mouse IgG1 monoclonal anti-Glucagon | Sigma | Cat# G2654-.2ML |
| Mouse IgG1 monoclonal anti-INSULIN | Sigma | Cat# I2018-.2ML |
| Rabbit monoclonal anti-C-Peptide | Cell Signaling | Cat# 4593S |
| Goat anti mouse IgG1–488 | Jackson Immunoresearch | Cat# 115–545-205 |
| Goat anti mouse IgG1-PE | Jackson Immunoresearch | Cat# 115–115-205 |
| Goat anti mouse IgG1–647 | Jackson Immunoresearch | Cat# 115–605-205 |
| Goat anti rabbit alexa 647 | Invitrogen | Cat# A21245 |
| Goat anti rabbit IgG-PE | Jackson Immunoresearch | Cat# 111–116-144 |
| Goat anti Rat alexa flu 647 | Thermo Fischer scientific | Cat# A21247 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Activin A | R&D | Cat#338-AC |
| Chir99021 | Cayman | Cat# 13122 |
| BFGF | R&D | Cat# 233-FB |
| FGF7 | R&D | Cat# 251-KG-050/CF |
| SANT-1 | Sigma-Aldrich | Cat# S4572 |
| Retinoic Acid | Sigma-Aldrich | Cat# R2625 |
| LDN-193189 | STEMGENT | Cat# 04–0074 |
| Phorbol 12-myristate 13-acetate | Tocris | Cat# 1201 |
| Heparin | Sigma | Cat# H3149 |
| Zinc Sulfate | Sigma | Cat# 1724769 |
| T3 (250 mg) | Sigma | Cat# T6397 |
| ALK5 inhibitor | Enzo Life Sciences | Cat# ALX-270–445 |
| GSIXX | Calbiochem | 565789 |
| N-Acetyl-L-cysteine | Sigma | A9165 |
| Trolox | EMD | Cat#648471 |
| R428 | SelleckChem | S2841 |
| Critical Commercial Assays | ||
| CisBio insulin test | Cisbio | 62INSPEB |
| XF cell mito Stress test kit | Agilent Technologies | 103015–100 |
| XF glycolysis stress test kit | Agilent Technologies | 103020–100 |
| Deposited Data | ||
| Microarray data set | This paper | https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-8120 |
| Experimental Models: Cell Lines | ||
| hESC-Mel1 INS-GFP | Dr. Stanley EG | |
| hESC-Mel1 INS-GFP- HNF1A-HET-C22 | This paper | |
| hESC-Mel1 INS-GFP-HNF1A-KO-C5 | This paper | |
| hESC-Mel1 INS-GFP-LINKA-DEL-C211 | This paper | |
| hESC-H1 | Dr. Thomson J | |
| hESC-H1-HNF1A-HET-C3 | This paper | |
| hESC-H1-HNF1A-KO-C21 | This paper | |
| hESC-H1-HNF1A-LINKA-DEL-C20 | This paper | |
| Oligonucleotides | ||
| Primers for quantitative RT-PCR | This paper | Table S1 |
| Recombinant DNA | ||
| pCas9_GFP | Addgene | 44719 |
| gRNA empty vector | Addgene | 41824 |
| Software and Algorithms | ||
| FlowJo | Ashland | https://www.flowjo.com/solutions/flowjo/downloads |
| Wave Desktop | Agilent | https://www.agilent.com/en/products/cell-analysis/software-download-for-wave-desktop |
| GraphPad Prism 6 | GraphPad Software | https://www.graphpad.com/support/faqid/%201952 |
| XLSTAT | Addinsoft | https://www.xlstat.com/en/solutions/base |
| Illustrator | Adobe System Software | https://www.adobe.com/cn/products/cs6/illustrator.html |
| ApE plasmid Editor | M. Wayne Davis | http://biologylabs.utah.edu/jorgensen/wayned/ape/ |
| Leica Application Suite X | Leica | https://www.leicamicrosystems.com/products/microssoftware/details/product/leica- |
| Illustrator | Adobe System Software | https://www.adobe.com/cn/products/cs6/illustrator.html |
Acknowledgments
PG is supported by NIH grants UC4 DK104196 and R01 DK118155. DS is supported by American Diabetes Association grant #1-16-JDF-086, F.M. Kirby Foundation, Inc, the NIH UL1TR001878 and the University of Pennsylvania, Institute for Translational Medicine and Therapeutics’ (ITMAT) Program. The Vanderbilt research was performed using resources and/or funding provided by the NIDDK-supported Human Islet Research Network (HIRN, RRID:SCR_014393; https://hirnetwork.org; UC4 DK104211, DK108120, and DK112232), by DK106755 and DK20593, and by the Department of Veterans Affairs (BX000666). We thank Nripesh Prasad at HudsonAlpha Institute for Biotechnology, Huntsville, AL, USA, for assistance with the lncRNA data from the MODY-3 patient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors declare no conflict of interest.
DATA AND SOFTWARE AVAILABILITY
HNF1A allelic series microarray dataset have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-8120
References
- Ainscow EK, Zhao C and Rutter G. a (2000). Acute overexpression of lactate dehydrogenase-A perturbs beta-cell mitochondrial metabolism and insulin secretion. Diabetes, 49(7), pp. 1149–1155. [DOI] [PubMed] [Google Scholar]
- Akpinar P, Kuwajima S, Krützfeldt J and Stoff el M (2005). Tmem27: a cleaved and shed plasma membrane protein that stimulates pancreatic beta cell proliferation. Cell metab, 2(6), pp. 385–97. [DOI] [PubMed] [Google Scholar]
- Beckner ME, Fellows-Mayle W, Zhang Z, Agostino NR, Kant JA, Day BW and Pollack IF (2010). Identification of ATP citrate lyase as a positive regulator of glycolytic function in glioblastomas. Int J. Cancer, 126(10), pp. 2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner C, Bacon S and Concannon C (2010). INS-1 Cells Undergoing Caspase-Dependent Apoptosis Enhance the Regenerative Capacity of Neighboring Cells. Diabetes, 59, pp. 2799–2808.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brial F, Lussier CR, Belleville K, Sarret P and Boudreau F (2015). Ghrelin inhibition restores glucose homeostasis in hepatocyte nuclear factor-1a (MODY3)-deficient mice’, Diabetes, 64(9). [DOI] [PubMed] [Google Scholar]
- Byrne MM, Sturis J, Menzel S, Yamagata K, Fajans SS, Dronsfield MJ, Bain SC, Hattersley a T., Velho G, Froguel P, Bell GI and Polonsky KS (1996). Altered insulin secretory responses to glucose in diabetic and nondiabetic subjects with mutations in the diabetes susceptibility gene MODY3 on chromosome 12. Diabetes, 45(11), pp. 1503–10. [DOI] [PubMed] [Google Scholar]
- Cai L (2004). Metallothionein as an Adaptive Protein Prevents Diabetes and its Toxicity. Nonlinearity Biol Toxicol Med April;2(2): 89–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert AE, Chalastanis A, Wu Y, Hurley LA, Kouri FM, Bi Y, Kachman M, May JL, Bartom E, Hua Y, Mishra RK, Schiltz GE, Dubrovskyi O, Mazar AP, Peter ME, Zheng H, James CD, Burant CF, Chandel NS, Davuluri RV, Horbinski C and Stegh AH (2017). Cancer-Associated IDH1 Promotes Growth and Resistance to Targeted Therapies in the Absence of Mutation. Cell Rep 19(9), pp. 1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson LMS, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, Jacobson P, Lönroth H, Maglio C, Näslund I, Pirazzi C, Romeo S, Sjöholm K, Sjöström E, Wedel H, Svensson P-A and Sjöstr öm L (2012). Bariatric Surgery and Prevention of Type 2 Diabetes in Swedish Obese Subjects. N Engl J Med 367(8), pp. 695–704. [DOI] [PubMed] [Google Scholar]
- Chandra V, Albagli-Curiel O, Hastoy B, Piccand J, Randriamampita C, Vaillant E, Cavé H, Busiah K, Froguel P, Vaxillaire M, Rorsman P, Polak M and Scharfmann R (2014). RFX6 Regulates Insulin Secretion by Modulating Ca2+ Homeostasis in Human β Cells. Cell Rep, 9(6), pp. 2206–2218. [DOI] [PubMed] [Google Scholar]
- Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, Marselli L, Suleiman M, Ratner LE, Marchetti P and Accili D (2016). Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 101(3), pp. 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE and Ellard S (2013). Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum mutat, 34(5), pp. 669–85. [DOI] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G and Gruss P (2003) ‘Opposing actions of Arx and Pax4 in endocrine pancreas development.’, Genes dev, 17(20), pp. 2591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M, Gjernes E, Druelle N, Ravaud C, Vieira A, Ben-Othman N, Pfeifer A, Avolio F, Leuckx G, Lacas-Gervais S, Burel-Vandenbos F, Ambrosetti D, Hecksher-Sorensen J, Ravassard P, Heimberg H, Mansouri A and Collombat P (2013). The Inactivation of Arx in Pancreatic α-Cells Triggers Their Neogenesis and Conversion into Functional β-Like Cells’, PLoS Genet 9(10) p. e1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA and Weiss R (2015). Type 2 diabetes mellitus. Nat Rev Dis Primers pp. 1–23. [DOI] [PubMed]
- Dranka BP, Benavides GA, Diers AR, Giordano S, Blake R, Reily C, Zou L, Chatham JC, Hill BG, Landar A and Darley-usmar VM (2011). Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med, 51(9), pp. 1621–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard S (2000) ‘Hepatocyte nuclear factor 1 alpha (HNF-1 alpha) mutations in maturity-onset diabetes of the young.’, Hum mutat, 16(5), pp. 377–85. [DOI] [PubMed] [Google Scholar]
- Estrada K, Aukrust I, Bjørkhaug L, Burtt NP, Mercader JM, García-Ortiz H, Huerta-Chagoya A, Moreno-Macías H, Walford G, Flannick J, Williams AL, Gómez-Vázquez MJ, Fernandez-Lopez JC, Martínez-Hernández A, Jiménez-Morales S, Centeno-Cruz F, Mendoza-Caamal, et al. (2014). Association of a low-frequency variant in HNF1A with type 2 diabetes in a latino population the SIGMA Type 2 Diabetes Consortium. JAMA 311(22), pp. 2305–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajans SS and Bell GI (2011). MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes care, 34(8), pp. 1878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, Ma C, Fontanillas P, Moutsianas L, McCarthy DJ, Rivas MA, Perry JRB, Sim X, Blackwell TW, Robertson NR, Rayner NW, Cingolani P, Locke AE, Tajes JF, Highland HM, Dupuis J, Chines PS, Lindgren CM, Hartl C, Jackson AU, Chen H, Huyghe JR, Van De Bunt M, Pearson RD, Kumar A, et al. (2016). The genetic architecture of type 2 diabetes. Nature, 536(7614), pp. 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HL, Zhang L, Becker TC, et al. A Pdx-1-Regulated Soluble Factor Activates Rat and Human Islet Cell Proliferation. Mol Cell Biol 2016;36(23):2918–2930. doi: 10.1128/MCB.00103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage BK, Baker RK and Kieffer TJ (2014). Overexpression of PAX4 reduces glucagon expression in differentiating hESCs. Islets, 6(2), pp. e29236–1–e29236–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-gonzalez MA, Carette C, Bagattin A and Chiral M (2016) ‘A suppressor locus for MODY3- diabetes’, Scient, pp. 1–14. [DOI] [PMC free article] [PubMed]
- Goo CK, Lim HY, Ho QS, Too HP, Clement MV and Wong KP (2012) ‘PTEN/Akt Signaling Controls Mitochondrial Respiratory Capacity through 4E-BP1’, PLoS ONE, 7(9), pp. 1–12. doi: 10.1371/journal.pone.0045806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grarup N, Sandholt CH, Hansen T and Pedersen O (2014). Genetic susceptibility to type 2 diabetes and obesity: From genome-wide association studies to rare variants and beyond. Diabetologia, 57(8), pp. 1528–1541. [DOI] [PubMed] [Google Scholar]
- Guo M, Zhang T, Dong X, Xiang JZ, Lei M, Evans T, Graumann J and Chen S (2017). Using hESCs to Probe the Interaction of the Diabetes-Associated Genes CDKAL1 and MT1E’, Cell Rep 19(8), pp. 1512–1521. [DOI] [PubMed] [Google Scholar]
- Haliyur R, Brissova M, Powers AC, Haliyur R, Tong X, Sanyoura M, Shrestha S, Lindner J, Saunders DC, Aramandla R, Poffenberger G, Redick SD, Bottino R, Prasad N, Levy SE, Blind RD, Harlan DM, Philipson LH, Stein RW, Brissova M and Powers AC (2019). Human islets expressing HNF1A variant have defective b cell transcriptional regulatory networks Graphical abstract Find the latest version : Human islets expressing HNF1A variant have defective β cell transcriptional regulatory networks. The clin invest, 129(1), pp. 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries LW, Ellard S, Stride A, Morgan NG and Hattersley AT (2006). Isomers of the TCF1 gene encoding hepatocyte nuclear factor-1 alpha show differential expression in the pancreas and define the relationship between mutation position and clinical phenotype in monogenic diabetes. Hum Mol Genet, 15(14), pp. 2216–24. [DOI] [PubMed] [Google Scholar]
- Hattersley AT and Patel KA (2017). Precision diabetes: learning from monogenic diabetes. Diabetologia 60(5), pp. 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homo-Delarche F, Calderari S, Irminger JC, Gangnerau MN, Coulaud J, Rickenbach K, Dolz M, Halban P, Portha B and Serradas P (2006). Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes, 55(6), pp. 1625–1633. [DOI] [PubMed] [Google Scholar]
- Horikawa Y (2018) ‘Maturity-onset diabetes of theyoung (MODY) as a model for elucidating the multifactorial origin of type 2 diabetes mellitus. J Diabetes Investig pp. 1–9. [DOI] [PMC free article] [PubMed]
- Jo W, Endo M, Ishizu K, Nakamura A and Tajima T (2011). A novel PAX4 mutation in a Japanese patient with maturity-onset diabetes of the young. The Tohoku J Exp Med 223(2), pp. 113–118. [DOI] [PubMed] [Google Scholar]
- John AN, Ram R and Jiang F (2018). RNA-Seq Analysis of Islets to Characterise the Dedifferentiation in Type 2 Diabetes Model Mice db / db. Endocr Pathol September;29(3):207–221. [DOI] [PubMed] [Google Scholar]
- Jonas J, Sharma A, Ilkova H, Patanè G, Laybutt R, Bonner-weir S, Weir C, Hasenkamp W, Patane G and Weir GC (1999). Chronic Hyperglycemia Triggers Loss of Pancreatic Beta Cell Differentiation in an Animal Model of Diabetes. J Biol Chem 274(20), pp. 14112–14121. [DOI] [PubMed] [Google Scholar]
- Katsarou A, Gudbjörnsdottir S, Rawshani A, Da belea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA and Lernmark A (2017). Type 1 diabetes mellitus. Nat Rev Dis Primers 3, pp. 1–18. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM and Diabetes Prevention Program Research Group (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346(6), pp. 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire K, Thorrez L and Schuit F (2016). Disallowed and Allowed Gene Expression : Two Faces of Mature Islet Beta Cells. Annu Rev Nutr, 36, pp. 45–71. [DOI] [PubMed] [Google Scholar]
- Li X, Shu Y, Xiang AH, Trigo E, Kuusisto J, Hartiala J, Swift AJ, Kawakubo M, Stringham HM, Bonnycastle LL, Lawrence JM, Laakso M, Allayee H, Buchanan TA and Watanabe RM (2009). Additive Effects of Genetic Variation in GCK and G6PC2 on Insulin Secretion and Fasting Glucose. Diabetes December; 58(12): 2946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Hu Q, Li C, Xing Z, Ma G, Wang C, Li J, Ye Y, Yao J, Liang K, Wang S, Park PK, Marks JR, Zhou Y, Zhou J, Hung MC, Liang H, Hu Z, Shen H, Hawke DH, Han L, Zhou Y, Lin C and Yang L (2017). The LINK-A lncRNA interacts with PtdIns(3,4,5)P3to hyperactivate AKT and confer resistance to AKT inhibitors. Nat Cell Biol, 19(3), pp. 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marselli L, Thorne J, Dahiya S, Sgroi DC, Sharma A, Bonner-Weir S, Marchetti P and Weir GC (2010). Gene expression profiles of beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS ONE July 13;5(7):e11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef SJ, Li X, Schiesser JV, Hirst CE, Yu QC, Lim SM, Nostro MC, Elliott D. a, Sarangi F, Harrison LC, Keller G, Elefanty a G. and Stanley EG (2012). INS(GFP/w) human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia, 55(3), pp. 694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Chesi A, Cousminer DL, Hawa MI, Bradfield JP, Hodge KM, Guy VC, Hakonarson H, Mauricio D, Schloot NC, Yderstræde KB, Voight BF, Schwartz S, Boehm BO, Leslie RD, Grant SFA, Kalkwarf HJ, Lappe JM, Gilsanz V, Oberfield SE, Shepherd JA, Kelly A and Zemel BS (2017). Relative contribution of type 1 and type 2 diabetes loci to the genetic etiology of adult-onset, non-insulin-requiring autoimmune diabetes. BMC Med 15(1), p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro MJ, Dharmadhikari G, Grün D, Groen N, Dielen T, Jansen E, van Gurp L, Engelse MA, Carlotti F, de Koning EJP and van Oudenaarden A (2016). A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Syst, 3(4), pp. 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard SB, Lund NS, Larsen A, Pedersen N, Rungby J and Smidt K (2015). Exogenous metallothionein potentiates the insulin response at normal glucose concentrations in INS-1E Beta-Cells without disturbing intracellular ZnT8 expression. Basic Clin Pharmacol Toxicol 116(2), pp. 173–177. [DOI] [PubMed] [Google Scholar]
- Nyunt O, Wu JY, McGown IN, Harris M, Huynh T, Leong GM, Cowley DM and Cotterill AM (2009). Investigating maturity onset diabetes of the young. Clin Biochem Rev 30(2), pp. 67–74. [PMC free article] [PubMed] [Google Scholar]
- Olsson AH, Yang BT, Hall E, Taneera J, Salehi A, Nitert MD and Ling C (2011) ‘Decreased expression of genes involved in oxidative phosphorylation in human pancreatic islets from patients with type 2 diabetes’, Eur J Endocrinol 165(4), pp. 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen KR (2018). Monogenic diabetes in adults : what are the new developments?. Curr Opin Genet Dev 2018 June;50:103–110. [DOI] [PubMed] [Google Scholar]
- Petra D, Stepanka P, Zdenek S, Stanislava K, Barbora O, Ondrej C and Jan L (2011). HNF1A mutation presenting with fetal macrosomia and hypoglycemia in childhood prior to onset of overt diabetes. J Pediatr Endocrinol Metab 2011;24(5–6):377–9. [PubMed] [Google Scholar]
- Piccand J, Strasser P, Hodson DJ, Meunier A, Ye T, Keime C, Birling MC, Rutter GA and Gradwohl G (2014). Rfx6 Maintains the Functional Identity of Adult Pancreatic β Cells. Cell Rep 9(6), pp. 2219–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D and Fontés G (2011). Glucolipotoxicity of the Pancreatic Beta Cell. BBA 1801(3), pp. 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky KS (2012).The past 200 years in diabetes. N Engl J Med 367(14), pp. 1332–40. [DOI] [PubMed] [Google Scholar]
- Pontoglio M, Sreenan S, Roe M, Pugh W, Ostrega D, Doyen a, Pick a J., Baldwin a, Velho G, Froguel P, Levisetti M, Bonner-Weir S, Bell GI, Yaniv M and Polonsky KS (1998). Defective insulin secretion in hepatocyte nuclear factor 1alpha-deficient mice. J Clin Invest 101(10), pp. 2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen TJ, Khan AM, Barton G, Butcher SA, Rutter GA, (2010). Identification of genes selectively disallowed in the pancreatic islet March-April; 2(2):89–95. [DOI] [PubMed] [Google Scholar]
- Sachdeva MM, Claiborn KC, Khoo C, Yang J, Groff DN, Mirmira RG and Stoffers DA (2009). Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. PNAS, 106(45), pp. 19090–19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfmann R and Pechberty S (2014). Development of a conditionally immortalized human pancreatic β cell line. J Clin Invest 124(5), pp. 2087–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servitja J-M, Pignatelli M, Maestro MA, Cardalda C, Boj SF, Lozano J, Blanco E, Lafuente A, McCarthy MI, Sumoy L, Guigó R and Ferrer J (2009). Hnf1alpha (MODY3) controls tissue-specific transcriptional programs and exerts opposed effects on cell growth in pancreatic islets and liver. Mol Cell Biol 29(11), pp. 2945–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Lee K, Yang D, Evans T, Chen S, Huangfu D, Shi Z, Lee K, Yang D, Amin S, Verma N, Li QV and Zhu Z (2017). Genome Editing in hPSCs Reveals GATA6 Haploinsufficiency and a Genetic Interaction with GATA4 in Human Pancreatic development. Cell Stem Cell 2017 May 4;20(5):675–688.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelin M, Rupnik M and Cencic A (2010). Pancreatic beta cell lines and their applications in diabetes mellitus research. Altex, 27(2), pp. 105–13. [DOI] [PubMed] [Google Scholar]
- Smith SB, Gasa R, Watada H, Wang J, Griffen SC and German MS (2003). Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J Biol Chem 278(40), pp. 38254–38259. [DOI] [PubMed] [Google Scholar]
- Spijker HS, Ravelli RBG, Mommaas-Kienhuis AM, van Apeldoorn AA, Engelse MA, Zaldumbide A, Bonner-Weir S, Rabelink TJ, Hoeben RC, Clevers HC, Mummery CL, Carlotti F, de Koning EJP 2013. Conversion of mature human beta-cells into glucagon-producing alpha-cells. Diabetes 62, p. 2471–80 10 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu DE, Hughes N, Kaplan B, Stanley C. a and De León DD (2012). Novel presentations of congenital hyperinsulinism due to mutations in the MODY genes: HNF1A and HNF4A. J Clin Endocrinol Metab 97(10), pp. E2026–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stijnen P, Ramos-Molina B, O’Rahilly S and Creemers JWM (2016). PCSK1 mutations and human endocrinopathies: From obesity to gastrointestinal disorders. Endo Rev, 37(4), pp. 347–371. [DOI] [PubMed] [Google Scholar]
- Taneera J, Fadista J, Ahlqvist E, Atac D, Ottosson-Laakso E, Wollheim CB and Groop L (2014). Identification of novel genes for glucose metabolism based upon expression pattern in human islets and effect on insulin secretion and glycemia. Hum Mol Gen, 24(7), pp. 1945–1955. [DOI] [PubMed] [Google Scholar]
- Teslaa T and Teitell MA (2014). Techniques to monitor glycolysis. Methods in Enzymol, 542, pp. 91–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA (1998). Embryonic Stem Cell Lines Derived from Human Blastocysts. Science, 282(5391), pp. 1145–1147. [DOI] [PubMed] [Google Scholar]
- Tiyaboonchai A, Cardenas-Diaz FL, Ying L, Maguire JA, Sim X, Jobaliya C, Gagne AL, Kishore S, Stanescu DE, Hughes N, De Leon DD, French DL and Gadue P (2017). GATA6 Plays an Important Role in the Induction of Human Definitive Endoderm, Development of the Pancreas, and Functionality of Pancreatic β Cells. Stem Cell Rep, 8(3), pp. 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA-K, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist P-H, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J and Ponten F (2015). Tissue-based map of the human proteome’, Science, 347(6220), pp. 1260419–1260419. [DOI] [PubMed] [Google Scholar]
- Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll S. a, Langenberg C, Hofmann OM, Dupuis J, Qi L, Segrè AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Bengtsson Boström, et al. (2010. ). Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis.’, Nat genet, 42(7), pp. 579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Antinozzi P. a, Hagenfeldt K. a, Maechler P and Wollheim CB (2000). Molecular targets of a human HNF1 alpha mutation responsible for pancreatic beta-cell dysfunction. EMBO J, 19(16), pp. 4257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Maechler P, Hagenfeldt K. a and Wollheim CB (1998). Dominant-negative suppression of HNF-1alpha function results in defective insulin gene transcription and impaired metabolism-secretion coupling in a pancreatic beta-cell line. EMBO J, 17(22), pp. 6701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Elghazi L, Martin S, Martins I, Srinivasan RS, Geng X, Sleeman M, Collombat P, Houghton J and Sosa-Pineda B (2008). Ghrelin is a novel target of Pax4 in endocrine progenitors of the pancreas and duodenum. Dev Dynamics, 237(1), pp. 51–61. [DOI] [PubMed] [Google Scholar]
- Wobser H, Düssmann H, Kögel D, Wang H, Reim ertz C, Wollheim CB, Byrne MM and Prehn JHM (2002). Dominant-negative suppression of HNF-1 alpha results in mitochondrial dysfunction, INS-1 cell apoptosis, and increased sensitivity to ceramide-, but not to high glucose-induced cell death. J Biol Chem 277(8) pp. 6413–21. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, Signorini S, Stoffel M, Bell GI (1996). Mutations in the hepatocyte nuclear factor-4a gene in maturity-onset diabetes of the young (MODY1). Nature 1996 December 5;384(6608):458–60. [DOI] [PubMed] [Google Scholar]
- Ye W, Zheng Y, Zhang S, Yan L, Cheng H and Wu M (2016). Oxamate improves glycemic control and insulin sensitivity via inhibition of tissue lactate production in db/db mice. PLoS ONE, 11(3), pp. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Guo M, Zhou T, Tan L, Chong CN, Zhang T, Dong X, Xiang JZ, Yu AS, Yue L, Qi Q, Evans T, Graumann J and Chen S (2016). An Isogenic Human ESC Platform for Functional Evaluation of Genome-wide-Association-Study-Identified Diabetes Genes and Drug Discovery. Cell Stem Cell 19(3), pp. 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB and Lowell BB (2001). Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell, 105(6), pp. 745–755. [DOI] [PubMed] [Google Scholar]
- Zhu Z, González F and Huangfu D (2014). The i CRISPR platform for rapid genome editing in human pluripotent stem cells. Methods Enzymol 2014;546:215–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito E, Chin KT, Blais J, Harding HP and Ron D (2010). ERO1-β, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cel Bio, 188(6), pp. 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.